Abstract
Prescription monitoring programs (PMPs) are designed to reduce medication diversion by identifying individuals obtaining the same medication from multiple providers (termed multiple provider episodes [MPEs]). This study determined whether recent changes to California’s PMP influenced: 1) the extent that practitioners issue prescriptions for a variety of Schedule II opioids; and 2) the incidence of MPEs involving these opioids. Intervention time series of California’s PMP data was used to determine the effect of requiring practitioners to transition from using triplicate prescription forms for Schedule II medications to security forms for all controlled substances. Outcome measures included changes in number of prescriptions issued for Schedule II long-acting or short-acting (SA) opioids and the MPEs involving these medications. Requiring a security form was associated with a sustained prescribing increase for SA hydromorphone, meperidine, and SA oxycodone; no prescribing changes were found for SA fentanyl, methadone, and SA morphine, or for any long-acting opioids. The same policy change, however, increased MPEs involving all opioids. Further effort is required to determine how California’s PMP can continue to ensure availability of prescription opioids for medical use while better mitigating their diversion.
Perspective
Statistical model-building was used to evaluate the influence of changes to California’s prescription monitoring program. The extent that practitioners prescribe Schedule II opioids and the incidence of people receiving prescriptions from multiple providers were measured. Such research illustrates the viability of evaluating drug control program impact on prescribing practice and potential diversion behaviors.
Keywords: California, prescription monitoring programs, opioid analgesics, multiple provider episodes, diversion, abuse
Currently, there is a dearth of research that has examined the effects of state prescription monitoring programs (PMPs). The relatively few studies detailing the impact of PMPs have consistently demonstrated an immediate and sustained reduction in prescribing or availability of monitored medications, as well as a concomitant increase in the medical use of alternative, and perhaps less effective, medications not subject to the PMP requirements.5,22,24-26,28-31,33 This phenomenon is known as the “substitution effect.”30 It was not until 2004 that 2 separate studies described a PMP’s influence not only on prescribing but also on a measure of possible abuse and diversion (ie, “pharmacy hopping”).24,26 Both studies determined that New York’s PMP decreased the prescribing of benzodiazepines and contributed to a substitution effect, but also reduced the occurrence of “pharmacy hopping.”
Prior PMP research has examined data when these programs were characterized as multiple-copy prescription programs (MCPPs). MCPPs required healthcare practitioners to use government-issued serialized duplicate or triplicate forms to prescribe Schedule II controlled substances, as well as other medications of interest such as benzodiazepines in New York.9 Since the early 1990s, PMPs have increasingly utilized electronic data transmission (EDT) systems. As with MCPPs, EDT systems are intended to reduce the incidence of abuse-related behaviors, including the use of multiple practitioners to obtain different prescriptions for the same medication.10 EDT systems tend to circumvent the restrictions imposed by MCPPs and are believed to better control prescription medication diversion.4,8,19 However, there is yet no empirical evidence to substantiate this judgment.
California has the distinction of being the first state to implement a PMP, which is now an EDT program—the Controlled Substance Utilization Review and Evaluation System (CURES). Table 1 describes historical developments related to California’s PMP. The legislative changes enacted through Senate Bill 151 were partially based on the belief that clinicians who treat patients’ prolonged pain avoided prescribing Schedule II opioid analgesics because using triplicate prescription forms was considered onerous. Instead, practitioners prescribed Schedule III opioids that were not subject to this requirement.17 As a result, the legislation was intended by its sponsors to remove potential barriers to prescribing Schedule II medications and improve pain care.
Table 1.
History of California’s Prescription Monitoring Program
| Date Effective | Brief Description of Policy Content |
|---|---|
| 1939 | PMP began Oldest, continually operational, multiple-copy prescription program utilizing paper-based triplicate forms. Applied only to selected “narcotics” (ie, opium, hashish, marijuana, cocaine) and limited physicians to issuing not more than 100 prescriptions in a 90-day period |
| 1972 | Mandated Schedule II “narcotics” |
| 1981 | Mandated any non-narcotic Schedule II drug |
| 1992 | Established Controlled Substances Prescription Advisory Council to study the PMP |
| 1996 | Established an electronic system (CURES) to monitor prescribing or dispensing of Schedule II medications, while retaining the requirement of a triplicate prescription form |
| January 1, 2005 | California Senate Bill 151 eliminated the requirement of the 66-year-old triplicate form and replaced it with a secure tamper-resistant form for all medications in Schedules II-V |
Abbreviations: PMP, prescription monitoring programs; CURES, Controlled Substance Utilization Review and Evaluation System.
