Spinal metastases frequently arise in patients with cancer. This article describes the neurologic, oncologic, mechanical, and systemic decision framework used at Memorial Sloan-Kettering Cancer Center to determine the optimal therapy for patients with spinal metastases.
Abstract
Background.
Spinal metastases frequently arise in patients with cancer. Modern oncology provides numerous treatment options that include effective systemic, radiation, and surgical options. We delineate and provide the evidence for the neurologic, oncologic, mechanical, and systemic (NOMS) decision framework, which is used at Memorial Sloan-Kettering Cancer Center to determine the optimal therapy for patients with spine metastases.
Methods.
We provide a literature review of the integral publications that serve as the basis for the NOMS framework and report the results of systematic implementation of the NOMS-guided treatment.
Results.
The NOMS decision framework consists of the neurologic, oncologic, mechanical, and systemic considerations and incorporates the use of conventional external beam radiation, spinal stereotactic radiosurgery, and minimally invasive and open surgical interventions. Review of radiation oncology and surgical literature that examine the outcomes of treatment of spinal metastatic tumors provides support for the NOMS decision framework. Application of the NOMS paradigm integrates multimodality therapy to optimize local tumor control, pain relief, and restoration or preservation of neurologic function and minimizes morbidity in this often systemically ill patient population.
Conclusion.
NOMS paradigm provides a decision framework that incorporates sentinel decision points in the treatment of spinal metastases. Consideration of the tumor sensitivity to radiation in conjunction with the extent of epidural extension allows determination of the optimal radiation treatment and the need for surgical decompression. Mechanical stability of the spine and the systemic disease considerations further help determine the need and the feasibility of surgical intervention.
Implications for Practice:
Treatment of spinal metastatic tumors requires a multidisciplinary approach which integrates radiation and medical oncology, surgery, and interventional radiology. The NOMS framework described in this manuscript incorporates the neurologic, oncologic, mechanical, and systemic considerations to facilitate decision making in the care of patients with spinal metastases. Furthermore, this framework allows dynamic integration of novel systemic and radiation options which is crucial in these rapidly evolving disciplines. The article summarizes the supporting literature for this framework and provides the results of implementation of the NOMS paradigm in the care of cancer patients.
Introduction
Spinal metastases occur in 20% of all patients with cancer [1, 2], with 5%–10% of patients with cancer developing spinal cord compression [3, 4]. The treatment of spinal metastases is palliative, with the goals of providing pain relief, maintenance or recovery of neurologic function, local durable tumor control, spinal stability, and improved quality of life. Over the past decade, treatment has evolved from simple decisions regarding the need for either surgery or conventional external beam radiation (cEBRT) to complex multimodality assessments that require the integration of new technologies such as stereotactic radiosurgery (SRS) and percutaneous cement augmentation.
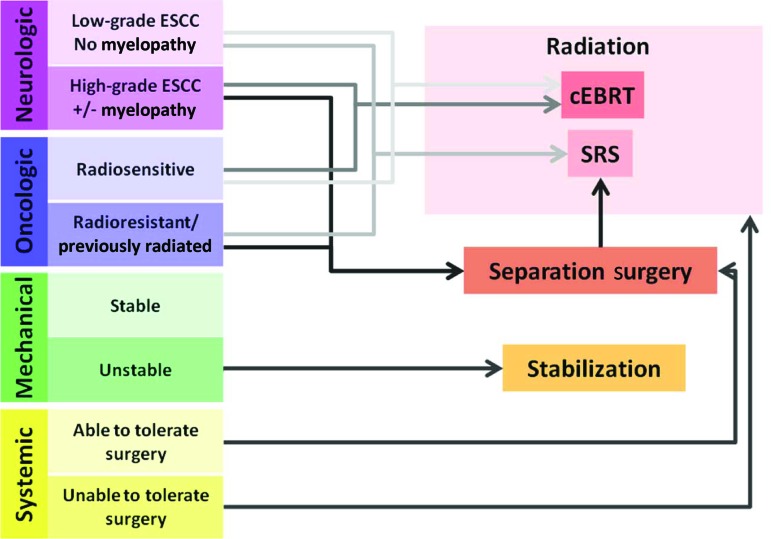
Over the past 15 years, the multidisciplinary spine team at Memorial Sloan-Kettering Cancer Center (MSKCC) has developed and used a decision framework for metastatic spine disease, NOMS, which incorporates four fundamental assessments: neurologic, oncologic, mechanical instability, and systemic disease. The goal of NOMS is to provide a dynamic framework for the treatment of spine metastases that integrates these four sentinel decision points to determine the use of radiation, surgery, and/or systemic therapy. NOMS assessment provides the ability to incorporate advances in interventional radiology, radiation and medical oncology, and surgical techniques to optimize patient outcomes. Furthermore, NOMS provides physicians with a common language across disciplines to help develop treatment plans for individual patients and foster outcome analysis across institutions.
Briefly, in NOMS, the neurologic consideration is an assessment of the degree of epidural spinal cord compression, myelopathy, and/or functional radiculopathy. The oncologic consideration is predicated on the expected tumoral response and durability of response to available treatments, such as conventional external beam radiation therapy, SRS, surgery, chemotherapy, hormones, immunotherapy, or biologics. Mechanical instability is a separate consideration defined for pathologic fractures; the treatment considerations include brace application, percutaneous cement and/or pedicle screw augmentation, or open surgery. The fourth consideration is the extent of systemic disease and medical comorbidities to evaluate the ability of the patient to tolerate a proposed treatment and the overall expected patient survival based on extent of disease and tumor histology.
Because radiation and surgery are presently the two most effective treatments for spinal metastases, the focus of this paper will be the application of NOMS in determining the optimal combination of radiation and surgery. It must be stressed, however, that the current indications for radiation, surgery, and medical management are expected to evolve so that future treatment options for metastatic tumors will change. Over the past 15 years, the spine team at MSKCC has employed the NOMS framework. Although the four assessments have remained constant, the treatments have changed dramatically with the integration of new technologies and new outcomes data.
Neurologic Assessment
The neurologic and oncologic indications are considered together. The neurologic considerations focus on the degree of spinal cord compromise and include a radiographic assessment of the degree of epidural spinal cord compression (ESCC) and the clinical assessment of myelopathy and/or functional radiculopathy. The clinical and radiographic assessments are clearly related, with only patients with radiographic cord compression being at risk of exhibiting neurological deficits attributable to epidural tumor. Thus, although myelopathy is a critical neurologic assessment, much of the decision making under the neurologic consideration is based on the degree of ESCC.
