Abstract
Impairments in cognitive control are a defining feature of schizophrenia. Aspects of cognitive control include proactive control, the maintenance of task rules or goals to bias attention and maintain preparedness, and reactive control, the engagement of attention in reaction to changing cognitive demands. Proactive control is thought to be particularly impaired in schizophrenia. We sought to examine proactive and reactive control in schizophrenia, as measured by reaction time (RT) variability and especially long RTs, thought to represent lapses in proactive control, during the Stroop paradigm. Furthermore we sought to examine the neural underpinnings of lapses in proactive control and the subsequent engagement of reactive control in those with schizophrenia compared to healthy controls, using fMRI. We found that patients with schizophrenia displayed greater RT variability and more especially long RTs than controls, suggesting that proactive control is weaker in the schizophrenia compared with the control group. All participants engaged regions of the cognitive control network during long RTs, consistent with an engagement of reactive control following a failure in proactive control on these trials. The schizophrenia group, however, displayed significantly diminished activity in these regions compared to controls. Our results suggest increased failures in proactive but also impaired reactive control in schizophrenia compared to healthy subjects.
Introduction
It is estimated that there are currently 24 million people worldwide who have been diagnosed with schizophrenia. One of the most debilitating aspects of the disease, and the most difficult to treat pharmacologically, are deficits in cognition (Cohen & Servan-Schreiber, 1992). Cognitive changes are evident before the onset of psychosis. Patients who later go on to develop schizophrenia have lower test scores and IQ scores as early as age seven (Seidman, Buka, Goldstein, & Tsuang, 2006). This deficit appears to amplify around the time of disease onset (Bilder et al., 2006, see also Fuller et al., 2002).
Cognitive control is defined as a set of cognitive and neural operations linked to the function of a distributed neural network that involves the dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC) and the inferior parietal lobes (IPL; Niendam et al., 2012). Disrupted cognitive control has been shown across a range of cognitive domains in schizophrenia (Lesh, Niendam, Minzenberg, & Carter, 2011) and reductions in the function of the frontal-cingulate-parietal cognitive control network have been reliably demonstrated in schizophrenia at the meta analytic level (Minzenberg, Laird, Thelen, Carter, & Glahn, 2009).
An influential model of cognitive control suggests that it can operate on different time scales, either proactively or reactively (Braver, Paxton, Locke, & Barch, 2009; Paxton, Barch, Racine, & Braver, 2008). Proactive control is conceptualized as maintenance of goal-relevant information to optimally bias attention, perception and response preparation ahead of a cognitively demanding event or the maintenance of task set. In contrast, reactive control reflects transient, ‘on the fly’ engagement of control processes at the onset of challenging task demands. It has also been suggested that proactive cognitive control may be relatively more disrupted than reactive control in schizophrenia (Edwards, Barch, & Braver, 2010; Lesh, Westphal, Niendam, Yoon, Minzenberg, Ragland, Solomon & Carter, 2013).
Intra-individual reaction time (RT) variability has been used extensively as a measure of cognitive instability (Fjell, Westlye, Amlien, & Walhovd, 2011), attention lapsing and mind wandering (see Tamm et al, 2012 for a review) in disorders such as Attention Deficit/Hyperactivity Disorder (ADHD: Buzy, Medoff, & Schweitzer, 2009; Epstein et al., 2010; Fassbender et al., 2009; Hervey et al., 2006; Leth-Steensen, Elbaz, & Douglas, 2000) and may serve as a mechanism for examining the basis behind cognitive control deficits. High levels of RT variability have also been associated with an inability to effectively engage cognitive control in situations of increased cognitive demands (Bellgrove, Hester, & Garavan, 2004), sustained attention impairments (Hervey, et al., 2006; Leth-Steensen, et al., 2000) and problems in regulating energetic states (Andreou et al., 2007; Kuntsi, Oosterlaan, & Stevenson, 2001; Scheres, Oosterlaan, & Sergeant, 2001; Sergeant, Geurts, Huijbregts, Scheres, & Oosterlaan, 2003). Increased RT variability in comparison to healthy control subjects has been noted in diseases associated with deficits in cognition, including ADHD (Buzy, et al., 2009; Castellanos & Tannock, 2002; Heathcote, Brown, & Cousineau, 2004; Loo et al., 2003), high functioning autism (Verte, Geurts, Roeyers, Oosterlaan, & Sergeant, 2005), traumatic brain injury (Segalowitz, Dywan, & Unsal, 1997; Stuss et al., 1989; Stuss, Stethem, Picton, Leech, & Pelchat, 1989; Tinius, 2003; Whyte, Polansky, Fleming, Coslett, & Cavallucci, 1995), early stage Alzheimer’s dementia (Duchek et al., 2009; Gorus, De Raedt, Lambert, Lemper, & Mets, 2008), schizophrenia (Kaiser et al., 2008; van den Bosch & Rombouts, 1997; Vinogradov, Poole, Willis-Shore, Ober, & Shenaut, 1998) and bipolar disorder with psychotic symptoms (Bora, Vahip, & Akdeniz, 2006). We hypothesize that increased RT variability and particularly long RT are an index of poor proactive control. Effective proactive control should result in a strong representation of the task set, resulting in consistent and relatively faster RTs to task stimuli. Under situations of ineffective proactive control longer RTs to task stimuli would be expected, which would increase the need for activation of reactive control in order to support correct responding.
Although the majority of studies in the past have used standard deviation of RT to compare RT variability between controls and clinical groups, this comparison may be problematic as the standard deviation of RT is highly correlated with the mean RT (Adams, Roberts, Milich, & Fillmore, 2011; Epstein et al., 2011; Wagenmakers & Brown, 2007), which can often differ significantly in clinical groups. For this reason, some groups (e.g., Bellgrove, et al., 2004; Simmonds et al., 2007) have favored the use of the coefficient of variation of RT (RT standard deviation/mean RT). However, more recently statistical approaches that formally model RT distributions have become available. The ex-Gaussian representation of RT variability consists of a normal distribution with a right sided, exponential “tail”. This right-sided tail captures a number of long RT, which can skew the standard mean RT measure, making the mean RT appear longer. The ex-Gaussian model permits the measurement of the mean (mu) and standard deviation (sigma) of the normal (Gaussian) component, and the mean and standard deviation (tau) of the exponential component. Tau is often considered to represent trials on which subjects were engaging in lapses of task engagement (Lee et al., 2012; Leth-Steensen, et al., 2000; Tamm et al., 2012), whereas the standard deviation of the normal component is considered to capture response preparation/execution problems (Bellgrove, et al., 2004; Schall, 2003; Schall & Hanes, 1998; Vaurio, Simmonds, & Mostofsky, 2009). Thus tau may represent trials on which participants experience lapses in proactive control and hence needed to rely upon more reactive, compensatory control mechanisms to support correct responding. Of the two studies we found that have employed ex-Gaussian techniques in schizophrenia one found elevated tau but not sigma or mu (Rentrop et al, 2010) and the other found elevated tau and mu but not sigma (Kieffaber et al, 2006).
