This article discusses the basics of helical tomotherapy (HT) for head and neck cancer (HNC) treatment. HT allows for precise dose distribution, patient setup, and dose delivery, making it a valuable tool for difficult HNC irradiation. The literature regarding HT's potential benefit in HNC is also reviewed.
Keywords: Head and neck cancer, Helical tomotherapy
Abstract
A decade after its first introduction into the clinic, little is known about the clinical impact of helical tomotherapy (HT) on head and neck cancer (HNC) treatment. Therefore, we analyzed the basics of this technique and reviewed the literature regarding HT's potential benefit in HNC. The past two decades have been characterized by a huge technological evolution in photon beam radiotherapy (RT). In HNC, static beam intensity-modulated radiotherapy (IMRT) has shown superiority over three-dimensional conformal RT in terms of xerostomia and is considered the standard of care. However, the next-generation IMRT, the rotational IMRT, has been introduced into the clinic without any evidence of superiority over static beam IMRT other than being substantially faster. Of these rotational techniques, HT is the first system especially developed for IMRT in combination with image-guided RT. HT is particularly promising for the treatment of HNC because its sharp dose gradients maximally spare the many radiosensitive organs at risk nearby. In addition, HT's integrated computed tomography scan assures a very precise dose administration and allows for some adaptive RT. Because HT is specifically developed for IMRT in combination with (integrated) image-guidance, it allows for precise dose distribution (“dose painting”), patient setup, and dose delivery. As such, it is an excellent tool for difficult HNC irradiation. The literature on the clinical results of HT in HNC all show excellent short-term (≤2 years) results with acceptable toxicity profiles. However, properly designed trials are still warranted to further substantiate these results.
Implications for Practice:
This article highlights the advantages of helical tomotherapy in the treatment of head and neck cancer as it combines three recent evolutions and challenges in radiotherapy in an integrated system. (1) A rotational IMRT technique that is very efficient in generating homogeneous dose distributions to the target volume while sparing the organs at risk more precisely than conventional IMRT approaches. (2) Three-dimensional image guidance permits a more precise administration of the radiation than the classical two-dimensional imaging. This allows for reduction of the safety margins, which in turn, reduces toxicity. (3) Adaptive radiotherapy is still very labor intensive and no software is able to provide daily online adaptation. Tomotherapy offers a platform that allows for pioneering attempts at adaptive radiotherapy with the possibility of recalculating the administered treatment based on the daily CT scan. Properly designed trials are still warranted to further substantiate the results of this promising treatment modality.
Introduction
Despite major progress in locoregional control, the treatment outcomes of locoregionally advanced head and neck cancer (HNC) remain poor, with a 5-year survival rate of approximately 35% due to the development of distant metastases, locoregional failures, second primaries, and/or comorbidities [1–3]. Altered radiotherapy (RT) schemes and the addition of cytotoxic chemotherapy or biological agents have all shown benefit in HNC [2–12]. Despite these combined modality approaches, which are often accompanied by substantial acute and late toxicities, 10%–30% of these patients still develop locoregional recurrence, most often in the high-dose RT zone [13–18]. Even with very conformal RT techniques, recurrences in the margin of the high-dose region account only for 0%–25% of the total number of recurrences. Recurrences in the elective treated neck are extremely uncommon and largely dependent on adequate irradiation. New RT techniques, which were developed to lower the toxicity, might allow for further treatment intensification and reirradiation in previous irradiated regions.
RT in HNC is particularly challenging due to the proximity of radiosensitive organs, which are at risk of being irradiated (e.g., parotid glands, submandibular and minor salivary glands, larynx and swallowing structures, such as the constrictor muscles, the base of the tongue, the glottic and supraglottic larynx). Therefore, patients with HNC may greatly benefit from radiotherapeutic technological evolutions. The introduction of helical tomotherapy (HT; Fig. 1) during the past decade is particularly promising for the treatment of HNC. HT is an intensity-modulated radiotherapy (IMRT) technique that combines the characteristics of a linear accelerator with the particularities of a computed tomography (CT) scan [19]. HT is the first system especially developed for IMRT in combination with image-guided RT (IGRT). This article provides a review of the basics behind this technique.
Figure 1.
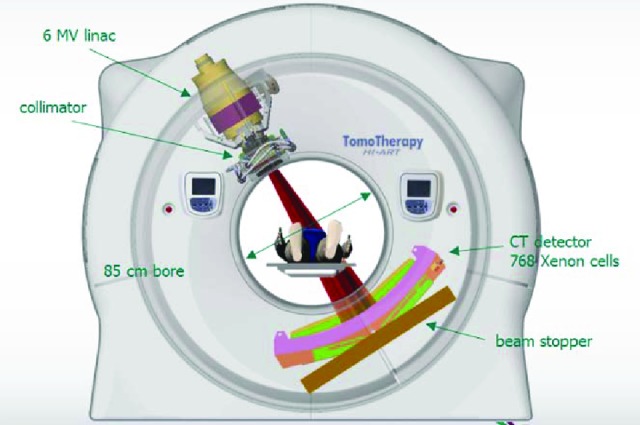
A schematic overview of the helical tomotherapy (HT) machine. The HT machine looks and works like a spiral computed tomography scan: The patient moves into the machine while the radiation source continuously turns around the patient. Courtesy of Accuray.
Abbreviations: CT, computed tomography; MV, megavolt; linac, linear accelerator.
New Radiotherapy Techniques
In the past two decades, large radiation fields (conventional RT) have been replaced by radiation fields shaped to the target volume (three-dimensional [3D] conformal RT), followed by the introduction of IMRT in different forms [20, 21]. The inverse planning techniques of IMRT no longer place fields around the target volumes; rather, they “shape” the dose around the target volume. With these sophisticated RT techniques, the necessity arises for more accurate definition of target volumes and image guiding.
The variable intensity created by IMRT combined with image guidance (IGRT) makes it possible to administer a highly conformal and a more homogeneous dose to the target volumes in comparison with conventional and 3D conformal RT [22]. This technique might lead to improved outcomes—not only in terms of toxicity but also in terms of locoregional control—as recently was shown in a randomized trial in nasopharyngeal cancer patients by Peng et al. [23]. The sharp dose falloff of IMRT allows for better sparing of the organs at risk [24, 25] and has shown to be leading to a decrease in acute and late toxicity in randomized trials [23, 26–29]. For this reason, more than 10 years after its introduction in clinical practice, IMRT has become the standard of care in the treatment of HNC.
Reduction of toxicity may create an opportunity for treatment intensification by radiotherapy, alone or combined with radiosensitizers, with the aim of better tumor control. Treatment intensification by IMRT can easily be achieved by the application of different doses to different adjacent risk zones at the same time, so-called dose painting [30, 31]. The precise coregistration of new imaging techniques, such as magnetic resonance imaging (MRI) and positron emission tomography (PET) scan, with the reference or planning CT scan makes it possible to even better delineate the tumor (i.e., the gross tumor volume) and also to define the more resistant/aggressive regions within it. The majority of locoregional HNC failures occur in the high-dose region, so dose painting could permit very localized dose escalation (or simultaneous integrated boost), with the aim of higher tumor control without increase of toxicity [32, 33]. However, “dose painting by numbers” requires careful daily image guidance and probably even adaptive RT, as discussed later. Currently, this technique is still considered to be experimental.
In the past few years, static-beam IMRT has been gradually replaced by rotational IMRT, based on Brahme's theory of more degrees of freedom resulting in more conformal dose distributions and thus achieving better dose painting [34]. These rotational techniques, including HT, were introduced without evidence-based superiority over static-beam IMRT [35, 36].