This study was designed to use time series model-building to analyze CURES data to determine whether California’s recent legislative change requiring using tamper-resistant security prescription forms, rather than triplicate forms, significantly influenced the extent that practitioners prescribe various Schedule II opioids. In addition, similar analyses examined the incidence of people obtaining the same opioid from more than 1 prescriber (termed multiple provider episodes [MPEs]).
Methods
Data Acquisition and Administrative Approvals
The details of creating a de-identified CURES database through an agreement with the Office of the Attorney General of California, as well as the variables coded, are described elsewhere.32 Institutional review boards of the University of Wisconsin, the UC Davis Medical Center, and the VA Northern California Health Care System approved this study.
Study Design
CURES data were analyzed to determine the effect of policy change both on the prescribing of opioids and on the rate of MPEs for an 82-month period between January 2000 and October 2006.
Dependent Variables
Prescription Data
Trends for 9 Schedule II opioids were evaluated: Short-acting (SA) fentanyl; long-acting (LA) fentanyl; SA hydromorphone; meperidine (which is not indicated for treating chronic pain due to the accumulation of an excitotoxic metabolite1); methadone; SA morphine; LA morphine; SA oxycodone; and LA oxycodone. Medication classifications were developed according to product information.21 A substitution effect could not be examined because of insufficient trend data for Schedule III hydrocodone- and codeine-combination products; CURES began requiring reporting of these medications after 2005. Longitudinal trends for the total number of prescriptions written for each opioid were analyzed separately.
Multiple Provider Episodes
MPEs were defined as individuals receiving prescriptions for the same medication from 2 or more practitioners within a 30-day period. Prescriptions issued by 2 different practitioners but filled at the same pharmacy were excluded, which allowed for clinically justifiable situations where patients: 1) substituted clinicians; or 2) obtained medications from a practitioner covering for the patient’s customary prescriber. Each month of the trend represented the total number of MPEs involving a study opioid, and separate analyses of MPEs were conducted for each opioid.
Independent Variable
The date on which the prescription form requirement changed represented the independent variable, or intervention event. On January 1, 2005, the policy change officially went into effect and required replacing the triplicate form for Schedule II medications with a tamper-resistant security form covering medications in multiple schedules. However, the new law also permitted practitioners to use either triplicate or tamper-resistant forms during a 6-month ramp up period prior to phasing out the triplicate form.
Statistical Analyses and Model-Building Procedures
Interrupted time series analyses were used to examine the temporal effects of the prescription form change on both the prescribing of Schedule II opioids and the frequency of MPEs involving these medications. The intervention (ie, policy change requiring the use of security prescription forms) breaks the time series into a pre- and a post-intervention phase. Statistically comparing the 2 phases estimates both the shape and magnitude of the intervention effect.
ARIMA Model-Building
An Autoregressive Integrated Moving Average (ARIMA) model-building strategy was necessary to accurately estimate the policy effects. In addition to anticipating the policy change intervention effects, statistical models must recognize and correct for the characteristics of these longitudinal series of prescription and MPE data.3,16 Processes inherent in a general ARIMA model can be expressed as ARIMA (p,d,q), and each integer is quantified below.
Characteristics of the Prescribing Trend Data
National prescribing of most opioids has tended to drift nonrandomly upward over time, regardless of specific policy development in various states, due to the increased acceptance and use of these medications for treating pain.12,13 As a result, the number of prescriptions for a given month was considered correlated with the total prescriptions issued in the preceding month, leading to an initial order of autoregression value of “1” (ARIMA[1,d,q]). Also, all data series were initially treated as nonstationary, requiring statistical transformation (referred to as “differencing”) in response to this natural upward drift (ARIMA[p,1,q]). Finally, random disturbances or noise can cause data series values to diverge from the mean by chance, and such events can influence the medical use of these drugs for pain, often independent of the evaluated intervention. All statistical models mathematically accounted for the random, yet perhaps consequential, impact of trend disturbances (ARIMA[p,d,1]). Consequently, the initial model tested for the number of prescriptions for each study drug was ARIMA(1,1,1).
