This retrospective study aimed to determined the proportion of patients with advanced head and neck cancer who continued to use opioids after the completion of radiation therapy. CAGE positivity was found to be a risk factor for opioid use beyond 3 months after the completion of radiation therapy and for duration of opioid treatment, indicating that routine screening and meticulous follow-up are needed for these patients.
Keywords: Head and neck cancer, Opioids, CAGE, Treatment-related side effects, Radiation
Abstract
Introduction.
The factors associated with successful opioid discontinuation after cancer treatment are not well-known. We determined the proportion of patients with advanced head and neck cancer who continued using opioids 3 months after the completion of radiation therapy with or without chemotherapy.
Methods.
We included 70 patients with head and neck cancer referred to our institution's supportive care center between January 1, 2008, and December 31, 2010. Patients who no longer used opioids 3 months after the completion of radiation therapy were classified as stoppers; patients who continued using opioids were considered nonstoppers. We compared demographics, cancer-related characteristics, alcoholism, substance abuse history, use of psychoactive drugs, and opioid-related factors between stoppers and nonstoppers.
Results.
In all, 44 of 70 patients (63%) and 23 of 70 patients (33%) continued opioids 3 months and 6 months after the completion of radiation therapy, respectively. A total of 18 of 44 nonstoppers (41%) and 3 of 26 stoppers (12%) were positive for alcoholism based on the CAGE questionnaire (i.e., Cut down, Annoying, Guilty, Eye opener; odds ratio: 5.3). Demographic and clinical characteristics did not differ between stoppers and nonstoppers. The median duration of any type of opioid use of CAGE-positive patients was significantly longer than that of CAGE-negative patients (median: 261 days vs. 93 days; hazard ratio: 2.5).
Conclusion.
CAGE positivity is a risk factor for opioid use beyond 3 months after the completion of radiation therapy and for duration of opioid treatment. Routine CAGE screening and meticulous follow-up are needed for these patients.
Implications for Practice:
Patients with head and neck cancer receiving chemoradiation suffer from side effects of treatment, especially pain. Severe pain is usually managed with opioids; however, in cancer survivors, specific groups of patients with risk factors cannot take opioids even to resolve treatment-related side effects. In this study, 63% and 33% of patients still used opioids 3 months and 6 months after the completion of radiation therapy, respectively. Patients who continued opioids at 3 months had higher positive CAGE than those who stopped opioids. CAGE-positive patients had longer median overall survival than CAGE-negative patients. Routine screening of CAGE for alcoholism, careful assessment of pain syndrome, longitudinal monitoring, and psychological intervention are needed for these patients.
Introduction
Chemoradiation therapy with or without induction chemotherapy is the standard of care for locally advanced head and neck cancer [1]. Patients who undergo chemoradiation therapy inevitably have frequent and acute severe toxicities; about 80% of these patients experience mucositis and 70% experience radiation-induced pain [2]. Severe treatment-related side effects can not only lower a patient's quality of life but also unexpectedly interrupt his or her treatment, which can impair local control of the disease and reduce the overall survival duration [3, 4]. Thus, managing the side effects of chemoradiation therapy is key to successfully completing curative treatment and improving quality of life in patients with locally advanced head and neck cancer.
Effective pain management has been reported to improve the rate of completion of curative radiation therapy of patients with locally advanced head and neck cancer. Pain control in more than half of these patients is achieved with opioids [5, 6], the mainstay of analgesia in managing moderate to severe pain related to cancer and its treatments. Approximately one third of patients reported persistent orofacial pain even months after treatment [7–9]. Persistent pain requiring opioids in cancer survivors without evidence of disease may be related to chronic tissue damage from cancer or its treatments, comorbidities such as periodontal disease, psychological factors, or opioid dependency/abuse [10, 11]. The role of opioids in the management of this chronic pain syndrome has not been established.
In patients without cancer, possible risk factors for opioid abuse and/or dependency include young age, male sex, alcoholism, psychiatric disease, and/or previous history of substance abuse [12–15]. In patients with cancer, young age, alcoholism, psychiatric disease, global symptom severity, and history of smoking are possible risk factors [16–20]. In previous studies, we found that alcoholism was associated with frequent opioid prescription and higher symptom expression. In addition, patients with head and neck cancer were referred to palliative care to manage their severe symptoms earlier than other patients with cancer [19, 21].
Considering these factors, it is not unreasonable to suspect that patients with locally advanced head and neck cancer who receive opioids for the pain induced by curative radiation therapy have a higher risk developing of opioid-related problems later in life. However, no studies have identified the factors associated with long-term opioid use in such patients. Therefore, we conducted the present study to determine the proportion of patients with locally advanced head and neck cancer who received curative radiation therapy and continued to use opioids, as well as to identify the factors associated with long-term opioid use in these patients. We hypothesized that a patient with higher risk of chemical coping (as determined by the CAGE screening questionnaire for alcoholism; see Methods) would have more difficulty in stopping opioids after the completion of treatment.