Rotational IMRT Techniques
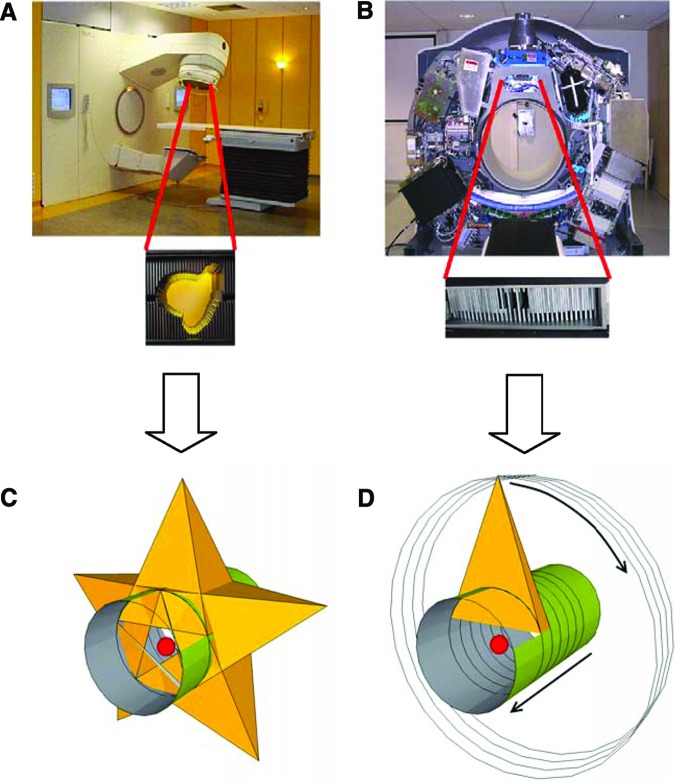
There are two major strategies for rotational RT: cone beam and fan beam. Cone beam is volumetric rotational IMRT, which was first introduced as intensity modulated arc therapy (IMAT) by Yu [37]. Fan beam is also referred to as serial tomotherapy [38] or helical tomotherapy [13] (Fig. 2).
Figure 2.
Cone beam versus fan beam. (A): Conventional linear accelerator with cone-beam multileaf collimator. (B): Helical tomotherapy machine (view without cover) with binary fan-beam multileaf collimator. (C): Cone beam continuously irradiates the entire target volume. (D): In helical tomotherapy, the fan beam irradiates the target volume slice by slice; different subvolumes (e.g., red sphere) receive their dose at different times, resulting in a higher dose rate per subvolume.
IMAT and its variations are classified as arc-based approaches to IMRT, as they can be delivered on a conventional linear accelerator with a conventional multileaf collimator (MLC). IMAT treats the whole tumor volume at once during the gantry rotation while modifying the dose rate, the gantry speed, and the shape of the beam [39–42]. For each delivery system, there are commercially available planning solutions. Tomotherapy, on the other hand, is specifically designed for IMRT. Serial tomotherapy was the starting point in IMRT, introduced in the 1990s [38]. With an add-on binary multileaf collimator and a dedicated inverse treatment planning system, it delivers a number of arcs of limited width with a conventional accelerator, in between which the treatment couch is moved longitudinally [43].
In contrast to the other rotational techniques, helical tomotherapy is delivered by a CT-like machine [19] (Figs. 1 and 2). Introduced in 2003 as Tomotherapy HiArt, it combines a continuous helical-administered IMRT with high-precision IGRT by an integrated CT scan. Modulation is obtained by changes in beam aperture and intensity every 7 degrees of the gantry rotation, resulting in 51 coplanar beam projections per 360-degree rotation. Sixty-four binary leaves are used to modulate the slit beam. The binary reference indicates that the leaves are either open or blocking the beam in operation (Fig. 2B). The machine introduced in 2010 (TomoHD, TomoTherapy, Sunnyvale, CA) has a higher maximum gantry speed (12 seconds per gantry rotation instead of 15 seconds). Helical and fixed radiation beams are integrated in this system. For treatment in direct mode, a fixed modulated slit beam is used while the patient is sliding through the bore of the treatment unit (to mirror a sliding widow approach).
The Importance of 3D IGRT in IMRT
With the introduction of these sophisticated RT techniques, there was a need for better image guidance (IGRT). As RT and IGRT both leveled up from two-dimensional (2D) to 3D imaging, the precision of IMRT was improved, allowing reduction of margins leading to a decrease of toxicity [44]. Besides the clear benefit of more precise delineation of the target volumes and the organs at risk since the introduction of multimodality imaging (CT, MRI, PET), 3D/volumetric IGRT is also very important for daily accurate repositioning of the patient and the patient's target volumes and organs at risk. Generally, IMRT safety margins are small, often copied from the literature, and not adapted to the institute's own situation; in addition, IMRT dose gradients are steep, especially near organs at risk. Therefore, even small inaccuracies in repositioning may influence target volume (TV) coverage and by this possibly also the cure rate. To avoid these geographical misses, careful imaging protocols that are adapted to institution-specific planning TV margins are required.
2D imaging (e.g., electronic portal imaging [EPI]) repositions the patient mainly on bony landmarks, the body contour, or radio-opaque implanted markers. On the other hand, 3D imaging (e.g., cone-beam CT) gives a view of the target volumes and organs at risk within the patient. As a result, not only the setup margin (part of the PTV compensating for random setup errors) but also the internal margin (part of the PTV taking organ movements into account) and soft-tissue changes (i.e., the treatment-induced systematic error) can be evaluated [45, 46]. Therefore, appropriate IGRT can lead to setup margin reduction. Even a small reduction of the TV radius (r) results in a substantial reduction of healthy tissues irradiated with high doses (4/3 πr3). The understanding of tissue changes during RT makes adaptive RT possible.
Besides 3D kilovolt (kV) imaging (making use of diagnostic x-rays), there is also megavolt (MV) imaging applying the actual treatment beam for imaging purposes, as in HT. An additional advantage of the MV energy of the beam is the absence of dental fillings artifacts due to the predominant Compton effect in photon interactions [47].
There are also some disadvantages to 3D IGRT, including the following:
The CT scan is not able to visualize intratreatment movements because it can only be used prior to each RT session and not during treatment delivery itself.
Its dose is not routinely integrated into the planning, which may lead to extra dosing above the planned dose.
Specifically for the MV CT scan, there is a lower soft-tissue contrast.
For HT, 3D IGRT uses 768 integrated xenon-filled ion chambers functioning as an imaging detector, which converts the MV fan beam into a MV-CT scanning beam (Fig. 1). For imaging, HT uses a 3.5-MV beam instead of 6 MV during treatment. As such, doses comparable to 2D EPI are obtained for 3D imaging [48]. The acquired MV-CT scan is automatically coregistered with the planning reference CT scan and can further be manually fine-tuned (Fig. 3). These extra manual corrections can be guided by the target volumes, the organs at risk, and the dose levels that can be visualized during the fusion. The treatment couch will then automatically adjust the patient's position. An overview of the different kinds of IGRT is given in the supplemental online data.
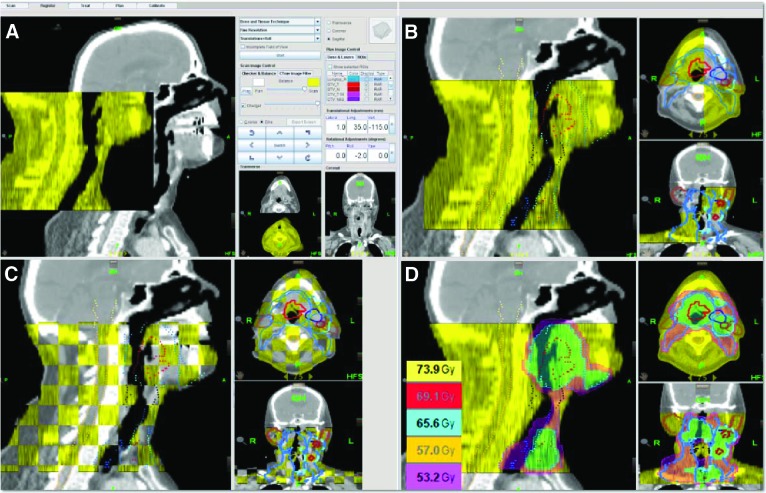
Figure 3.