Characteristics of the Trends in Multiple Provider Episodes
As with the prescribing trends, initial analyses of the pre- and post-intervention autocorrelations and partial autocorrelations were used to help inform the ARIMA parameter values that best characterized the trend lines for the MPEs involving each study drug.3,11 Unlike the trends for prescribing data, however, it was not possible to anticipate the ARIMA properties for this variable, and different model parameters were expected.
Characteristics of the Intervention Effects
Although the tamper-resistant form for all prescription medications was mandated on January 1, 2005, a ramp up period began 6 months prior, when practitioners could use either the security or triplicate forms to prescribe Schedule II medications. It was expected, therefore, that the ramp up period would likely have a gradual but notable influence on prescribing. A First-Order Modeling approach was used to characterize a gradual effect beginning in July, 2004 and continuing over the 6-month period before the complete policy implementation. Given these monthly data, the intervention variable (X) was assigned the value X = 1/6 in July 2004, X = 2/6 in August 2004, etc., until X = 1 in December 2004.
There also was a need to operationalize the independent variable either as a Step Function, for which the policy effect remains throughout the postintervention, or as a Pulse Function representing a transient influence. Communication with state law enforcement, regulatory agencies, and licensees led us to anticipate a Step Function as best representing the impact of policy adoption on opioid prescribing. Absent information to predetermine policy impact on MPEs, we initially predicted the same effect that was conceptualized for prescribing. Since differencing changes the time course of the intervention effect itself, this characteristic was accounted for when interpreting models that required differencing.
ARIMA modeling was performed using PASW (formerly SPSS, Inc., Chicago, IL) Trends, v.18. A Bonferroni correction for multiple outcome testing was used to adjust for the individual analyses for each dependent variable. As a result, a P value < .005 was considered statistically significant.
Results
Policy Change Effects on Trends for Opioid Prescribing
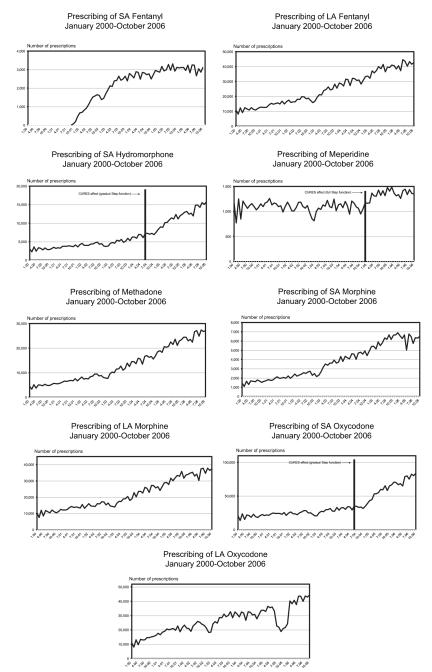
Throughout the 7-year study period, over 15,500,000 prescriptions were written for the 9 opioids analyzed (Table 2). SA oxycodone products accounted for the largest cumulative number of prescriptions, followed by LA oxycodone, LA fentanyl, and LA morphine. Fig 1 demonstrates the prescribing trends for each opioid. As stated previously, initial analysis of the autocorrelations and partial autocorrelations3,11 helped determine that ARIMA(1,1,1) was an optimal model for all study drugs except meperidine, but further model-building was conducted to determine whether more explanatory coefficients could be obtained. However, as hypothesized, additional analyses confirmed that a single ARIMA(1,1,1) model best explained the impact of the policy change for 2 of the 3 study opioids that showed statistical significance.
Table 2.
Frequency of Prescriptions and Percent Involvement in Multiple Provider Episodes Involving Schedule II Opioids
| Drug Name | Rx Numbers | % Multiple Provider Episodes |
|---|---|---|
| SA Oxycodone | 4,479,682 | 10.9% |
| LA Oxycodone | 2,851,864 | 8.7% |
| LA Fentanyl | 2,745,591 | 8.1% |
| LA Morphine | 2,389,252 | 8.5% |
| Methadone | 1,496,601 | 8.6% |
| SA Hydromorphone | 823,866 | 15.2% |
| SA Morphine | 412,646 | 10.0% |
| SA Fentanyl | 181,288 | 11.4% |
| Meperidine | 125,861 | 7.0% |
| Total Rx Count | 15,506,651 | 9.6% |
Abbreviations: LA, long-acting; SA, short-acting.