Methods
This retrospective study was reviewed and approved by the institutional review board of The University of Texas MD Anderson Cancer Center with a waiver of consent. We reviewed the medical records of patients with head and neck cancer who were referred to MD Anderson's outpatient supportive care center (SCC) for palliative care between January 1, 2008 and December 31, 2010. We included patients who received curative chemoradiation or radiation therapy for head and neck cancer, visited the SCC within 1 month after completing chemoradiation or radiation therapy, and had no evidence of disease 3 months after the completion of chemoradiation or radiation therapy. We excluded patients whose disease was refractory to treatment or relapsed within 3 months after the completion of chemoradiation or radiation therapy, patients who received curative surgery before or after radiation therapy, and patients who underwent salvage cervical lymph node dissection for residual disease after radiation therapy.
Patients for whom the absence of a prescription for opioids indicated they had stopped using opioids (prescription at MD Anderson Cancer Center and chart description in transcribed document for outside MD Anderson Cancer Center) within 3 months after the completion of radiation therapy were considered to be “stoppers.” Patients for whom the presence of a prescription for opioids indicated they had not stopped using opioids at 3 months after the completion of radiation therapy were considered to be “nonstoppers.” The 3-month cutoff time was based on the findings of previous studies indicating that a majority of patients return to their baseline level of pain and function within 3 months after treatment [6, 7, 22–24]. In addition, we also conducted an analysis of stoppers versus nonstoppers at the 6-month period to determine if identified risk factors at 3 months are persistent at 6 months, as well as to determine duration of treatment among those patients who ultimately succeeded becoming stoppers.
Data Collection
We reviewed the patients' medical records for the following data: (a) demographic factors, including age, sex, and race; (b) cancer-related factors, including types, stage, human papilloma virus (HPV) tumor stain results, and treatment; (c) clinical factors, including the CAGE (i.e., Cut down, Annoying, Guilty, Eye-opener) score for alcoholism, Eastern Cooperative Oncology Group performance status, Edmonton Symptom Assessment Scale (ESAS) score, and use of psychoactive drugs (sedatives, hypnotics, antianxiolytics, neuroleptics, and psychostimulants); (d) opioid-related factors, including type and dose of opioids, oral morphine equivalent daily dose (MEDD, mg/day) at the time of referral and around the completion of radiation therapy, peak MEDD during treatment, starting date of opioids, and stopping date of opioids; (e) presence of radiation-induced mucositis at 3 months based on clinical examination; (f) past medical history, including psychiatric disease, substance abuse (other than alcoholism), smoking history, and if available, preadolescent sexual abuse history; and (g) date of relapse or occurrence of secondary malignancy.
Clinical Assessment
All patients referred to the SCC underwent screening for alcoholism using the CAGE questionnaire. This is a validated questionnaire that consists of four questions, with two or more “yes” answers considered to be positive for alcoholism [25–27]. All patients were also assessed with ESAS for their symptom assessment. ESAS is a highly validated tool for multiple symptom assessment and has been used clinically [28, 29].
We calculated the MEDD of opioids as the sum of the amounts of regular opioids and as-needed opioids used over 24 hours. Standard conversion ratios were used to calculate equivalency between morphine and different opioids. We used a 1:1 ratio to calculate equivalency between morphine and hydrocodone [30–32].
Statistical Analysis
Descriptive statistics were used for all factors. Frequencies and percentages were used to summarize the patients' categorical demographic data and clinical characteristics. Means, standard deviations, medians, and ranges were used to summarize the patients' continuous demographic data and clinical characteristics.
We used the two-sample t test or Mann-Whitney test to compare the continuous variables, such as age, ESAS symptom distress scores during or after radiation therapy, and MEDD of opioids, between the two groups, and the test was chosen depending on normality. For discrete variables such as sex, clinical characteristics (history of psychiatric disease, smoking, and/or substance abuse), HPV positivity, and type of opioid (strong or weak), we used the χ2 test or Fisher's exact test. Strong opioids were fentanyl, hydromorphone, methadone, morphine, and oxycodone; weak opioids were hydrocodone, propoxyphene, and tramadol. We defined the duration of opioid use as the time from the completion of radiation therapy to the last day of opioid use. We defined disease-free survival (DFS) duration as the time from the first day of any anticancer treatment to the day of last follow-up, diagnosis of recurrent disease, or diagnosis of second primary cancer.
DFS was analyzed using Kaplan-Meier methodology to determine if stopper status at 3 and 6 months was associated with a difference in DFS; significance was assessed via the log-rank test. Duration of opioid use was analyzed with backwards stepwise Cox regressions to determine patient characteristics associated with longer use of opioids. All available patient characteristics were included in the full models for weak and strong opioids.
Sample Size Justification
This study was based on a convenient sample of 70 patients with head and neck cancer who had a DFS duration of more than 3 months. We calculated the proportion of patients who continued using opioids more than 3 months after the completion of radiation therapy (nonstoppers) along with the corresponding 95% confidence interval (CI). We assumed that approximately 20% of the patients would be nonstoppers based on our previous study on CAGE positivity [33], which corresponded to a 95% CI of ±9.4% when n = 70. This is a conservative estimate because patients may be nonstoppers for reasons other than CAGE positivity.