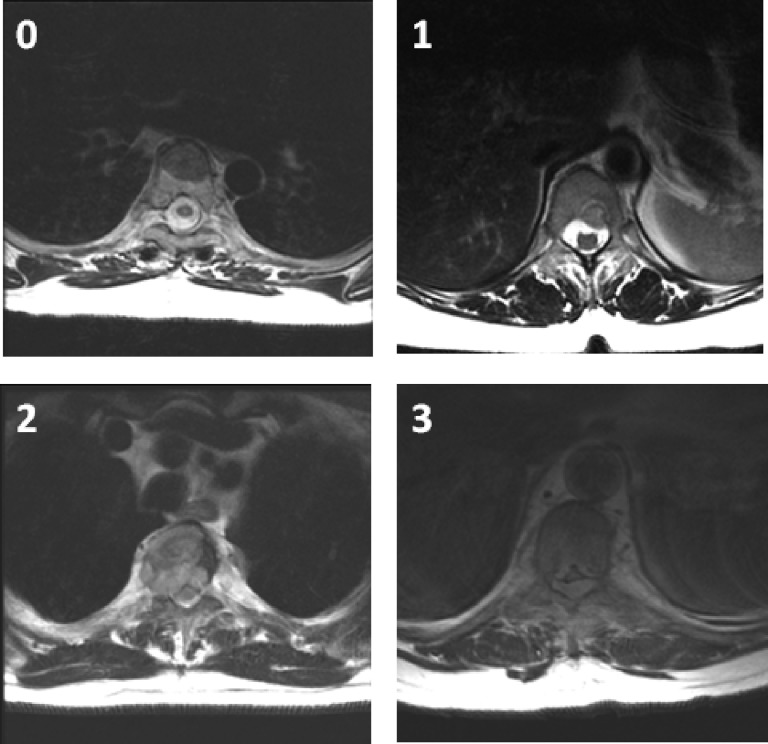
A six-point grading system was designed and validated by the Spine Oncology Study Group (SOSG) to describe the degree of ESCC [5]. This system uses axial T2-weighted images at the site of most severe compression (Fig. 1). In the absence of mechanical instability, Grades 0, 1a, and 1b are considered for radiation as initial treatment. Grades 2 and 3 describe high-grade ESCC and, unless the tumor is highly radiosensitive, require surgical decompression prior to radiation therapy. The role of surgery and radiosurgery in patients with grade 1c epidural tumors remains to be clearly defined, but the integration of high-dose hypofractionated radiation may allow administration of SRS while avoiding spinal cord toxicity.
Figure 1.
A six-point grading system by the Spine Oncology Study Group [5] uses axial T2-weighted images at the site of most severe compression to describe the degree of epidural spinal cord compression: 0, tumor is confined to bone only; 1, tumor extension into the epidural space without deformation of the spinal cord; 2, spinal cord compression but cerebrospinal fluid is visible; and 3, spinal cord compression without visible cerebrospinal fluid. The grade 1 delineation is further subdivided into 1a–1c: 1a, epidural impingement but no deformation of the thecal sac; 1b, deformation of the thecal sac but without spinal cord abutment; and 1c, deformation of the thecal sac with spinal cord abutment but without compression.
Oncologic Assessment
The oncologic consideration is the responsiveness of a tumor to currently available treatments. At present, radiation is the most effective and least invasive modality for local tumor control. Therefore, much of the oncologic consideration is devoted to determining the radiation sensitivity of the tumor. Tumors are considered to be radiosensitive or radioresistant based on their response to cEBRT, which is delivered in one or two radiation beams without precise conformal techniques. The fraction dose that can be delivered using cEBRT is significantly limited by the spinal cord within the radiation field. Recent technological advances allow image-guided delivery of conformal radiation doses with high spatial precision, known as image-guided radiation therapy (IGRT). IGRT can deliver high doses of radiation in close proximity to the spinal cord while maintaining radiation exposure of the spinal cord and other adjacent vital structures within the limits of safety. SRS, which delivers high doses of tightly focused radiation, relies on IGRT platforms and can be administered as a single fraction or in 3–5 fractions using a hypofractionated schedule.
A review of the literature shows that tumor histology is perhaps the most important factor in determining response to cEBRT. Among patients who underwent cEBRT in the setting of spinal metastases, the mean ambulation rate was 81% (range: 58%–100%) [6]. However, only 6%–67% percent of nonambulatory patients recovered ambulation, with reports in the 60% range thought to be attributable to the large number of favorable histologies in those series [7]. Literature analysis reveals that all authors classify lymphoma, seminoma, and myeloma as radiosensitive histologies (Table 1) and supports the use of cEBRT to treat these tumors, regardless of the degree of ESCC or neurologic deficit [7–14]. On the other hand, solid tumors exhibit a wide range of radiosensitivity. Radiosensitive solid tumor histologies include breast, prostate, ovarian, and neuroendocrine carcinomas. Renal, thyroid, hepatocellular, colon, and non-small cell lung carcinomas, sarcoma, and melanoma represent radioresistant tumors [7–15]. Solid tumors with radioresistant histologies generally require SRS to achieve durable local control, whereas radiosensitive solid tumors may be treated with cEBRT or SRS.
Table 1.
Summary of expected radiation response based on histology
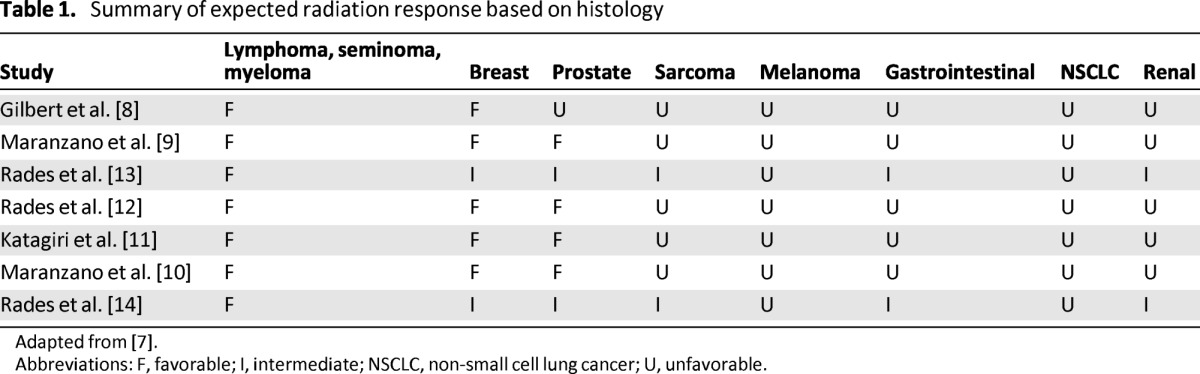
Adapted from [7].
Abbreviations: F, favorable; I, intermediate; NSCLC, non-small cell lung cancer; U, unfavorable.
Radiosensitive Tumors
Patients with radiosensitive tumors may be treated with cEBRT regardless of the ESCC grade. Conventional EBRT provides both symptomatic relief and satisfactory local control rates for patients with radiosensitive tumors. This approach is effective, regardless of the degree of ESCC, and has been shown to improve ambulatory status, provide durable local tumor control, and provide pain relief. A prospective study conducted by Maranzano and Latini showed myeloma, breast, and prostate cancer had respective response durations of 16, 12, and 10 months, with 67% of nonambulatory patients secondary to breast metastases regaining ambulation [16]. Katagiri et al. found that 72% of patients with favorable histologies exhibited combined improvement in their motor strength, functional ability, and pain scores [11]. Several additional studies confirm that patients with favorable histologies are more likely to have good postradiation ambulation and remain ambulatory longer than patients with unfavorable primary histologies [8, 12]. The appropriate radiation dose and fractionation vary according to the goal of treatment. Although short-course radiation (800 cGy × 1 and 400 cGy × 5) provides short-term palliation, long-course radiation with higher total doses provides more durable tumor control [12, 17].
