Abstract
Background.
Prostate cancer (PC) is the most commonly diagnosed noncutaneous malignancy in American men. PC, which exhibits a slow growth rate and multiple potential target epitopes, is an ideal candidate for immunotherapy. GVAX for prostate cancer is a cellular immunotherapy, composed of PC-3 cells (CG1940) and LNCaP cells (CG8711). Each of the components is a prostate adenocarcinoma cell line that has been genetically modified to secrete granulocyte-macrophage colony-stimulating factor. Hypothesizing that GVAX for prostate cancer could be effective in a neoadjuvant setting in patients with locally advanced disease, we initiated a phase II trial of neoadjuvant docetaxel and GVAX. For the trial, the clinical effects of GVAX were assessed in patients undergoing radical prostatectomy (RP).
Methods.
Patients received docetaxel administered at a dose of 75 mg/m2 every 3 weeks for 4 cycles. GVAX was administered 2–3 days after chemotherapy preoperatively for four courses of immunotherapy. The first dose of GVAX was a prime immunotherapy of 5×108 cells. The subsequent boost immunotherapies consisted of 3×108 cells. After RP, patients received an additional six courses of immunotherapy. Pathologic complete response, toxicity, and clinical response were assessed. The primary endpoint of the trial was a pathologic state of pT0, which is defined as no evidence of cancer in the prostate.
Results.
Six patients completed neoadjuvant docetaxel and GVAX therapy. No serious drug-related adverse events were observed. Median change in prostate-specific antigen (PSA) following neoadjuvant therapy was 1.47 ng/ml. One patient did not undergo RP due to the discovery of positive lymph nodes during exploration. Of the five patients completing RP, four had a downstaging of their Gleason score. Undetectable PSA was achieved in three patients at 2 months after RP and in two patients at 3 years after RP.
Conclusions.
Neoadjuvant docetaxel/GVAX is safe and well tolerated in patients with high-risk locally advanced PC. No evidence of increased intraoperative hemorrhage or increased length of hospital stay postoperatively was noted. These results justify further study of neoadjuvant immunotherapy.
Discussion
Through prostate-specific antigen (PSA) screening, there has been a shift in detection towards earlier stage prostate cancer (PC) over the past 15 years [1, 2]. Despite this change, 15% of patients still present with locally advanced disease, of whom 50% will develop biochemical failure within 10 years [3, 4]. Based on phase III trials with docetaxel [5] and phase II trials with GVAX [6], we initiated a phase II trial evaluating neoadjuvant docetaxel/GVAX in patients with high-risk locally advanced PC.
In this study, docetaxel and GVAX therapies were well-tolerated. No grade 3 or 4 toxicities were identified. All six patients completed therapy. Neoadjuvant docetaxel/GVAX did not affect surgical therapy as evidenced by low intraoperative blood loss, a short median length of hospital stay, and the absence of intraoperative complications. During the study, an interim analysis of a separate GVAX-related protocol raised potential safety concerns. Thus, based on an Independent Data Monitoring Committee recommendation, our study was terminated. Six patients completed the trial and here we report their outcomes.
One patient was found to have regional metastatic disease intraoperatively and RP was aborted. Of the 5 patients completing radical prostatectomy (RP), three patients had an undetectable PSA 2 months postoperatively. Without receiving adjuvant or salvage therapy, two patients had no evidence of disease 3 years postoperatively. Although no pathological complete responses were obtained, four of the six patients had a downstaging in their Gleason sum by pathologic review of their RP specimen compared with their biopsy specimen.
Figure 1.
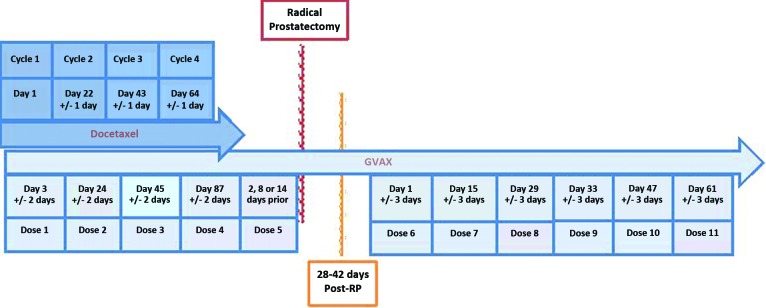
Study Schema.