Results
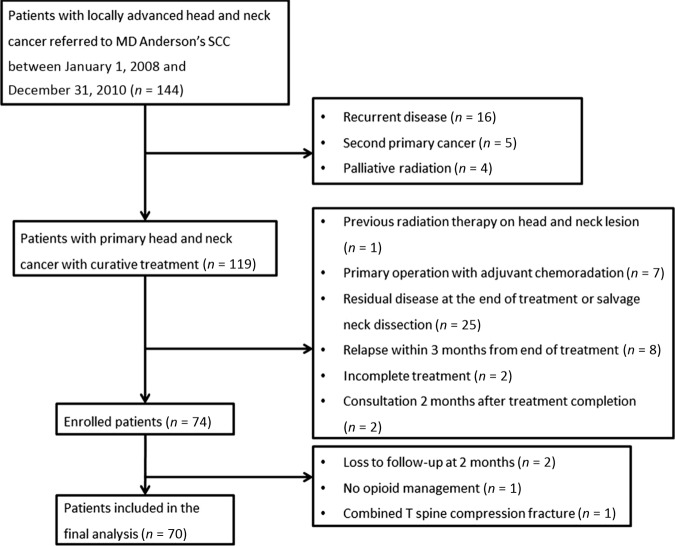
Between January 1, 2008, and December 31, 2010, a total of 144 patients with advanced head and neck cancer were seen at our SCC for consultation. Seventy of these patients—44 (63%) nonstoppers and 26 (37%) stoppers—met the eligibility criteria were included in the present study (Fig. 1). Ten patients (14%) were already on opioids during their first visit to our cancer center.
Figure 1.
Study flowchart.
Abbreviation: SCC, supportive care center.
Patients' demographic data are given in Table 1. The median age was 56 years (interquartile range: 50–64 years), and 11 of 70 patients had a history of substance abuse. The CAGE positivity rate of nonstoppers (41%) was significantly higher than that of stoppers (12%; odds ratio [OR]: 5.3; p = .014). Patients' clinical characteristics are given in Table 2. The primary site and stage of cancer, dose of radiation, and rate of radiation-induced mucositis 3 months after the completion of radiation therapy did not differ significantly between stoppers and nonstoppers.
Table 1.
Demographic data of opioid stoppers and nonstoppers 3 months after the completion of radiation therapy
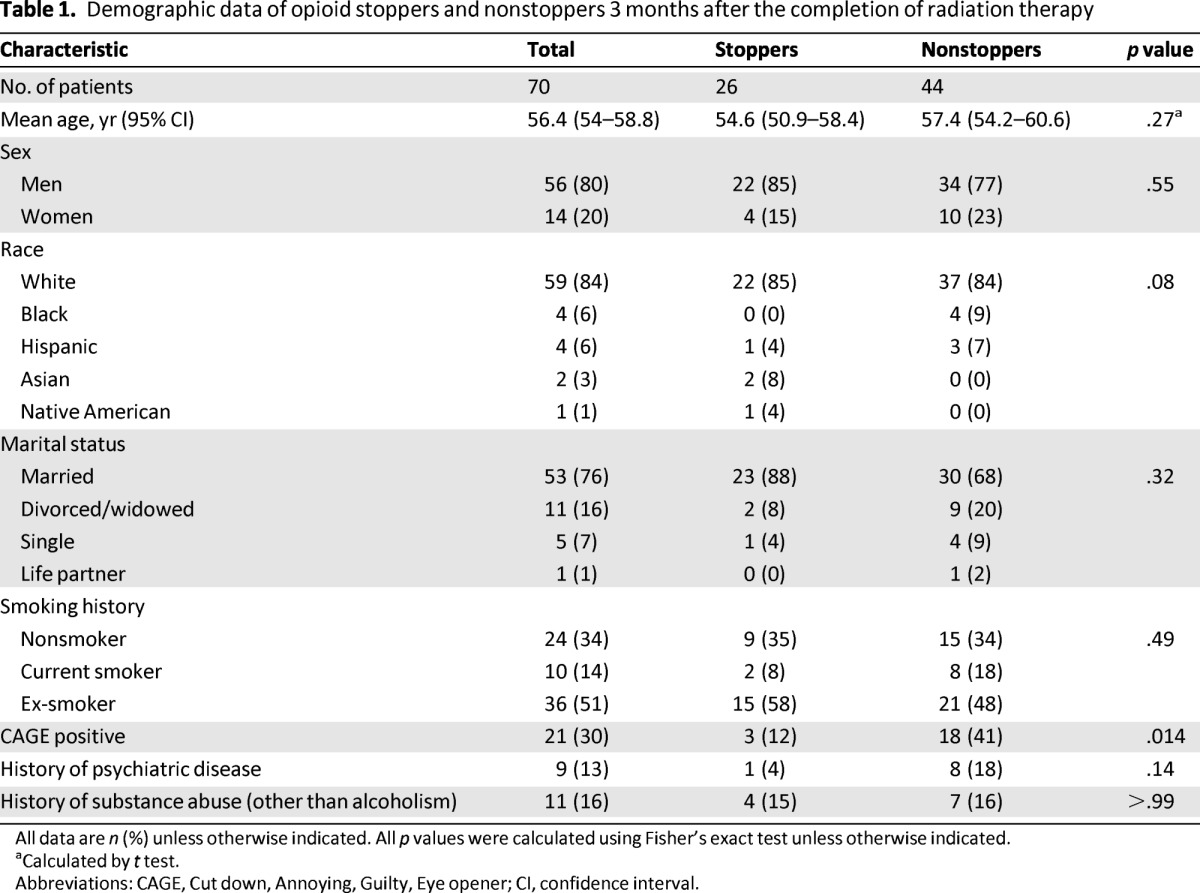
All data are n (%) unless otherwise indicated. All p values were calculated using Fisher's exact test unless otherwise indicated.
