This multicenter, retrospective analysis assessed the efficacy and safety of ipilimumab in 33 patients with unresectable or metastatic mucosal melanoma. The study provides evidence that ipilimumab can result in durable antitumor effects in a subset of patients with mucosal melanoma, although the response rate was low.
Keywords: Mucosal melanoma, Ipilimumab, CTLA-4, Immunotherapy, Cancer-testis antigens
Abstract
The outcome of patients with mucosal melanoma treated with ipilimumab is not defined. To assess the efficacy and safety of ipilimumab in this melanoma subset, we performed a multicenter, retrospective analysis of 33 patients with unresectable or metastatic mucosal melanoma treated with ipilimumab. The clinical characteristics, treatments, toxicities, radiographic assessment of disease burden by central radiology review at each site, and mutational profiles of the patients' tumors were recorded. Available peripheral blood samples were used to assess humoral immunity against a panel of cancer-testis antigens and other antigens. By the immune-related response criteria of the 30 patients who underwent radiographic assessment after ipilimumab at approximately week 12, there were 1 immune-related complete response, 1 immune-related partial response, 6 immune-related stable disease, and 22 immune-related progressive disease. By the modified World Health Organization criteria, there were 1 immune-related complete response, 1 immune-related partial response, 5 immune-related stable disease, and 23 immune-related progressive disease. Immune-related adverse events (as graded by Common Terminology Criteria for Adverse Events version 4.0) consisted of six patients with rash (four grade 1, two grade 2), three patients with diarrhea (one grade 1, two grade 3), one patient with grade 1 thyroiditis, one patient with grade 3 hepatitis, and 1 patient with grade 2 hypophysitis. The median overall survival from the time of the first dose of ipilimumab was 6.4 months (range: 1.8–26.7 months). Several patients demonstrated serologic responses to cancer-testis antigens and other antigens. Durable responses to ipilimumab were observed, but the overall response rate was low. Additional investigation is necessary to clarify the role of ipilimumab in patients with mucosal melanoma.
Implications for Practice:
Melanoma arising from the mucosal surfaces of the body is rare, and patients with this disease have a poor prognosis. Although significant progress has been made in developing new therapies for patients with metastatic melanoma arising from the skin, very little is known about the effects of treatments for patients with mucosal melanoma. Ipilimumab is a promising immunotherapy that has been shown to improve the overall survival of patients with cutaneous melanoma. The efficacy of ipilimumab in patients with mucosal melanoma, however, remains unknown. This article describes the efficacy and toxicities of ipilimumab for patients with mucosal melanoma and shows that, although the overall response rate was low, some patients can achieve durable responses with a reasonable side effect profile. This is the largest study of any specific systemic therapy for patients with mucosal melanoma, although further study is needed.
Introduction
Mucosal melanoma (MM) is a rare subset of melanoma that is clinically and biologically distinct from cutaneous melanoma. Although complete surgical resection is the optimal management of localized MM, given the sensitive anatomic locations where MM arises such as the head and neck, vulvovaginal, or anorectal mucosae, resection with adequate margins is often not possible. Relapse rates after surgery are high [1, 2] and most patients ultimately require systemic therapy. No prospective clinical trials for patients with MM have been published, and the most effective systemic therapy remains undefined.
Ipilimumab (Bristol-Myers Squibb, Princeton, NJ, http://www.bms.com) is a monoclonal antibody that enhances antitumor immunity by blocking the negative regulatory function of cytotoxic T-lymphocyte antigen 4. The overall survival benefit of ipilimumab for patients with cutaneous melanoma has been demonstrated in two phase III studies [3, 4]. Ipilimumab was approved by the U.S. Food and Drug Association in March 2011 and has become a standard of care for patients with unresectable or metastatic melanoma. However, whether patients with MM benefit from ipilimumab is unknown. Very few patients with MM were treated in the clinical trials during the development of ipilimumab. Only preliminary data of patients with MM treated with ipilimumab in the expanded access programs have been presented [5, 6]. As the clinical use of ipilimumab continues to expand, defining its efficacy in patients with MM is essential.
We conducted a multicenter, retrospective analysis of 33 patients with unresectable or metastatic MM treated with ipilimumab. Herein, we report the clinical activity and toxicity observed with this therapeutic approach. In an exploratory manner, we also preliminarily describe antibody responses to cancer-testis antigens and other antigens during treatment with ipilimumab.
Patients and Methods
After institutional review board approval, the databases of three institutions (Memorial Sloan-Kettering Cancer Center, Dana-Farber Cancer Institute, and Massachusetts General Hospital) were queried for all patients with unresectable or metastatic melanoma of primary mucosal origin treated with single-agent ipilimumab. Patients receiving ipilimumab as part of reinduction therapy were excluded. Demographic and other clinical characteristics were determined from institutional databases.