NOTE. LA hydromorphone was excluded from analysis because prescription numbers were insignificant. LA levorphanol was excluded from analysis due to periodic missing data throughout the study period.
Figure 1.
Trends in number of prescriptions issued for opioid medications within California CURES program. Date and effect, indicated by vertical bar and notation, provided for interrupted time series findings significant at P < .005 level.
Beginning with the 6-month ramp up period, when practitioners could use either triplicate or security forms, a gradual and sustained increase in prescribing for SA hydromorphone and SA oxycodone was found during the postintervention period (ARIMA(1,1,1) = 5.215, P < .001 and ARIMA(1,1,1) = 5.504, P < .001, respectively). Beginning January 1, 2005, however, there was an immediate and generally sustained increase in the prescribing of meperidine when the forms became mandatory (ARIMA(0,0,1) = 10.256, P < .001). No significant effects were found for the prescribing of SA and LA fentanyl, methadone, SA and LA morphine, and LA oxycodone. The prescribing trends for these medications tended to rise throughout the study period without notable differences in trend slopes between the pre- and post-intervention phases.
Policy Change Effects on Multiple Provider Episodes Involving Opioids
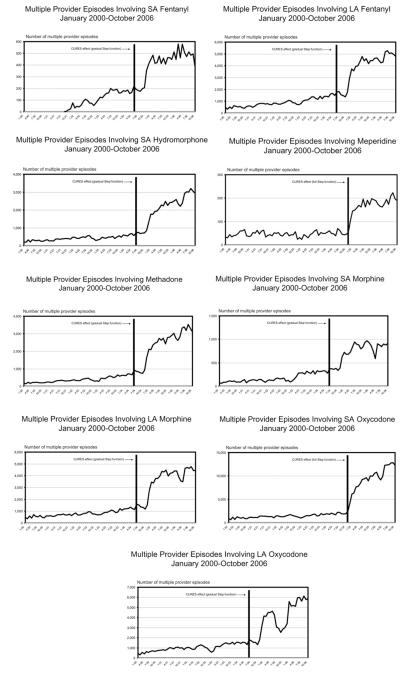
During the study time frame, MPEs ranged from a high of 15.2% involving all prescriptions for SA hydromorphone to 7.0% for meperidine, with MPEs characterizing 9.6% of all prescriptions for the analyzed opioids (see Table 2).
As apparent from Fig 2, the incidence of MPEs involving each study medication tended to be a generally increasing phenomenon, but insufficient to require differencing. As expected, the best-fitting ARIMA model for MPEs was not the same as that used to explain prescribing trends. The same ARIMA(1,0,0) model, however, best characterized the trends for MPEs involving each medication (see Table 3). The observed policy effect also was a gradual step function in most instances—the occurrence of MPEs for each study drug eventually increased and was sustained after transitioning from triplicate to security prescription forms. For meperidine (ARIMA(1,0,0) = 11.390, P < .001) and SA oxycodone (ARIMA(1,0,0) = 3.458, P < .001), the increase in MPEs coincided immediately with the security form mandate (on January 1, 2005). Such postintervention increases maintained their significance despite perceptible declines and rebounds occurring for most medications later in the postintervention phase.
Figure 2.
Trends for occurrence of multiple provider episodes within California CURES program. Date and effect, indicated by vertical bar and notation, provided for interrupted time series findings significant at P < .005 level.
Table 3.
Significant Parameter Estimates for Policy Change Effects on Multiple Provider Episodes
| Medication | Model Type | ß | SE | t-ratio | Significance |
|---|---|---|---|---|---|
| SA Fentanyl | ARIMA(1,0,0) | 349.282 | 40.450 | 8.635 | .001 |
| LA Fentanyl | ARIMA(1,0,0) | 3076.314 | 394.381 | 7.800 | .001 |
| SA Hydromorphone | ARIMA(1,0,0) | 1301.383 | 313.120 | 4.156 | .001 |
| Meperidine | ARIMA(1,0,0) | 126.728 | 11.390 | 11.126 | .001 |
| Methadone | ARIMA(1,0,0) | 1718.412 | 338.130 | 5.082 | .001 |
| SA Morphine | ARIMA(1,0,0) | 627.492 | 62.672 | 10.012 | .001 |
| LA Morphine | ARIMA(1,0,0) | 3131.985 | 276.599 | 11.323 | .001 |
| SA Oxycodone | ARIMA(1,0,0) | 4580.153 | 1324.397 | 3.458 | .001 |
| LA Oxycodone | ARIMA(1,0,0) | 3457.444 | 558.850 | 6.187 | .001 |
Abbreviations: SA, short-acting; LA, long-acting.
