This systematic review examines the clinical effectiveness and cost-effectiveness of the use of adjuvant imatinib mesylate for treating patients with localized primary gastrointestinal stromal tumors (GISTs). Adjuvant imatinib was found to be more cost-effective for treating localized primary GISTs than surgery alone. In addition, 3 years of adjuvant imatinib may be more cost-effective than 1 year of adjuvant therapy.
Keywords: Gastrointestinal stromal tumor, Imatinib, Adjuvant, Economics, Cost-effectiveness
Abstract
Objective.
This article presents the clinical effectiveness and cost-effectiveness of the use of adjuvant imatinib mesylate for treating patients with localized primary gastrointestinal stromal tumors (GISTs) and discusses the impact of prolonged treatment with adjuvant imatinib on health care costs.
Methods.
A systematic review of the medical literature was conducted to explore recently reported clinical trials demonstrating the clinical benefit of adjuvant imatinib in GISTs, along with analyses discussing the economic impact of adjuvant imatinib.
Results.
Two phase III trials have demonstrated a significant clinical benefit of adjuvant imatinib treatment in GIST patients at risk of recurrence after tumor resection. Guidelines now suggest adjuvant treatment for at least 3 years in patients at high risk of recurrence. Despite this clinical effectiveness, prolonged use of adjuvant imatinib can lead to an increase in the risk for adverse events and to increased costs for both patients and health care systems. However, the increased cost is partially offset by cost reductions associated with delayed or avoided GIST recurrences. Three years of adjuvant treatment in high-risk patients was concluded to be cost-effective. Therefore, the careful selection of patients who are most likely to benefit from treatment can lead to improved clinical outcomes and significant cost savings.
Conclusion.
Although introducing adjuvant imatinib has an economic impact on health plans, this effect seems to be limited. Several analyses have demonstrated that adjuvant imatinib is more cost-effective for treating localized primary GISTs than surgery alone. In addition, 3 years of adjuvant imatinib is more cost-effective than 1 year of adjuvant therapy.
Implications for Practice:
Results from two phase III randomized trials with adjuvant imatinib in gastrointestinal stromal tumors (GISTs) have a positive impact on practice guidelines. The significant relapse-free survival and overall survival benefits and the low toxicity profile associated with 3-year adjuvant imatinib, together with the apparent increase in tumor recurrence after patients had completed 3 years of therapy, served as the foundation for the National Comprehensive Cancer Network (NCCN) and ESMO recommendation of at least 3 years of adjuvant imatinib for patients with KIT-positive GISTs at high risk of recurrence after surgery. Moreover, 3 years of adjuvant imatinib is more cost-effective than 1 year of adjuvant therapy. The adjuvant imatinib should not be used for patients with imatinib-resistant tumor genotypes (e.g., PDGFRAD842V mutation found mainly in gastric GISTs), even for patients with high-risk GISTs.
Introduction
Imatinib mesylate, an oral tyrosine kinase inhibitor of KIT and platelet-derived growth factor receptor-α, is the standard of care in the first-line management of unresectable and/or metastatic gastrointestinal stromal tumors (GISTs) [1, 2]. For patients with primary localized GISTs, the standard curative treatment is surgery [1, 2]. However, for patients treated surgically–even with complete resection—the rate of recurrence may be as high as 33% at 5 years [3, 4]. This recurrence is associated with substantial medical care costs. On the basis of data from the Surveillance, Epidemiology, and End Results cancer registry (1993–2002), Rubin et al. estimated that GIST recurrence was associated with an additional $101,700 for the cost of care over the 5-year period after initial resection [5]. Therefore, treatments to delay or reduce recurrence could lead to substantial reductions in the disease burden [5].
Two phase III trials have demonstrated a significant clinical benefit of adjuvant imatinib treatment for patients with GISTs who are at risk of recurrence after tumor resection. In the phase III American College of Surgeons Oncology Group (ACOSOG) Intergroup Z9001 study, adjuvant imatinib was shown to significantly delay recurrence compared with placebo when given for a year after surgical resection [6]. In addition, the phase III trial by the Scandinavian Sarcoma Group and the Sarcoma Group of the Arbeitsgemeinschaft Internistische Onkologie (SSGXVIII/AIO) demonstrated that, in patients with a high risk of GIST recurrence, adjuvant imatinib treatment for 3 years had greater clinical benefit than treatment for 1 year—not only delaying recurrences but also improving overall survival of patients [7]. The National Comprehensive Cancer Network (NCCN) guidelines now recommend adjuvant imatinib for at least 3 years for patients with high-risk GISTs [8].
Pharmacoeconomic studies have demonstrated the cost-effectiveness of first-line imatinib for the treatment of patients with advanced metastatic GISTs [9]. On the basis of the proven clinical effectiveness of adjuvant imatinib therapy for GISTs from the two phase III trials mentioned previously, pharmacoeconomic models of imatinib in the adjuvant setting also have been developed. Results from these pharmacoeconomic analyses generally support the belief that adjuvant imatinib is cost-effective and increases the life expectancy of patients with GISTs [10–13].
This review summarizes the clinical studies supporting adjuvant imatinib for GISTs, provides an overview of the cost-effectiveness analyses of adjuvant imatinib, and discusses the possible impact of these results on criteria for selecting patients for adjuvant treatment.
Clinical Effectiveness of Adjuvant Imatinib for GISTs
The efficacy of adjuvant imatinib for the treatment of patients with GISTs following surgical resection was first demonstrated in the phase III ACOSOG Z9001 study. This was a double-blind, randomized, placebo-controlled study in patients with a histological diagnosis of KIT-positive, localized primary GISTs measuring at least 3 cm [6]. After complete resection of the tumor, 1 year of adjuvant imatinib was shown to significantly improve recurrence-free survival (RFS) rates compared with placebo (98% vs. 83%, hazard ratio [HR]: 0.35, 95% confidence interval [CI]: 0.22–0.53; p < .0001) [6]. In addition, a statistically significant clinical benefit in RFS was seen for patients at high risk of recurrence (tumor size ≥10 cm) [6].