Diagnostic molecular pathology by mass spectroscopy genotyping was performed to determine the presence of genetic mutations involving the BRAF, NRAS, and c-KIT genes. Fluorescence in situ hybridization was performed as previously described [7] to assess the presence of any c-KIT copy number genetic alterations (amplification).
Efficacy and Toxicity Assessment
Tumor responses were assessed radiographically by a single radiologist at each institution (M.B. or N.R.), with each radiologist blinded to clinical outcomes. The immune-related (ir) response criteria (RC) and modified World Health Organization (mWHO) response criteria were applied to determine each patient's response [8]. Patients were designated as having a complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) by each set of radiographic criteria at the time of the first radiographic assessment and at each subsequent radiographic assessment. Overall survival (OS) time was calculated by Kaplan-Meier methodology from the time of the first dose of ipilimumab until the date last documented alive (censored) or date of death by any cause. Toxicity was determined by investigator-assessed chart review and graded using Common Terminology Criteria for Adverse Events [9] (version 4.0).
Serologic Analysis
The sera of treated patients were analyzed for antibody titers against a panel of known antigens by enzyme-linked immunosorbent assay (ELISA) as previously described [10]. The panel consisted mostly of cancer/testis antigens (NY-ESO-1, MAGE-A1, MAGE-A3, MAGE-A4, MAGE-A10, MAGE-C1/CT7, GAGE-2, MAGE-C2/CT10, CT45, HORMAD1/CT46, CT47, CXorf48, CXorf61, SAGE1, PASD1, CSAG2, NXF2, ACTL8, SSX1, SSX2, SSX4, and XAGE-1) and other tumor-related antigens (p53, ERG, GAG-HERV-K, SOX2, ATF2, UBQLN2, Melan-A, PLAC1, FAM178B, TRIM21, TCEA2, LIN28-A, Trp-2, HERV-W, and GAS7). Results were considered positive if antibody titers were greater than 1:100 and were considered to be significantly increased if the titers rose by more than a factor of 5 between any two time points. Seroreactivity against dihydrofolate reductase was used as a negative control. Positive results were confirmed in duplicate.
Results
Thirty-three patients were included in the final analysis. Clinical characteristics of the patients are indicated in Table 1. Of note, the most common primary sites were sinonasal (12 patients), anorectal (8 patients), and vulvovaginal (8 patients). Twenty-five (76%) patients had received systemic therapy for their metastatic disease prior to ipilimumab.
Table 1.
Patient clinical and treatment characteristics (n = 33)
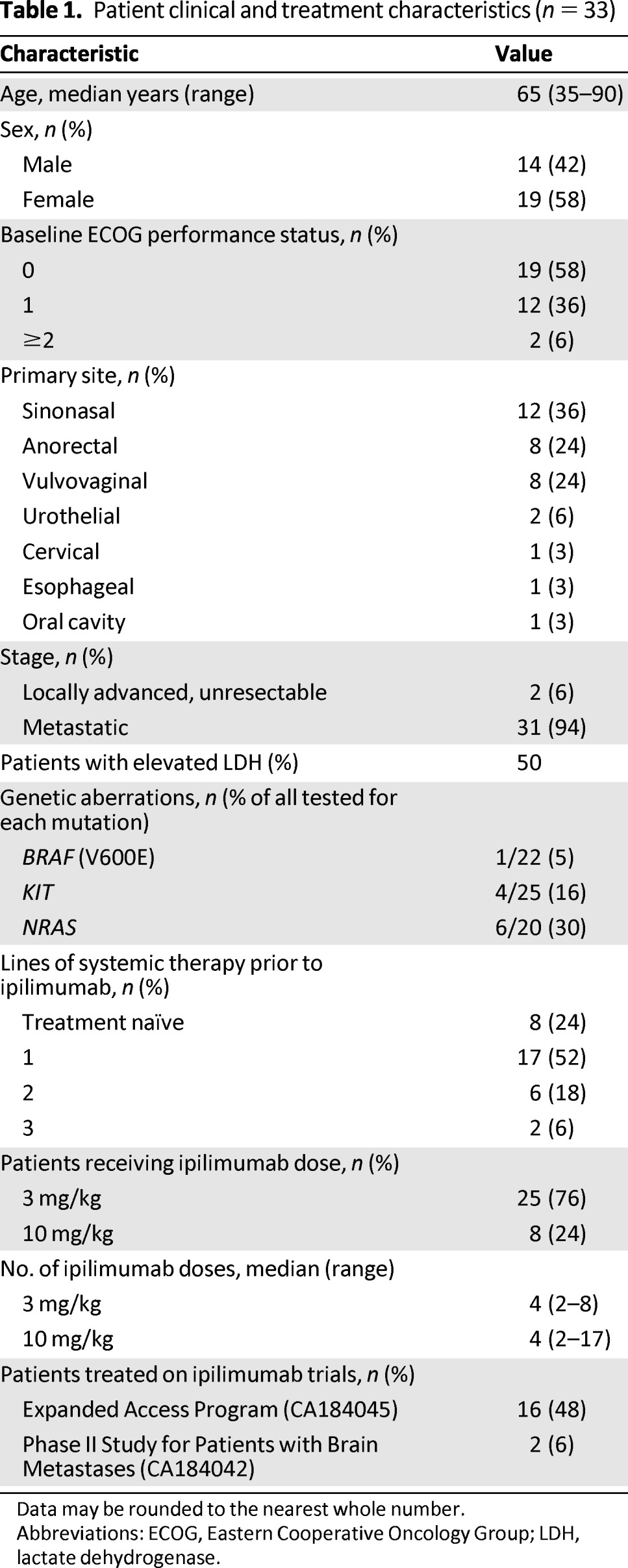
Data may be rounded to the nearest whole number.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase.