Discussion
This study contributes to a nascent literature that explores the impact of electronic PMPs14,20,23 by examining policy change effects for California’s CURES program, the oldest and largest PMP. Requiring a security form in 2005, replacing its historical triplicate prescription form, had variable effects on the prescribing of each study opioid. Prescribing for SA hydromorphone, meperidine, and SA oxycodone showed significant increases subsequent to the policy change. Alternatively, SA and LA fentanyl, methadone, SA and LA morphine, and LA oxycodone trends remained relatively unchanged throughout the study.
Only certain SA opioids underwent significant increases in prescribing in recent years, compared with prescribing during the preintervention phase. This finding possibly relates to the fewer regulatory requirements associated with prescribing Schedule III opioids (which are SA formulations) during the Schedule II triplicate program. Thus, changing the law could have led to SA Schedule II opioids being utilized instead of Schedule III medications. Sponsors of SB 151 intended to repeal potential barriers to prescribing Schedule II medications, and the noted rise in SA opioid prescribing could be further, indirect, evidence that eliminating the triplicate requirement removed obstacles involved in prescribing Schedule II relative to Schedule III opioids. This interpretation also implies that a substitution effect may well have been in place and was subsequently corrected by SB 151.
Prior to transitioning from triplicate to security forms, law enforcement advocates argued that PMPs effectively suppressed excessive Schedule II opioid prescribing—all states with the highest rates of Schedule II opioid prescribing lacked such programs.18,27 Thus, there was great concern about shifting California from a more rigorous system (ie, where all prescribers of Schedule II medications were required to engage with the state Department of Justice through registration and serialization of prescription forms that regulated only this drug class) to a security form system for all prescribed medications that eschewed such requirements. That prescribing rates for SA oxycodone escalated more abruptly than any LA opioid also could be interpreted to suggest that the triplicate program’s impact had more to do with discriminating between Schedule II and III medications rather than regulatory obstacles directly (eg, Department of Justice registration and serialization); in addition, it is possible that other contemporaneous but unevaluated activities, such as changes in reimbursement policy or media coverage surrounding LA oxycodone products, could partially explain this effect.
CURES data also showed that MPEs represented almost 10% of all Schedule II opioid prescriptions issued during the 7-year study period. This finding is similar to published results about the percentage of aberrant drug-related behaviors evidenced from the chronic pain population receiving opioids.7 All trends for MPEs tended to drastically increase following the transition to the security prescription form, and the effect was maintained throughout the postintervention phase. Although data in Table 2 suggest that MPEs are not necessarily proportional to aggregate prescription volume, visual inspection of the figures indicates that the opioids involved most often in MPEs conformed to their prescription frequencies during the postintervention phases.
These series of results suggest that security forms generally were not as effective at preventing MPEs, compared to triplicate prescription forms. The reasons for this are not clear. However, only slightly more than 50% of California physicians subscribed to the program that issued triplicate prescriptions,8 and perhaps those with the triplicate forms were cautious about using them due to their law enforcement implication. In addition, the increase in MPEs involving Schedule II opioids may have resulted from practitioners choosing to prescribe these medications, rather than opioids in Schedule III, because the perceived barriers to prescribing were lessened. It is plausible that converting to security forms suddenly enlarged the prescriber population, which may at least partially account for the increase in prescribing and MPEs. Although neither the prescribing patterns nor MPEs associated with Schedule III opioids could be evaluated longitudinally, this scenario is consistent with the finding that the greatest number of MPEs involved SA oxycodone and replicates recent study results from Massachusetts.14 Neither study, however, could determine how much of the increase in MPEs seen with SA oxycodone was compensated by decreased involvement of hydrocodone- or codeine-combination formulations.