Image-guided radiotherapy by helical tomotherapy. (A and B): The pretreatment megavolt computed tomography (CT) scan (yellow) is automatically fused with the reference CT scan (grey). (C and D): Extra manual corrections can be done based on the target volumes ([C]: red and blue), the organs at risk ([C]: e.g., spinal cord in orange), and the dose levels (D), which can be visualized to guide the fusion.
Current research focuses on real-time adapted treatment planning using the image-of-the-day to calculate the dose distribution or adapt the treatment based on this instant information. The challenge is to define clinically meaningful thresholds for replanning and to develop fast and accurate dose calculation algorithms. Moreover, obtaining reliable density information from these in-room CT data sets, necessary for a correct dose calculation, has proven to be challenging in clinical routine.
Adaptive Radiotherapy
Adaptive radiation therapy (ART) is another interesting development that aims at optimal compensation of uncertainties, including organ deformation and interfraction organ motion as well as dosimetric errors incurred in previous fractions. Effective ART strategies based on closed-loop feedback systems capable of taking into account the dose delivery history and the patient's on-treatment geometric model have to be created. de la Zerda et al. [49] have classified these strategies as “adapting to changing geometry” and “adapting to geometry and the delivered dose.” The first strategy focuses on organ deformation observed just before treatment, whereas the latter strategy also uses the dose delivery history to optimize the dose distribution accumulated over the entire course of treatment by adapting at each fraction.
Current research focuses on real-time adapted treatment planning using the image-of-the-day to calculate the dose distribution or adapt the treatment based on this instant information [50, 51]. The challenge is to define clinically meaningful thresholds for replanning and to develop fast and accurate dose calculation algorithms. Moreover, obtaining reliable density information from these in-room CT data sets, necessary for a correct dose calculation, has proven to be challenging in clinical routine [52–55]. Scatter artifacts and beam parameters influencing the stability of Hounsfield units used for dose calculation need to be monitored closely and correction methods might be required. Even more challenging might be the need and accuracy related to deformable registration algorithms necessary for summation of the different treatment parts to a global treatment plan [56–58].
For all these reasons, “real-time” or “online” adaptive IMRT is still considered to be under development, and it should not be used on a routine basis yet. A more widely accepted approach is “offline” replanning on a newly acquired planning CT scan after significant changes in anatomy have been detected.
HT in Daily Practice
The preparation for HT treatment is the same as for other RT techniques. The patient has to be positioned on a well-fitting head/neck support and a customized thermoplastic mask is made. A high-resolution contrast-enhanced CT scan, with typically 2- to 3-mm slice thickness is performed in treatment position. Reference laser lines are drawn on the mask. The planning reference CT scan, together with the planning MRI and PET-CT scan (if felt necessary), are introduced and coregistered in a third-party software module for the delineation of target volumes and organs at risk. With these coregistrations, the target volumes and the organs at risk can be contoured more precisely. A coregistration before the start of induction chemotherapy or before operation is done, when appropriate, to delineate target volumes based on the initial situation. Consequently, the CT scan and the contours are fed into the HT planning system. The different dose constraints, tolerances, and dose-volume relationships are imported into the system. The high performance 14-blade computing cluster searches for the best compromise, guided by penalties and priorities that can permanently be evaluated and adjusted. Once the plan is approved, an individual patient-oriented quality control is performed on a phantom.
A daily MV-CT scan is made prior to each session to compare the position of the target volumes and the organs at risk with their position on the planning reference CT scan (the so-called matching or registration). Based on the translations into three dimensions (x-, y-, and z-axes) and the rotation around the z-axis (the “roll”), the patient's positioning is fully automated and adjusted with millimeter-precision [59, 60].
During the treatment, the patient moves into the HT machine with a fixed table speed comparable to a CT scan, while the radiation field is rotating around the patient with a fixed width of 1 cm, 2.5 cm, or 5 cm. As such, the patient is irradiated by multiple overlapping helical slices (like a corkscrew). The degree of overlap between two slices is defined by the pitch—that is, the couch travel during one 360-degree rotation of the source in function of the beam width. Meanwhile, the beam aperture and intensity of the individual beamlets change every 7 degrees in function of the modulation needed. This dividing of the treatment into multiple small beams (beamlets) by a 64-leaf binary multileaf collimator allows targeted radiotherapy with high-dose gradients in different directions and forms. Even in complicated treatments, a dose difference (gradient) of 50% can be obtained over a few millimeters (Fig. 4). Therefore, HT is ideal for dose painting, with better sparing of the healthy tissue out of the high-dose zone and with the possibility to treat different target volumes to different doses in function of the suspected tumor load and radiosensitivity. The head and neck region, with its difficult anatomy and close relationship of critical organs to the tumor, is therefore ideal to be treated by this technique.
Figure 4.
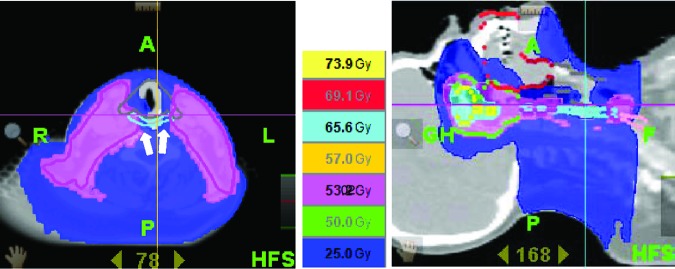
Excellent sparing of at-risk organs obtained with helical tomotherapy. Steep dose gradients (e.g., white arrows) make it possible to spare parts of the swallowing apparatus: the constrictor muscles (light blue), the glottic and supraglottic larynx (grey), the base of tongue (dark red), and the oral mucosa (red).
Besides the more precise and more homogeneous irradiation of the target volume, HT can make the difference for various reasons in different locations of the head and neck region. For example, in the nasopharynx/sinus region, HT can limit/guide the dose to the optical structures, including the optical nerves, optical chiasm, retinae, lachrymal glands (including those in the eyelids), and lenses, as well as the parotids, the brain, the brainstem, the nasal mucosae, the cochleae, and the inner ears. At the level of the neck and in function of the localization and size of the primary tumor and involved lymph nodes, HT can also limit/guide the dose to the lips, oral mucosae, tongue, submandibular glands, constrictor muscles, base of tongue, glottis, thyroid gland, and the upper part of the esophagus. The sparing of the swallowing apparatus may be particularly interesting, as late dysphagia has become a major side effect of combined chemoradiation and of altered fractionation, with a major impact on the quality of life [61–64].
The Limitations of HT
A first concern is the higher theoretical risk on radioinduced malignancies—a late effect of all forms of ionizing radiation—due to the increased low-dose irradiated volume and the higher “integral dose” that occur with IMRT [65, 66]. This total administered dose rises because of the spreading out of the dose over more healthy tissue (360 degrees) to lower the high-dose zone in the organs at risk nearby the target volumes. IMRT also causes more scatter and leakage contribution through the collimator system and the leaves than 3D conformal RT. However, HT is specifically developed for IMRT, has no flattening filter, and shows five times less leakage through the leaves (0.5% vs. 2.5% mainly due to the extra shielding) than the classic linear accelerators. Therefore, the integral dose with HT is nearly equal to 6-MV 3D-conformal RT and 6-MV IMRT [67–69].
Older literature data from Verellen et al., published in 1999, reported an eightfold increase in the risk of radiation-induced secondary cancers when comparing old serial tomotherapy with parallel-opposed, wedged treatment fields [65]. However, these data are outdated because manufacturers have taken extra precautions to optimize the new-generation machines/techniques. In 2006, Hall suggested an increase in this risk from 1%–2% with conventional RT to 2%–5% with IMRT [66].