On the basis of available tissue and timing of ipilimumab treatment, some patients had undergone molecular analysis. Twenty-two patients were tested for BRAF mutations; one patient had a BRAF V600E mutation. Twenty-five patients underwent analysis for c-KIT genetic aberrations; two patients had exon 11 L576P mutations, one had an exon 11 V560D mutation, and one had genetic amplification but no mutation. Twenty patients underwent analysis for NRAS mutations; six patients had NRAS mutations, as indicated in Table 1.
Eighteen patients received ipilimumab as part of two Bristol-Myers Squibb clinical protocols: 16 patients were part of an expanded-access protocol (CA184045) and two were part of a protocol for patients with brain metastases (CA184042). If patients treated on these protocols achieved stable disease or better at week 12, they were permitted to receive maintenance ipilimumab on week 24 and thereafter every 12 weeks. Fifteen patients received ipilimumab off protocol as part of standard clinical practice at 3 mg/kg every 3 weeks for a maximum of four doses.
Response Analysis
Out of the 33 patients included in the study, 30 were evaluable for radiographic assessment of changes to their total tumor burden following ipilimumab. Of the three patients who were not radiographically evaluable, one patient did not have a radiographic assessment after ipilimumab due to deterioration from complications of chronic obstructive pulmonary disease neither related to ipilimumab nor melanoma; one had progressive central nervous system disease requiring treatment and did not undergo subsequent systemic disease radiographic assessment; and one had a nonmeasurable esophageal primary lesion at baseline and after ipilimumab, making assessment of the change in total tumor burden not possible.
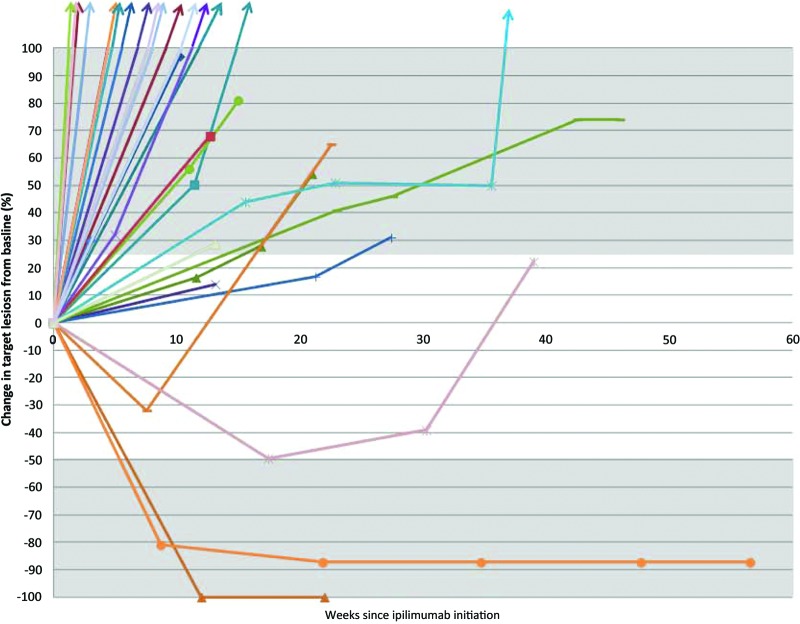
By the irRC, at the time of the first radiographic assessment (approximately week 12), there were one irCR, one irPR, and six irSD cases. Both the irCR and irPR were confirmed on subsequent scans as required by the irRC. Twenty-two patients had PD at the time of the first scan; however, only 10 of these patients with PD had a follow-up scan to document progression as required by irRC (Fig. 1). The overall response rate by irRC in evaluable patients was 6.7% (2 of 30 patients; 95% confidence interval [CI]: 0.8%–22.1%). By the mWHO criteria, at the time of the first radiographic assessment (approximately week 12), one CR, one PR, and five SD cases were observed. Twenty-three patients had PD at the time of the first scan. One patient classified as irSD was reclassified as having PD by mWHO given the appearance of new lesions. The overall response rate in evaluable patients by mWHO (6.7%, 2 of 30 patients) was identical to the response rate by the irRC.