Previous studies utilizing the ex-Gaussian model of RT have associated increased intra-individual RT variability (IIV) and increased levels of tau with an inability to suppress task irrelevant networks (often referred to as the default mode network or DMN) during cognitively demanding situations (Broyd et al., 2009; Fassbender, et al., 2009). The DMN is a group of regions, primarily distributed along the medial wall of the brain, which is active when individuals are engaged in non-task related thought or mind wandering (Binder et al., 1999; Raichle et al., 2001; Weissman, Roberts, Visscher, & Woldorff, 2006). Activity in these regions is often reported to be anti-correlated with activity in cognitive control regions; when engaging in a cognitively demanding activity, DMN is suppressed and cognitive control regions are activated and weak anti-correlations between DMN and control regions during the resting state as well as task performance have been associated with poor task performance on cognitive tasks (Castellanos et al., 2008; Kelly, Uddin, Biswal, Castellanos, & Milham, 2008). Lapses in attention have also been linked to insufficient DMN suppression (Weissman, et al., 2006). The DMN has been shown in some studies to be hyper-active during task performance in schizophrenia and while this has been invoked to explain the intrusion of internal mental activity that might be experienced by the subject as psychotic symptoms (Pomarol-Clotet et al., 2008; Whitfield-Gabrieli et al., 2009) it has also been suggested to be related to the impaired cognition associated with the illness (Harrison, Yucel, Pujol & Pantelis, 2007; Whitfield-Gabrielli et al, 2009).
In the present study we sought to investigate the dynamics of cognitive control instability and the relative integrity of proactive and reactive cognitive control using RT distributional analysis and the Stroop Task during fMRI. We examined trials with uncharacteristically long RT, as measured by tau via the ex-Gaussian distribution of RT. We hypothesized that patients with schizophrenia would display increased tau compared with controls as found in previous studies in schizophrenia (Rentrop et al, 2010; Kieffaber et al, 2006), reflecting lapses in proactive cognitive control in the patient group. We also expected that across groups, trials with long RT would be associated with increased activity in lateral and medial frontal cortex, due to increased engagement of reactive control during these trials. We further hypothesized that this long RT related neural activity would be relatively attenuated in individuals with schizophrenia due to deficits in reactive control. These findings would suggest that patients with schizophrenia exhibit an overreliance on a poorly functioning reactive control network when carrying out tasks of cognition.
Ed Smith was a pioneer is using functional imaging methods to interrogate the functional architecture of human cognition and later in his career he sought to use this same approach to understanding those aspects of cognitive and emotional processes that are impaired in schizophrenia, and related to symptoms of the illness as well as those that are intact. In doing so he brought a new sophistication to functional imaging studies of the illness. He was also an influential participant in CNTRICS, where the Stroop task was recommended for the measurement of cognitive control, and of the RDoC initiative that followed, where cognitive control was identified as a key construct that is impaired in psychopathology. We dedicate this article to him.
Method
Participants
Twenty-five first episode patients who met DSM-IV criteria for a schizophrenia spectrum disorder (19 schizophrenia, 5 schizoaffective, 1 schizophreniform) and 26 control subjects were recruited. The schizophrenia subjects were outpatients, recently admitted to a University based specialty clinic for early psychosis, and were tested within one year of the onset of their first psychotic symptoms. Fifteen healthy controls and 14 schizophrenia patients were included in a previous study comparing the AX-CPT and Stroop (Lesh, Westphal, Niendam, Yoon, Minzenberg, Ragland, Solomon & Carter, 2013). Characteristics for patients and controls are reported in Table 1. All participants were assessed using the Structured Clinical Interview for the DSM-IV-TR (SCID-I/P; First, Spitzer, Gibbon, & Williams, 2002). Clinicians with masters or doctoral degrees, trained to high reliability (kappa>0.80; range 0.80–1.0), conducted clinical interviews. Schizophrenia participants were followed longitudinally and diagnoses were confirmed 12 months after ascertainment. Clinical ratings were collected in the patient sample using the Scale for the Assessment of Negative Symptoms (SANS; NC. Andreasen, 1983), Scale for the Assessment of Positive Symptoms (SAPS; NC. Andreasen, 1984), and Brief Psychiatric Rating Scale (BPRS; Lukoff, Nuechterlein, & Ventura, 1986). Exclusion criteria for both groups included Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) IQ score below 70, alcohol or drug dependence or abuse within 3 months before testing, positive urine toxicology screen for illicit drugs, prior head trauma worse than a Grade I concussion, or contraindication to MRI scanning. Controls were additionally excluded for any lifetime diagnosis of an Axis I or Axis II disorder or any first-degree relatives with a psychotic disorder. After complete description of the study to the subjects, written informed consent was obtained. The University of California, Davis Institutional Review Board, approved the study.
Table 1.
Demographic and clinical data.
| Measure | Group
|
|
|---|---|---|
| Control (n=26) | Schizophrenia (n=25) | |
| Demographic and Clinical Data | ||
| Age: Mean (SD) | 19.3 (3.6) | 19.9 (3.8) |
| Gender (% male)* | 39 | 72 |
| Handedness (% right) | 89 | 100 |
| Subject Education: Mean (SD) | 12.4 (3.1) | 11.7 (2.3) |
| Parental Education: Mean (SD) | 15.0 (2.7) | 15.4 (2.6) |
| IQ (WASI): Mean (SD) | 112.2 (13.7) | 103.5 (12.8) |
| Duration of Illness (days): Mean (SD) | - | 214 (96) |
| SANS: Mean (SD) | - | 8.4 (4.4) |
| SAPS: Mean (SD) | - | 6.8 (3.6) |
| BPRS: Mean (SD) | - | 44.3 (12.5) |
| GAF: Mean (SD) | - | 44.0 (8.6) |
| Antipsychotic Type at Scan | ||
| Atypical | - | 15 |
| Typical | - | 1 |
| Unmedicated | - | 9 |
SD, standard deviation
p < 0.05
Stroop Task
All subjects performed a single-trial version of the Stroop task. Stimuli consisted of one of three words (RED, GREEN, and BLUE) that were printed in one of those three ink colors. Trials were either congruent (e.g., the word “RED” in red ink), or incongruent (e.g., the word “RED” in green ink). Subjects responded with a button press corresponding to the ink color of the word and were told to ignore the word itself. Stimuli were displayed for 1 second and were separated by a 2 second inter-trial interval consisting of a fixation cross. Each subject completed three blocks of 88 trials with approximately 73 % congruent trials. Stimuli were pseudorandomized such that the first and last four trials of each scanning run were congruent trials. Subjects were told to maximize speed while minimizing errors.
Behavioral Analysis
The numerical algorithm Quantile Maximum Probability Estimator (QMPE; Heathcote, et al., 2004) was used to estimate the ex-Gaussian parameters. IIV was estimated within each condition (I and C) for all subjects. In addition to examining the variability of Gaussian component (σ) and the exponential component (τ) separately we used a combination of the two as our estimate of the intra-individual RT variability as expressed by the following equation:
Repeated measures ANOVA examined effects of Group (SZ, control) and Stimulus (C, I) as well any possible Group X Condition interaction on tau and IIV separately. Accuracy and RT were also analyzed using ANOVA with stimulus (C, I) as a within-subjects factor and group (SZ, control) as a between-subjects factor.
Image Acquisition and Analysis
Acquisition
Imaging data were obtained using a 1.5T General Electric MRI scanner. Coplanar T1-weighted and T2-weighted structural images were acquired prior to each fMRI sequence. T2-weighted EPI sessions were acquired with the following settings: TR=1500 ms, echo time=32 ms, flip angle=90°, field of view=22cm, matrix size=64×64. EPI images consisted of 20 contiguous and interleaved 4.0-mm axial slices with a 3.44-mm × 3.44-mm × 4.0mm voxel size.
