Abstract
Background.
From 1988 to 1999, the Radiation Therapy Oncology Group (RTOG) conducted four prospective studies (8802, 8903, 9506, 9706) of patients with clinical stage T2–4a muscle-invasive bladder cancer. Treatment was selective bladder preservation using transurethral surgery (TURBT) plus cisplatin-based induction and consolidation chemoradiation regimens, reserving radical cystectomy for invasive tumor recurrence. We investigated vascular endothelial growth factor (VEGF) pathway biomarkers in this unique clinical dataset (median follow-up of 3.1 years).
Methods.
A total of 43 patients with tissue available from the entry TURBT were included in this analysis. Expression of VEGF ligands and receptors were quantified and scored by the AQUA platform (HistoRX, now Genoptix, Carlsbad, CA) and analyzed after median split.
Results.
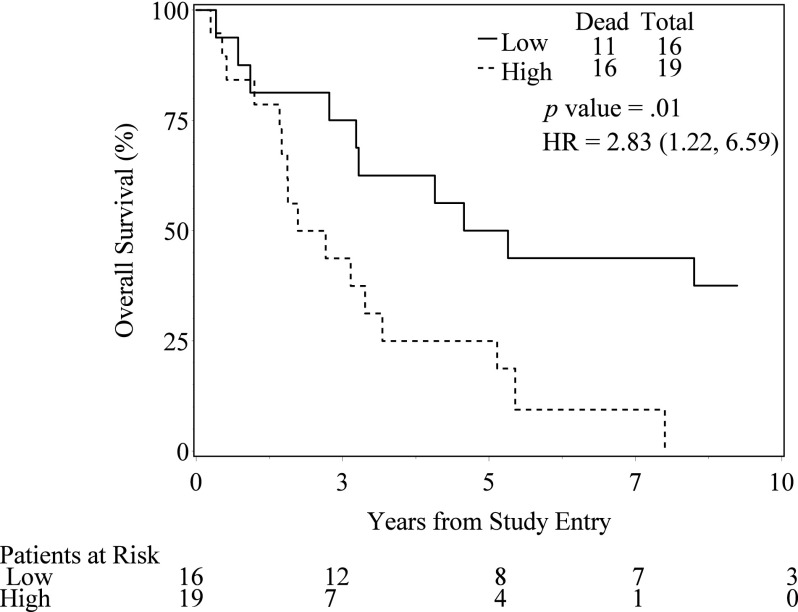
VEGF expression levels were not associated with increased rates of complete response to induction chemoradiation. Higher levels of cytoplasmic VEGF-B, VEGF-C, and VEGF-R2 were associated with decreased overall survival rates. The 3-year overall survival estimates for high and low expressers were 43.7% and 75% for VEGF-B cytoplasm (p = .01), 40.2% and 86.7% for VEGF-C cytoplasm (p = .01), and 49.7% and 66.7% for VEGF-R2 cytoplasm (p = .02). Higher expression levels of cytoplasm VEGF-B were associated with higher rates of distant failure (p = .01).
Conclusions.
Although VEGF ligands and receptors do not appear to be associated with complete response to induction chemoradiation for muscle-invasive bladder cancer, we report significant associations with overall survival and distant failure for certain VEGF family members.
Discussion
Tumor angiogenesis underlies the pathogenesis of many malignancies. The proangiogenic VEGF is a key molecule in the tumor angiogenesis pathway. Prior studies have shown that the deregulation of several angiogenic molecules influences urothelial carcinogenesis and that VEGF is implicated in bladder cancer recurrence. Our finding of the associations of VEGF-B expression with distant failure and overall survival is consistent with previous reports describing overexpression of VEGF-B in lung adenocarcinoma brain metastases tissue. Patients with overexpression of VEGF-B may benefit from the addition of anti-VEGF agents or other systemic therapies to their therapeutic regimens to reduce the risk of distant metastasis and to improve survival. The majority of downstream angiogenic effects of VEGF—including endothelial cell proliferation, invasion, and migration—are mediated by VEGFR-2. Therefore, it is not surprising that we also found VEGF-R2 to be associated with reduced overall survival rates. Consistent with previous reports suggesting that VEGF-C expression is associated with stage, grade, tumor size, lymph node metastasis, and worse overall prognosis, we identified VEGF-C to be associated with overall survival in our bladder cancer cohort who were managed with selective bladder preservation.
Figure 1.
Overall Survival by VEGF-B Expression.
In addition to the retrospective nature of this study, several limiting factors should be considered when interpreting the results. Although tissue from only about 15% of patients enrolled in the four RTOG trials was available, pretreatment characteristics and all other outcomes were similar between patients for whom tissue was available and those who did not have tissue available. Given the unique nature of our patient cohort, we were not able to include an external validation set to confirm our results. Due to limited sample size, only univariate analysis was performed and reported p values were accordingly not adjusted for multiple testing.
In summary, VEGF biomarkers did not predict for chemoradiation sensitivity for patients undergoing bladder preservation. However, high VEGF-B expression was associated with increased rates of distant failure and poor overall survival. VEGF-C and VEGF-R2 were associated with poor overall survival. Thus, the VEGF-B, -C, and -R2 markers appear to identify patients with particularly good or bad outcomes. VEGF-B might be a predictive factor for distant failure and could become a valued biomarker to encourage early systemic therapy. However, confirmation of these results from a larger prospective trial is needed. Furthermore, VEGF markers appear to define a patient subset, which might benefit from formal evaluation of anti-VEGF molecular targeted therapies, such as monoclonal antibodies or receptor tyrosine kinase inhibitors in combination with early systemic cytotoxic therapy.
Supplementary Material
Acknowledgments
This trial was conducted by the Radiation Therapy Oncology Group (RTOG), and was supported by RTOG grant U10 CA21661 and CCOP grant U10 CA37422 from the National Cancer Institute (NCI). The publication was supported by the 2010 Pennsylvania Department of Health Formula Grant 4100054841 (to AG), and funds from the Ohio State University Comprehensive Cancer Center (to AC). This manuscript's contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute. We thank Vaia Dedousi-Huebner for writing and editorial assistance.
Footnotes
ClinicalTrials.gov Identifier: NA Sponsor(s): NA
Principal Investigator: NA IRB Approved: Yes
Disclosures
Author disclosures available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.