Figure 1.
Spider-plot demonstrating changes in tumor burden over time for each of the 30 radiographically evaluable patients. Tumor burden was assessed as the sum of the products of the perpendicular diameters of each target lesion as per the immune-related response criteria and modified World Health Organization response criteria. The gray shaded areas indicate the cutoffs for partial response (≥50% tumor burden reduction) or progressive disease (≥25% tumor burden increase).
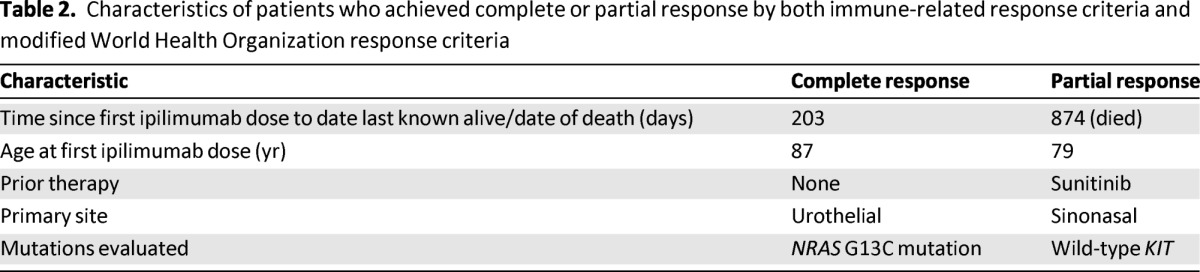
The patient who achieved a complete response by both irRC and mWHO had MM arising from the urinary bladder with metastatic sites involving the perivesical wall, retroperitoneum, liver, and lung. Ipilimumab was administered at 3 mg/kg, and the complete response has been durable, currently ongoing at 22 weeks (Fig. 2). This patient had not received any treatment for metastatic disease prior to ipilimumab and suffered from hypophysitis requiring ongoing treatment with replacement hydrocortisone and thyroid hormone. The patient who achieved a PR by both irRC and mWHO had previously been treated with sunitinib and achieved a response to ipilimumab that lasted 56 weeks. Ipilimumab therapy in this patient was associated with a persistent grade 1 rash. This patient subsequently developed progressive disease and died 2.4 years after starting ipilimumab (Table 2). One patient previously treated with cisplatin, vinblastine, and dacarbazine and also imatinib nearly met criteria for an irPR with a reduction in baseline tumor burden of −49.4% but was classified as achieving irSD. This patient ultimately progressed after 39 weeks.
Figure 2.
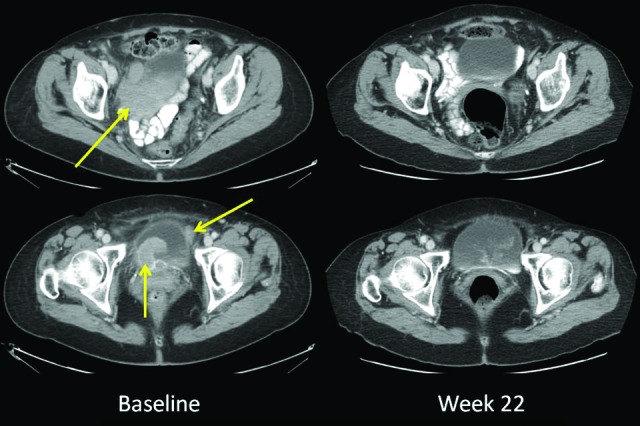
Representative images of a patient achieving a complete response both by immune-related response criteria and modified World Health Organization response criteria. The patient had a primary mucosal melanoma involving the bladder with perivesical metastases. Small hepatic, pulmonary, and retroperitoneal metastases were also present. The complete response has been durable, ongoing at 22 weeks.
Table 2.
Characteristics of patients who achieved complete or partial response by both immune-related response criteria and modified World Health Organization response criteria
Overall Survival Analysis
After a median follow-up of 9.9 months (range: 5.8–20.2 months) for survivors, the median overall survival for the entire cohort was 6.4 months (Fig. 3). Mutational status, ipilimumab dose, baseline lactate dehydrogenase, baseline Eastern Cooperative Oncology Group performance status, and number of prior therapies were not associated with overall survival by univariate analysis. The median baseline absolute lymphocyte count (ALC) was 780 cells/μL; the baseline ALC was not associated with OS.