Preprocessing
Preprocessing and functional analysis was completed using Statistical Parametric Mapping-8 (SPM8, http://www.fil.ion.ucl.ac.uk/SPM8), including slice timing correction and spatial realignment. Spatial normalization was performed with the FMRIB Software Library (FSL v5.0, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) using a linear transformation of each subjects’ mean EPI image to their SPGR image, followed by nonlinear warping to the Montreal Neurological Institute (MNI) T1 template. Partial smoothing using a Gaussian 8 mm full-width half-maximum kernel was subsequently performed in SPM8. Individual fMRI runs were removed from the analysis if translational movement exceeded 4-mm or rotational movement exceeded 3 degrees of within-run movement.
Whole-Brain Analysis
We employed multiple regression in the general linear model framework using SPM8. All task regressors were modeled and only correct responses were included in the reported contrasts. Stroop regressors included a short RT, long RT, and an error regressor that was included as a regressor of no interest. In order to have a sufficient number of trials to accurately model the effects of interest, an RT cutoff using a multiple of tau was used that resulted in an average of 30 long RT trials across all subjects (tau multiple = 2.2). The short RT regressor was composed of all other correct trials. Translational and rotational movement data were included as covariates. Group-level random-effects comparisons between groups were computed by subtracting the short from long RT regressors (Long-short RT contrast). All between-group contrasts were thresholded at the voxel level with p<0.01 and clusters were considered significant if they survived cluster level FWE correction of p<0.05.
Region of Interest Analyses
In addition to whole-brain analyses, a priori hypotheses regarding the DLPFC, IPL, ACC, and precuneus prompted an exploratory interrogation of four bilateral regions of interest (ROI). DLPFC, IPL and ACC regions have been consistently implicated in cognitive control functioning, and the precuneus has additionally been linked to increased IIV and tau in other clinical disorders (Castellanos et al, 2008). The DLPFC ROI consisted of bilateral Brodmann Area 9 anatomical masks obtained from the Wake Forest University PickAtlas (Maldjian, Laurienti, & Burdette, 2004; Maldjian, Laurienti, Kraft, & Burdette, 2003). The remaining ROIs were obtained using bilateral ACC, IPL, and precuneus regions defined in the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002). Beta weights for each ROI were extracted and analyzed from the long and short RT contrasts, separately, and evaluated with repeated measures ANOVA.
Finite Impulse Response (FIR) Analysis
A secondary analysis using a FIR basis set was conducted with the goal of confirming RT-related activity differences between groups as well as exploring the nature of these differences over time without making assumptions about the shape of the RT-related response. Rather than binning RTs based upon a tau cutoff as implemented in the primary analysis, we used RT as a continuous parametric modulator to measure the relationship between RT variability and BOLD activity. Similar to the methods of Yarkoni and colleagues (2009), all correct trials (Incongruent and Congruent) were modeled as a single task effect of interest, representing task versus fixation-cross baseline. For each subject, RTs were demeaned and divided by the standard deviation. These standardized RTs were implemented as parametric modulators at each of 7 discrete time points, encompassing a time window of 10.5 seconds post-stimulus onset. In order to compliment our primary analysis and identify where in the time-course the two groups differ, whole-brain analyses were conducted at each time point separately. As a correction for multiple comparisons, we used a height threshold of p<0.01 (as in the primary analysis) but with a bonferroni-corrected FWE cluster threshold of p<0.007 (correcting for seven time bins). To further explore the pattern of RT-related activity, beta weights reflecting the 7 FIR regressors were extracted from each ROI specified in the primary analysis.
Results
Participant Characteristics
Table 1 presents relevant demographic and clinical characteristics for each group. Patients and controls did not differ on age, handedness, participant education, or parental education (p > 0.24). However, significantly more males were present in the patient group (Fisher’s Exact Test: p = 0.025) and patients had significantly lower estimated IQ (t(46) = 2.30, p = 0.026).
Behavioral Results
The examination of accuracy, measured by examining percent correct responses, revealed a main effect of Stimulus (F(1,49)=19.70; p < 0.0001) such that all subjects were more accurate in response to congruent compared to incongruent stimuli (95.91 vs. 93.52%, respectively). There was a marginally significant main effect of Group (F(1,49)=3.95; p=0.053) such that controls were more accurate overall than the schizophrenia group (95.90 vs. 93.48%, respectively). There was no significant Stimulus x Group interaction (p>0.3). In terms of RT to correct responses, there was also a main effect of Stimulus (F(1,49)=133.12; p < 0.0001), such that all subjects responded more quickly to congruent compared to incongruent stimuli (690.18 vs. 774.26 ms, respectively). There was also a main effect of Group (F(1,49)=11.29; p=0.002) such that controls responded more quickly in general than the schizophrenia group (688.72 vs. 798.26 ms, respectively). There was also a Stimulus x Group interaction (F(1,49)=4.53; p = 0.04), which was driven by a greater slowing to incongruent stimuli in the SZ group compared to controls (748.29 to 848.23 ms vs. 634.31 to 703.14 ms, respectively).
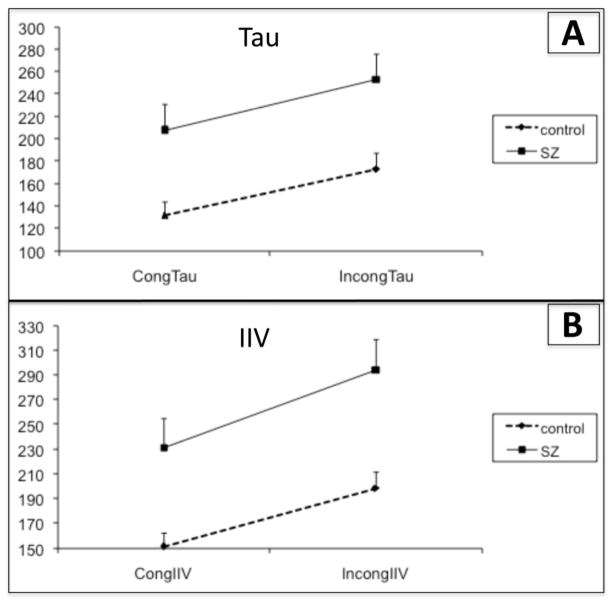
When examining IIV, repeated measures ANOVA revealed a main effect of Stimulus (F(1,49) = 86.43, p<0.0001) and Group (F(1,49) = 11.46, p=0.001), such that all subjects displayed an increase in IIV in the Incongruent compared to Congruent stimuli. The schizophrenia group also displayed greater IIV than controls in general. There was no Group X Stimulus interaction (p=0.19, See Fig. 1A). A similar result emerged when examining tau; there was a main effect of Stimulus (F(1,49) = 29.80, p<0.0001) and of Group (F(1,49) = 9.80, p=0.003), with all participants displaying larger tau during Incongruent stimuli and the schizophrenia group displaying increased tau compared to controls. Again, there was no Stimulus X Group interaction (p=0.78, See Fig. 1B).
Figure 1. Intra-individual RT variability (IIV) and tau during the Stroop task.
A) Tau measured in ms for congruent (cong) and incongruent (incong) stimuli in the control and schizophrenia (SZ) groups. All participants displayed and increase in tau with increasing difficulty (in incongruent compared to congruent stimuli). The SZ group had an overall increase in tau compared to the control group.