A subgroup analysis of this trial demonstrated that tumor mutation status can predict response to imatinib [14]. A significant impact of adjuvant therapy was seen in the high-risk group of patients with exon 11 KIT mutations, with negligible impact in patients with a PDGFRA D842V mutation [14]. Results from the phase III ACOSOG Z9001 study prompted the approval of imatinib for adjuvant use in many countries [15–17]. However, although adjuvant imatinib was effective in preventing recurrence while patients were on treatment, tumor recurrences sharply increased 6 months after completion of therapy, suggesting 1 year of adjuvant imatinib may not be sufficient [6].
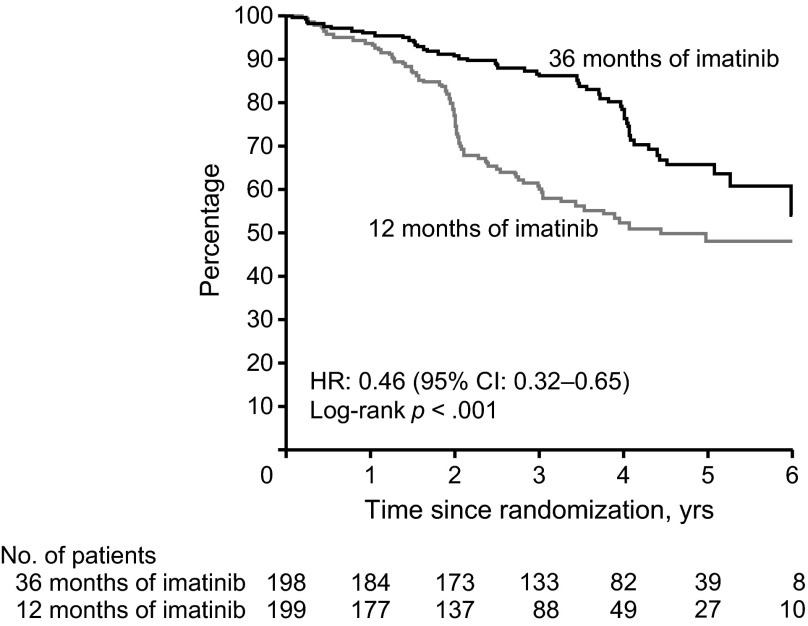
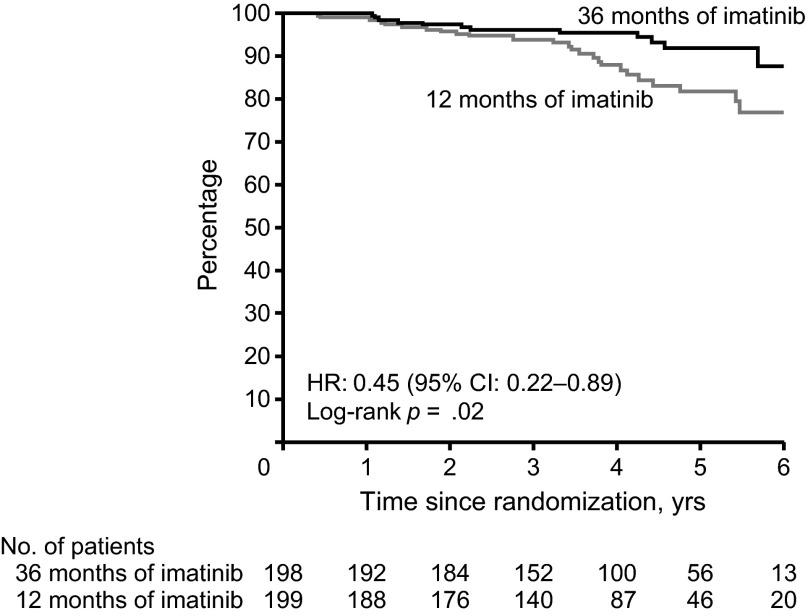
The recent SSGXVIII/AIO trial prospectively evaluated the impact of a longer duration of treatment, comparing 3 years versus 1 year of adjuvant imatinib therapy in patients who had a high risk for tumor recurrence after surgery, according to the U.S. National Institutes of Health (NIH) consensus criteria [7]. The trial demonstrated that, compared with 1 year of adjuvant imatinib, 3 years of therapy significantly improved RFS rates (5-year RFS: 47.9% vs. 65.6%, HR: 0.46, 95% CI: 0.32–0.65; p < .001; Fig. 1) and overall survival (OS) rates (5-year OS: 81.7% vs. 92.0%, HR: 0.45, 95% CI: 0.22–0.89; p = .02; Fig. 2) [7]. It is important to note that in this trial very few patients relapsed while on adjuvant therapy [7]. Tumor recurrences apparently increased after patients stopped adjuvant imatinib, even in the 3-year therapy group, suggesting that 3 years of treatment may not be enough in some patients. Durations longer than 3 years should be considered for patients with a high risk of recurrence.
Figure 1.
Recurrence-free survival for 3 years versus 1 year of adjuvant imatinib therapy from the phase III trial by the Scandinavian Sarcoma Group and the Sarcoma Group of the Arbeitsgemeinschaft Internistische Onkologie. Reprinted from [22] with permission from the American Medical Association.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Figure 2.
Overall survival for 3 years versus 1 year of adjuvant imatinib therapy from the phase III trial by the Scandinavian Sarcoma Group and the Sarcoma Group of the Arbeitsgemeinschaft Internistische Onkologie. Reprinted from [22] with permission from the American Medical Association.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Furthermore, imatinib was generally well-tolerated [7]. Almost all patients experienced at least one adverse event during the SSGXVIII/AIO study, but most of these events were mild in severity (Table 1) [7]. Patients on 3-year adjuvant imatinib experienced more grade 3 or 4 adverse events than those on 1-year adjuvant imatinib (32.8% vs. 20.1%, respectively) [7]. In addition, more patients discontinued imatinib in the 3-year group than in the 1-year group for a reason other than GIST recurrence (25.8% vs. 12.6%, respectively), most commonly due to adverse events (13.6% vs. 7.5%, respectively) [7]. The incidence of cardiac adverse events (2.0% vs. 4.1%, respectively) or second cancer (6.6% vs. 7.2%, respectively) was low and comparable in both groups [7].