The absence of a pT0 in this study is similar to those of other neoadjuvant studies evaluating docetaxel, gefitinib, and mitoxantrone [7–10]. Some suggest that absence of pT0 in a patient is due to the fact that the optimal dose and duration of neoadjuvant agents remain to be optimized [10]. Prior studies evaluating neoadjuvant hormonal ablation prior to RP demonstrated significant pathologic downstaging yet failed to demonstrate meaningful improvement in biochemical recurrence (BCR) [11]. Taken together, these results suggest that achieving pT0 status and remaining disease free after RP may be unrelated. It is clear that neoadjuvant therapy can affect tumor biology, but ultimately the benefit of neoadjuvant therapy must be judged by disease-free survival or its surrogate, biochemical recurrence-free survival.
These results must be interpreted within the limitations of our study. Our cohort is small and is not powered to make conclusions with respect to pathologic response and tolerance of therapy. However, these patients underwent nonstandard therapy and the safe results from this study provide some evidence that GVAX for PC, or similar agents, could be evaluated in a larger cohort. Moreover, the approval of the immunotherapy vaccine sipuleucel-T by the U.S. Food and Drug Administration [12] and a recent study demonstrating enhanced survival in patients with relapsing acute myeloid leukemia treated with GVAX [13] should encourage the re-evaluation of immune therapy for patients with PC.
Supplementary Material
Footnotes
ClinicalTrials.gov Identifier: NCT00089856 Sponsor: Cell Genesys
Principal Investigator: Jacqueline Vuky IRB Approved: Yes
Disclosures
Author disclosures available online.
Reference
- 1.Catalona WJ, Richie JP, Ahmann FR, et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: Results of a multicenter clinical trial of 6,630 men. J Urol. 1994;151:1283–1290. doi: 10.1016/s0022-5347(17)35233-3. [DOI] [PubMed] [Google Scholar]
- 2.Soh S, Kattan MW, Berkman S, et al. Has there been a recent shift in the pathological features and prognosis of patients treated with radical prostatectomy? J Urol. 1997;157:2212–2218. [PubMed] [Google Scholar]
- 3.Partin AW, Yoo J, Carter HB, et al. The use of prostate specific antigen, clinical stage and Gleason score to predict pathological stage in men with localized prostate cancer. J Urol. 1993;150:110–114. doi: 10.1016/s0022-5347(17)35410-1. [DOI] [PubMed] [Google Scholar]
- 4.Catalona WJ, Smith DS. Cancer recurrence and survival rates after anatomic radical retropubic prostatectomy for prostate cancer: Intermediate-term results. J Urol. 1998;160:2428–2434. doi: 10.1097/00005392-199812020-00012. [DOI] [PubMed] [Google Scholar]
- 5.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 6.Higano CS, Corman JM, Smith DC, et al. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer. 2008;113:975–984. doi: 10.1002/cncr.23669. [DOI] [PubMed] [Google Scholar]
- 7.Beer TM, Garzotto M, Lowe BA, et al. Phase I study of weekly mitoxantrone and docetaxel before prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res. 2004;10:1306–1311. doi: 10.1158/1078-0432.ccr-1021-03. [DOI] [PubMed] [Google Scholar]
- 8.Febbo PG, Richie JP, George DJ, et al. Neoadjuvant docetaxel before radical prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res. 2005;11:5233–5240. doi: 10.1158/1078-0432.CCR-05-0299. [DOI] [PubMed] [Google Scholar]
- 9.Dreicer R, Magi-Galluzzi C, Zhou M, et al. Phase II trial of neoadjuvant docetaxel before radical prostatectomy for locally advanced prostate cancer. Urology. 2004;63:1138–1142. doi: 10.1016/j.urology.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 10.Vuky J, Porter C, Isacson C, et al. Phase II trial of neoadjuvant docetaxel and gefitinib followed by radical prostatectomy in patients with high-risk, locally advanced prostate cancer. Cancer. 2009;115:784–791. doi: 10.1002/cncr.24092. [DOI] [PubMed] [Google Scholar]
- 11.Klotz LH, Goldenberg SL, Jewett MA, et al. Long-term followup of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. J Urol. 2003;170:791–794. doi: 10.1097/01.ju.0000081404.98273.fd. [DOI] [PubMed] [Google Scholar]
- 12.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 13.Borrello IM, Levitsky HI, Stock W, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting cellular immunotherapy in combination with autologous stem cell transplantation (ASCT) as postremission therapy for acute myeloid leukemia (AML) Blood. 2009;114:1736–1745. doi: 10.1182/blood-2009-02-205278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