aCalculated by t test.
Abbreviations: CAGE, Cut down, Annoying, Guilty, Eye opener; CI, confidence interval.
Table 2.
Clinical characteristics of opioid stoppers and nonstoppers 3 months after the completion of radiation therapy
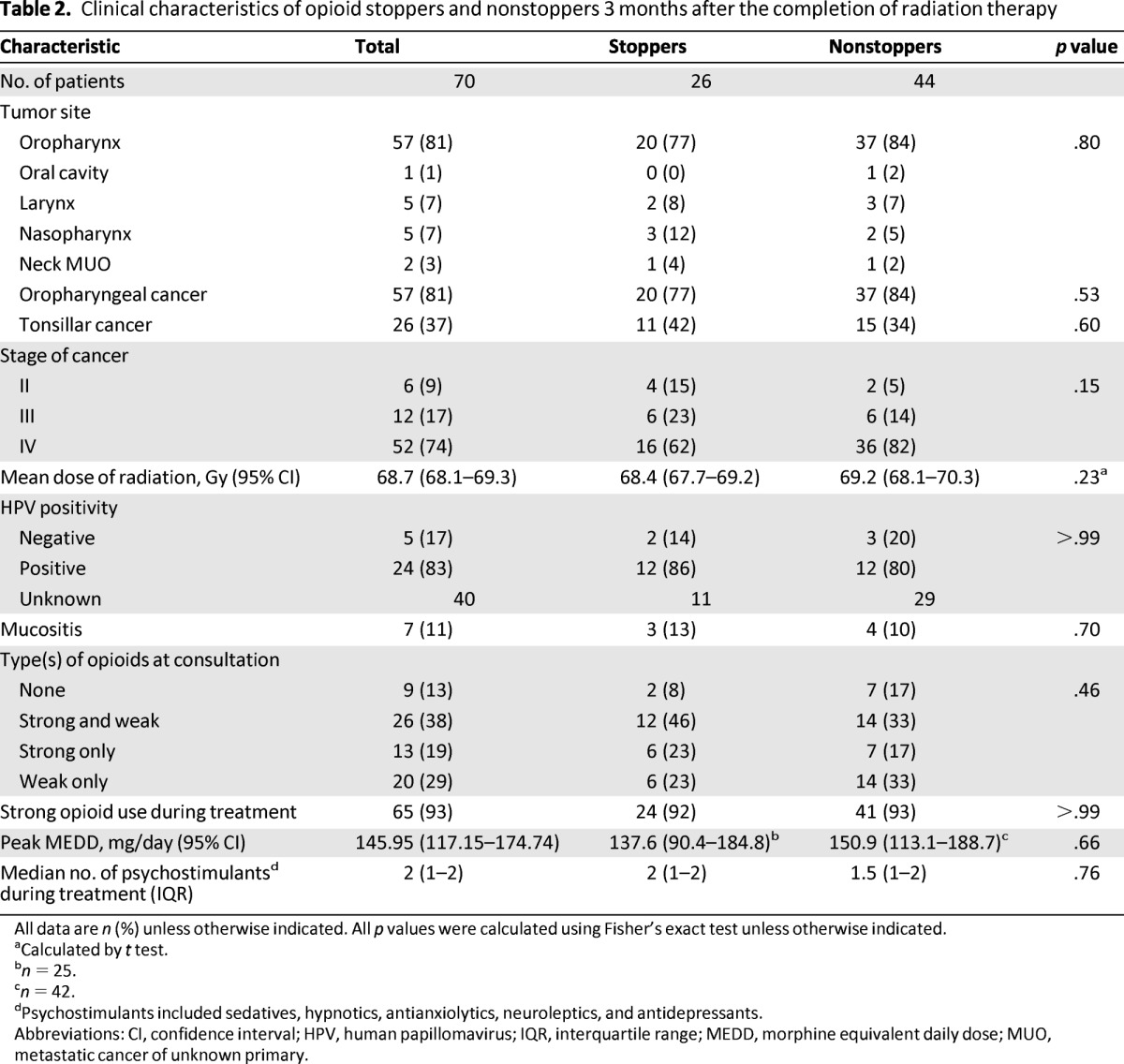
All data are n (%) unless otherwise indicated. All p values were calculated using Fisher's exact test unless otherwise indicated.
aCalculated by t test.
bn = 25.
cn = 42.
dPsychostimulants included sedatives, hypnotics, antianxiolytics, neuroleptics, and antidepressants.