B) IIV measured in ms for congruent and incongruent stimuli in the control and SZ groups. All participants displayed and increase in IIV with increasing difficulty (in incongruent compared to congruent stimuli). The SZ group had an overall increase in IIV compared to the control group.
Correlations between RT variability and accuracy to congruent and incongruent stimuli separately in all subjects together revealed robust evidence of a relationship between these variables. Increased tau was associated with fewer correct responses to both congruent and incongruent stimuli. IIV displayed a similar pattern of correlations (see Table 2 for all significant correlations). In the schizophrenia group alone, in response to congruent stimuli, increased tau and IIV was associated with fewer percent correct responses (see Table 2).
Table 2.
Correlations between RT variability and task performance
| Group
|
|||||||
|---|---|---|---|---|---|---|---|
| All Together n=51 |
control only n=26 |
SZ only n=25 |
|||||
|
| |||||||
| Stroop Stimuli | Performance | Tau [r] | IIV [r] | Tau [r] | IIV [r] | Tau [r] | IIV [r] |
| Congruent | % Correct
|
−0.52**** | −0.55**** | n.s. | n.s. | −0.51** | −0.54** |
| incongruent | % Correct | −0.32* | −0.31* | n.s. | n.s. | n.s. | n.s. |
p < 0.05;
p < 0.01;
p < 0.001;
p < 0.0001
Imaging Results
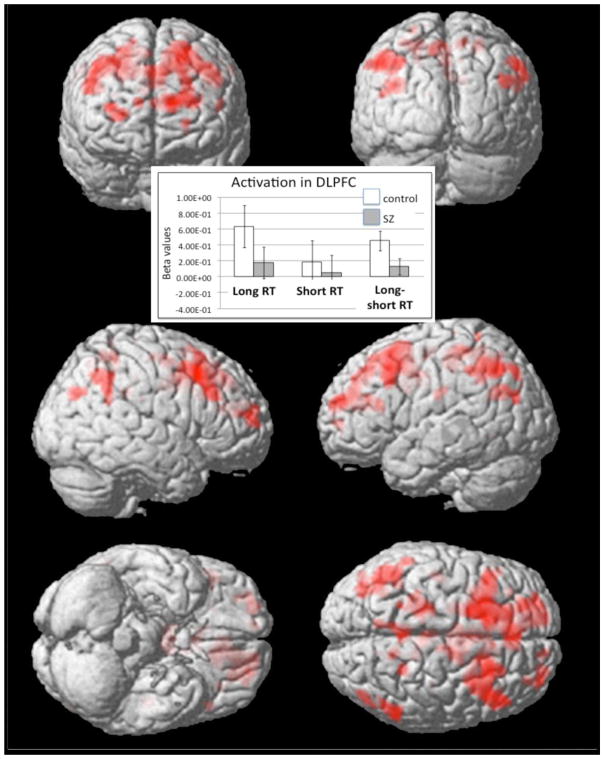
Within-group analyses examining long vs. short RT in the control group revealed increased activity in a broad network of regions including motor regions and cognitive control regions such as bilateral DLPFC, and ACC (see Fig 2 and Table 3). The same within-group contrast in the schizophrenia group resulted in a smaller set of significant regions including left motor regions and right IPL.
Figure 2. Within-group contrast displaying activations for Long vs. Short Reaction Times (RTs).
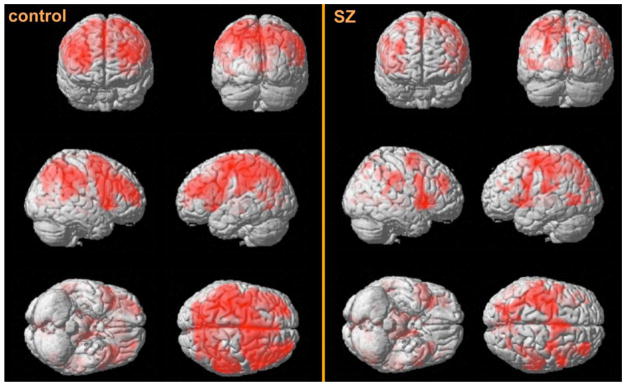
Separate within-group contrasts were performed for the control and SZ groups separately. Areas of activity during Long RT in controls included motor cortex, insula as well as regions of the cognitive control network such as bilateral prefrontal cortex. Regions of activity in the SZ group included left precentral gyrus, right IPL, right insula and left thalamus.
Table 3.
Regions of significant activation (height p<0.01; FWE cluster corrected p<0.05).
| Region | Brodmann’s Area | MNI coordinates
|
T voxel | ||||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Control | |||||||
| L. Supplementary Motor Areaa | 6 | −2 | 2 | 54 | 8.51 | ||
| L. Precentral Gyrus | 6 and 4 | −38 | −28 | 58 | 8.22 | ||
| L. Precuneus | 7 | −12 | −72 | 50 | 7.03 | ||
| L. Inferior Frontal Gyrus | 9 | −44 | 4 | 28 | 6.76 | ||
| L. Middle Frontal Gyrus | 46 | −46 | 34 | 22 | 6.51 | ||
| R. Insula | 13 | 34 | 20 | 4 | 6.18 | ||
| L. Insula | 13 | −32 | 18 | 6 | 6.07 | ||
| R. Inferior Frontal Gyrus | 44 | 58 | 10 | 16 | 6.02 | ||
| R. Middle Frontal Gyrus | 10 | 32 | 56 | 16 | 5.94 | ||
| Schizophrenia | |||||||
| L. Precentral Gyrus | 6 | −40 | −18 | 62 | 8.64 | ||
| R. Insula | 13 | 34 | 22 | −2 | 7.44 | ||
| R. Inferior Parietal Cortex | 40 | 34 | −52 | 36 | 5.06 | ||
| L. Thalamus | - | −12 | −24 | −2 | 4.81 | ||
|
| |||||||
| Control > Schizophrenia | |||||||
| L. Superior Frontal Gyrusb | 6 | −18 | 8 | 60 | 3.89 | ||
| L. Middle Frontal Gyrus | 9 | −44 | 18 | 38 | 3.37 | ||
| Schizophrenia > Control | |||||||
| No significant clusters | - | - | - | - | - | ||
MNI, Montreal Neurological Institute; Area; L, left; R, right
At the given threshold, a very large single cluster was identified. To better distinguish individual regions, a more stringent threshold of voxel-wise FWE p<0.05 and cluster size > 20 was applied. These significant regions are listed below the global maximum significant cluster.
One large significant cluster survived correction with the peak in superior frontal gyrus and extending down through middle frontal gyrus.
Between-group analyses revealed greater activity in the control group during long versus short RT in the left lateral frontal cortex (see Fig. 3). Region of interest analysis of DLPFC revealed a significant group (control or schizophrenia) by RT length (long or short) interaction (F(1,49) = 4.22, p < 0.05), a significant main effect of RT length (F(1,49) = 13.517, p = 0.001), and no main effect of group (F(1,49) = 0.818, p = 0.37). ANOVA of IPL revealed a trend level group by RT length interaction (F(1,49) = 3.705, p = 0.06), a significant main effect of RT length (F(1,49) = 26.924, p < 0.001), and a significant main effect of group (F(1,49) = 4.300, p < 0.05). ANOVA of ACC revealed a trend level group by RT length interaction (F(1,49) = 3.668, p = 0.06), no main effect of RT length (F(1,49) = 2.168, p = 0.15), and no main effect of group (F(1,49) = 1.079, p = 0.30). ANOVA of precuneus revealed no group by RT length interaction (F(1,49) = 2.768, p = 0.10), a significant main effect of RT length (F(1,49) = 18.497, p < 0.001), and a significant main effect of group (F(1,49) = 5.465, p < 0.05).