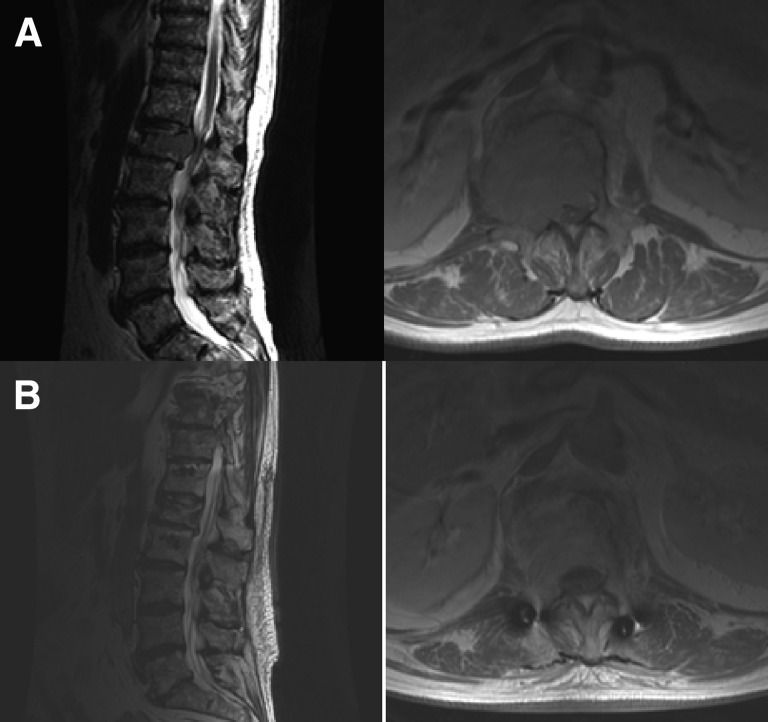
With such favorable responses to cEBRT, patients with radiosensitive spine metastases are treated with cEBRT, often avoiding surgical intervention. Furthermore, the literature supports the use of cEBRT even when there is evidence of high-grade ESCC from radiosensitive tumors due to the ability of cEBRT to cause mitotic cell death within the tumor and subsequent spinal cord decompression [16, 18] without causing damage to surrounding neurologic tissues (Fig. 2).
Figure 2.
This 67-year-old man with no cancer history presented with back pain. Pain was secondary to an L1 tumor (A). Percutaneous needle biopsy provided the diagnosis of multiple myeloma. The patient was started on high-dose dexamethasone and underwent conventional external beam radiation. (B): The epidural tumor entirely resolved 11 weeks later.
Radioresistant Tumors Without High-Grade ESCC
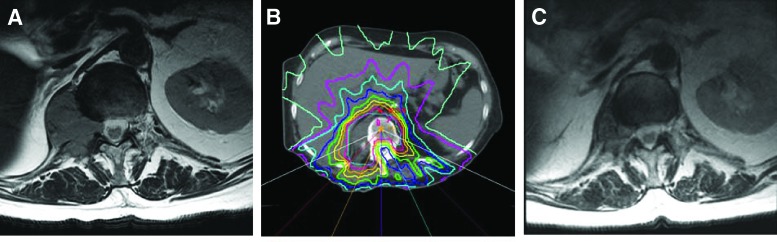
Patients with radioresistant tumors and ESCC grades 0, 1a, and 1b can be treated with IGRT and do not require surgical decompression. Radioresistant tumors do not have acceptable response rates to cEBRT. Maranzano et al. demonstrated a response rate of only 20% for tumors such as hepatocellular carcinoma, with a durability of 1–3 months [16]. Katagiri et al. showed a 33% success rate in treating radioresistant histologies [11]. This is due to limitation of cEBRT in delivering tumoricidal doses of radiation to radioresistant tumors without high risk of spinal cord or adjacent organ (e.g., kidney) toxicity. On the other hand, growing evidence suggests that despite some histologies being radioresistant to cEBRT, durable local tumor control can be achieved in these tumors using SRS. Series reporting outcomes for high-dose SRS have demonstrated radiographic and clinical responses of greater than 85% regardless of tumor histology [7]. SRS is also effective for alleviating pain, with studies showing either a partial or complete pain response in 85%–92% of patients treated with spine radiosurgery [19–22]. Yamada et al. used SRS to treat 103 patients with radioresistant oligometastatic tumors [23]. Local control was 92% at a median follow-up time of 16 months. This study included a dose escalation from 18 to 24 Gy. A subgroup analysis revealed even greater local control rates in those patients receiving 24 Gy. A recent review of 413 patients treated with SRS continues to demonstrate this dose response, with patients treated with 24 Gy having a recurrence rate of 3% at 3-year follow-up, independent of histology (Fig. 3) [24].
Figure 3.
Imaging studies from a 77-year-woman. (A): The patient had renal cell carcinoma with T12 metastasis that was causing radicular pain. (B): The patient underwent single-fraction stereotactic radiosurgery (2400 cGy). By 2 weeks after treatment, the pain completely resolved and the patient did not require pain medications. (C): Follow-up magnetic resonance imaging performed 3 months after stereotactic radiosurgery showed significant reduction in tumor size.
These findings represent a change from previous treatment regimens in which patients with radioresistant spinal metastases were often referred for excisional surgery in the hope of improving local control due to the historically poor responses to cEBRT. SRS, which is an outpatient procedure, may be a better first-line treatment than the extensive surgical interventions [6]. Generally, SRS-related complications are mild and include esophagitis, mucositis, dysphagia, diarrhea, paresthesia, transient laryngitis, and transient radiculitis [23, 25–28]. The most serious complication, radiation-induced spinal cord injury, is exceedingly rare. One multicenter publication found only 6 of 1,075 patients developed radiation-induced myelopathy after spinal radiosurgery [29]. Another complication of SRS that is becoming apparent is delayed vertebral body fracture [30].
Radioresistant Tumors With High-Grade ESCC
Patients with radioresistant tumors and ESCC grades 2 and 3 require surgical decompression and stabilization prior to IGRT. In the setting of spinal cord compression secondary to metastatic solid tumors, a prospective randomized trial conducted by Patchell et al. showed that surgical decompression followed by cEBRT yielded significantly superior results when compared to cEBRT alone. Statistically significant improvement in outcomes were found in the surgery group in terms of survival, overall ambulation, maintenance of ambulation, recovery of ambulation, narcotic requirement, and bowel and bladder continence. Furthermore, there was no difference in length of hospitalization between the surgery group and radiation group [31].