Table 1.
Adverse events occurring in >30% of patients
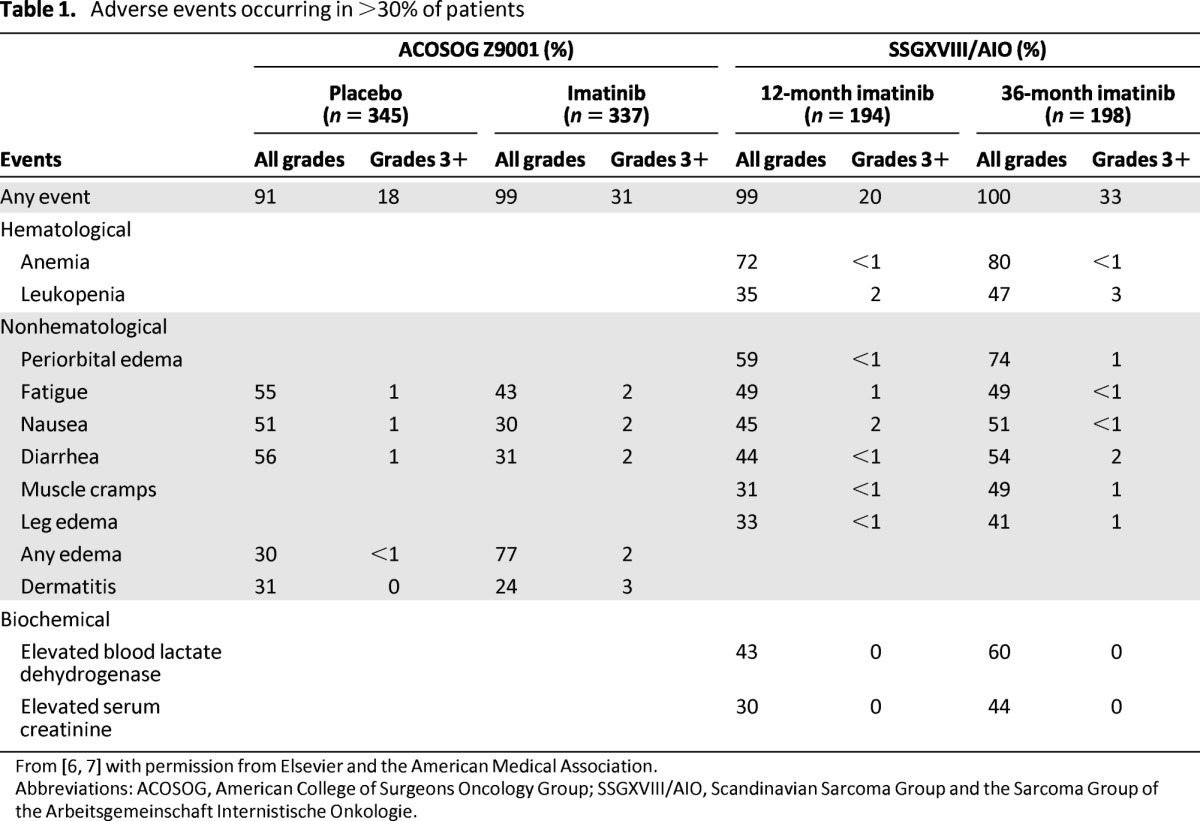
Similar safety profiles were reported in the ACOSOG Z9001 and SSGXVIII/AIO trials (Table 1) [6, 7]. In the SSGXVIII/AIO trial, a higher frequency of recorded grade 3 or 4 adverse events and a higher discontinuation rate in the 3-year group were expected, given that these patients had 3 times longer imatinib exposure compared with patients in the 1-year group. In addition, when compared with the incidence rate of grade 3 or 4 events reported in the ACOSOG Z9001 trial for patients on placebo (18%) or 1-year imatinib treatment (31%) [6], grade 3 or 4 adverse events with 3-year adjuvant imatinib therapy (33%) were infrequent overall in the SSGXVIII/AIO trial [7]. Discretion is warranted for this comparison because of the differences in study design and patient populations of these two trials.
Results from the SSGXVIII/AIO trial have had a positive impact on practice guidelines. The significant RFS and OS benefits and the low toxicity profile associated with 3-year adjuvant imatinib, together with the apparent increase in tumor recurrence after patients had completed 3 years of therapy [7], served as the foundation for the NCCN recommendation of at least 3 years of adjuvant imatinib for patients with KIT-positive GISTs at high risk of recurrence after surgery [8]. In the United States and Europe, imatinib is indicated for the adjuvant treatment of patients following complete resection of KIT-positive GISTs [15–17]. However, the optimal treatment duration is still unknown [15]. In line with the NCCN recommendation, the approval of imatinib for adjuvant treatment in Europe is also for patients with a significant risk of recurrence after tumor resection [17]. The European guidelines also state that patients at low or very low risk of recurrence should not be prescribed adjuvant imatinib because the benefits of this therapy have not been established for this patient population [17].
The clinical effectiveness of adjuvant imatinib in the treatment of patients with surgically resected localized GISTs also is confirmed by the relatively low number of patients needed to treat (NNT) to prevent one recurrence or death [18–20]. On the basis of results from the ACOSOG Z9001 study, it was estimated that, after 1 year of treatment, seven patients would need to be treated with adjuvant imatinib to avoid one recurrence or death [18]. In a follow-up analysis using the results from the SSGXVIII/AIO trial, the NNT was shown to decrease with longer follow-up; the NNT decreased to four when patients receive 3 years of adjuvant imatinib treatment [20]. In relation to other adjuvant cancer therapies, the effectiveness of imatinib in this setting is apparent. As a comparison, the NNTs for other adjuvant cancer therapies when given for 1 year were as follows: NNT of 100 for trastuzumab in breast cancer; NNT of 20 for cisplatin with 5-fluorouracil in gastric cancer [18].