Figure 3.
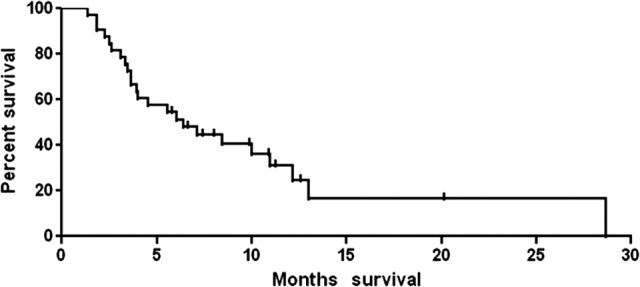
Overall survival of cohort (n = 33).
Toxicity Analysis
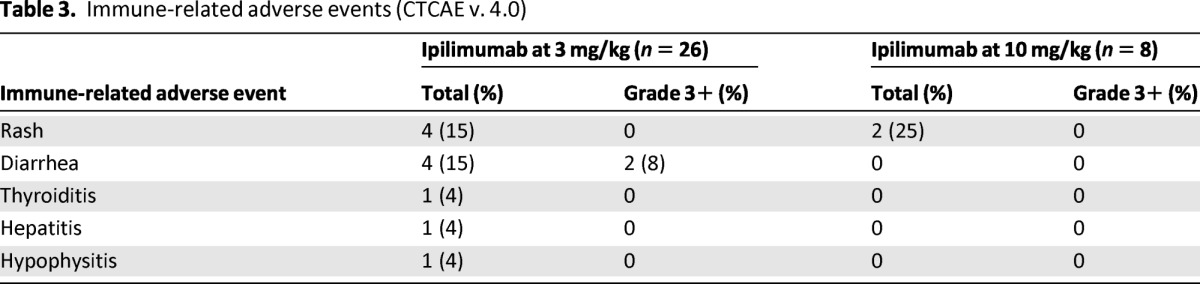
Rash was the most common immune-related adverse event (irAE), affecting six patients (Table 3). Although most cases were grade 1, two patients had grade 2 rashes. Three patients had diarrhea. Two of these three patients suffered grade 3 diarrhea. Less common irAEs included one patient with thyroiditis (grade 1), one patient with hypophysitis (grade 2), and one patient with hepatitis (grade 3). No infrequent irAEs such as uveitis or pneumonitis were experienced. Some patients required systemic steroids to treat their irAEs; however, all toxicities resolved without significant long-term sequelae. Although the numbers were small, there did not seem to be an ipilimumab dose-dependent effect on the occurrence of irAEs.
Table 3.
Immune-related adverse events (CTCAE v. 4.0)
Serologic Analysis
Nine patients treated at Memorial Sloan-Kettering Cancer Center had sera available for analysis prior to and after at least one ipilimumab dose. Of these patients, one experienced seroconversion to the defined cancer-testis antigen NY-ESO-1 after two doses of ipilimumab. This patient did not display antibody reactivity to the other tested antigens; although stable disease was achieved at first radiographic assessment (week 15), disease progression ultimately occurred. Another patient with progressive disease was seropositive against the p53, GAG-HERV-K, and CSAG2 antigens both before and after ipilimumab. A third patient was seropositive for GAG-HERV-K before and after ipilimumab and had an increase in antibody titers against the CT45 antigen during ipilimumab treatment; this third patient had progressive disease. The other six patients evaluated were not reproducibly seropositive to the antigens in the defined panel.
In addition to the nine patients who had sera samples available before and after ipilimumab, an additional patient had serum available for analysis at the time of the third dose and 2 weeks after the fourth dose of ipilimumab. This patient was seropositive for antibodies directed against NY-ESO-1 and p53 but had progressive disease. Another patient with progressive disease had serum available 6 months after the fourth dose of ipilimumab demonstrating seroreactivity against the antigen CT45.
Discussion
Our retrospective study evaluating the activity of ipilimumab in 33 patients with advanced MM provides evidence that although the response rate is low, ipilimumab can result in durable antitumor effects in a subset of patients with MM. The overall durable response rate (CR and PR) by both irRC and mWHO in our study was 6.7%. These results are consistent with response rates by mWHO of 4.2%–10.9% reported in prior trials of ipilimumab monotherapy for patients predominantly with cutaneous melanoma [4, 11–13]. Although the overall patient numbers are relatively low, the rate of irAEs we observed appears to be consistent with prior trials as well, including the 8% rate (10 out of 131 patients) of grade 3+ toxicity in patients with MM reported from the ipilimumab expanded access program [6]. The median overall survival of 6.4 months seen in our cohort is consistent with the median overall survival of 6.7 months presented for patients with MM treated with ipilimumab in the Italian expanded access program [5].