Figure 3. Between-group contrast displaying activations for Long vs. Short RT in the control vs. SZ group.
The control group displayed greater activity than the SZ group in left superior frontal gyrus and left middle frontal gyrus. The SZ group did not significantly activate any regions more than controls. The inset displays a graph plotting beta values for long and short RT contrasts separately in our a priori DLPFC ROI. The graph demonstrates significant between-group differences in the long RT contrast only.
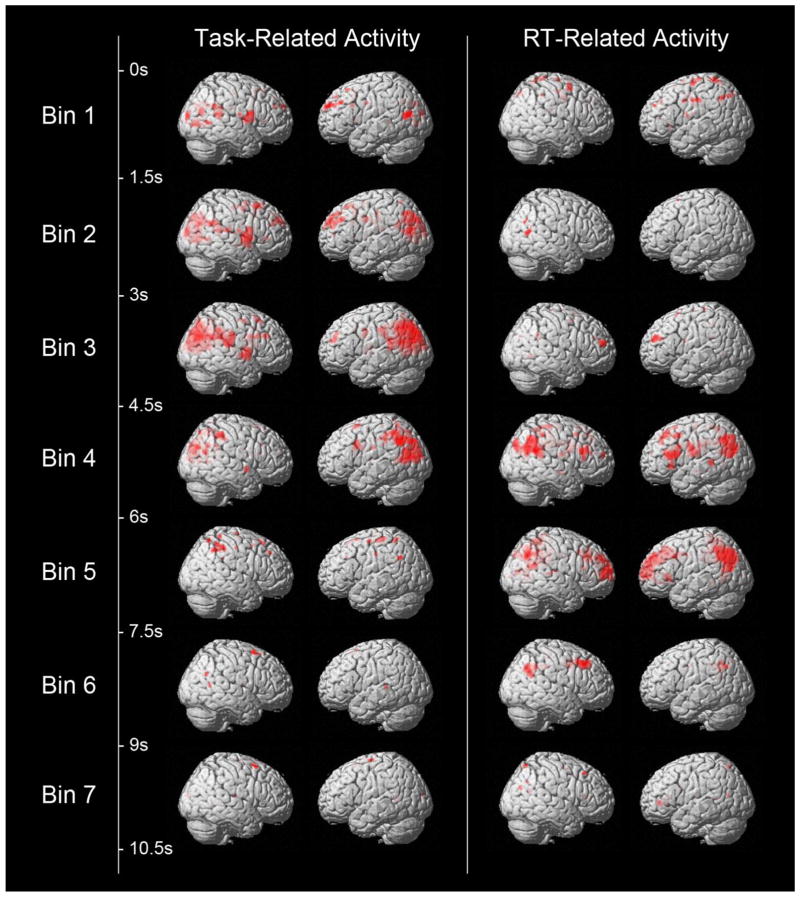
As a follow-up analysis, a FIR basis set was used with RT as a continuous parametric modulator to determine overall task- and RT-related activity differences between groups. For the 7 time bins reflecting overall task-related activity, healthy controls showed regions of significantly greater activity in bins 2, 3, and 4, reflecting a time window of 1.5 to 6 seconds post-stimulus onset. Significant clusters included primary visual cortex, precuneus, and IPL. There were no regions that showed greater task-related activity in patients compared to controls. For the 7 time bins reflecting RT-related activity, healthy controls showed regions of significantly greater activity in bins 4 and 5, reflecting a time window of 4.5 to 7.5 seconds post-stimulus onset. Significant clusters included left IPL, precuneus, middle cingulate gyrus, and DLPFC (see Fig. 4).
Figure 4. Between-group contrast displaying activations at seven time bins for Task-related activations (on left) and RT-related activations (on right) from the Finite Impulse Response (FIR) Analysis.
Controls displayed more Task-related activity during time bins two to four, reflecting a time window of 1.5 to 6 seconds post-stimulus onset. Activated clusters included primary visual cortex, precuneus, and IPL. The schizophrenia group did not activate any region over controls in any time bin. Controls displayed more RT-related activity during time bins four and five, reflecting a time window of 4.5 to 7.5 seconds post-stimulus onset. Activated regions included left IPL, precuneus, middle cingulate gyrus, and DLPFC. Once again the schizophrenia group did not activate any region over controls in any time bin.
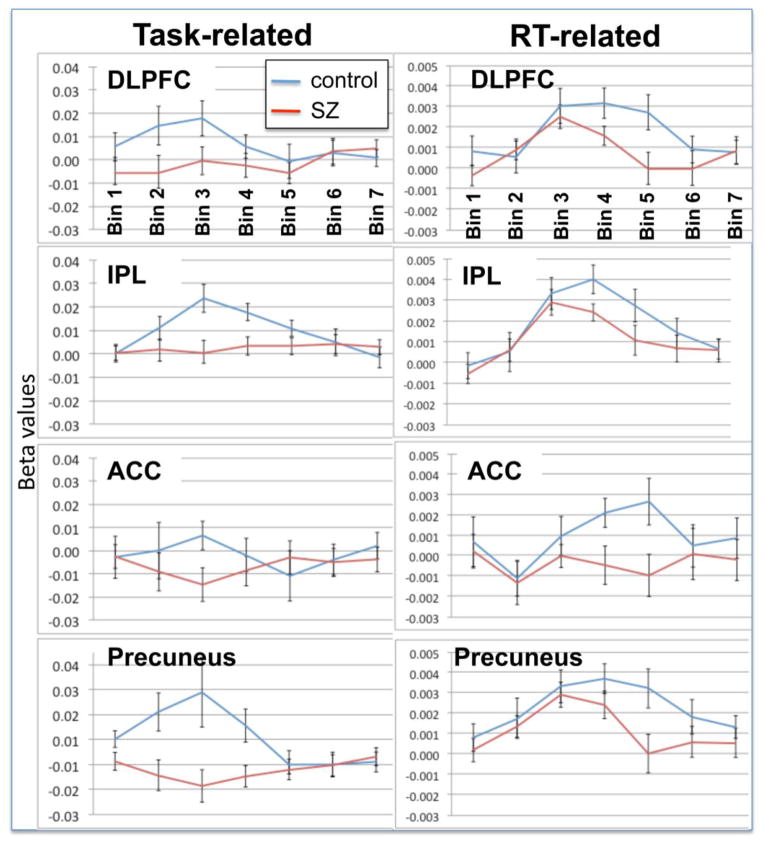
As a final examination of the pattern of RT-related activity in each group across time, we plotted the Task- and RT-related activity in a-priori ROIs used in our primary analysis (see Fig. 5). These plots reflect a similar result to the RT-related effects reported above; although there were no significant between-group differences in activation in early time bins (one to three), controls displayed greater activity in DLPFC, IPL and the ACC during bins four and five. The Task-related plots also suggest somewhat increased activity in cognitive-control-related regions during early time bins in the control compared with the schizophrenia group.
Figure 5. Task- and RT-related time series plots of beta values in a priori ROIs from the FIR analysis.
These plots echo results from the whole-brain FIR analysis demonstrating increased RT-related activity in cognitive control-related regions in the control compared to the schizophrenia group in later time bins (4 and 5) in the absence of any significant differences in the early time bins (1 to 3).