Currently, the primary goals of surgery include preservation or restoration of mechanical stability and circumferential decompression of the spinal cord to preserve neurologic function and allow delivery of tumoricidal radiation doses to the entire tumor volume while avoiding toxicity to the spinal cord. The assumed maximal safe radiation dose to a single voxel on the spinal cord (cordDmax) is 14 Gy [23]. SRS outcome analysis revealed that all treatment failures had less than 15 Gy to some portion of the planning target volume [32]. Therefore, in the absence of separation between the tumor margin and the spinal cord, the requisite 15 Gy cannot be delivered to the entire tumor margin without risking spinal cord toxicity. On the basis of this logic, to avoid underdosing any portion of the planned target volume, a small (2 mm) separation between the tumor and the spinal cord is required. Therefore, patients with radioresistant tumors causing high-grade ESCC undergo surgery to provide separation between the tumor and the spinal cord and permit optimal SRS dosing to the tumor. The term “separation surgery” was devised by Benzel and Angelov to describe such operations, in which only minimal tumor resection is carried out to separate the tumor margin from the spinal cord, leaving the bulk of the tumor mass to be treated with radiation.
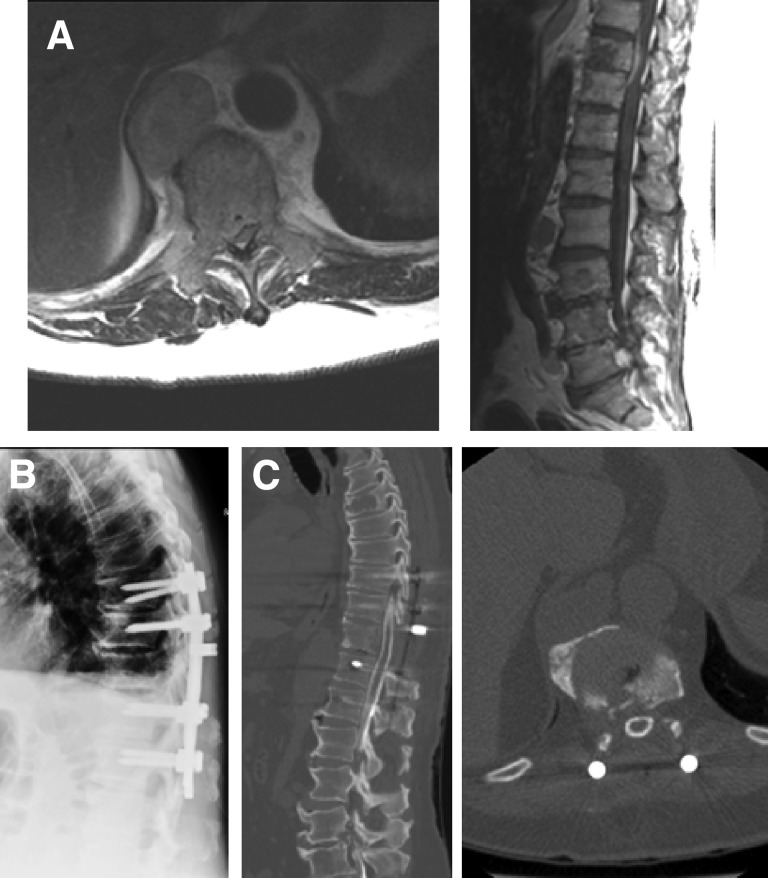
Postoperative SRS provides durable local tumor control rates, which are similar to the results of SRS treatment for low-grade ESCC tumors. Rock et al. reported a 92% local control rate in patients treated with radiosurgery following open surgical procedures [33]. Moulding et al. reviewed the outcomes in 21 patients with radioresistant metastases causing high-grade ESCC who underwent single-fraction SRS after instrumented separation surgery [34]. The 1-year local progression risk after receiving 24 Gy dose was estimated to be 6.3%. In a series of 186 patients with mostly radioresistant histologies who underwent separation surgery followed by high-dose single fraction (24 Gy) or hypofractionated SRS (8–10 Gy × 3), the 1-year local progression rates were 4.1% and 9.0%, respectively. These results were achieved regardless of tumor histology and the degree of preoperative ESCC (Fig. 4).
Figure 4.
Imaging studies from a 65 year-old man with renal cell carcinoma metastatic to T10 resulting in Grade 3 epidural spinal cord compression and myelopathy (A). T10 laminectomy, bilateral T9–T10 and T10–T11 facetectomies, transpedicular resection of ventral epidural tumor, and T8–T12 posterolateral instrumentation and fusion were performed (B). Postoperative myelogram shows complete circumferential decompression of the spinal cord (C). Postoperatively the patient received 2850 cGy in three fractions.
The ability to deliver tumoricidal radiation doses safely and effectively with SRS to gross residual tumor volumes has changed the goals of surgery. Durable local tumor control with postoperative SRS obviates the need for extensive tumor resection. Currently, in place of maximal tumor excision, surgery only needs to provide separation between the tumor and the spinal cord to optimize the delivery of SRS. More aggressive tumor resection often requires both anterior and posterior decompression and stabilization, associated with prolonged anesthesia time and greater potential morbidity. No comparison studies between these two approaches have been done. However, our experience has shown that patients better tolerate a limited decompression over attempts at maximal, gross total tumor resection. In a recent review, the symptomatic fixation failure rate from separation surgery was only 2.8%.
The combination of neurologic and oncologic assessments can help one decide whether the patient may undergo immediate radiation or if surgical decompression is required. With use of current technology, only those patients harboring radioresistant tumors with high-grade ESCC require surgical intervention prior to radiation therapy from a neurologic and oncologic perspective. Radiation therapy provides all other patients with adequate local tumor control, pain control and maintenance, or recovery of neurologic function. Surgery can be avoided in these patients unless there is progression of tumor or neurologic deficit during radiation, prior external beam radiation to overlapping ports, or spinal instability. The type of radiation offered depends on tumor histology, with the evidence supporting the use of cEBRT for radiosensitive tumors and SRS for radioresistant histologies.
Mechanical Assessment
Mechanical instability represents an independent indication for surgical stabilization or percutaneous cement augmentation, regardless of the ESCC grade and radiosensitivity of the tumor. Radiation, although effective for local tumor control, has no impact on spinal stability. Mechanical instability serves as an indication for surgery regardless of the neurologic or oncologic assessment. The SOSG has defined spinal instability as the “loss of spinal integrity as a result of a neoplastic process that is associated with movement-related pain, symptomatic or progressive deformity, and/or neural compromise under physiologic loads” [35]. The assessment of spinal instability is dependent on both clinical and radiographic criteria.
Clinically, patients with spinal instability present with severe movement-related pain that is characteristic of the specific spinal level involved. Instability pain must be distinguished from biologic pain. Biologic pain presents in the evenings and mornings and readily responds to steroids and radiation [36]. Understanding the differences between these two pain syndromes and knowing the clinical symptoms of mechanical instability is crucial to identifying those patients who require surgical stabilization to prevent neurologic injury and achieve pain control.