Limited published data exist regarding the efficacy of therapies commonly used for cutaneous melanoma in patients with MM. One prior publication reported the efficacy of dacarbazine-based chemotherapy regimens in patients with noncutaneous melanoma [14]. Although MM was found to be a poor prognostic factor in multivariate analysis in this study, patients had received a variety of dacarbazine-based regimens, and the specific efficacy for patients with MM was not independently reported.
Smaller series have reported the efficacy of biochemotherapy in patients with MM. The response rate we observed in our study is lower than the response rates of 36%–47% reported in single-institution studies of biochemotherapy (cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon-alpha) for patients with MM [15–17]. The patient population treated in these studies was largely treatment naïve and younger than our patient population, which may have led to a selection bias favoring the efficacy of biochemotherapy. Furthermore, these prior studies of biochemotherapy for patients with MM were small, ranging from 11 to 18 patients each.
Significant efforts in genetically profiling MM have revealed important genetic differences between MM and cutaneous melanoma. Whereas almost 50% of patients with cutaneous melanoma will harbor a mutation in the serine-threonine protein kinase BRAF [18, 19], BRAF mutations have been found less frequently in patients with MM [20, 21]. Instead, patients with MM have a high proportion of activating mutations and/or amplifications involving the receptor tyrosine kinase gene, c-KIT [22, 23].
Our study of patients with MM revealed a similar pattern with a higher level of c-KIT genetic aberrations (16% of tumors tested) compared with BRAF mutations (5% of tumors tested). Importantly, however, this study selected patients with MM treated with ipilimumab. This may have led to an underrepresented number of patients with BRAF or KIT mutations who may have instead been treated with BRAF inhibitors such as vemurafenib or KIT inhibitors such as imatinib. We did not observe correlations between ipilimumab response and the underlying genetic subtype of MM, possibly due to the small number of patients within each group.
The proportion of NRAS mutations (30% of tumors tested) observed in our cohort was higher than expected. Since the presence of NRAS mutations has been associated with inferior OS in patients with advanced melanoma in some series [24], although speculative, this may have influenced the lower median OS time observed (6.4 months) in our cohort compared with the median OS time of 10.1 months in a phase III evaluation of ipilimumab [4]. Biological differences between MM and cutaneous melanoma, independent of mutational status, or differences in baseline patient characteristics may also be involved in this observed difference in median OS. It is also possible that our observed lower OS time may have been due to the fact that many patients were treated on the compassionate, expanded access trial of ipilimumab and may not have otherwise been considered appropriate clinical trial candidates. Further, the baseline ALC of 780 cells per microliter suggests that the cohort was generally lymphopenic at the start of ipilimumab therapy. Because a low ALC has been described to be a poor prognostic characteristic for patients with malignancy, it is possible this may be an additional reflection of the poor underlying health of our cohort prior to ipilimumab therapy [25]. We speculate that this may also be related to the shorter median OS time seen in this small cohort compared with patients with cutaneous melanoma treated in prior phase III trials.
Our investigation is limited by several factors. First, the retrospective nature may have led to an underreporting of irAEs, which were considered when they were clearly documented in the medical record. Further, our cohort size of 33 patients is small. Sample size limitations may have therefore obscured real differences that exist among patients with various genetic abnormalities or other clinical characteristics, possibly relevant to ipilimumab response. Nevertheless, mutational status was not shown to be associated with ipilimumab response in another study, similar to our results [26]. Finally, our cohort was heterogeneous, and patients received treatment with various ipilimumab doses and schedules.
Despite these inherent limitations, to our knowledge, this investigation represents the first published clinical experience reporting the outcomes of patients with MM treated with ipilimumab. It is the largest of any prior study evaluating systemic therapy for patients with MM [15–17], and three cancer centers were involved to minimize biases inherent in single-institutional studies.
As advances in the molecular profiling of MM lead to novel targeted therapeutic strategies, understanding the role of ipilimumab in the treatment of MM is essential. Ipilimumab appears to have activity in a subset of patients with MM, and patients with this melanoma subtype should not be excluded from future immunotherapy clinical trials. Immunologic mechanisms yet to be discovered that are unique to MM may exist that could ultimately be manipulated to maximize responses to immunotherapy.
Our preliminary descriptive serologic analyses revealed that patients with mucosal melanoma can have measurable immune responses to known cancer-testis antigens and other antigens. The seroconversion observed in one patient to NY-ESO-1 and the increase in antibody reactivity to CT45 after ipilimumab therapy is suggestive, but not conclusive, of ipilimumab's immunomodulatory role in this patient population. Serum samples were not available from the two responding patients to compare changes in antibody titers between responders and nonresponders. Inferences correlating antibody responses with clinical outcomes would therefore require a larger patient cohort. These serologic analyses were intended to be very exploratory and hypothesis generating.