Critically, during the period of these dramatically increased rates of MPEs, California’s PMP did not permit prescribers to access the data at the time of treating a patient. As with many states, California now has a “point of care” online system for checking PMP information and it will be important to assess whether this mitigates the rise in MPEs. Also important is further defining the characteristics of the MPEs identified in this study, to determine the distribution of individuals by number of prescribers, as a means of exploring the extent of questionable activity.13
Results from this research contribute to the gradually accumulating evidence about the utility of using sophisticated statistical methodologies to evaluate the extent that changes to PMP requirements affect not only medication prescribing but also the incidence of possible aberrant drug-seeking behaviors. There are relatively few analytical studies and they generally have not evaluated data from EDT systems. All PMPs now utilize EDT systems, so the findings and conclusions from most earlier published studies are not necessarily relevant for existing programs. However, understanding the impact of current PMP characteristics will be valuable in guiding policy-makers, law enforcement officials, regulators, and healthcare professionals when conceptualizing efforts to better mitigate nonmedical use of prescription medications while ensuring their adequate availability for legitimate clinical treatment.
Various limitations characterize this study. First, these results relate to California’s CURES program and may not be generalizable. Second, this was a retrospective study focusing on a single, albeit potentially important, intervention event that could influence clinical practice. Further explanation could be derived though additional multivariate methods, by controlling for additional confounds and estimating their predictive significance. Third, the study intervention characterized a unique situation: Changing from triplicate forms covering Schedule II medications to security forms for all prescription medications. Future research could focus on other distinctive PMP characteristics to determine their effects. Fourth, it was not possible to directly evaluate a substitution effect because prescription information for Schedule III medications was not collected until 2005. Fifth, although prescribing increased throughout the study period for most opioids, it was not feasible to determine whether such prescribing was appropriate. Finally, given the disparate motivations for seeking prescription opioids from more than 1 practitioner, it remains difficult to characterize the MPEs identified in this study. Researchers typically have equated doctor shopping or pharmacy hopping with abuse-related behaviors for illicit purposes,15 but in some instances such behaviors could indicate efforts to obtain better pain management or symptom relief.2 Understanding the motivation for potential aberrant drug-related behaviors remains critical for ascertaining apposite clinical implications.
Conclusion
California’s transition from triplicate to security prescription forms seems to have, at least in part, delivered on its objective of leveling barriers involved in prescribing Schedule II opioids. However, LA opioids remained largely unaffected by the change. Transitioning to tamper-resistant security forms also was associated with rising MPEs. The reasons for this occurrence remain unclear and must be investigated. Since data were not available to compare MPEs associated with Schedule III opioid compounds, we cannot disregard a possible epiphenomenon in which prescribing from multiple practitioners simply shifted from 1 schedule to another.
Given the proliferation of state PMPs since 2005,6 it becomes increasingly important to quantify the effectiveness of these systems, as well as their impact on patient care, especially during a time of diminishing state fiscal resources and the need to justify continued funding. Determining specific effects of current EDT systems is essential to inform the creation of future PMPs or modifying existing systems. In this way, the ideal goal of PMPs—providing an effective diversion control mechanism while minimizing deleterious effects on legitimate prescribing—may be more effectively achieved.19 Understanding these programs’ ramifications for legitimate clinical practice and diversion mitigation will help ensure proper focus on the dual public health objectives of the nonmedical use of prescription opioid medications and appropriate pain management.
Acknowledgments
Mr. Casamalhuapa and Mr. Baxi had full access to all of the data in the study, while Dr. Wilsey takes responsibility for the integrity of the data and Dr. Gilson takes responsibility for the accuracy of the data analysis. Dr. Gilson is grateful to Paul Rathouz, PhD, Professor and Department Chair of Biostatistics and Medical Informatics, University of Wisconsin School of Medicine and Public Health, for his valuable assistance in reviewing and confirming interpretation of the results. Dr. Rathouz’s assistance was supported by the National Cancer Institute of the National Institutes of Health under the University of Wisconsin Comprehensive Cancer Center Support grant P30CA014520.
The authors gratefully acknowledge funding for this project by the Robert Wood Johnson Foundation. The authors also acknowledge support from the Regents of the University of California, on behalf of its Davis Campus. Database architect support was derived through Grant Number UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. No funder had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
A.M.G. reports the following conflicts of interest and financial disclosures: Honoraria from Covidian and Meda Pharmaceuticals, research grant from King Pharmaceuticals, and unrestricted educational grant support on behalf of the Board of Regents of the University of Wisconsin from Purdue Pharma. S.M.F., B.L.W., C.C., and H.B. have no conflicts of interest or financial disclosures to report.