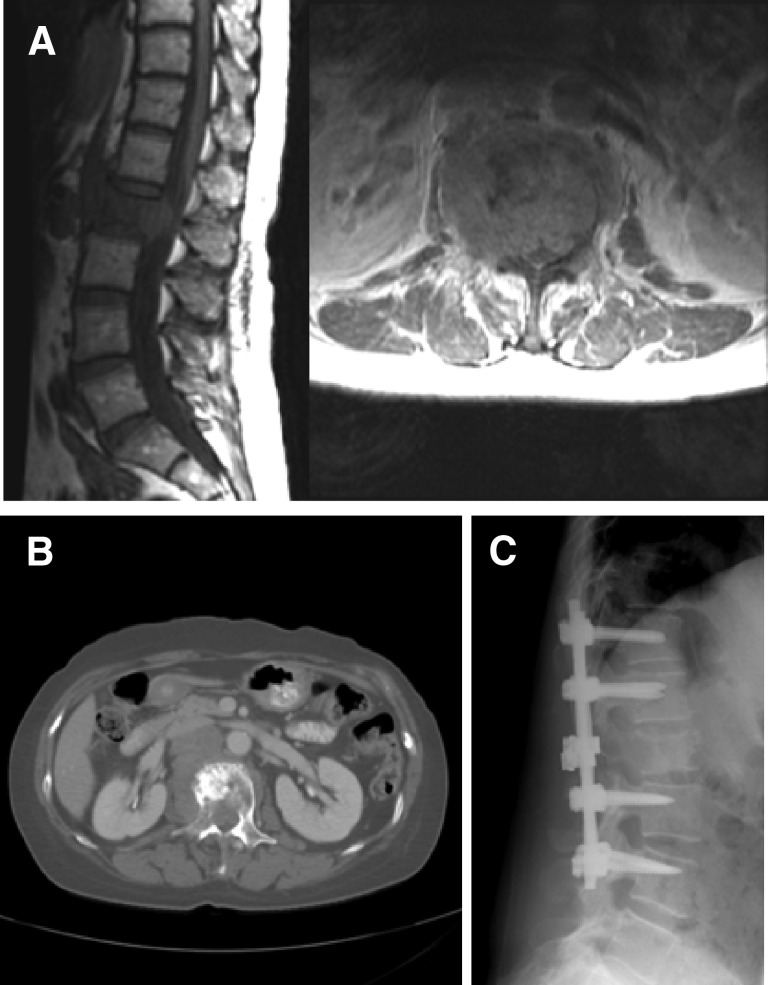
Patients with instability at the atlantoaxial junction present with pain on rotation, flexion, and extension. Patients with C2 fractures with normal spinal alignment or minimal subluxation often heal without surgical intervention. These patients may be placed in a hard cervical collar during and for 6 weeks after radiotherapy, with a 95% chance of fracture healing [37]. However, patients with fracture subluxations >5 mm or >3.5 mm subluxation and 11-degree angulation between C1 and C2 with movement-related neck pain require instrumented spine fixation [37, 38]. Subaxial cervical instability is manifested by pain with flexion and extension that often corresponds to dynamic instability of the spine on imaging and tumor extension into the joint [39]. Instability pain in the thoracic spine is often elicited with extension, causing unremitting pain as the patient straightens an unstable kyphosis. In the lumbar spine, instability may present with mechanical radiculopathy, manifested with severe radicular pain upon standing. Tumor infiltration of the lumbar vertebral body and the corresponding joint result in the inability of the vertebra to support biologic axial loads, leading to collapse of the neural foramen when standing and compression of the exiting nerve root (Fig. 5). All patients with clear manifestations of cervical, thoracic, or lumbar mechanical instability require a surgical stabilization because mechanical pain does not improve with steroids and radiation does not restore spinal stability.
Figure 5.
Imaging studies from a 60-year-old woman. (A): The patient had colon adenocarcinoma metastatic to L2, resulting in severe mechanical radiculopathy. (B): The tumor was lytic, with extension into bilateral posterior elements. (C): L2 laminectomy and T12–L4 posterolateral instrumentation and fusion were performed, with complete resolution of the mechanical pain.
Painful pathologic compression fractures in the absence of gross spinal instability or significant posterior element involvement can be treated with cement augmentation procedures, such as vertebroplasty or kyphoplasty [40–42]. Fourney et al. reported that 84% of patients demonstrated marked or complete pain relief at a median follow-up of 4.5 months, and their visual analogue pain scores were durable at 1 year [43]. A recent systematic review of literature performed by the members of the SOSG resulted in a strong recommendation for the use of vertebroplasty or kyphoplasty in the setting of symptomatic osteolytic tumors [44]. Furthermore, a multicenter randomized controlled trial comparing kyphoplasty to nonsurgical management showed that kyphoplasty was associated with a significant improvement in the Roland-Morris disability questionnaire (RDQ) at 1 month after the procedure. The improvement in the RDQ and Short Form 36 persisted for 6 months after the procedure but lost statistical significance at 1 year [45].
To aid clinicians in the diagnosis of neoplastic instability, the SOSG devised an 18-point Spinal Instability Neoplastic Score (SINS) (Table 2) [35, 46]. This grading scheme includes six parameters: location, pain, alignment, osteolysis, vertebral body collapse, and posterior elements involvement. Lesions with a low SINS (0–6) are generally stable and do not require surgical stabilization, whereas a high SINS (13–18) reliably predicts the need for surgical stabilization to restore spinal stability. Intermediate SINS tumors require further assessment to determine the need for surgery.
Table 2.