Although the risk of radiation-induced cancer is real, for adults it has to be put into its proper perspective: the theoretical data mentioned are outweighed by the global risk of the HNC population to develop second primary squamous cell HNC of 25% at 5 years to 40% at 10 years [70, 71]. Moreover, radiation-induced solid cancers often appear after 15–20 years. Patients with HNC are often older and in poor general condition, so one may argue that the benefit of reduced toxicity by HT outbalances the risk of a secondary cancer. However, with the increase of virus-associated tumors, which are more frequently occurring in younger patients with fewer comorbidities who are less prone to develop secondary tumors, this possible increased risk needs to be balanced with the treatment benefit.
Another disadvantage of HT is the treatment time. The 5–7 minutes for a simultaneous integrated boost treatment with the 2.5-cm collimator width HT in a patient with HNC is shorter than static-beam IMRT (8–12 minutes), but definitely longer than the 3-minute treatment time with RapidArc (Varian, Palo Alto, CA) [72–73]. This treatment time can be significantly shortened when using the 5.0-cm collimator width, at the price of less conformity/homogeneity of the target volumes and/or less sparing of the organs at risk. Treatment time may be important for the patient's comfort as well as tumor control. In an in vitro study on nasopharyngeal carcinoma cell lines, Zheng et al. showed that longer fraction delivery time results in less tumor cell kill, probably due to sublethal damage repair during the irradiation [74]. However, with HT, although the full treatment may take up to 5–10 minutes, each slice of tissue is treated with a very high dose rate, thus preventing sublethal damage repair by excluding any dose-rate effect (Fig. 2). Further, Shaikh et al. estimated an increase in the tumor control probability of 2%–3% with HT compared with the faster 3D conformal RT due to the lower dose rate of the latter, and an additional 2%–3% compared with static-beam IMRT [75].
The fixed beam width of 2.5 cm or 5 cm (not the 1-cm width) causes a less sharp dose gradient in the craniocaudal direction [76]. In the future, the introduction of the per-treatment variation of the beam width and the couch speed in function of the TV during the treatment should resolve this problem [77].
Results of Helical Tomotherapy in HNC
Analytical Study
Much controversy has occurred over the article by Bortfeld and Webb, in which the authors compared single-arc IMRT with helical tomotherapy [78]. They concluded: “Single-Arc holds potential as an efficient IMRT technique especially for relatively simple cases. In very complex cases, Single-Arc may unduly compromise the quality of the dose distribution, if one tries to keep the treatment time below 2 min or so. As with all IMRT techniques, it is important to carefully explore for each individual case the tradeoff between plan quality and the efficiency of its delivery carefully for each individual case.” One could calculate that for a theoretical PTV of 10 × 10 × 10 cm, 900 beamlets (elementary beams used for intensity modulation) are possible for dose optimization in a classical IMRT against 17,000 for HT. Taking modulation of the individual beamlets and the pitch into account with HT, the differences in possibilities become even bigger: 36,000 variables for optimization for RapidArc against 1,500,000 for HT. The clinical value of these differences remains to be assessed.
Planning Studies
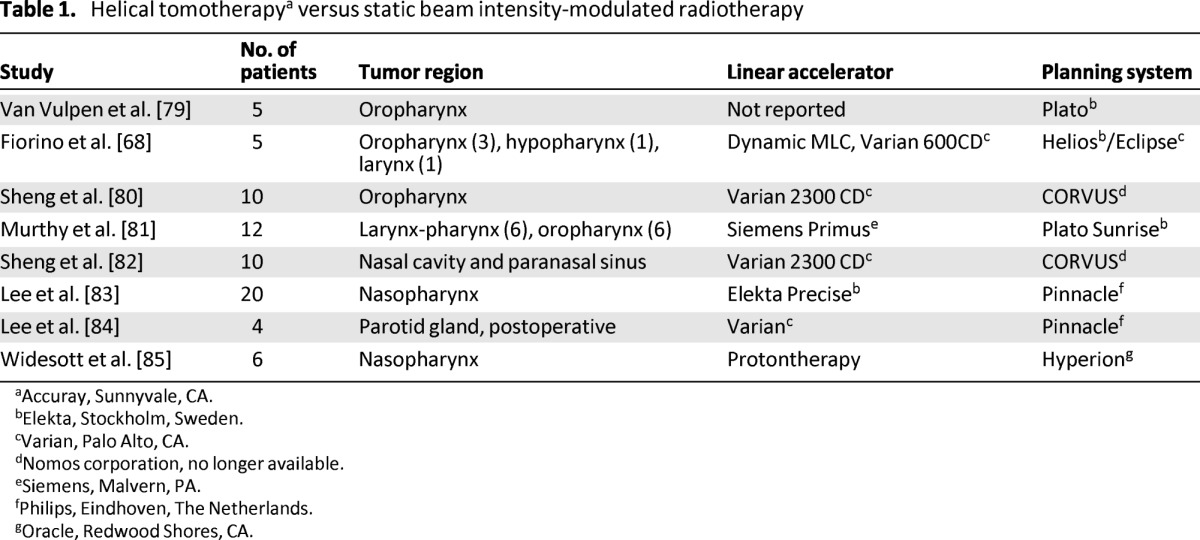
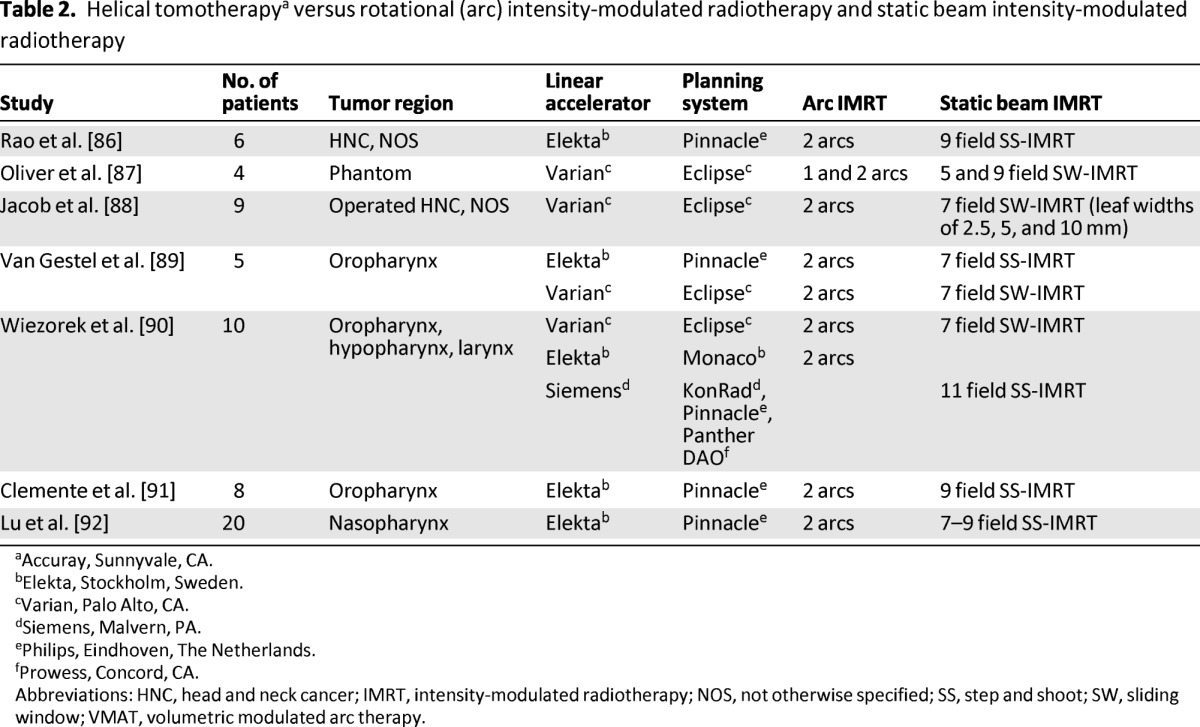
The issue of planning studies is controversial. The comparison of one planning system with the other is subject to variations in the quality/experience of the planner, the efforts made to push each system to its limits, the number of organs at risk that are contoured and/or spared, and the version of the software and machine used. The most relevant planning comparisons in HNC are summarized in Tables 1 and 2.