References
- 1.American Pain Society . Principles of analgesic use in the treatment of acute pain and cancer pain. Sixth American Pain Society; Glenview, IL: 2008. [Google Scholar]
- 2.Ballantyne JC, LaForge KS. Opioid dependence and addiction during opioid treatment of chronic pain. Pain. 2007;129:235–255. doi: 10.1016/j.pain.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 3.Box GEP, Jenkins GM, Reinsel GC. Time series analysis: Forecasting and control. 3 Prentice Hall; Upper Saddle River, NJ: 1994. [Google Scholar]
- 4.Brushwood DB. Maximizing the value of electronic prescription monitoring programs. J Law Med Ethics. 2003;31:41–54. doi: 10.1111/j.1748-720x.2003.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 5.Curtis LH, Stoddard J, Radeva JI, Hutchison S, Dans PE, Wright A, Woosley RL, Schulman KA. Geographic variation in the prescription of Schedule II opioid analgesics amoung outpatients in the United States. Health Serv Res. 2006;41:837–855. doi: 10.1111/j.1475-6773.2006.00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drug Enforcement Administration [Accessed November 14, 2011];Q & A State prescription drug monitoring programs. 2010 http://www.deadiversion.usdoj.gov/faq/rx_monitor.htm.
- 7.Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 2008;9:444–459. doi: 10.1111/j.1526-4637.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- 8.Fishman SM, Papazian JS, Riches PS, Gilson AM. Regulating opioid prescribing through prescription monitoring programs: Balancing drug diversion and treatment of pain. Pain Med. 2004;5:309–324. doi: 10.1111/j.1526-4637.2004.04049.x. [DOI] [PubMed] [Google Scholar]
- 9.Gilson AM. Laws and policies involving pain management. In: Ballantyne JC, Rathmell JP, Fishman SM, editors. Bonica’s Management of Pain. Lippincott Williams & Wilkins; Hagerstown, MD: 2010. [Google Scholar]
- 10.Gilson AM, Kreis PG. The burden of the nonmedical use of prescription opioid analgesics. Pain Med. 2010;10(Suppl 2):89–100. doi: 10.1111/j.1526-4637.2009.00668.x. [DOI] [PubMed] [Google Scholar]
- 11.Glass GV, Willson VL, Gottman JM. Design and analysis of time-series experiments. Information Age Publishing; Charlotte, NC: 2008. [Google Scholar]
- 12.Joranson DE, Carrow GM, Ryan KM, Schaefer L, Gilson AM, Good P, Eadie J, Peine S, Dahl JL. Pain management and prescription monitoring. J Pain Symptom Manage. 2002;23:231–238. doi: 10.1016/s0885-3924(01)00410-9. [DOI] [PubMed] [Google Scholar]
- 13.Joranson DE, Ryan KM, Gilson AM, Dahl JL. Trends in medical use and abuse of opioid analgesics. JAMA. 2000;283:1710–1714. doi: 10.1001/jama.283.13.1710. [DOI] [PubMed] [Google Scholar]
- 14.Katz N, Panas L, Kim M, Auder AD, Bilansky A, Eadie J, Kreiner P, Paillard FC, Thomas C, Carrrow G. Usefulness of prescription monitoring programs for surveillance: Analysis of Schedule II opioid prescription data in Massachusetts, 1996-2006. Pharmacoepidemiol Drug Saf. 2010;19:115–123. doi: 10.1002/pds.1878. [DOI] [PubMed] [Google Scholar]
- 15.Lineberry TW, Bostwick M. Taking the physician out of “physician shopping”: A case series of clinical problems associated with internet purchases of medication. Mayo Clin Proc. 2004;79:1031–1034. doi: 10.4065/79.8.1031. [DOI] [PubMed] [Google Scholar]
- 16.McDowall D, McCleary R, Meidinger EE, Hay RA., Jr . Interrupted time series analysis. Sage Publications; Beverly Hills, CA: 1980. [Google Scholar]
- 17.Medical Board of California Clarification of Senate Bill. 2004. p. 151.