Despite adjuvant imatinib therapy, patients may still develop recurrent disease, either while on or after completing treatment, which may be indicative of a different pathogenesis—primary or secondary resistance, respectively. In the case of recurrence during therapy, imatinib dose increase or second-line therapy may be warranted. For recurrence after completion of therapy, restarting imatinib at the standard dose may be effective. A subgroup analysis of the SSGXVIII/AIO trial recently demonstrated that most patients who received prior adjuvant imatinib treatment respond to imatinib rechallenge for treating recurrence [21]. Of the 46 evaluable patients who were treated with imatinib for recurring GISTs, 32.6% achieved a complete response. This rate is very high and may be related to close follow-up of patients after stopping imatinib therapy and early detection of recurrence with low tumor burden. Furthermore, 30.4% achieved a partial response and 21.7% had stable disease, yielding a clinical benefit rate (CBR) of 84.8% [21]. The CBR was similar between patients assigned to the 1-year and 3-year groups (87.9% and 76.9%, respectively; p = .385) [21].
Economic Value of Adjuvant Imatinib for GISTs
A number of publications have described the cost-effectiveness of imatinib in the adjuvant setting. Clearly, incremental prescription drug costs are observed with the benefits of adjuvant treatment of any duration (Table 2) [22]. However, the increased cost has been shown to be partially offset by the reduction in costs associated with delayed GIST recurrence. Furthermore, the overall expenditure to provide adjuvant imatinib treatment to patients with GISTs has been shown to have a limited budget impact on health plans [19, 22].
Table 2.
Associated monthly costs for gastrointestinal stromal tumors and recurrence
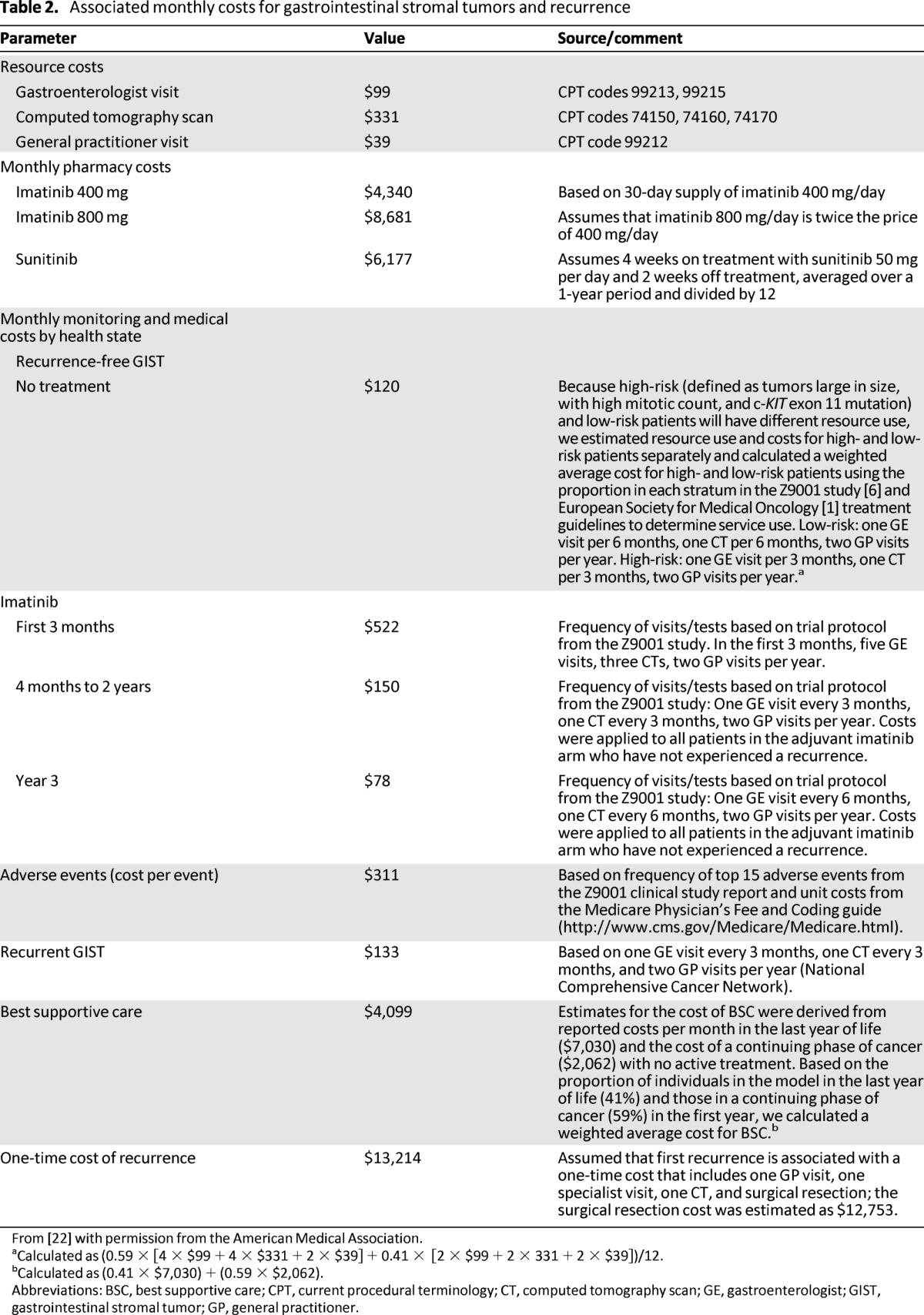
From [22] with permission from the American Medical Association.
aCalculated as (0.59 × [4 × $99 + 4 × $331 + 2 × $39] + 0.41 × [2 × $99 + 2 × 331 + 2 × $39])/12.
bCalculated as (0.41 × $7,030) + (0.59 × $2,062).