CT45 has been described as a cancer testis antigen expressed in classical Hodgkin lymphoma, melanoma, and other malignancies [27, 28]. Antibody responses to this antigen are rare. Interestingly, we found two patients in our small cohort who were seropositive for CT45. Whether this unexpected finding represents a unique immunologic response in patients with mucosal melanoma that could be relevant for future immunotherapeutic approaches requires further study. GAG-HERV-K is a retroviral antigen that has been described in men with prostate cancer [29]. Neither of the two patients with detectable antibody titers against GAG-HERV-K had a known history of prostate cancer, but both were older men for whom this diagnosis was possible.
Although some patients achieved durable responses, the overall response rate in this cohort was low. This may be related to the unique biology of MM, but it requires further study. We expect the role of ipilimumab for patients with MM will be further clarified after results are ultimately available from the currently ongoing prospective study of ipilimumab for patients with MM (NCT 01355120). Additional research is also necessary to define the possibly distinct immunologic mechanisms that may be involved in immunotherapeutic approaches for patients with metastatic mucosal melanoma.
Acknowledgments
This study was supported by grants from the National Institutes of Health (RC2CA148468), the Melanoma Research Alliance, Swim Across America, the Cancer Research Institute, the Lita Annenberg Hazen Foundation, and the Commonwealth Foundation for Cancer Research. This investigation was supported by grant UL1-TR000457 of the Clinical and Translational Science Center at Weill Cornell Medical College.
We thank the following individuals for their contributions to this work: Erika Ritter, Rita Chiu, and Christine Sedrak for their assistance performing the serologic assays; Kita Bogatch for database management and providing details of clinical information on the patients treated at Memorial Sloan-Kettering Cancer Center; and Jianda Yuan for his leadership and direction of the immune-monitoring facility at Memorial Sloan-Kettering Cancer Center.
Footnotes
Editor's Note: See the accompanying commentary on pages 658–660.
Author Contributions
Conception/Design: Michael A. Postow, Jason J. Luke, Richard D. Carvajal
Provision of study material or patients: Michael A. Postow, Jason J. Luke, Donald P. Lawrence, Nageatte Ibrahim, Keith T. Flaherty, Ryan J. Sullivan, Patrick A. Ott, Sandra D'Angelo, Mark A. Dickson, Gary K. Schwartz, Paul B. Chapman, Jedd D. Wolchok, F. Stephen Hodi, Richard D. Carvajal
Collection and/or assembly of data: Michael A. Postow, Jason J. Luke, Mark Bluth, Nikhil Ramaiya, Sacha Gnjatic
Data analysis and interpretation: Michael A. Postow, Jason J. Luke, Katherine Panageas, Sacha Gnjatic, Richard D. Carvajal
Manuscript writing: Michael A. Postow, Jason J. Luke, Richard D. Carvajal
Final approval of manuscript: Michael A. Postow, Jason J. Luke, Katherine Panageas, Donald P. Lawrence, Nageatte Ibrahim, Keith T. Flaherty, Ryan J. Sullivan, Patrick A. Ott, Margaret K. Callahan, James J. Harding, Sandra D'Angelo, Mark A. Dickson, Gary K. Schwartz, Paul B. Chapman, Sacha Gnjatic, Jedd D. Wolchok, F. Stephen Hodi, Richard D. Carvajal
Disclosures
Michael A. Postow: Bristol-Myers Squibb (C/A); Margaret K. Callahan: Bristol-Myers Squibb (RF); Jedd D. Wolchok: Bristol-Myers Squibb (C/A, RF); F. Stephen Hodi: Bristol-Myers Squibb (C/A, RF); Richard D. Carvajal: Bristol-Myers Squibb (RF). The other authors indicated no financial relationships.