Table 1.
Helical tomotherapya versus static beam intensity-modulated radiotherapy
Accuray, Sunnyvale, CA.
bElekta, Stockholm, Sweden.
cVarian, Palo Alto, CA.
dNomos corporation, no longer available.
eSiemens, Malvern, PA.
fPhilips, Eindhoven, The Netherlands.
gOracle, Redwood Shores, CA.
Table 2.
Helical tomotherapya versus rotational (arc) intensity-modulated radiotherapy and static beam intensity-modulated radiotherapy
Accuray, Sunnyvale, CA.
bElekta, Stockholm, Sweden.
cVarian, Palo Alto, CA.
dSiemens, Malvern, PA.
ePhilips, Eindhoven, The Netherlands.
fProwess, Concord, CA.
Abbreviations: HNC, head and neck cancer; IMRT, intensity-modulated radiotherapy; NOS, not otherwise specified; SS, step and shoot; SW, sliding window; VMAT, volumetric modulated arc therapy.
Clinical Studies
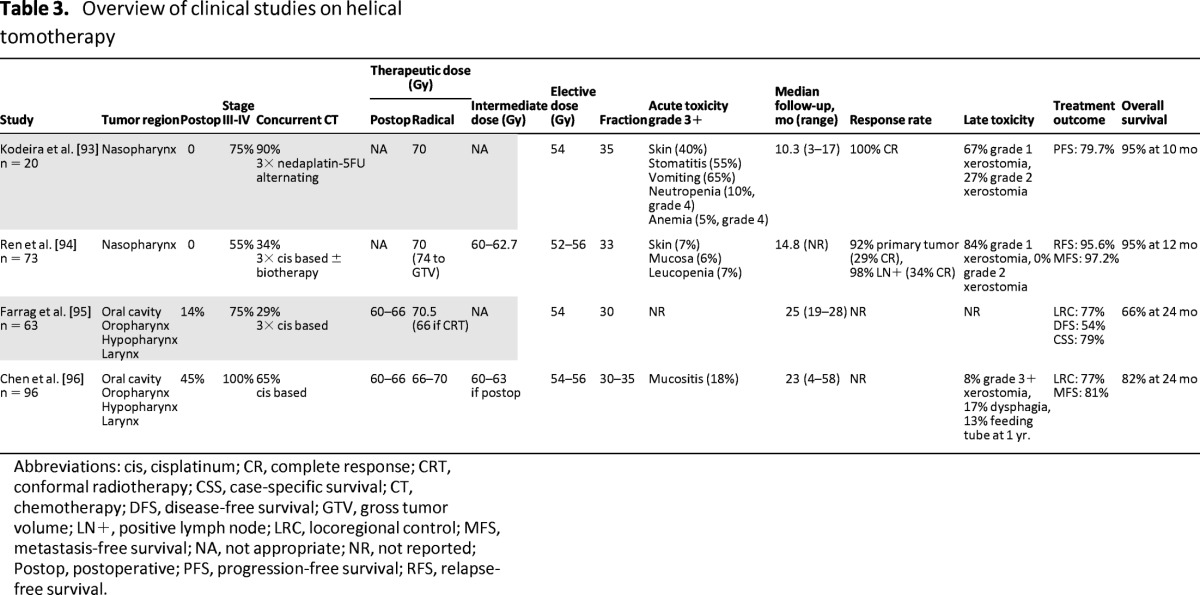
Only a few articles have been published about the clinical results of HT in HNC. They all show excellent short-term (≤2 years) results with acceptable toxicity profiles. However, because all these studies are small single-center studies, their results should be interpreted with caution and cannot be extrapolated to the benefit of HT in general. An overview of these studies can be found in Table 3.
Table 3.
Overview of clinical studies on helical tomotherapy
Abbreviations: cis, cisplatinum; CR, complete response; CRT, conformal radiotherapy; CSS, case-specific survival; CT, chemotherapy; DFS, disease-free survival; GTV, gross tumor volume; LN+, positive lymph node; LRC, locoregional control; MFS, metastasis-free survival; NA, not appropriate; NR, not reported; Postop, postoperative; PFS, progression-free survival; RFS, relapse-free survival.
Particularly interesting is the retrospective comparison by Chen et al. [96], in which they compared HT with step-and-shoot (SS) IMRT in 149 patients with locally advanced squamous cell carcinoma of the oropharynx, oral cavity, and larynx/hypopharynx. Although dosimetrical analysis revealed that the use of HT resulted in significant improvements with respect to mean dose (23.5 vs. 27.9 Gy, p = .03) and V30 (30.1 vs. 43.9 Gy, p = .01) to the contralateral parotid gland, the incidence of grade 3+ xerostomia in the late setting was 10% and 8% among patients treated by HT and SS-IMRT, respectively (p = .46). This study failed to show significant differences in any of the quality-of-life endpoints (p > .05 for all). However, in this retrospective study, there was a clear imbalance in study populations between both treatment modalities. Besides the heterogeneity with respect to primary sites of disease, tumor burden, and follow-up, there was a clear imbalance in the population undergoing concomitant chemoradiation (53% for SS-IMRT vs. 65% for HT). Moreover, retrospective analyses of toxicity in general are less reliable and this particular study was too underpowered to draw any definitive conclusions.
Conclusion
HT, like other rotational IMRT techniques, can be considered to be a logical evolution of static-beam IMRT. Moreover, HT is specifically developed for IMRT in combination with (integrated) IGRT. As an integrated system, this approach allows for precise dose distribution (dose painting), patient setup, and dose delivery. The literature on the clinical results of HT in HNC all show excellent short-term (≤2 years) results with acceptable toxicity profiles. For all these reasons, we consider HT to be an excellent tool for difficult head and neck cancer irradiation.
See www.TheOncologist.com for supplemental material available online.
Supplementary Material
Author Contributions
Conception/design: Dirk Van Gestel, Dirk Verellen, Danielle Van den Weyngaert, Jan B. Vermorken, Vincent Gregoire
Provision of study materials or patients: Dirk Van Gestel, Olivier Vanderveken, Carl Van Laer, Danielle Van den Weyngaert, Jan B. Vermorken
Collection and/or assembly of data: Dirk Van Gestel, Dirk Verellen, Lien Van De Voorde, Bie de Ost, Geert De Kerf
Data analysis and interpretation: Dirk Van Gestel, Dirk Verellen, Lien Van De Voorde, Jan B. Vermorken, Vincent Gregoire
Manuscript writing: Dirk Van Gestel, Dirk Verellen, Bie de Ost, Geert De Kerf, Olivier Vanderveken, Carl Van Laer, Jan B. Vermorken, Vincent Gregoire
Final approval of manuscript: Dirk Van Gestel, Dirk Verellen, Lien Van De Voorde, Bie de Ost, Geert De Kerf, Olivier Vanderveken, Carl Van Laer, Danielle Van den Weyngaert, Jan B. Vermorken, Vincent Gregoire
Disclosures
Dirk Van Gestel: Accuray/TomoTherapy (H). The other authors indicated no financial relationships.