Abbreviations: BSC, best supportive care; CPT, current procedural terminology; CT, computed tomography scan; GE, gastroenterologist; GIST, gastrointestinal stromal tumor; GP, general practitioner.
On the basis of results from the ACOSOG Z9001 study, a decision analytic model estimated that approximately 12%–22% of the cost associated with adjuvant imatinib (prescription drug costs and costs of monitoring patients on adjuvant imatinib) is offset by delaying and avoiding GIST recurrences [22]. Assuming any change in the use of imatinib is in accordance with current guideline recommendations, the model predicted that the introduction of adjuvant imatinib would lead to a net budgetary impact of less than $0.01 per member per month in the third year (Table 3) [22].
Table 3.
Economic impact in the first 3 years of imatinib as adjuvant therapy for localized resected gastrointestinal stromal tumors
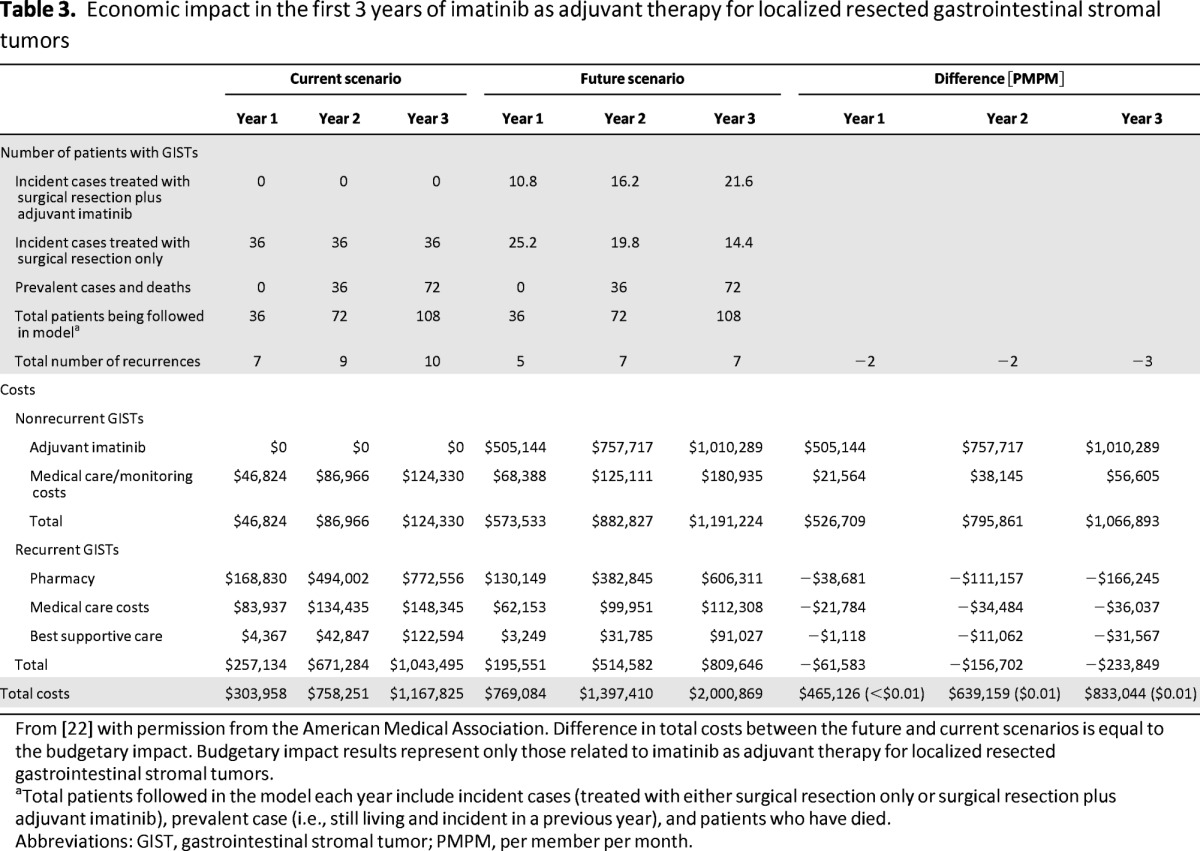
From [22] with permission from the American Medical Association. Difference in total costs between the future and current scenarios is equal to the budgetary impact. Budgetary impact results represent only those related to imatinib as adjuvant therapy for localized resected gastrointestinal stromal tumors.
aTotal patients followed in the model each year include incident cases (treated with either surgical resection only or surgical resection plus adjuvant imatinib), prevalent case (i.e., still living and incident in a previous year), and patients who have died.
Abbreviations: GIST, gastrointestinal stromal tumor; PMPM, per member per month.
The cost-effectiveness of a treatment can be assessed by determining how much the drug or treatment costs per quality-adjusted life-year (QALY) gained. A QALY is a measure of the extra years of life a person might gain as a result of treatment that is then adjusted based on the quality of those years. This type of measure is particularly important when considering treatments for chronic conditions. An economic comparison between two treatments can be performed by determining the incremental cost-effectiveness ratio (ICER), which is the cost-to-benefit ratio of two treatments, usually QALY. Several analyses have demonstrated that adjuvant imatinib is more cost-effective for treating localized primary GISTs than surgery alone, and 3 years of adjuvant imatinib is more cost-effective than 1 year of adjuvant therapy [10–13].
The cost-effectiveness of 1 year of adjuvant imatinib treatment versus surgery alone also was demonstrated in the Canadian and Russian health care systems [10, 12]. The Russian analysis concluded that 1 year of adjuvant imatinib resulted in a gain of 1.15 QALYs at the cost of €12,246, whereas the Canadian analysis concluded that adding imatinib resulted in a gain of 0.745 QALYs at an expected additional per-patient lifetime cost of $26,800 for 1 year of treatment. These analyses were based on the available results from the ACOSOG Z9001 study, which had a relatively short follow-up time (19.7 months) and did not show an OS benefit with adjuvant imatinib therapy [6].