C/A: Consulting/advisory relationship; RF: Research funding; E: Employment; H: Honoraria received; OI: Ownership interests; IP: Intellectual property rights/inventor/patent holder; SAB: scientific advisory board
Reference
- 1.Patel SG, Prasad ML, Escrig M, et al. Primary mucosal malignant melanoma of the head and neck. Head Neck. 2002;24:247–257. doi: 10.1002/hed.10019. [DOI] [PubMed] [Google Scholar]
- 2.Yeh JJ, Shia J, Hwu WJ, et al. The role of abdominoperineal resection as surgical therapy for anorectal melanoma. Ann Surg. 2006;244:1012–1017. doi: 10.1097/01.sla.0000225114.56565.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 4.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Vecchio M, Simeone E, Chiarion Sileni V, et al. Efficacy and safety of ipilimumab in patients with pretreated, mucosal melanoma: Experience from Italian clinics participating in the European Expanded Access Programme. ESMO. 2012 Abstract 1130P. [Google Scholar]
- 6.Lawrence D, McDermott D, Hamid O, et al. Treatment of patients (pts) with stage III or IV melanoma on an ipilimumab (Ipi) expanded access program (EAP): Results for 3mg/kg cohort. Presented at the Ninth International Congress of the Society for Melanoma Research; November 8, 2012; Hollywood, CA. [Google Scholar]
- 7.Antonescu CR, Busam KJ, Francone TD, et al. L576P KIT mutation in anal melanomas correlates with KIT protein expression and is sensitive to specific kinase inhibition. Int J Cancer. 2007;121:257–264. doi: 10.1002/ijc.22681. [DOI] [PubMed] [Google Scholar]
- 8.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 9.Bethesda, MD: National Cancer Institute; 2009. May, [Accessed May 12, 2013]. Cancer Therapy Evaluation Program (CTEP) Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Available at http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010–06-14_QuickReference_5x7.pdf. [Google Scholar]
- 10.Gnjatic S, Old LJ, Chen YT. Autoantibodies against cancer antigens. Methods Mol Biol. 2009;520:11–19. doi: 10.1007/978-1-60327-811-9_2. [DOI] [PubMed] [Google Scholar]
- 11.Hersh EM, O'Day SJ, Powderly J, et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naive patients with advanced melanoma. Invest New Drugs. 2011;29:489–498. doi: 10.1007/s10637-009-9376-8. [DOI] [PubMed] [Google Scholar]
- 12.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: A randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 13.Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–5598. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 14.Yi JH, Yi SY, Lee HR, et al. Dacarbazine-based chemotherapy as first-line treatment in noncutaneous metastatic melanoma: Multicenter, retrospective analysis in Asia. Melanoma Res. 2011;21:223–227. doi: 10.1097/CMR.0b013e3283457743. [DOI] [PubMed] [Google Scholar]
- 15.Kim KB, Sanguino AM, Hodges C, et al. Biochemotherapy in patients with metastatic anorectal mucosal melanoma. Cancer. 2004;100:1478–1483. doi: 10.1002/cncr.20113. [DOI] [PubMed] [Google Scholar]
- 16.Harting MS, Kim KB. Biochemotherapy in patients with advanced vulvovaginal mucosal melanoma. Melanoma Res. 2004;14:517–520. doi: 10.1097/00008390-200412000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Bartell HL, Bedikian AY, Papadopoulos NE, et al. Biochemotherapy in patients with advanced head and neck mucosal melanoma. Head Neck. 2008;30:1592–1598. doi: 10.1002/hed.20910. [DOI] [PubMed] [Google Scholar]
- 18.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 20.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 21.Maldonado JL, Fridlyand J, Patel H, et al. Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst. 2003;95:1878–1890. doi: 10.1093/jnci/djg123. [DOI] [PubMed] [Google Scholar]
- 22.Curtin JA, Busam K, Pinkel D, et al. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24:4340–4346. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 23.Rivera RS, Nagatsuka H, Gunduz M, et al. C-kit protein expression correlated with activating mutations in KIT gene in oral mucosal melanoma. Virchows Arch. 2008;452:27–32. doi: 10.1007/s00428-007-0524-2. [DOI] [PubMed] [Google Scholar]
- 24.Jakob JA, Bassett RL, Ng CS, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer. 2012;118:4014–4023. doi: 10.1002/cncr.26724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ray-Coquard I, Cropet C, Van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69:5383–5391. doi: 10.1158/0008-5472.CAN-08-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahabi V, Whitney G, Hamid O, et al. Assessment of association between BRAF-V600E mutation status in melanomas and clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61:733–737. doi: 10.1007/s00262-012-1227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YT, Chadburn A, Lee P, et al. Expression of cancer testis antigen CT45 in classical Hodgkin lymphoma and other B-cell lymphomas. Proc Natl Acad Sci U S A. 2010;107:3093–3098. doi: 10.1073/pnas.0915050107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen YT, Hsu M, Lee P, et al. Cancer/testis antigen CT45: Analysis of mRNA and protein expression in human cancer. Int J Cancer. 2009;124:2893–2898. doi: 10.1002/ijc.24296. [DOI] [PubMed] [Google Scholar]
- 29.Ishida T, Obata Y, Ohara N, et al. Identification of the HERV-K gag antigen in prostate cancer by SEREX using autologous patient serum and its immunogenicity. Cancer Immun. 2008;8:15. [PMC free article] [PubMed] [Google Scholar]