Spinal Instability Neoplastic Score
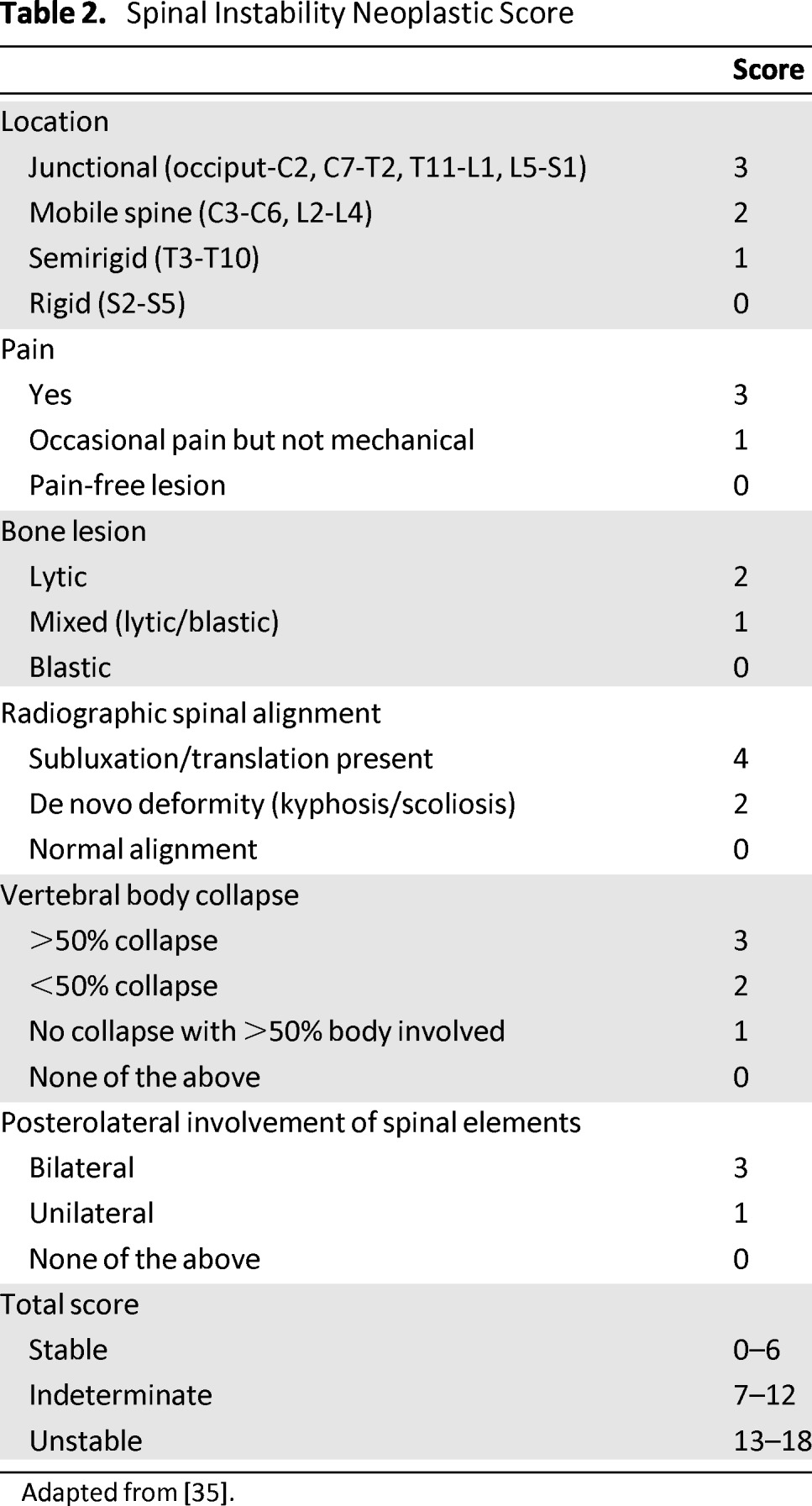
Adapted from [35].
Systemic Assessment
All treatment decisions are predicated on the patient's ability to tolerate the proposed intervention based on the extent of systemic comorbidities and tumor burden. The systemic disease assessment determines what a patient can tolerate physiologically and is dependent on extent of tumor dissemination, medical comorbidities, and tumor histology. Optimal metastatic staging workup is histology dependent and should be determined by the patient's oncologist. Surgical risk stratification may be performed by the patient's oncologist or internist. These factors are used in concert to determine if the proposed treatment can be administered with acceptable risk to the patient.
Understanding tumor biology and behavior is also critical when determining appropriate treatments. It has been shown that metastatic spine invasion by certain tumor histologies is indicative of shortened survival and may preclude benefit from some interventions. For example, in multiple series, non-small cell lung carcinoma, colon carcinoma, and carcinoma of unknown primary origin have median survival rates of approximately 4 months from the time of surgery [47]. Because of shortened survival, patients harboring aggressive tumors may not benefit from extensive interventions that require prolonged hospital stays or intensive physical therapy. If the complication risk secondary to systemic comorbidities precludes surgical intervention, radiation and medical therapeutics can often be administered to patients, even in significantly advanced stages of their illness.
Numerous prognostic scoring systems exist to facilitate the estimation of the expected survival of patients with spinal metastases. These scores may be used to help determine whether the patient is an appropriate surgical candidate. Unfortunately, multiple reviews have shown that physicians frequently tend to overestimate the expected survival time; furthermore, the always-evolving armamentarium of anticancer pharmacotherapy continuously alters survival expectations. Therefore, we generally avoid rigid survival prediction systems in favor of individualized discussion with the patient's oncologist. Because surgery for patients with spinal metastases serves a palliative purpose, we concentrate on whether the patients would have an opportunity to adequately recover from the indicated surgery and/or radiation in order to continue systemic therapy. Generally, as long as reasonable pharmacotherapy is available for the postoperative period to attempt systemic tumor control and systemic progression does not appear to be rapid enough to prevent postoperative recovery, the patient will be considered for surgery.
Conclusions
Modern framework for treatment of metastatic spine tumors must emphasize durable tumor control while minimizing treatment-related morbidity, while giving consideration to effective pharmacologic, radiation, and surgical treatment options to achieve this goal. NOMS provides a framework that facilitates decision-making and can optimize patient care (Table 3, Fig. 6). The durable tumor control rates achieved with cEBRT for radiosensitive tumors and with IGRT for radioresistant tumors make radiation therapy the treatment of choice in achieving durable local tumor control. In light of the great results after radiation therapy, the goals of surgery have changed. Although historically surgeons aimed to achieve maximal tumor resection to optimize tumor control, the goal of modern surgery for spinal metastases is to provide a separation of the tumor from the spinal cord to optimize the radiation dose that can be safely delivered to the tumor volume. Minimizing the extent of surgical intervention makes surgery safer for the patients. Consideration of spinal stability, the degree of epidural tumor extension in conjunction with the radiosensitivity of the tumor, and systemic comorbidities allows the correct determination of the optimal combination of radiation modality and surgery.
Table 3.
Current NOMS decision framework
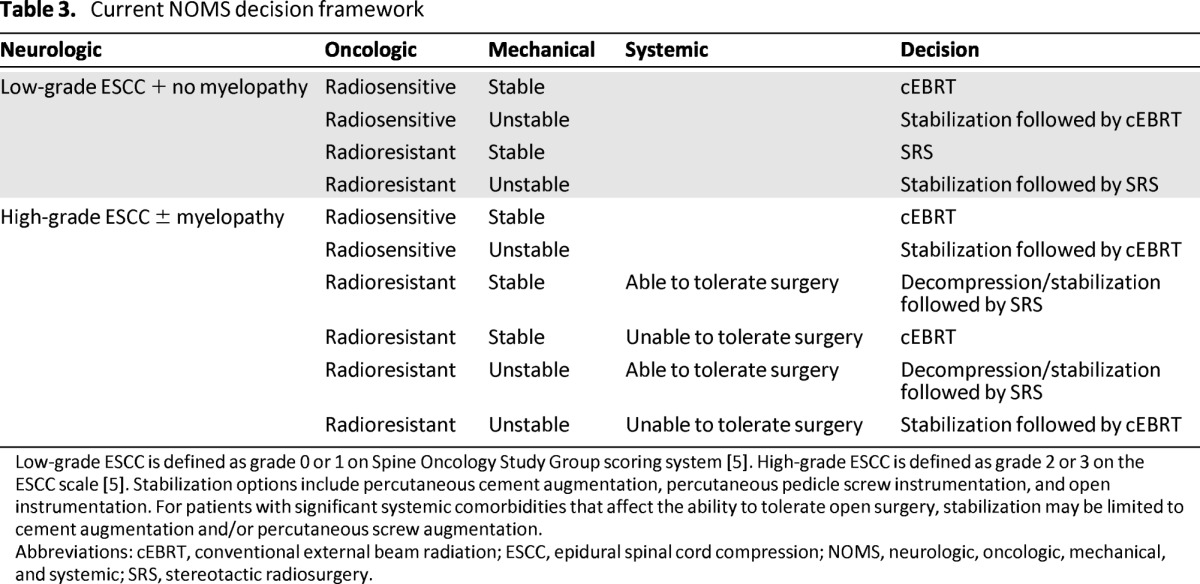
Low-grade ESCC is defined as grade 0 or 1 on Spine Oncology Study Group scoring system [5]. High-grade ESCC is defined as grade 2 or 3 on the ESCC scale [5]. Stabilization options include percutaneous cement augmentation, percutaneous pedicle screw instrumentation, and open instrumentation. For patients with significant systemic comorbidities that affect the ability to tolerate open surgery, stabilization may be limited to cement augmentation and/or percutaneous screw augmentation.