Abbreviations: CI, confidence interval; HPV, human papillomavirus; IQR, interquartile range; MEDD, morphine equivalent daily dose; MUO, metastatic cancer of unknown primary.
The HPV positivity rates of the 14 stoppers and 15 nonstoppers for whom HPV status data were available did not differ significantly, even when the rates were analyzed according to disease location (p = .8). The rates of strong opioid use during anticancer treatment, peak MEDDs, and types of opioids used at the time of consultation between stoppers and nonstoppers did not differ significantly.
Among 52 patients (74%) who were seen at SCC within 2 weeks after completion of radiation therapy, the severity of pain (6 [4–8] for stoppers vs. 7 [5–8] for nonstoppers; p = .7) and all other ESAS symptoms were not significantly different between stoppers and nonstoppers. The median durations of strong opioid use and any opioid use of all patients were 91 days (95% CI: 75–127 days) and 140 days (95% CI: 93–184 days), respectively. The median duration of any opioid use of CAGE-positive patients (261 days, 95% CI: 155–832 days) was significantly longer than that of CAGE-negative patients (93 days, 95% CI: 74–149 days; p= .008; Fig. 2). The frequency of history of substance abuse was not significantly different between stoppers and nonstoppers based on univariate χ2 tests at 3 and 6 months.
Figure 2.
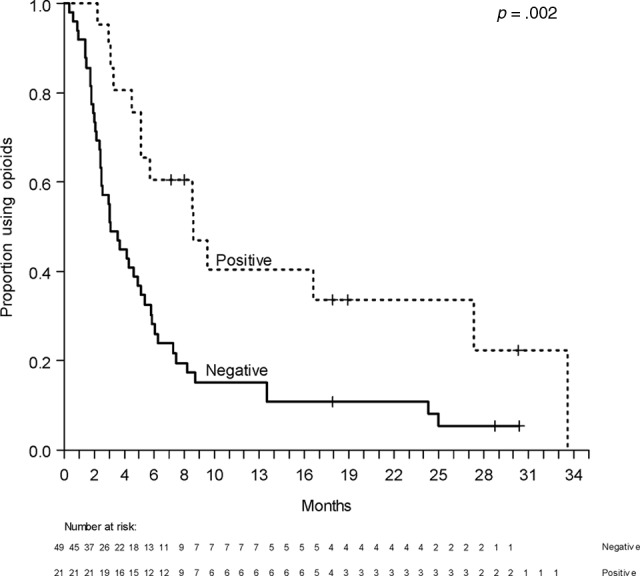
Duration of any type of opioid use according to CAGE status.
In the final parsimonious multivariable Cox model with backward stepwise regression, substance abuse (HR: 0.45, p = .047) and radiation dose (HR: 0.89, p = .017) were independently associated with increased time to stopping strong opioids. The median duration of strong opioid use was 155 days (95% CI: 45–760 days) among patients with a history of substance abuse and 90 days (95% CI: 73–126 days) for patients without a history of substance abuse (HR: 0.54, p = .047).
The median follow-up duration of all patients from the completion of radiation therapy was 764 days (95% CI: 686–871 days). During the follow-up period, 13 patients had disease relapse or second primary cancers. The DFS did not differ significantly between stoppers and nonstoppers.
Twenty-two nonstoppers (53%) were still using opioids 6 months after they completed radiation therapy. The CAGE positivity rate of nonstoppers (12 of 23, 52%) was significantly higher than that of stoppers (8 of 46, 17%; OR: 5.2, p = .005). Patients whose MEDD increased by 1 mg within 3 months had a 2.8% higher probability of continuing opioid use at 6 months (OR: 1.028, p = .005).
Discussion
Our study found that more than 50% of patients were unable to stop opioid therapy more than 3 months after completion of radiotherapy with or without chemotherapy. At 6 months, 23 patients (33%) still were not able to discontinue opioids. Our findings suggest that a large proportion of patients with advanced head and neck cancer remain on opioid therapy for months after completion of curative treatment. These patients may benefit from close monitoring by an interdisciplinary team, such as supportive care.
Acute radiation-induced mucositis typically occurs 2–3 weeks after treatment initiation, worsens over the course of radiation, and improves 4–6 weeks after treatment [34]. Although acute mucositis has been well characterized, there is limited research on the late effects of radiation-induced mucositis. Patients may experience chronic mucosal pain and sensitivity, which could limit their oral intake and food choices. Xerostomia, thick secretions, halitosis, oral infections, trismus, taste alterations, osteoradionecrosis, anxiety, and depression may further increase the symptom burden for these patients, months after treatment. In this study, we examined a novel marker of complex orofacial pain management: longer-term opioid use. Future research should address the risks and benefits of chronic opioid therapy for these patients.
We did not identify the demographic variables associated with opioid cessation within 3 months after the completion of radiation therapy. In contrast, in studies of opioid use in cancer-free patients, younger age was associated with opioid dependency [14, 15]. These conflicting results may be explained by the narrow age range in the present study (median age: 56 years; interquartile range: 50–64 years).