A cost-effectiveness analysis of adjuvant imatinib therapy versus surgery alone was carried out by the manufacturer of imatinib (Novartis Pharmaceuticals Corporation, East Hanover, NJ, http://www.us.novartis.com) [23]. This analysis compared the cost per QALY gained with 3 years of adjuvant imatinib versus surgery alone. The 3-year recurrence rates were estimated based on extrapolation of the 1-year RFS from the ACOSOG Z9001 study and compared with the cost of 3 years of treatment. For patients with GISTs with intermediate to high risk of recurrence, as defined by the Miettinen and Lasota/NCCN–Armed Forces Institute of Pathology (AFIP) criteria, the study showed that the ICER for 3 years of adjuvant imatinib treatment versus surgery alone was £23,601 (∼$37,000 or ∼€30,000 based on current rates) per QALY gained [23]. The widely used cost-effectiveness threshold is $100,000/QALY gained, below which there is considered to be value associated with the intervention [24]. The National Institute for Clinical Excellence (NICE), to which this analysis was submitted for reimbursement of coverage of adjuvant imatinib in the United Kingdom, found it subject to a number of limitations: the “significant-risk” patient group was only a subset of the total population from the ACOSOG Z9001 study and was retrospectively categorized, the 3-year recurrence rates were extrapolated from the 1-year RFS rates of the ACOSOG Z9001 study, and there was no OS benefit. Because of these limitations, the guidance issued by NICE in June 2010 did not recommend imatinib as adjuvant treatment after resection of GISTs [23].
Recently, the cost-effectiveness of treating patients with 3 years versus 1 year of adjuvant imatinib was evaluated from a payer's perspective in the United States. The study addressed a number of the limitations from the previously mentioned analysis [13]. The SSGXVIII/AIO trial had a median follow-up time of 54 months and demonstrated a significant OS benefit with 3 years versus 1 year of adjuvant imatinib in a prospectively defined high-risk patient population [7]. First recurrence and mortality rates for 1- and 3-year imatinib therapy were obtained from the trial, and cost and utilities were derived from the literature. The lifetime Markov model, which was developed to reflect the natural course of the disease in surgically resected patients with high-risk GISTs, predicted RFS and OS rates comparable to those reported in the SSGXVIII/AIO trial. The model predicted the total lifetime cost per patient with GIST was $302,100 with 3 years of adjuvant imatinib therapy versus $217,800 with 1 year of therapy [13]. In addition, patients on 3 years of adjuvant imatinib had higher QALYs versus those on 1 year of therapy (8.53 vs. 7.18 per patient, respectively). As a result, the incremental cost-effectiveness ratio of 3 years versus 1 year of adjuvant imatinib was $62,600 per QALY gained. At a $100,000/QALY threshold, 3 years of adjuvant imatinib was 100% cost-effective when compared with 1 year of adjuvant imatinib [13].
Although many patients experienced adverse events in the SSGXVIII/AIO trial, imatinib was generally well-tolerated and most of the events were mild in severity. Despite a higher incidence of grade 3 or 4 adverse events with the longer duration of treatment, there were no unexpected adverse events seen in the 3-year adjuvant treatment group [7]. This result is not surprising, and the increased medical care cost for monitoring and treating grade 3 or 4 adverse events is likely to be offset by the reduced cost for treating recurrence.
Patient Selection for Adjuvant Imatinib for GISTs
In clinical practice, a number of factors need to be considered when making a recommendation to start patients on adjuvant imatinib treatment. Currently, imatinib is given to most patients with advanced or metastatic GISTs, as well as those with complete surgical resection who are at significant risk of recurrence. To ensure rational use of adjuvant imatinib and to forego both reasonably avoidable costs and adverse events (AEs), careful patient selection is important.
As noted previously, recurrent disease develops in many patients even after complete resection [3, 4]. Accurate prediction of a patient's risk of recurrence may help identify those patients for adjuvant imatinib therapy. A number of risk stratification schemes have been developed: the U.S. NIH consensus criteria, Miettinen and Lasota/NCCN-AFIP criteria, and modified NIH consensus criteria (Joensuu criteria). These schemes assign risk to patients with GISTs based on a number of established independent risk factors, including mitotic rate, tumor size, tumor location, and tumor rupture [25–28]. Although the modified NIH classification appears to be the best criteria for identifying a single high-risk group for consideration of adjuvant therapy [29], models that address the continuous and non-linear nature of tumor size and mitotic count, such as the nomograms developed by Gold et al. [30] and Rossi et al. [31] and the prognostic contour maps from nonlinear modeling [29], may provide more accurate estimations for the risk of recurrence and are appropriate for individualizing risk stratification for GISTs.
Currently available data support 3 years of adjuvant treatment for patients at a high risk of recurrence [7]. Results from several phase II studies [32–38] support the idea that at least 2 years of adjuvant imatinib for intermediate-risk GISTs is beneficial and may be considered. However, patients with a very low risk or low risk tumors are likely to be cured by surgery alone and should not receive adjuvant imatinib [17].
Although imatinib benefits most patients with advanced GISTs, some patients show resistance to the drug. Activating mutations of KIT or PDGFRA are found in the vast majority of GISTs, and the mutational status of these oncoproteins has been shown to be predictive of clinical response to imatinib [39]. Most GISTs express oncoproteins that are intrinsically sensitive to imatinib therapy (e.g., KIT exon 11 mutations) [39], resulting in an excellent overall clinical response that supports the use of adjuvant imatinib in these patients. However, a small number of GISTs express oncoproteins that are either intrinsically resistant to imatinib or are associated with poor clinical response. For patients with imatinib-resistant tumor genotypes (e.g., PDGFRA D842V mutation found mainly in gastric GISTs) [39], adjuvant imatinib should not be used, even for patients with high-risk GISTs. Taken together, these findings suggest that GIST mutation status can predict response to adjuvant imatinib and that genotyping can help determine the likelihood that a patient will respond to treatment.