C/A: Consulting/advisory relationship; RF: Research funding; E: Employment; H: Honoraria received; OI: Ownership interests; IP: Intellectual property rights/inventor/patent holder; SAB: scientific advisory board
Reference
- 1.Vermorken JB. Medical treatment in head and neck cancer. Ann Oncol. 2005;16:ii258–ii264. doi: 10.1093/annonc/mdi735. [DOI] [PubMed] [Google Scholar]
- 2.Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to loco-regional treatment for head and neck squamous cell carcinoma: Three meta-analyses of update individual data. Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 3.Pignon JP, Le Maitre A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet. 2006;368:843–854. doi: 10.1016/S0140-6736(06)69121-6. [DOI] [PubMed] [Google Scholar]
- 5.Budach W, Her T, Budach V, et al. A meta-analysis of hyperfractionated and accelerated radiotherapy and combined chemotherapy and radiotherapy regimens in unresected locally advanced squamous cell carcinoma of the head and neck. BMC Cancer. 2006;6:28. doi: 10.1186/1471-2407-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachaud JM, Cohen-Jonathan E, Alzieu C, et al. Combined post-operative radiotherapy and weekly cisplatin infusion for locally advanced head and neck cancer carcinoma. Final report of a randomized trial. Int J Radiat Oncol Biol Phys. 1996;36:999–1004. doi: 10.1016/s0360-3016(96)00430-0. [DOI] [PubMed] [Google Scholar]
- 7.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 8.Cooper JS, Pajak TF, Forastiere A, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 9.Bernier J, Cooper YS. Chemoradiation after surgery for high risk head and neck cancer patients: how strong is the evidence? The Oncologist. 2005;10:215–224. doi: 10.1634/theoncologist.10-3-215. [DOI] [PubMed] [Google Scholar]
- 10.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 11.Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–1704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 12.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and flurouracil alone or with docetaxel in head and neck cancer. N Engl j Med. 2007;357:1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 13.Chen AM, Farwell DG, Luu Q, et al. Misses and near-misses after postoperative radiation therapy for head and neck cancer: Comparison of IMRT and non-IMRT techniques in the CT-simulation era. Head Neck. 2010;32:1452–1459. doi: 10.1002/hed.21343. [DOI] [PubMed] [Google Scholar]
- 14.Chao KS, Ozyigit G, Tran BN, et al. Patterns of failure in patients receiving definitive and postoperative IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2003;55:312–321. doi: 10.1016/s0360-3016(02)03940-8. [DOI] [PubMed] [Google Scholar]
- 15.Dawson LA, Anzai Y, Marsh L, et al. Patterns of local-regional recurrence following parotid-sparing conformal and segmental intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2000;46:1117–1126. doi: 10.1016/s0360-3016(99)00550-7. [DOI] [PubMed] [Google Scholar]
- 16.O'Sullivan B, Warde P, Grice B, et al. The benefits and pitfalls of ipsilateral radiotherapy in carcinoma of the tonsillar region. Int J Radiat Oncol Biol Phys. 2001;51:332–343. doi: 10.1016/s0360-3016(01)01613-3. Erratum in: Int J Radiat Oncol Biol Phys 2001;51:1465. [DOI] [PubMed] [Google Scholar]
- 17.Duprez F, Bonte K, De Neve W, et al. Regional relapse after intensity-modulated radiotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;79:450–458. doi: 10.1016/j.ijrobp.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Gupta T, Jain S, Agarwal JP, et al. Prospective assessment of patterns of failure after high-precision definitive (chemo)radiation in head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2011;80:522–531. doi: 10.1016/j.ijrobp.2010.01.054. [DOI] [PubMed] [Google Scholar]
- 19.Mackie TR, Holmes T, Swerdloff S, et al. Tomotherapy: A new concept for the delivery of dynamic conformal radiotherapy. Med Phys. 1993;20:1709–1719. doi: 10.1118/1.596958. [DOI] [PubMed] [Google Scholar]
- 20.Bernier J, Hall EJ, Giaccia A. Radiation oncology: A century of achievements. Nat Rev Cancer. 2004;4:1–11. doi: 10.1038/nrc1451. [DOI] [PubMed] [Google Scholar]
- 21.Verellen D, De Ridder M, Linthout N, et al. Innovations in image-guided radiotherapy. Nat Rev Cancer. 2007;7:949–960. doi: 10.1038/nrc2288. [DOI] [PubMed] [Google Scholar]
- 22.Clark CH, Bidmead AM, Mubata CD, et al. Intensity-modulated radiotherapy improves target coverage, spinal cord sparing and allows dose escalation in patients with locally advanced cancer of the larynx. Radiother Oncol. 2004;70:189–198. doi: 10.1016/j.radonc.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Peng G, Wang T, Yang KY, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104:286–293. doi: 10.1016/j.radonc.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Grégoire V, De Neve W, Eisbruch A, et al. Intensity-modulated radiation therapy for head and neck carcinoma. The Oncologist. 2007;12:555–564. doi: 10.1634/theoncologist.12-5-555. [DOI] [PubMed] [Google Scholar]
- 25.Mendenhall WM, Amdur RJ, Palta JR. Intensity-modulated radiotherapy in the standard management of head and neck cancer: Promises and pitfalls. J Clin Oncol. 2006;24:2618–2623. doi: 10.1200/JCO.2005.04.7225. [DOI] [PubMed] [Google Scholar]
- 26.Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25:4873–4879. doi: 10.1200/JCO.2007.11.5501. [DOI] [PubMed] [Google Scholar]
- 27.Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy versus conventional radiotherapy for early-stage nasopharyngeal carcinoma: Initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66:981–991. doi: 10.1016/j.ijrobp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Nutting C, Morden J, Harrington K, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta T, Agarwal J, Jain S, et al. Three-dimensional conformal radiotherapy (3D-CRT) versus intensity modulated radiation therapy (IMRT) in squamous cell carcinoma of the head and neck: A randomized controlled trial. Radiother Oncol. 2012;104:343–348. doi: 10.1016/j.radonc.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Ling CC, Humm J, Larson S, et al. Towards multidimensional radiotherapy (MD-CRT): Biological imaging and biological conformality. Int J Radiat Oncol Biol Phys. 2000;47:551–560. doi: 10.1016/s0360-3016(00)00467-3. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Xing L. Towards biologically conformal radiation therapy (BCRT): Selective IMRT dose escalation under the guidance of spatial biology distribution. Med Phys. 2005;32:1473–1484. doi: 10.1118/1.1924312. [DOI] [PubMed] [Google Scholar]
- 32.Mohan R, Wu Q, Manning M, et al. Radiobiological considerations in the design of fractionation strategies for intensity-modulated radiation therapy of head and neck cancers. Int J Radiat Oncol Biol Phys. 2000;46:619–630. doi: 10.1016/s0360-3016(99)00438-1. [DOI] [PubMed] [Google Scholar]
- 33.Wu Q, Mohan R, Morris M, et al. Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas. I: Dosimetric results. Int J Radiat Oncol Biol Phys. 2003;56:573–585. doi: 10.1016/s0360-3016(02)04617-5. [DOI] [PubMed] [Google Scholar]
- 34.Brahme A. Optimization of stationary and moving beam radiationtherapy techniques. Radiother. Oncol. 1988;12:129–140. doi: 10.1016/0167-8140(88)90167-3. [DOI] [PubMed] [Google Scholar]