The proportion of CAGE-positive patients (30%) in the present study was higher than that of previous studies of prevalence of alcoholism in patients with cancer [19, 20]. In previous studies, CAGE positivity was associated with head and neck cancer. It is probably because its etiology is closely related to smoking and alcohol consumption [35].
In the present study, the proportion of CAGE-positive patients who stopped using opioids 3 months after completing radiation therapy was lower than that of CAGE-negative patients. However, peak MEDD was not associated with stopping opioids within 3 months of radiation therapy completion. We reported similar results in our previous studies [19, 20]. Dev et al. [20] reported that CAGE positivity was associated with inappropriate opioid escalation or abuse. Parson et al. [19] reported that CAGE-positive patients were more frequently on opioids and had higher symptom expression but not higher MEDD.
The association between CAGE positivity and not stopping opioids within 3 months after the completion of radiation therapy may be explained by the fact that alcohol and opioids have similar reward and reinforcement systems [36–38]. Opioids bind the mu receptors in the mesolimbic system, resulting in affected rewards, and alcohol through the release of endorphins also stimulates mesolimbic system. Several clinical trials reported that opioid antagonists affect opioid and alcohol dependence [39]. Another possible explanation of the association between CAGE positivity and not stopping opioids within 3 months after radiation therapy completion is the association between alcoholism and the abuse of other substances [40]. Additional studies of opioid abuse in a larger group of patients might reveal information that more fully explains the relationship between CAGE positivity and opioid use; in the meantime, the use of the CAGE questionnaire may help clinicians to identify head and neck cancer survivors who are at risk of opioid-related problems. Importantly, CAGE positivity is likely only one of many contributors to prolonged opioid use, and continual analgesia use months after treatment does not necessarily mean that the patient is misusing opioids. Other factors such as persistent mucosal damage, comorbid conditions, and psychological issues may also explain why patients remain on opioids for a long term. Future studies should assess CAGE and these other potential factors prospectively.
In our study, the substance abuse rate (16%; 11 of 70 patients) was higher than those previously reported for patients with cancer (<5%) and the general population (8.9%), even when we excluded alcoholism [41–43] The abuse of one substance has been reported to be positively associated with opioid dependency and multiple substance abuse. In addition, a history of substance abuse was also associated with a long duration of strong opioid use—a finding that has been previously reported for both patients with cancer and cancer-free patients [15, 44, 45]. Screening for a history of substance abuse in patients with head and neck cancer can help clinicians to identify patients who have a high risk of developing opioid-related problems and monitor such patients accordingly.
Our study had several limitations. First, the retrospective nature of this study limited the quality of our data collection. For instance, we were only able to ascertain the presence of opioid prescriptions based on chart documentation and pharmacy records; we may have missed opioid prescriptions provided by outside physicians and coded patients as opioid stoppers. This would have resulted in an underestimation of the proportion of nonstoppers. We were also unable to retrieve the specific site and nature of orofacial pain. Furthermore, aberrant behaviors may be underdetected and underdocumented. Prospective studies may help overcome these methodological issues to a certain extent. Second, we only included patients referred because of high symptom expression, complex pain syndromes, and higher doses of opioid, thus representing a more challenging population for symptom management than those seen at a typical head and neck clinic. Third, the sample size was small and thus likely underpowered to detect differences between stoppers and nonstoppers. Larger prospective studies are needed to provide further insights.
In conclusion, the findings of the present study suggest that a large proportion of patients with head and neck cancer seen by palliative care may require long-term opioids after completion of curative therapies. Such patients may benefit from careful assessment of pain syndrome, longitudinal monitoring, screening for alcoholism, psychosocial interventions, and interprofessional support. Future prospective studies are also required to examine other factors contributing to long-term opioid use and optimal management of orofacial pain in these patients.
Acknowledgments
This work was supported by the National Institutes of Health (grants RO1NRO10162–01A1, RO1CA122292–01, and RO1CA124481–01 to E.B.) and MD Anderson Cancer Center (support grant CA016672).
Author Contributions
Conception/Design: Jung Hye Kwon, David Hui, Gary Chisholm, Eduardo Bruera
Provision of study material or patients: Jung Hye Kwon, David Hui, Eduardo Bruera
Collection and/or assembly of data: Jung Hye Kwon
Data analysis and interpretation: Jung Hye Kwon, David Hui, Gary Chisholm, Eduardo Bruera
Manuscript writing: Jung Hye Kwon, David Hui, Gary Chisholm, Eduardo Bruera
Disclosures
The authors indicated no financial relationships.