Discussion
In this study we sought to examine the nature of impaired cognitive control in schizophrenia using a RT distributional analysis. We found an increase in RT variability, reflected by increased IIV in patients with schizophrenia compared to controls. Consistent with previous studies of RT variability in schizophrenia (Rentrop et al, 2010; Kieffaber et al, 2006) we also found an increase in the degree of unusually long RT in the patients with schizophrenia compared to control subjects. Increased numbers of unusually long RT are thought to reflect proactive cognitive control failures. This is borne out in our data, as both increased IIV and tau were associated with poorer performance in all subjects. Across all stimulus types and in both groups long reactions times were associated with increased recruitment of a frontal-parietal cognitive control network. Furthermore, consistent with the results of the behavioral analysis showing an increased IIV and high levels of very long RT’s in the patient group, patients with schizophrenia showed reduced recruitment of the DLPFC during these trials.
Although the relationship between fMRI activity in prefrontal and parietal regions and attention control is not unambiguous (e.g. is increased activity in these regions related to less efficiency or greater attention control?) increased activity in these regions has consistently been associated with better performance for response preparation and conflict management (MacDonald et al, 2000; Sakai and Passingham, 2003; Hester, Murphy, Foxe, Foxe, Javitt and Garavan, 2004; Fassbender, Foxe and Garavan, 2006). Subjects tend to increase activity in these regions when they are given an opportunity to prepare for an up-coming response in a cognitive paradigm (Fassbender, Foxe and Garavan, 2006). Furthermore, activation to cued correct responses is usually greater in these regions compared to un-cued correct responses (Hester, Murphy, Foxe, Foxe, Javitt and Garavan, 2004). Finally, appropriate post-error behavioral adjustment has also been associated with greater activity in dlPFC (Garavan, Ross, Murphy, Roche and Stein, 2002). Given this evidence, we therefore interpret increased activity in these regions to reflect engagement of cognitive control processes in either a sustained or reflexive manner.
Another concern with interpreting these results is the possibility that greater BOLD activity during long RT trials simply reflects greater “time-on-task,” reflecting longer periods of sustained neural activity and larger BOLD amplitude (i.e., Grinband et al., 2011). However, as Yeung, Cohen and Botvinick (2011) point out, the argument that the BOLD response builds with increasing RT does not account for any underlying cognitive process – as they argue “it is vital to provide some account of the relationship between RT and observed neural activity”. Additionally, the fact that the control group showed more activity in the long RT condition even while patients showed longer overall reaction times is inconsistent with the “time-on-task” explanation. Finally, supplemental analyses using RT as a parametric modulator offered additional converging evidence.
Additional analyses, conducted to further examine of the temporal nature of between-group differences in the engagement of cognitive control regions during long RT revealed results consistent with our primary analysis. Task-related brain activity in the time window of 1.5 to six seconds revealed greater activity in the control compared to the schizophrenia group in the primary visual cortex, precuneus, and IPL, indicating less initial orientation to and visual processing of the stimulus in the schizophrenia group. Importantly for our question of interest, the Task by RT interaction revealed significant between-group differences only in the time window from 4.5 to 7.5 seconds, whereby controls activated the cognitive control network more than the schizophrenia group. These results suggest that there were no between-group differences during early epochs but that differences lay in sustaining control-related activity in the schizophrenia group. We believe that this supports our hypothesis of a weakened ability to recruit cognitive-control-related activity reactively in the schizophrenia group during trials on which they have lapsed in attention or displayed poor proactive control. The time series Task-related FIR plots in a priori ROIs also suggests that the control group may display increased proactive control compared to the schizophrenia group, as they demonstrated somewhat elevated cognitive control-related activity in early time bins.
Previous studies examining increased RT variability in healthy subjects have also found that increased RT variability is associated with activity in cognitive control-related brain regions (Bellgrove, et al., 2004; Simmonds, et al., 2007). However, these studies did not utilize ex-Gaussian measures of variability or specifically examine brain activity associated with long RT. An additional study, which examined whole-brain correlations between length of RT and activity in brain regions across a number of different cognitive paradigms (Yarkoni, Barch, Gray, Conturo, & Braver, 2009) found remarkably similar results to the abovementioned studies; longer RT was associated with increased activity in bilateral frontal, medial prefrontal cortex, parietal and thalamic regions. We feel that our results are consistent with these results in that long RT were associated with increased recruitment of reactive cognitive control systems in order to support correct responding on these trials.
As mentioned, long RT have been associated with failures in cognitive control, specifically failures in proactive processes that lead to increased response selection difficulty and the recruitment of reactive control processes to support correct responding. The results of our fMRI analysis are consistent with this interpretation. Our finding of hypoactivity in fronto-parietal regions during long RT in the schizophrenia group is also consistent with the theory that schizophrenia patients show deficits in activating the reactive control network on trials during which demands for reactive control are highest.
Long RTs are also assumed to reflect instances of lapses in task engagement or “mind wandering”. Along with reductions in cognitive control related brain activity, attention lapsing has also been linked to intrusions or the inefficient suppression of task irrelevant networks during cognitively demanding performance (Fassbender, et al., 2009; Weissman, et al., 2006). However, we did not find any suppression of these networks associated with long RT in this study. Neither did we find any between-group differences in suppression of the precuneus, a focus of default network dysfunction in other clinical disorders (e.g., Castellanos, et al., 2008). It is possible that this reflects the rapid event-related design of our task. Task irrelevant network intrusions associated with proactive control lapses might be seen during the inter-stimulus interval following the trial or trials preceding long RT trials or even error trials and might not be captured by the present analysis. RT distributional analyses of data using designs that better separate out proactive and reactive aspects of cognitive control, such as the AXCPT (Barch, Braver, Carter, Poldrack & Robbins, 2009) could shed light on this issue.
It is possible that some other explanation could exist for our finding of between-group difference in RT/BOLD response such as decreased motivation or increased levels of distractibility in our schizophrenia compared to control group. Since the Stroop task does not have built in differential deficit control it is difficult to completely rule out this possibility in accounting for a proactive control deficit. In the present study while patients were marginally less accurate than controls overall (93.52%, vs 95.91 respectively) their overall high level of performance on the Stroop task in this first episode sample would suggest that patients were motivated and engaged with the task. Furthermore in numerous studies using the AX CPT in the present population and others have shown a consistent differential deficit in proactive cognitive control (Yoon et al., 2008; Edwards Barch and Braver, 2010; Lesh et al., 2013). Increased distractibility could be considered to be theoretically related to our hypothesis of proactive control impairments and would result in the same requirement for engagement of reactive control in order to correctly respond following a period of “attention lapse”. Future studies should attempt to tease out these differing aspects of attention control by using a task that explicitly controls for general deficits in motivation as well as being more suited to examining error versus correct trials across relatively extended intertrial intervals.
In summary the results of the present study inform our understanding of the nature of impaired cognitive control by showing an enhanced IIV and skewing of the RT distribution during Stroop task performance in first episode schizophrenia. This pattern of results is consistent with an increase in lapses of proactive cognitive control in these patients. In addition the pattern of reduced cognitive control-related brain activity during long RT trials in the patient group suggest that cognitive and neural processes underlying reactive control are also likely to be impaired in schizophrenia. This highlights the value of the RT distributional approach in parsing out the trials on which highest demands for reactive control are present in individual patients to address the integrity of this important aspect of higher cognition. Future research using more targeted experimental paradigms as well as methods such as EEG should focus on the role of intrusions of task irrelevant network activity in lapses of proactive control in schizophrenia.