Most GISTs express oncoproteins that are intrinsically sensitive to imatinib therapy (e.g., KIT exon 11 mutations) resulting in an excellent overall clinical response that supports the use of adjuvant imatinib in these patients. However, a small number of GISTs express oncoproteins that are either intrinsically resistant to imatinib or are associated with poor clinical response.
Tumor response to imatinib therapy based on tumor genotype is also dependent on initial treatment dose (400 mg vs. 800 mg daily) [40]. Patients with tumors expressing KIT exon 9 mutations had a significantly superior response to the higher dose of imatinib in studies in the metastatic setting, suggesting that these patients should be treated with a higher dose upfront [40]. The optimal dosage for adjuvant therapy in patients with KIT exon 9-mutant GIST, however, remains unclear.
Conclusions
Many patients with resected primary GISTs experience disease recurrence within 5 years. The emergence of imatinib therapy for the treatment of advanced GISTs has provided a basis for the development of imatinib as adjuvant treatment for GISTs. Clinical trials have supported the efficacy and safety of imatinib following surgical resection. The ACOSOG Z9001 pivotal trial established the RFS benefit of adjuvant imatinib for 1 year in patients with resected GISTs, and results from the phase III SSGXVIII/AIO trial demonstrated a long-term RFS benefit of 3-year versus 1-year adjuvant imatinib therapy. In addition, for the first time, an OS benefit with 3-year adjuvant imatinib therapy was established. The clinical effectiveness of adjuvant imatinib also is confirmed by the low NNT associated with 1-year adjuvant imatinib to prevent one recurrence or death.
Although introducing adjuvant imatinib has an economic impact on health plans, the effect appears to be limited. Several analyses have demonstrated that adjuvant imatinib is more cost-effective for treating localized primary GISTs than surgery alone, and 3 years of adjuvant imatinib is more cost-effective than 1 year of therapy. The appropriate selection of patients with relatively high risk of recurrence and more sensitive mutations for adjuvant imatinib treatment is a cost-saving approach.
Acknowledgments
We thank Jinling Wu, M.D., Ph.D., for editorial assistance with this manuscript, which was supported by Novartis Pharmaceuticals.
Author Contributions
Conception/Design: Piotr Rutkowski, Alessandro Gronchi
Manuscript writing: Piotr Rutkowski, Alessandro Gronchi
Final approval of manuscript: Piotr Rutkowski, Alessandro Gronchi
Disclosures
Piotr Rutkowski: Novartis Pharmaceuticals (CA, H), Pfizer (H); Alessandro Gronchi: Novartis Pharmaceuticals (CA, H).
C/A: Consulting/advisory relationship; RF: Research funding; E: Employment; H: Honoraria received; OI: Ownership interests; IP: Intellectual property rights/inventor/patent holder; SAB: scientific advisory board
Reference
- 1.The ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23:vii49–vii55. doi: 10.1093/annonc/mds252. [DOI] [PubMed] [Google Scholar]
- 2.Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: Update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8:S1–S41. doi: 10.6004/jnccn.2010.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: Recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassan I, You YN, Shyyan R, et al. Surgically managed gastrointestinal stromal tumors: A comparative and prognostic analysis. Ann Surg Oncol. 2008;15:52–59. doi: 10.1245/s10434-007-9633-z. [DOI] [PubMed] [Google Scholar]
- 5.Rubin JL, Sanon M, Taylor DC, et al. Epidemiology, survival, and costs of localized gastrointestinal stromal tumors. Int J Gen Med. 2011;4:121–130. doi: 10.2147/IJGM.S16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeMatteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: A randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–1104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joensuu H, Eriksson M, Sundby HK, et al. One vs. three years of adjuvant imatinib for operable gastrointestinal stromal tumor: A randomized trial. JAMA. 2012;307:1265–1272. doi: 10.1001/jama.2012.347. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. Clinical practice guidelines in oncology: Soft tissue sarcoma. [Accessed July 23, 2012]. Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 9.Blanke CD, Huse DM. Cost effectiveness of tyrosine kinase inhibitor therapy in metastatic gastrointestinal stromal tumors. J Med Econ. 2010;13:681–690. doi: 10.3111/13696998.2010.534670. [DOI] [PubMed] [Google Scholar]
- 10.Krysanov I, Zorin N, Pyadushkina E, et al. Cost-effectiveness analysis of adjuvant therapy with imatinib mesylate in patients after resection of localized primary resection of localized primary gastrointestinal stromal tumor. Value Health. 2010;13:A265–A266. [Google Scholar]
- 11.National Institute for Health and Clinical Excellence Single Technology Appraisal Submission. The clinical and cost effectiveness of imatinib as adjuvant treatment for adult patients who are at significant risk of relapse following resection of KIT positive gastrointestinal stromal tumours. [Accessed July 23, 2012]. Available at http://www.hta.ac.uk/erg/reports/2173.pdf.
- 12.Pawar V, El Ouagari K, Rubin J, et al. Cost-effectiveness of adjuvant imatinib in patients with surgically resected localized gastrointestinal stromal tumors (GIST): Canadian societal perspective. Presented at: The European Multidisciplinary Cancer Congress; October 8–12, 2010; Stockholm, Sweden. [Google Scholar]
- 13.Sanon M, Taylor D, Parthan A, et al. Cost-effectiveness of 3-years of adjuvant imatinib in gastrointestinal stromal tumors (GIST) in the United States. J Med Econ. 2013;16:150–159. doi: 10.3111/13696998.2012.709204. [DOI] [PubMed] [Google Scholar]
- 14.Corless CL, Ballman KV, Antonescu C, et al. Relation of tumor pathologic and molecular features to outcome after surgical resection of localized primary gastrointestinal stromal tumor (GIST): Results of the intergroup phase III trial ACOSOG Z9001. J Clin Oncol. 2010;28(suppl):10006. [Google Scholar]
- 15.Novartis Pharmaceuticals Corporation. Gleevec/Glivec prescribing information. [Accessed July 23, 2012]. Available at: http://www.pharma.us.novartis.com/product/pi/pdf/gleevec_tabs.pdf.