Reference
- 1.Seiwert TY, Salama JK, Vokes EE. The chemoradiation paradigm in head and neck cancer. Nat Clin Pract Oncol. 2008;4:156–171. doi: 10.1038/ncponc0750. [DOI] [PubMed] [Google Scholar]
- 2.Trotti A, Bellm Lam, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother Oncol. 2003;66:253–262. doi: 10.1016/s0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 3.Bese NS, Hendry J, Jeremic B. Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. Int J Radiat Oncol Biol Phys. 2007;68:654–661. doi: 10.1016/j.ijrobp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Robertson C, Robertson AG, Hendry JH, et al. Similar decreases in local tumor control are calculated for treatment protraction and for interruptions in the radiotherapy of carcinoma of the larynx in four centers. Int J Radiat Oncol Biol Phys. 1998;40:319–329. doi: 10.1016/s0360-3016(97)00716-5. [DOI] [PubMed] [Google Scholar]
- 5.Zenda S, Matsuura K, Tachibana H, et al. Multicenter phase II study of an opioid-based pain control program for head and neck cancer patients receiving chemoradiotherapy. Radiother Oncol. 2011;101:410–414. doi: 10.1016/j.radonc.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Bensadoun RJ, Franquin JC, Ciais G, et al. Low-energy He/Ne laser in the prevention of radiation-induced mucositis. A multicenter phase III randomized study in patients with head and neck cancer. Support Care Cancer. 1999;7:244–252. doi: 10.1007/s005200050256. [DOI] [PubMed] [Google Scholar]
- 7.Chen SC, Lai YH, Liao CT, et al. Changes of symptoms and depression in oral cavity cancer patients receiving radiation therapy. Oral Oncol. 2010;46:509–513. doi: 10.1016/j.oraloncology.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 8.Epstein JB, Hong C, Logan RM, et al. A systematic review of orofacial pain in patients receiving cancer therapy. Support Care Cancer. 2010;18:1023–1031. doi: 10.1007/s00520-010-0897-7. [DOI] [PubMed] [Google Scholar]
- 9.List MA, Siston A, Haraf D, et al. Quality of life and performance in advanced head and neck cancer patients on concomitant chemoradiotherapy: A prospective examination. J Clin Oncol. 1999;17:1020–1028. doi: 10.1200/JCO.1999.17.3.1020. [DOI] [PubMed] [Google Scholar]
- 10.Epstein JB, Elad S, Eliav E, et al. Orofacial pain in cancer: Part II—Clinical perspectives and management. J Dent Res. 2007;86:506–518. doi: 10.1177/154405910708600605. [DOI] [PubMed] [Google Scholar]
- 11.Fischer DJ, Klasser GD, Epstein JB. Cancer and orofacial pain. Oral Maxillofac Surg Clin North Am. 2008;20:287–301. doi: 10.1016/j.coms.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Larance B, Degenhardt L, Lintzeris N, et al. Definitions related to the use of pharmaceutical opioids: Extramedical use, diversion, non-adherence and aberrant medication-related behaviours. Drug Alcohol Rev. 2011;30:236–245. doi: 10.1111/j.1465-3362.2010.00283.x. [DOI] [PubMed] [Google Scholar]
- 13.Passik SD, Kirsh KL, McDonald MV, et al. A pilot survey of aberrant drug-taking attitudes and behaviors in samples of cancer and AIDS patients. J Pain Symptom Manage. 2000;19:274–286. doi: 10.1016/s0885-3924(00)00119-6. [DOI] [PubMed] [Google Scholar]
- 14.Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105:1776–1782. doi: 10.1111/j.1360-0443.2010.03052.x. [DOI] [PubMed] [Google Scholar]
- 15.Edlund MJ, Steffick D, Hudson T, et al. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129:355–362. doi: 10.1016/j.pain.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Passik SD, Kirsh KL, Donaghy KB, Portenoy RK. Pain and aberrant drug-related behaviors in medically ill patients with and without histories of substance abuse. Clin J Pain. 2006;22:173–181. doi: 10.1097/01.ajp.0000161525.48245.aa. [DOI] [PubMed] [Google Scholar]
- 17.Passik SD, Schreiber J, Kirsh KL, Portenoy RK. A chart review of the ordering and documentation of urine toxicology screens in a cancer center: Do they influence patient management? J Pain Symptom Manage. 2000;19:40–44. doi: 10.1016/s0885-3924(99)00137-2. [DOI] [PubMed] [Google Scholar]
- 18.Lawlor P, Walker P, Bruera E, et al. Severe opioid toxicity and somatization of psychosocial distress in a cancer patient with a background of chemical dependence. J Pain Symptom Manage. 1997;13:356–361. doi: 10.1016/s0885-3924(97)00081-x. [DOI] [PubMed] [Google Scholar]
- 19.Parsons HA, Delgado-Guay MO, El Osta B, et al. Alcoholism screening in patients with advanced cancer: Impact on symptom burden and opioid use. J Palliat Med. 2008;11:964–968. doi: 10.1089/jpm.2008.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dev R, Parsons HA, Palla S, et al. Undocumented alcoholism and its correlation with tobacco and illegal drug use in advanced cancer patients. Cancer. 2011;117:4551–4556. doi: 10.1002/cncr.26082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon JH, Hui D, Chisholm G, et al. Clinical characteristics of cancer patients referred early to supportive and palliative care. J Palliat Med. 2013;16:148–155. doi: 10.1089/jpm.2012.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ackerstaff AH, Tan IB, Rasch CR, et al. Quality-of-life assessment after supradose selective intra-arterial cisplatin and concomitant radiation (RADPLAT) for inoperable stage IV head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2002;128:1185–1190. doi: 10.1001/archotol.128.10.1185. [DOI] [PubMed] [Google Scholar]