- 35.Palma DA, Verbakel WF, Otto K, et al. New developments in arc radiation therapy: A review. Cancer Treat Rev. 2010;36:393–399. doi: 10.1016/j.ctrv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Bentzen SM. Randomized controlled trials in health technology assessment: Overkill or overdue? Radiother Oncol. 2008;86:142–147. doi: 10.1016/j.radonc.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu C. Intensity-modulated arc therapy with dynamic multileaf collimation: An alternative to tomotherapy. Phys Med Biol. 1995;40:1435–1449. doi: 10.1088/0031-9155/40/9/004. [DOI] [PubMed] [Google Scholar]
- 38.Carol M. Peacock: A system for planning and rotational delivery of intensity-modulated fields. Int J Imag Sys Tech. 1995;6:56–61. [Google Scholar]
- 39.Wolff D, Stieler F, Hermann B, et al. Clinical implementation of volumetric intensity-modulated arc therapy (VMAT) with ERGO++ Strahlenther Onkol. 2010;186:280–288. doi: 10.1007/s00066-010-2071-z. [DOI] [PubMed] [Google Scholar]
- 40.Stieler F, Wolff D, Schmid H, et al. A comparison of several modulated radiotherapy techniques for head and neck cancer and dosimetric validation of VMAT. Radiother Oncol. 2011;101:388–393. doi: 10.1016/j.radonc.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 41.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2007;35:310–317. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 42.Gevaert T, Engels B, Garibaldi C, et al. Implementation of HybridArc treatment technique in preoperative radiotherapy of rectal cancer: Dose patterns in target lesions and organs at risk as compared to helical tomotherapy and RapidArc. Rad Oncol. 2012;7:120. doi: 10.1186/1748-717X-7-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verellen D, Linthout N, Van Den Berge D, et al. Initial experience with intensity-modulated conformal radiation therapy for treatment of the head and neck region. Int J Radiat Oncol Biol Phys. 1997;39:99–114. doi: 10.1016/s0360-3016(97)00304-0. [DOI] [PubMed] [Google Scholar]
- 44.Van Asselen B, Dehnad H, Raaijmakers CPJ, et al. The dose to the parotid glands with IMRT for oropharyngeal tumors: The effect of reduction of positioning margins. Radiother Oncol. 2002;64:197–204. doi: 10.1016/s0167-8140(02)00152-4. [DOI] [PubMed] [Google Scholar]
- 45.Kreeft A, Rasch C, Muller S, et al. Cine MRI of swallowing in patients with advanced oral or oropharyngeal carcinoma: A feasibility study. Eur Arch Otorhinolaryngol. 2012;269:1703–1711. doi: 10.1007/s00405-011-1861-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Kranen S, van Beek S, Mencarelli A, et al. Correction strategies to manage deformations in head-and-neck radiotherapy. Radiother Oncol. 2010;94:199–205. doi: 10.1016/j.radonc.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 47.Yang C, Liu T, Jennelle RL, et al. Utility of megavoltage fan-beam CT for treatment planning in a head-and-neck cancer patient with extensive dental fillings undergoing helical TomoTherapy. Med Dosim. 2010;35:108–114. doi: 10.1016/j.meddos.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Beavis AW. Is TomoTherapy the future of IMRT? Br J Radiol. 2004;77:285–295. doi: 10.1259/bjr/22666727. [DOI] [PubMed] [Google Scholar]
- 49.de la Zerda A, Armbruster B, Xing L. Formulating adaptive radiation therapy (ART) treatment planning into a closed-loop control framework. Phys Med Biol. 2007;52:4137–4153. doi: 10.1088/0031-9155/52/14/008. [DOI] [PubMed] [Google Scholar]
- 50.Yan D, Lockman D, Martinez A, et al. Computed tomography guided management of interfractional patient variation. Semin Radiat Oncol. 2005;15:168–179. doi: 10.1016/j.semradonc.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Lof J, Lind B, Brahme A. An adaptive control algorithm for optimization of intensity modulated radiotherapy considering uncertainties in beam profiles, patient set-up and internal organ motion. Phys Med Biol. 1998;43:1605–1628. doi: 10.1088/0031-9155/43/6/018. [DOI] [PubMed] [Google Scholar]
- 52.van Elmpt W, Nijsten S, Mijnheer B, et al. The next step in patient-specific QA: 3D dose verification of conformal and intensity-modulated RT based on EPID dosimetry and Monte Carlo dose calculations. Radiother Oncol. 2008;86:86–92. doi: 10.1016/j.radonc.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Duchateau M, Tournel K, Verellen D, et al. The effect of tomotherapy imaging beam output instabilities on dose calculation. Phys Med Biol. 2010;55:329–336. doi: 10.1088/0031-9155/55/11/N03. [DOI] [PubMed] [Google Scholar]
- 54.Siewerdsen JH, Daly MJ, Bakhtiar B, et al. A simple, direct method for x-ray scatter estimation and correction in digital radiography and cone-beam CT. Med Phys. 2006;33:187–197. doi: 10.1118/1.2148916. [DOI] [PubMed] [Google Scholar]
- 55.Graham SA, Moseley DJ, Siewerdsen JH, et al. Compensators for dose and scatter management in cone-beam computed tomography. Med Phys. 2007;34:2691–2703. doi: 10.1118/1.2740466. [DOI] [PubMed] [Google Scholar]
- 56.Kashani R, Hub M, Kessler ML, et al. Technical note: A physical phantom for assessment of accuracy of deformable alignment algorithms. Med Phys. 2007;37:2785–2788. doi: 10.1118/1.2739812. [DOI] [PubMed] [Google Scholar]
- 57.Jaffray D, Lindsay P, Brock K, et al. Accurate accumulation of dose for improved understanding of radiation effects in normal tissue. Int J Radiat Oncol Biol Phys. 2010;76:S135–S139. doi: 10.1016/j.ijrobp.2009.06.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schultheiss TE, Tome WA. It is not appropriate to “deform” dose along with deformable image registration in adaptive radiotherapy. Medl Phys. 2012;39:6531–6533. doi: 10.1118/1.4722968. [DOI] [PubMed] [Google Scholar]
- 59.Shubert LK, Westerly DC, Tomé WA, et al. A comprehensive assessment by tumor site of patient setup using daily MVCT imaging from more than 3,800 helical tomotherapy treatments. Int J Radiat Oncol Biol Phys. 2009;73:1260–1269. doi: 10.1016/j.ijrobp.2008.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin L, Shi C, Eng T, et al. Evaluation of inter-fractional setup shifts for site-specific helical tomotherapy treatments. Tecnol Cancer Res Treat. 2009;8:115–122. doi: 10.1177/153303460900800204. [DOI] [PubMed] [Google Scholar]
- 61.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: Which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60:1425–1439. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 62.Dirix P, Nuyts S. Evidence-based organ-sparing radiotherapy in head and neck cancer. Lancet Oncol. 2010;11:85–91. doi: 10.1016/S1470-2045(09)70231-1. [DOI] [PubMed] [Google Scholar]
- 63.Nguyen NP, Moltz CC, Frank C, et al. Dysphagia following chemoradiation for locally advanced head and neck cancer. Ann Oncol. 2004;15:383–388. doi: 10.1093/annonc/mdh101. [DOI] [PubMed] [Google Scholar]
- 64.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26:3770–3776. doi: 10.1200/JCO.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- 65.Verellen D, Vanhavere F. Risk assessment of radiation-induced malignancies based on whole-body equivalent estimates for IMRT treatment in the head and neck region. Radiother Oncol. 1999;53:199–203. doi: 10.1016/s0167-8140(99)00079-1. [DOI] [PubMed] [Google Scholar]