Abbreviations: cEBRT, conventional external beam radiation; ESCC, epidural spinal cord compression; NOMS, neurologic, oncologic, mechanical, and systemic; SRS, stereotactic radiosurgery.
Figure 6.
Schematic depiction of the neurologic, oncologic, mechanical, and systemic (NOMS) decision framework.
Abbreviations: cEBRT, conventional external beam radiation; SRS, stereotactic radiosurgery.
Acknowledgments
I.L. and D.G.R. contributed equally to this work.
Author Contributions
Conception/Design: Ilya Laufer, Eric Lis, Brett Cox, Michael Stubblefield, Yoshiya Yamada, Mark Bilsky
Provision of study material or patients: Ilya Laufer, Eric Lis, Brett Cox, Michael Stubblefield, Yoshiya Yamada, Mark Bilsky
Collection and/or assembly of data: Ilya Laufer, Yoshiya Yamada, Mark Bilsky
Data analysis and interpretation: Ilya Laufer, David Rubin, Yoshiya Yamada, Mark Bilsky
Manuscript writing: Ilya Laufer, David Rubin, Yoshiya Yamada, Mark Bilsky
Final approval of manuscript: Ilya Laufer, David Rubin, Eric Lis, Michael Stubblefield, Yoshiya Yamada, Mark Bilsky
Disclosures
Ilya Laufer: SpineWave, Development of vertebral replacement device (C/A); Yoshiya Yamada: Varian Medical Systems (C/A); Mark Bilsky: DePuy Spine (IP).
C/A: Consulting/advisory relationship; RF: Research funding; E: Employment; H: Honoraria received; OI: Ownership interests; IP: Intellectual property rights/inventor/patent holder; SAB: scientific advisory board
Reference
- 1.Cobb CA, 3rd, Leavens ME, Eckles N. Indications for nonoperative treatment of spinal cord compression due to breast cancer. J Neurosurg. 1977;47:653–658. doi: 10.3171/jns.1977.47.5.0653. [DOI] [PubMed] [Google Scholar]
- 2.Walsh GL, Gokaslan ZL, McCutcheon IE, et al. Anterior approaches to the thoracic spine in patients with cancer: Indications and results. Ann Thorac Surg. 1997;64:1611–1618. doi: 10.1016/s0003-4975(97)01034-5. [DOI] [PubMed] [Google Scholar]
- 3.Barron KD. Experiences with metastatic neoplasms involving the spinal cord. Neurology. 1959;9:91–106. doi: 10.1212/wnl.9.2.91. [DOI] [PubMed] [Google Scholar]
- 4.Bach F. Metastatic spinal cord compression. Occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta neurochirurgica. 1990;107:37–43. doi: 10.1007/BF01402610. [DOI] [PubMed] [Google Scholar]
- 5.Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010;13:324–328. doi: 10.3171/2010.3.SPINE09459. [DOI] [PubMed] [Google Scholar]
- 6.Bilsky M, Laufer I, Burch S. Shifting paradigms in the treatment of metastatic spine disease. Spine. 2009;34:S101–S107. doi: 10.1097/BRS.0b013e3181bac4b2. [DOI] [PubMed] [Google Scholar]
- 7.Gerszten PC, Mendel E, Yamada Y. Radiotherapy and radiosurgery for metastatic spine disease: What are the options, indications, and outcomes? Spine. 2009;34:S78–S92. doi: 10.1097/BRS.0b013e3181b8b6f5. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: Diagnosis and treatment. Ann Neurol. 1978;3:40–51. doi: 10.1002/ana.410030107. [DOI] [PubMed] [Google Scholar]
- 9.Maranzano E, Bellavita R, Rossi R, et al. Short-course versus split-course radiotherapy in metastatic spinal cord compression: Results of a phase III, randomized, multicenter trial. J Clin Oncol. 2005;23:3358–3365. doi: 10.1200/JCO.2005.08.193. [DOI] [PubMed] [Google Scholar]
- 10.Maranzano E, Latini P, Perrucci E, et al. Short-course radiotherapy (8 Gy × 2) in metastatic spinal cord compression: An effective and feasible treatment. Int J Radiat Oncol Biol Phys. 1997;38:1037–1044. doi: 10.1016/s0360-3016(97)00128-4. [DOI] [PubMed] [Google Scholar]
- 11.Katagiri H, Takahashi M, Inagaki J, et al. Clinical results of nonsurgical treatment for spinal metastases. Int J Radiat Oncol Biol Phys. 1998;42:1127–1132. doi: 10.1016/s0360-3016(98)00288-0. [DOI] [PubMed] [Google Scholar]
- 12.Rades D, Fehlauer F, Schulte R, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006;24:3388–3393. doi: 10.1200/JCO.2005.05.0542. [DOI] [PubMed] [Google Scholar]
- 13.Rades D, Fehlauer F, Stalpers LJ, et al. A prospective evaluation of two radiotherapy schedules with 10 versus 20 fractions for the treatment of metastatic spinal cord compression: Final results of a multicenter study. Cancer. 2004;101:2687–2692. doi: 10.1002/cncr.20633. [DOI] [PubMed] [Google Scholar]
- 14.Rades D, Karstens JH, Alberti W. Role of radiotherapy in the treatment of motor dysfunction due to metastatic spinal cord compression: Comparison of three different fractionation schedules. Int J Radiat Oncol Biol Phys. 2002;54:1160–1164. doi: 10.1016/s0360-3016(02)02979-6. [DOI] [PubMed] [Google Scholar]
- 15.Rades D, Huttenlocher S, Bajrovic A, et al. Surgery followed by radiotherapy versus radiotherapy alone for metastatic spinal cord compression from unfavorable tumors. Int J Radiat Oncol Biol Phys. 2011;81:e861–e868. doi: 10.1016/j.ijrobp.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 16.Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: Final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32:959–967. doi: 10.1016/0360-3016(95)00572-g. [DOI] [PubMed] [Google Scholar]
- 17.Chow E, Harris K, Fan G, et al. Palliative radiotherapy trials for bone metastases: A systematic review. J Clin Oncol. 2007;25:1423–1436. doi: 10.1200/JCO.2006.09.5281. [DOI] [PubMed] [Google Scholar]
- 18.Bilsky MH, Lis E, Raizer J, et al. The diagnosis and treatment of metastatic spinal tumor. The Oncologist. 1999;4:459–469. [PubMed] [Google Scholar]
- 19.Gerszten PC, Burton SA, Ozhasoglu C, et al. Radiosurgery for spinal metastases: Clinical experience in 500 cases from a single institution. Spine. 2007;32:193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 20.Ryu S, Fang Yin F, Rock J, et al. Image-guided and intensity-modulated radiosurgery for patients with spinal metastasis. Cancer. 2003;97:2013–2018. doi: 10.1002/cncr.11296. [DOI] [PubMed] [Google Scholar]