- 23.van der Molen L, van Rossum MA, Burkhead LM, et al. A randomized preventive rehabilitation trial in advanced head and neck cancer patients treated with chemoradiotherapy: Feasibility, compliance, and short-term effects. Dysphagia. 2011;26:155–170. doi: 10.1007/s00455-010-9288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boscolo-Rizzo P, Maronato F, Marchiori C, et al. Long-term quality of life after total laryngectomy and postoperative radiotherapy versus concurrent chemoradiotherapy for laryngeal preservation. Laryngoscope. 2008;118:300–306. doi: 10.1097/MLG.0b013e31815a9ed3. [DOI] [PubMed] [Google Scholar]
- 25.Moore AA, Seeman T, Morgenstern H, et al. Are there differences between older persons who screen positive on the CAGE questionnaire and the Short Michigan Alcoholism Screening Test-Geriatric Version? J Am Geriatr Soc. 2002;50:858–862. doi: 10.1046/j.1532-5415.2002.50211.x. [DOI] [PubMed] [Google Scholar]
- 26.Ewing JA. Detecting alcoholism: The CAGE questionnaire. JAMA. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 27.Bisson J, Nadeau L, Demers A. The validity of the CAGE scale to screen for heavy drinking and drinking problems in a general population survey. Addiction. 1999;94:715–722. doi: 10.1046/j.1360-0443.1999.9457159.x. [DOI] [PubMed] [Google Scholar]
- 28.Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 29.Cummings G, Biondo PD, Campbell D, et al. Can the global uptake of palliative care innovations be improved? Insights from a bibliometric analysis of the Edmonton Symptom Assessment System. Palliat Med. 2011;25:71–82. doi: 10.1177/0269216310381449. [DOI] [PubMed] [Google Scholar]
- 30.McPherson ML. Bethesda, MD: American Society of Health-System Pharmacists; 2009. Demystifying opioid conversion calculations: A guide for effective dosing; p. 5. [Google Scholar]
- 31.Hallenbeck JL. New York: Oxford University Press; 2003. Palliative care perspectives; p. 71. [Google Scholar]
- 32.Mercadante S, Caraceni A. Conversion ratios for opioid switching in the treatment of cancer pain: A systematic review. Palliat Med. 2011;25:504–515. doi: 10.1177/0269216311406577. [DOI] [PubMed] [Google Scholar]
- 33.Bruera E, Moyano J, Seifert L, et al. The frequency of alcoholism among patients with pain due to terminal cancer. J Pain Symptom Manage. 1995;10:599–603. doi: 10.1016/0885-3924(95)00084-4. [DOI] [PubMed] [Google Scholar]
- 34.Epstein JB, Thariat J, Bensadoun RJ, et al. Oral complications of cancer and cancer therapy: From cancer treatment to survivorship. CA Cancer J Clin. 2012;62:400–422. doi: 10.3322/caac.21157. [DOI] [PubMed] [Google Scholar]
- 35.Anantharaman D, Marron M, Lagiou P, et al. Population attributable risk of tobacco and alcohol for upper aerodigestive tract cancer. Oral Oncol. 2011;47:725–731. doi: 10.1016/j.oraloncology.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav. 2004;81:339–358. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 37.McLellan AT, Lewis DC, O'Brien CP, et al. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- 38.Koob GF. Dynamics of neuronal circuits in addiction: Reward, antireward, and emotional memory. Pharmacopsychiatry. 2009;42:S32–S41. doi: 10.1055/s-0029-1216356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lobmaier PP, Kunøe N, Gossop M, et al. Naltrexone depot formulations for opioid and alcohol dependence: A systematic review. CNS Neurosci Ther. 2011;17:629–636. doi: 10.1111/j.1755-5949.2010.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stinson FS, Grant BF, Dawson DA, et al. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2005;80:105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Starr TD, Rogak LJ, Passik SD. Substance abuse in cancer pain. Curr Pain Headache Rep. 2010;14:268–275. doi: 10.1007/s11916-010-0118-6. [DOI] [PubMed] [Google Scholar]
- 42.Derogatis LR, Morrow GR, Fetting J, et al. The prevalence of psychiatric disorders among cancer patients. JAMA. 1983;249:751–757. doi: 10.1001/jama.249.6.751. [DOI] [PubMed] [Google Scholar]
- 43.U.S. Department of Health and Human Services. Results from the 2010 National Survey on Drug Use and Health: Summary of national findings. [Accessed November 27, 2012]. Available at http://www.samhsa.gov/data/nsduh/2k10nsduh/2k10results.htm.
- 44.Sullivan MD, Edlund MJ, Fan MY, et al. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: The TROUP Study. Pain. 150:332–339. doi: 10.1016/j.pain.2010.05.020. 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: A systematic review and literature synthesis. Clin J Pain. 2008;24:497–508. doi: 10.1097/AJP.0b013e31816b1070. [DOI] [PubMed] [Google Scholar]