- 16.Cohen MH, Cortazar P, Justice R, et al. Approval summary: Imatinib mesylate in the adjuvant treatment of malignant gastrointestinal stromal tumors. The Oncologist. 2010;15:300–307. doi: 10.1634/theoncologist.2009-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novartis. Glivec summary of product characteristics. 2010. [Accessed July 23, 2012]. Available at: http://www.medicines.org.uk/emc/medicine/15014.
- 18.Rubin J, Deflin M, Taylor D, et al. Comparison of the clinical benefit of a novel adjuvant therapy in gastrointestinal stromal tumors (GIST) with other adjuvant cancer therapies. Ann Oncol. 2009;20:vii29–vii114. [Google Scholar]
- 19.Valentim J, Coombs J, Sakano A, et al. Number needed to treat (NNT) to avoid one gastrointestinal stromal tumour (GIST) recurrence in Brazil: Cost comparison and budget impact analysis of adjuvant treatment with imatinib. Presented at: International Society for Pharmacoeconomics and Outcomes Research 12th Annual European Congress; October 24–27, 2009; Paris, France. [Google Scholar]
- 20.Deflin M, Parathan A, Taylor D, et al. Comparison of the clinical benefit of an adjuvant therapy in gastrointestinal stromal tumors (GIST) with other adjuvant cancer therapies. J Clin Oncol. 2012;30(suppl 4):129. [Google Scholar]
- 21.Reichardt P, Hartmann J, Sundby Hall K, et al. Response to imatinib rechallenge of GIST that recurs following completion of adjuvant imatinib treatment: The first analysis in the SSGXVIII/AIO trial patient population. European Multidisciplinary Cancer Congress 2011; September 23–27, 2011; Stockholm, Sweden. [Google Scholar]
- 22.Rubin JL, Taylor DC, Sanon M, et al. Budgetary impact of treatment with adjuvant imatinib for 1 year following surgical resection of Kit-positive localized gastrointestinal stromal tumors. J Manag Care Pharm. 2010;16:482–491. doi: 10.18553/jmcp.2010.16.7.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dretzke J, Round J, Connock M, et al. Imatinib as adjuvant treatment following resection of KIT-positive gastrointestinal stromal tumours. Health Technol Assess. 2010;14:63–70. doi: 10.3310/hta14suppl2/09. [DOI] [PubMed] [Google Scholar]
- 24.Sleijfer S, Verweij J. The price of success: Cost-effectiveness of molecularly targeted agents. Clin Pharmacol Ther. 2009;85:136–138. doi: 10.1038/clpt.2008.245. [DOI] [PubMed] [Google Scholar]
- 25.Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 26.Miettinen M, Lasota J. Gastrointestinal stromal tumors: Review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466–1478. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 27.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411–1419. doi: 10.1016/j.humpath.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 28.Rutkowski P, Bylina E, Wozniak A, et al. Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour: The impact of tumour rupture on patient outcomes. Eur J Surg Oncol. 2011;37:890–896. doi: 10.1016/j.ejso.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Joensuu H, Vehtari A, Riihimaki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: An analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265–274. doi: 10.1016/S1470-2045(11)70299-6. [DOI] [PubMed] [Google Scholar]
- 30.Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: A retrospective analysis. Lancet Oncol. 2009;10:1045–1052. doi: 10.1016/S1470-2045(09)70242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossi S, Miceli R, Messerini L, et al. Natural history of imatinib-naive GISTs: A retrospective analysis of 929 cases with long-term follow-up and development of a survival nomogram based on mitotic index and size as continuous variables. Am J Surg Pathol. 2011;35:1646–1656. doi: 10.1097/PAS.0b013e31822d63a7. [DOI] [PubMed] [Google Scholar]
- 32.Jiang WZ, Guan GX, Lu HS, et al. Adjuvant imatinib treatment after R0 resection for patients with high-risk gastrointestinal stromal tumors: A median follow-up of 44 months. J Surg Oncol. 2011;104:760–764. doi: 10.1002/jso.22010. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Gong JF, Wu AW, et al. Post-operative imatinib in patients with intermediate or high risk gastrointestinal stromal tumor. Eur J Surg Oncol. 2011;37:319–324. doi: 10.1016/j.ejso.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Huang H, Liang H, Zhan ZL, et al. Surgical outcomes of gastrointestinal stromal tumors of the stomach: A single unit experience in the era of targeted drug therapy. Med Oncol. 2012;29:941–947. doi: 10.1007/s12032-011-9888-x. [DOI] [PubMed] [Google Scholar]
- 35.Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): Early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009;99:42–47. doi: 10.1002/jso.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang D, Zhang Q, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: Long-term follow-up results of Radiation Therapy Oncology Group 0132. Ann Surg Oncol. 2012:1074–1080. doi: 10.1245/s10434-011-2190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McAuliffe JC, Hunt KK, Lazar AJ, et al. A randomized, phase II study of preoperative plus postoperative imatinib in GIST: Evidence of rapid radiographic response and temporal induction of tumor cell apoptosis. Ann Surg Oncol. 2009;16:910–919. doi: 10.1245/s10434-008-0177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang B, Sr, Lee J, Ryu M, et al. A phase II study of imatinib mesylate as adjuvant treatment for curatively resected high-risk localized gastrointestinal stromal tumors. J Clin Oncol. 2009;27(suppl):e21515. [Google Scholar]
- 39.Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 40.Debiec-Rychter M, Sciot R, Le Cesne A, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006;42:1093–1103. doi: 10.1016/j.ejca.2006.01.030. [DOI] [PubMed] [Google Scholar]