Table 4.
FIR analysis regions of significant activation.
| Task-related | MNI coordinates
|
||||
|---|---|---|---|---|---|
| Region | Brodmann’s Area | x | y | z | T voxel |
| Control > Schizophrenia | |||||
| Bin 1: No significant clusters | |||||
| Bin 2: R. Precuneusa | 7 | 16 | −64 | 32 | 4.51 |
| Bin 3: L. Cuneusb | 7/39 | −10 | −72 | 30 | 5.08 |
| Bin 4: L. Posterior Cingulatec | 30 | −18 | −60 | 14 | 4.65 |
| Bin 5–7: No significant clusters | |||||
|
| |||||
| Schizophrenia > Control | |||||
| Bins 1–7: No significant clusters | |||||
|
| |||||
| RT-related | |||||
| Control > Schizophrenia | |||||
| Bin 1–3: No significant clusters | |||||
| Bin 4: L. Middle/Posterior Cingulated | 24/31 | −4 | −26 | 34 | 3.76 |
| Bin 5: L. Inferior Parietal Cortex | 7/40 | −38 | −74 | 44 | 4.51 |
| L. Precuneus | 31 | 0 | −44 | 40 | 4.16 |
| L. Superior Frontal Gyruse | 9 | −20 | 26 | 38 | 3.61 |
| Bin 6–7: No significant clusters | |||||
|
| |||||
| Schizophrenia > Control | |||||
| Bins 1–7: No significant clusters | |||||
Note: height p<0.01; FWE cluster corrected p<0.007; MNI, Montreal Neurological Institute; Area; L, left; R, right
This cluster extended into left precuneus and bilateral cuneus.
This cluster extended into bilateral inferior parietal cortex and middle temporal gyrus.
This cluster extended laterally and superiorally into left inferior parietal cortex.
This cluster extended anteriorly into anterior cingulate, ventrally to thalamus, and posteriorly to precuneus.
This large cluster extended laterally into middle frontal gyrus, and medially into medial frontal gyrus.
Acknowledgments
This research was supported by grant RO1MH059883-11 awarded to C.S.C.
References
- Adams ZW, Roberts WM, Milich R, Fillmore MT. Does response variability predict distractibility among adults with attention-deficit/hyperactivity disorder? Psychol Assess. 2011;23(2):427–436. doi: 10.1037/a0022112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, IA: The University of Iowa; 1984. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: The University of Iowa; 1983. [Google Scholar]
- Andreou P, Neale BM, Chen W, Christiansen H, Gabriels I, Heise A, Kuntsi J. Reaction time performance in ADHD: improvement under fast-incentive condition and familial effects. Psychol Med. 2007;37(12):1703–1715. doi: 10.1017/S0033291707000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Carter CS, Poldrack RA, Robbins TW. CNTRICS final task selection: executive control. Schizophr Bull. 2009;35(1):115–35. doi: 10.1093/schbul/sbn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellgrove MA, Hester R, Garavan H. The functional neuroanatomical correlates of response variability: evidence from a response inhibition task. Neuropsychologia. 2004;42(14):1910–1916. doi: 10.1016/j.neuropsychologia.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Reiter G, Bates J, Lencz T, Szeszko P, Goldman RS, Kane JM. Cognitive development in schizophrenia: follow-back from the first episode. J Clin Exp Neuropsychol. 2006;28(2):270–282. doi: 10.1080/13803390500360554. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11(1):80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Bora E, Vahip S, Akdeniz F. Sustained attention deficits in manic and euthymic patients with bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(6):1097–1102. doi: 10.1016/j.pnpbp.2006.04.016. doi:S0278-5846(06)00165-5. [DOI] [PubMed] [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc Natl Acad Sci U S A. 2009;106(18):7351–7356. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33(3):279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Buzy WM, Medoff DR, Schweitzer JB. Intra-individual variability among children with ADHD on a working memory task: an ex-Gaussian approach. Child Neuropsychol. 2009;15(5):441–459. doi: 10.1080/09297040802646991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Milham MP. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63(3):332–337. doi: 10.1016/j.biopsych.2007.06.025. doi:S0006-3223(07)00615-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3(8):617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Servan-Schreiber D. Context, cortex, and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol Rev. 1992;99(1):45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners’ 3rd Edition Manual. Toronto: Multi-Health Systems; 2008. [Google Scholar]
- Duchek JM, Balota DA, Tse CS, Holtzman DM, Fagan AM, Goate AM. The utility of intraindividual variability in selective attention tasks as an early marker for Alzheimer’s disease. Neuropsychology. 2009;23(6):746–758. doi: 10.1037/a0016583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards BG, Barch DM, Braver TS. Improving prefrontal cortex function in schizophrenia through focused training of cognitive control. Front Hum Neurosci. 2010;4:32. doi: 10.3389/fnhum.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JN, Hwang ME, Antonini T, Langberg JM, Altaye M, Arnold LE. Examining predictors of reaction times in children with ADHD and normal controls. J Int Neuropsychol Soc. 2010;16(1):138–147. doi: 10.1017/S1355617709991111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JN, Langberg JM, Rosen PJ, Graham A, Narad ME, Antonini TN, Altaye M. Evidence for higher reaction time variability for children with ADHD on a range of cognitive tasks including reward and event rate manipulations. Neuropsychology. 2011;25(4):427–441. doi: 10.1037/a0022155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, Schweitzer JB. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First Michael B, Spitzer Robert L, Gibbon Miriam, Williams Janet BW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fjell AM, Westlye LT, Amlien IK, Walhovd KB. Reduced white matter integrity is related to cognitive instability. J Neurosci. 2011;31(49):18060–18072. doi: 10.1523/JNEUROSCI.4735-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R, Nopoulos P, Arndt S, O’Leary D, Ho BC, Andreasen NC. Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. Am J Psychiatry. 2002;159(7):1183–1189. doi: 10.1176/appi.ajp.159.7.1183. [DOI] [PubMed] [Google Scholar]
- Grinband J, Savitskaya J, Wager TD, Teichert T, Ferrera VP, Hirsch J. The dorsal medial frontal cortex is sensitive to time on task, not response conflict or error likelihood. Neuroimage. 2011;15;57(2):303–11. doi: 10.1016/j.neuroimage.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorus E, De Raedt R, Lambert M, Lemper JC, Mets T. Reaction times and performance variability in normal aging, mild cognitive impairment, and Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2008;21(3):204–218. doi: 10.1177/0891988708320973. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Yücel M, Pujol J, Pantelis C. Taskinduced deactivation of midline cortical regions in schizophrenia assessed with fMRI. Schizophr Res. 2007;91:82–6. doi: 10.1016/j.schres.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Heathcote A, Brown S, Cousineau D. QMPE: estimating Lognormal, Wald, and Weibull RT distributions with a parameter-dependent lower bound. Behav Res Methods Instrum Comput. 2004;36(2):277–290. doi: 10.3758/bf03195574. [DOI] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF, Tonev S, Eugene Arnold L, Keith Conners C, Hechtman L. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychol. 2006;12(2):125–140. doi: 10.1080/09297040500499081. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Roth A, Rentrop M, Friederich HC, Bender S, Weisbrod M. Intra-individual reaction time variability in schizophrenia, depression and borderline personality disorder. Brain Cogn. 2008;66(1):73–82. doi: 10.1016/j.bandc.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39(1):527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kieffaber PD, Kappenman ES, Bodkins M, Shekhar A, O’Donnell BF, Hetrick WP. Switch and maintenance of task set in schizophrenia. Schizophrenia Research. 2006;84(2–3):345–58. doi: 10.1016/j.schres.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Oosterlaan J, Stevenson J. Psychological mechanisms in hyperactivity: I. Response inhibition deficit, working memory impairment, delay aversion, or something else? J Child Psychol Psychiatry. 2001;42(2):199–210. [PubMed] [Google Scholar]