- 21.Ryu S, Rock J, Rosenblum M, et al. Patterns of failure after single-dose radiosurgery for spinal metastasis. J Neurosurg. 2004;101(suppl 3):402–405. [PubMed] [Google Scholar]
- 22.Gerszten PC, Burton SA, Ozhasoglu C, et al. Stereotactic radiosurgery for spinal metastases from renal cell carcinoma. J Neurosurg Spine. 2005;3:288–295. doi: 10.3171/spi.2005.3.4.0288. [DOI] [PubMed] [Google Scholar]
- 23.Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484–490. doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 24.Yamada Y, Cox B, Zelefsky MJ, et al. An analysis of prognostic factors for local control of malignant spine tumors treated with spine radiosurgery. Int J Radiat Oncol Biol Phys. 2011;81:S132–S133. [Google Scholar]
- 25.Benzil DL, Saboori M, Mogilner AY, et al. Safety and efficacy of stereotactic radiosurgery for tumors of the spine. J Neurosurg. 2004;101(suppl 3):413–418. [PubMed] [Google Scholar]
- 26.Degen JW, Gagnon GJ, Voyadzis JM, et al. CyberKnife stereotactic radiosurgical treatment of spinal tumors for pain control and quality of life. J Neurosurg Spine. 2005;2:540–549. doi: 10.3171/spi.2005.2.5.0540. [DOI] [PubMed] [Google Scholar]
- 27.Chang EL, Shiu AS, Mendel E, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine. 2007;7:151–160. doi: 10.3171/SPI-07/08/151. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton AJ, Lulu BA. A prototype device for linear accelerator-based extracranial radiosurgery. Acta Neurochir Suppl. 1995;63:40–43. doi: 10.1007/978-3-7091-9399-0_9. [DOI] [PubMed] [Google Scholar]
- 29.Gibbs IC, Patil C, Gerszten PC, et al. Delayed radiation-induced myelopathy after spinal radiosurgery. Neurosurgery. 2009;64:A67–A72. doi: 10.1227/01.NEU.0000341628.98141.B6. [DOI] [PubMed] [Google Scholar]
- 30.Rose PS, Laufer I, Boland PJ, et al. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J Clin Oncol. 2009;27:5075–5079. doi: 10.1200/JCO.2008.19.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet. 2005;366:643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 32.Lovelock DM. Correlation of local failure with measures of dose insufficiency in the high-dose single-fraction treatment of bony metastases. Int J Radiat Oncol Biol Phys. 2010;77:1282–1287. doi: 10.1016/j.ijrobp.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rock JP, Ryu S, Shukairy MS, et al. Postoperative radiosurgery for malignant spinal tumors. Neurosurgery. 2006;58:891–898. doi: 10.1227/01.NEU.0000209913.72761.4F. [DOI] [PubMed] [Google Scholar]
- 34.Moulding HD, Elder JB, Lis E, et al. Local disease control after decompressive surgery and adjuvant high-dose single-fraction radiosurgery for spine metastases. J Neurosurg Spine. 2010;13:87–93. doi: 10.3171/2010.3.SPINE09639. [DOI] [PubMed] [Google Scholar]
- 35.Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: An evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine. 2010;35:E1221–E1229. doi: 10.1097/BRS.0b013e3181e16ae2. [DOI] [PubMed] [Google Scholar]
- 36.Posner JB. Back pain and epidural spinal cord compression. Med Clin North Am. 1987;71:185–205. doi: 10.1016/s0025-7125(16)30865-3. [DOI] [PubMed] [Google Scholar]
- 37.Bilsky MH, Shannon FJ, Sheppard S, et al. Diagnosis and management of a metastatic tumor in the atlantoaxial spine. Spine. 2002;27:1062–1069. doi: 10.1097/00007632-200205150-00011. [DOI] [PubMed] [Google Scholar]
- 38.Sundaresan N, Galicich JH, Lane JM, et al. Treatment of odontoid fractures in cancer patients. J Neurosurg. 1981;54:187–192. doi: 10.3171/jns.1981.54.2.0187. [DOI] [PubMed] [Google Scholar]
- 39.Bilsky MH. Operative management of metastatic and malignant primary subaxial cervical tumors. J Neurosurg Spine. 2005;2:256–264. doi: 10.3171/spi.2005.2.3.0256. [DOI] [PubMed] [Google Scholar]
- 40.Burton AW, Mendel E. Vertebroplasty and kyphoplasty. Pain Physician. 2003;6:335–341. [PubMed] [Google Scholar]
- 41.Hentschel SJ, Burton AW, Fourney DR, et al. Percutaneous vertebroplasty and kyphoplasty performed at a cancer center: Refuting proposed contraindications. J Neurosurg Spine. 2005;2:436–440. doi: 10.3171/spi.2005.2.4.0436. [DOI] [PubMed] [Google Scholar]
- 42.Bartolozzi B, Nozzoli C, Pandolfo C, et al. Percutaneous vertebroplasty and kyphoplasty in patients with multiple myeloma. Eur J Haematol. 2006;76:180–181. doi: 10.1111/j.1600-0609.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 43.Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98(suppl 1):21–30. doi: 10.3171/spi.2003.98.1.0021. [DOI] [PubMed] [Google Scholar]
- 44.Mendel E, Bourekas E, Gerszten P, et al. Percutaneous techniques in the treatment of spine tumors: What are the diagnostic and therapeutic indications and outcomes? Spine. 2009;34:S93–S100. doi: 10.1097/BRS.0b013e3181b77895. [DOI] [PubMed] [Google Scholar]
- 45.Berenson J. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: A multicentre, randomised controlled trial. Lancet Oncol. 2011;12:225–235. doi: 10.1016/S1470-2045(11)70008-0. [DOI] [PubMed] [Google Scholar]
- 46.Fourney DR, Frangou EM, Ryken TC, et al. Spinal instability neoplastic score: An analysis of reliability and validity from the spine oncology study group. J Clin Oncol. 2011;29:3072–3077. doi: 10.1200/JCO.2010.34.3897. [DOI] [PubMed] [Google Scholar]
- 47.Wang JC, Boland P, Mitra N, et al. Single-stage posterolateral transpedicular approach for resection of epidural metastatic spine tumors involving the vertebral body with circumferential reconstruction: Results in 140 patients. J Neurosurg Spine. 2004;1:287–298. doi: 10.3171/spi.2004.1.3.0287. [DOI] [PubMed] [Google Scholar]