- 18.Office of National Drug Control Policy . National drug control strategy. The White House: 2004. NCJ 203722. [Google Scholar]
- 19.Pain & Policy Studies Group . Achieving Balance in Federal and State Pain Policy: A Guide to Evaluation. Fifth edition University of Wisconsin Paul P. Carbone Comprehensive Cancer Center; Madison, WI: 2008. [Google Scholar]
- 20.Pradel V, Frauger E, Thirion X, Ronfle E, Lapierre V, Masut A, Coudert C, Blin O, Micallef J. Impact of prescription monitoring program on doctor-shopping for high dosage buprenorphine. Pharmacoepidemiol Drug Saf. 2009;18:36–43. doi: 10.1002/pds.1681. [DOI] [PubMed] [Google Scholar]
- 21.Red Book . RED BOOK Ambulatory Care Drug Database Coding System. Reuters Thompson; Ann Arbor, MI: 2006. [Google Scholar]
- 22.Reidenberg MM. Effect of the requirement for triplicate prescriptions for benzodiazepines in New York State. Clin Pharmacol Ther. 1991;50:129–131. doi: 10.1038/clpt.1991.116. [DOI] [PubMed] [Google Scholar]
- 23.Reisman RM, Shenoy PJ, Atherly AJ, Flowers CR. Prescription opioid usage and abuse relationships: An evaluation of state prescription drug monitoring program efficacy. Subst Abuse. 2009;3:41–51. doi: 10.4137/sart.s2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross-Degnan D, Simoni-Wastila L, Brown JS, Mah M, Cosler LE. A controlled study of the effects of state surveillance on indicators of problematic and non-problematic benzodiazepine use in a medicaid population. Int J Psychiatry Med. 2004;34:103–123. doi: 10.2190/8FR4-QYY1-7MYG-2AGJ. [DOI] [PubMed] [Google Scholar]
- 25.Sigler KA, Guernsey BG, Ingrim NB, Buesing AS, Hokanson JA, Galvan E, Doutre WH. Effect of a triplicate prescription law on prescribing of Schedule II drugs. Am J Hosp Pharm. 1984;41:108–111. [PubMed] [Google Scholar]
- 26.Simoni-Wastila L, Ross-Degnan D, Mah C, Gao X, Brown J, Cosler LE, Fanning T, Gallagher P, Salzman G. A retrospective data analysis of the impact of the New York triplicate prescription program on benzodiazepine use in Medicaid patients with chronic psychiatric and neurologic disorders. Clin Ther. 2004;26:322–336. doi: 10.1016/s0149-2918(04)90030-6. [DOI] [PubMed] [Google Scholar]
- 27.United States General Accounting Office . Prescription drugs: State monitoring programs provide useful tool to reduce diversion. United States General Accounting Office; Washington, DC: 2002. GAO-02-634. [Google Scholar]
- 28.Van Haaren AM, Lapane KL, Hughes CM. Effect of triplicate prescription policy on benzodiazepine administration in nursing home residents. Pharmacotherapy. 2001;21:1159–1166. doi: 10.1592/phco.21.15.1159.33898. [DOI] [PubMed] [Google Scholar]
- 29.Wagner AK, Soumerai SB, Zhang F, Mah C, Simoni-Wastila L, Cosler LE, Fanning T, Gallagher P, Ross-Degnan D. Effects of state surveillance on new post-hospitalization benzodiazepine use. Int J Qual Health Care. 2003;15:423–431. doi: 10.1093/intqhc/mzg064. [DOI] [PubMed] [Google Scholar]
- 30.Wastila LJ, Bishop C. The influence of multiple copy prescription programs on analgesic utilization. J Pharm Care Pain Symptom Control. 1996;4:3–19. [Google Scholar]
- 31.Weintraub M, Singh S, Byrne L, Maharaj K, Guttmacher L. Consequences of the 1989 New York State Triplicate Benzodiazepine Prescription Regulations. JAMA. 1991;266:2392–2397. [PubMed] [Google Scholar]
- 32.Wilsey BL, Fishman SM, Gilson AM, Casamalhuapa C, Baxi H, Zhang H, Li CS. Profiling multiple provider prescribing of opioids, benzodiazepines, stimulants, and anorectics. Drug Alcohol Depend. 2010;112:99–106. doi: 10.1016/j.drugalcdep.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Zullich SG, Grasela TH, Jr, Fiedler-Kelly JB, Gengo FM. Impact of triplicate prescription program on psychotropic prescribing patterns in long-term care facilities. Ann Pharmacother. 1992;26:539–546. doi: 10.1177/106002809202600417. [DOI] [PubMed] [Google Scholar]