- Lee RW, Jacobson LA, Pritchard AE, Ryan MS, Yu Q, Denckla MB, Mahone EM. Jitter Reduces Response-Time Variability in ADHD: An Ex-Gaussian Analysis. J Atten Disord. 2012 doi: 10.1177/1087054712464269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011;36(1):316–338. doi: 10.1038/npp.2010.156npp2010156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesh TA, Westphal AJ, Niendam TA, Yoon JH, Minzenberg MJ, Ragland JD, Solomon M, Carter CS. Proactive and reactive cognitive control and dorsolateral prefrontal cortex dysfunction in first episode schizophrenia. Neuroimage: Clinical. 2013;2(0):590–599. doi: 10.1016/j.nicl.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychol (Amst) 2000;104(2):167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Loo SK, Specter E, Smolen A, Hopfer C, Teale PD, Reite ML. Functional effects of the DAT1 polymorphism on EEG measures in ADHD. J Am Acad Child Adolesc Psychiatry. 2003;42(8):986–993. doi: 10.1097/01.CHI.0000046890.27264.88. [DOI] [PubMed] [Google Scholar]
- Lukoff D, Nuechterlein KH, Ventura J. Manual for the Expanded Brief Psychiatric Rating Scale (BPRS) Schizophr Bull. 1986;12:594–602. [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21(1):450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12(2):241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton JL, Barch DM, Racine CA, Braver TS. Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cereb Cortex. 2008;18(5):1010–1028. doi: 10.1093/cercor/bhm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Salvador R, Sarro S, Gomar J, Vila F, Martinez A, McKenna PJ. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol Med. 2008;38(8):1185–1193. doi: 10.1017/S0033291708003565. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentrop M, Rodewald K, Roth A, Simon J, Walther S, Fiedler P, Weisbrod M, Kaiser S. Intra-individual variability in high-functioning patients with schizophrenia. Psychiatry Research. 2010;178(1):27–32. doi: 10.1016/j.psychres.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Schall JD. Neural correlates of decision processes: neural and mental chronometry. Curr Opin Neurobiol. 2003;13(2):182–186. doi: 10.1016/s0959-4388(03)00039-4. [DOI] [PubMed] [Google Scholar]
- Schall JD, Hanes DP. Neural mechanisms of selection and control of visually guided eye movements. Neural Netw. 1998;11(7–8):1241–1251. doi: 10.1016/s0893-6080(98)00059-8. [DOI] [PubMed] [Google Scholar]
- Scheres A, Oosterlaan J, Sergeant JA. Response execution and inhibition in children with AD/HD and other disruptive disorders: the role of behavioural activation. J Child Psychol Psychiatry. 2001;42(3):347–357. [PubMed] [Google Scholar]
- Segalowitz SJ, Dywan J, Unsal A. Attentional factors in response time variability after traumatic brain injury: an ERP study. J Int Neuropsychol Soc. 1997;3(2):95–107. [PubMed] [Google Scholar]
- Seidman LJ, Buka SL, Goldstein JM, Tsuang MT. Intellectual decline in schizophrenia: evidence from a prospective birth cohort 28 year follow-up study. J Clin Exp Neuropsychol. 2006;28(2):225–242. doi: 10.1080/13803390500360471. [DOI] [PubMed] [Google Scholar]
- Sergeant JA, Geurts H, Huijbregts S, Scheres A, Oosterlaan J. The top and the bottom of ADHD: a neuropsychological perspective. Neurosci Biobehav Rev. 2003;27(7):583–592. doi: 10.1016/j.neubiorev.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Fotedar SG, Suskauer SJ, Pekar JJ, Denckla MB, Mostofsky SH. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45(9):2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Stethem LL, Hugenholtz H, Picton T, Pivik J, Richard MT. Reaction time after head injury: fatigue, divided and focused attention, and consistency of performance. J Neurol Neurosurg Psychiatry. 1989;52(6):742–748. doi: 10.1136/jnnp.52.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Stethem LL, Picton TW, Leech EE, Pelchat G. Traumatic brain injury, aging and reaction time. Can J Neurol Sci. 1989;16(2):161–167. doi: 10.1017/s0317167100028833. [DOI] [PubMed] [Google Scholar]
- Tamm L, Narad ME, Antonini TN, O’Brien KM, Hawk LW, Jr, Epstein JN. Reaction time variability in ADHD: a review. Neurotherapeutics. 2012;9(3):500–508. doi: 10.1007/s13311-012-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinius TP. The intermediate visual and auditory continuous performance test as a neuropsychological measure. Arch Clin Neuropsychol. 2003;18(2):199–214. [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van den Bosch RJ, Rombouts RP. Coping and cognition in schizophrenia and depression. Compr Psychiatry. 1997;38(6):341–344. doi: 10.1016/s0010-440x(97)90930-5. [DOI] [PubMed] [Google Scholar]
- Vaurio RG, Simmonds DJ, Mostofsky SH. Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia. 2009;47(12):2389–2396. doi: 10.1016/j.neuropsychologia.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verte S, Geurts HM, Roeyers H, Oosterlaan J, Sergeant JA. Executive functioning in children with autism and Tourette syndrome. Dev Psychopathol. 2005;17(2):415–445. doi: 10.1017/s0954579405050200. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Poole JH, Willis-Shore J, Ober BA, Shenaut GK. Slower and more variable reaction times in schizophrenia: what do they signify? Schizophr Res. 1998;32(3):183–190. doi: 10.1016/s0920-9964(98)00043-7. [DOI] [PubMed] [Google Scholar]
- Wagenmakers EJ, Brown S. On the linear relation between the mean and the standard deviation of a response time distribution. Psychol Rev. 2007;114(3):830–841. doi: 10.1037/0033-295X.114.3.830. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio: Harcourt Assessment; 1999. [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte J, Polansky M, Fleming M, Coslett HB, Cavallucci C. Sustained arousal and attention after traumatic brain injury. Neuropsychologia. 1995;33(7):797–813. doi: 10.1016/0028-3932(95)00029-3. doi:0028-3932(95)00029-3. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Barch DM, Gray JR, Conturo TE, Braver TS. BOLD correlates of trial-by-trial reaction time variability in gray and white matter: a multi-study fMRI analysis. PLoS One. 2009;4(1):e4257. doi: 10.1371/journal.pone.0004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Cohen JD, Botvinick MM. Errors of interpretation and modeling: a reply to Grinband et al. Neuroimage. 2011;15;57(2):316–9. doi: 10.1016/j.neuroimage.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Minzenberg MJ, Ursu S, Walter R, Wendelken C, Ragland JD, Carter CS. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. The American Journal of Psychiatry. 2008;165:1006–1014. doi: 10.1176/appi.ajp.2008.07060945. [DOI] [PMC free article] [PubMed] [Google Scholar]