- 66.Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006;65:1–7. doi: 10.1016/j.ijrobp.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 67.Aoyama H, Westerly D, Mackie T, et al. Integral radiation dose to normal structures with conformal external beam radiation. Int J Radiat Oncol Biol Phys. 2006;64:962–967. doi: 10.1016/j.ijrobp.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 68.Fiorino C, Dell'Oca I, Pierelli A, et al. Significant improvement in normal tissue sparing and target coverage for head and neck cancer by means of helical tomotherapy. Radiother Oncol. 2006;78:276–282. doi: 10.1016/j.radonc.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 69.Ramsey C, Seibert R, Mahan S, et al. Out-of-field dosimetry measurements for a helical tomotherapy system. J Appl Clin Med Phys. 2006;7:1–11. doi: 10.1120/jacmp.v7i3.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwartz LH, Ozsahin M, Zhang GN, et al. Synchronous and metachronous head and neck carcinomas. Cancer. 1994;74:1933–1938. doi: 10.1002/1097-0142(19941001)74:7<1933::aid-cncr2820740718>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 71.Trotti A, Fu KK, Pajak TF, et al. Long-term outcomes of RTOG 90–03: A comparison of hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2005;63:S70–S71. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 72.Verbakel WF, Cuijpers JP, Hoffmans D, et al. Volumetric intensity-modulated arc therapy vs. conventional IMRT in head and neck cancer: A comparative planning and dosimetric study. Int J Radiat Oncol Biol Phys. 2009;74:252–259. doi: 10.1016/j.ijrobp.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 73.Murthy V, Gupta T, Kadam A, et al. Time trial: A prospective comparative study of the time-resource burden for three-dimensional conformal radiotherapy and intensity-modulated radiotherapy in head and neck cancers. J Cancer Res Ther. 2009;5:107–112. doi: 10.4103/0973-1482.52800. [DOI] [PubMed] [Google Scholar]
- 74.Zheng XK, Chen LH, Wang WJ, et al. Impact of prolonged fraction delivery times simulating IMRT on cultured nasopharyngeal carcinoma cell killing. Int J Radiat Oncol Biol Phys. 2010;78:1541–1547. doi: 10.1016/j.ijrobp.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 75.Shaikh M, Burmeister J, Joiner M, et al. Biological effect of different IMRT delivery techniques: SMLC, DMLC, and Helical TomoTherapy. Med Phys. 2010;37:762–770. doi: 10.1118/1.3284369. [DOI] [PubMed] [Google Scholar]
- 76.Perna L, Fiorino C, Cozzarini C, et al. Sparing the penile bulb in the radical irradiation of clinically localised prostate carcinoma: A comparison between MRI and CT prostatic apex definition in 3DCRT, Linac-IMRT and Helical Tomotherapy. Radiother Oncol. 2009;93:57–63. doi: 10.1016/j.radonc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 77.Sterzing F, Uhl M, Hauswald H, et al. Dynamic jaws and dynamic couch in helical tomotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1266–1273. doi: 10.1016/j.ijrobp.2009.07.1686. [DOI] [PubMed] [Google Scholar]
- 78.Bortfeld T, Webb S. Single Arc IMRT? Phys Med Biol. 2009;54:9–20. doi: 10.1088/0031-9155/54/1/N02. [DOI] [PubMed] [Google Scholar]
- 79.Van Vulpen M, Field C, Raaijmakers CP, et al. Comparing step-and-shoot IMRT with dynamic helical tomotherapy IMRT plans for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;62:1535–1539. doi: 10.1016/j.ijrobp.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 80.Sheng K, Molloy JA, Read PW. Intensity-modulated radiation therapy (IMRT) dosimetry of the head and neck: A comparison of treatment plans using linear accelerator-based IMRT and helical tomotherapy. Int J Radiat Oncol Biol Phys. 2006;65:917–923. doi: 10.1016/j.ijrobp.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 81.Murthy V, Master Z, Gupta T, et al. Helical TomoTherapy for head and neck squamous cell carcinoma: Dosimetric comparison with linear accelerator-based step-and-shoot IMRT. J Cancer Res Ther. 2010;6:194–198. doi: 10.4103/0973-1482.65245. [DOI] [PubMed] [Google Scholar]
- 82.Sheng K, Molloy J, Larner J, et al. A dosimetric comparison of non-coplanar IMRT versus helical tomotherapy for nasal cavity and paranasal sinus cancer. Radiother Oncol. 2007;82:174–178. doi: 10.1016/j.radonc.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 83.Lee TF, Fang FM, Chao PJ, et al. Dosimetric comparisons of helical tomotherapy and step-and-shoot intensity-modulated radiotherapy in nasopharyngeal carcinoma. Radiother Oncol. 2008;89:89–96. doi: 10.1016/j.radonc.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 84.Lee TK, Rosen II, Gibbons JP, et al. Helical TomoTherapy for parotid gland tumors. Int J Radiat Oncol Biol Phys. 2008;70:883–891. doi: 10.1016/j.ijrobp.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 85.Widesott L, Pierelli A, Fiorino C, et al. Intensity-modulated proton therapy versus helical tomotherapy in nasopharynx cancer: Planning comparison and NTCP evaluation. Int J Radiat Oncol Biol Phys. 2008;72:589–596. doi: 10.1016/j.ijrobp.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 86.Rao M, Yang W, Chen F, et al. Comparison of Elekta VMAT with helical tomotherapy and fixed field IMRT: Plan quality, delivery efficiency and accuracy. Med Phys. 2010;37:1350–1359. doi: 10.1118/1.3326965. [DOI] [PubMed] [Google Scholar]
- 87.Oliver M, Ansbacher W, Beckham WA. Comparing planning time, delivery time and plan quality for IMRT, RapidArc and Tomotherapy. J Appl Med Phys. 2009;10:3068. doi: 10.1120/jacmp.v10i4.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jacob V, Bayer W, Astner ST, et al. A planning comparison of dynamic IMRT for different collimator leaf thicknesses with helical tomotherapy and RapidArc for prostate and head and neck tumors. Strahlenther Onkol. 2010;186:502–510. doi: 10.1007/s00066-010-2124-3. [DOI] [PubMed] [Google Scholar]
- 89.Van Gestel D, van Vliet-Vroegindeweij C, Van den Heuvel F, et al. RapidArc, SmartArc and TomoHD compared with classical step and shoot and sliding window intensity modulated radiotherapy in an oropharyngeal cancer treatment plan comparison. Radiat Oncol. 2013;8:37. doi: 10.1186/1748-717X-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wiezorek T, Brachwitz T, Georg D, et al. Rotational IMRT techniques compared to fixed gantry IMRT and tomotherapy: Multi-institutional planning study for head-and-neck cases. Radiat Oncol. 2011;6:1–10. doi: 10.1186/1748-717X-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Clemente S, Wu B, Sanguineti G, et al. SmartArc-based volumetric modulated arc therapy for oropharyngeal cancer: A dosimetric comparison with both intensity-modulated radiation therapy and helical tomotherapy. Int J Radiat Oncol Biol Phys. 2011;80:1248–1255. doi: 10.1016/j.ijrobp.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 92.Lu SH, Cheng JC, Kuo SH, et al. Volumetric modulated arc therapy for nasopharyngeal carcinoma: A dosimetric comparison with TomoTherapy and step-and-shoot IMRT. Radiat Oncol. 2012;104:324–330. doi: 10.1016/j.radonc.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 93.Kodaira T, Tomita N, Tachibana H, et al. Aichi cancer center initial experience of intensity modulated radiationtherapy for nasopharyngeal cancer using helical TomoTherapy. Int J Radiat Oncol Biol Phys. 2009;73:1129–1134. doi: 10.1016/j.ijrobp.2008.06.1936. [DOI] [PubMed] [Google Scholar]
- 94.Ren G, Du L, Ma L, et al. Clinical observation of 73 nasopharyngeal carcinoma patients treated by helical tomotherapy: The China experience. Technol Cancer Res Treat. 2011;10:259–266. doi: 10.7785/tcrt.2012.500201. [DOI] [PubMed] [Google Scholar]
- 95.Farrag A, Voordeckers M, Tournel K, et al. Pattern of failure after helical TomoTherapy in head and neck cancer. Strahlenther Onkol. 2010;186:511–516. doi: 10.1007/s00066-010-2130-5. [DOI] [PubMed] [Google Scholar]
- 96.Chen AM, Marsano J, Perks J, et al. Comparison of IMRT techniques in the radiotherapeutic management of head and neck cancer: Is tomotherapy “better” than step-and-shoot IMRT? Technol Cancer Res Treat. 2011;10:171–177. doi: 10.7785/tcrt.2012.500192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.