Abstract
Background
Amyloid-related imaging abnormalities (ARIA) have been reported in Alzheimer’s disease (AD) patients treated with bapineuzumab, a humanized monoclonal antibody to amyloid-β. ARIA includes MRI signal abnormalities suggestive of vasogenic edema and sulcal effusions (ARIA-E) and hemosiderin deposits (ARIA-H). A better understanding of the incidence and risk factors for ARIA may further the development of amyloid-modifying treatments for AD.
Methods
Two neuroradiologists independently reviewed (kappa=0.76) and then reached consensus reads on over 2500 FLAIR-MRIs from 262 participants in three phase 2 studies of bapineuzumab. Subjects (n=210) were included in risk analyses if they had no evidence of ARIA-E on pre-treatment MRI, received bapineuzumab, and had at least one post-treatment MRI.
Findings
36/210 (17%) subjects developed ARIA-E during treatment; 28 of these 36 (78%) did not report associated symptoms. Adverse events reported in 8 symptomatic patients included headache, confusion, neuropsychiatric and gastrointestinal symptoms. 15/36 of the ARIA-E cases (42%) were detected only on central review. 13/15 received additional infusions while ARIA-E was present, without any associated symptoms reported. ARIA-E incidence increased with bapineuzumab dose (Hazard Ratio [HR] 2.24 per mg/kg increase in dose; p<0·001) and with APOE ε4 allele number (HR 2.55 per allele; p<0·001).
Interpretation
ARIA appears to represent a spectrum of imaging findings with variable clinical correlates, with some cases remaining asymptomatic even when treated through ARIA-E. The increased risk of ARIA with APOE ε4 and bapineuzumab dose, and the time course in relation to dosing, is consistent with alterations in vascular amyloid burden.
Introduction
Immunotherapy for Alzheimer’s disease is an area of active research, with a number of clinical trials currently investigating active or passive immunotherapeutic approaches to lower cerebral amyloid-β burden.1–4 Several of these studies have reported treatment-related abnormalities on brain imaging, but the pathophysiology underlying these changes remains uncertain.1–4 Furthermore, since these imaging abnormalities can be clinically silent, their exact incidence and the spectrum of clinical and imaging features remains unclear.
Imaging abnormalities associated with immunotherapy were first observed in a phase 1 study of bapineuzumab,3 a humanized monoclonal antibody against beta amyloid, and subsequently in the phase 2 bapineuzumab studies.1,2 The MRI abnormalities, seen on T2 weighted/fluid attenuation inversion recovery (FLAIR) sequences were initially referred to as “vasogenic edema”4. As additional cases were identified in subsequent trials, it became clear that there was a spectrum of imaging alterations associated with amyloid modifying treatments. A recent expert workgroup suggested the umbrella term: Amyloid-Related Imaging Abnormalities (ARIA),5 which includes FLAIR signal abnormalities thought to represent parenchymal vasogenic edema and sulcal effusions (ARIA-E), as well as abnormalities detectable on GRE/T2* sequences believed to represent microhemorrhages and hemosiderosis (ARIA-H).
This report focuses on incident ARIA in the setting of bapineuzumab; however, its findings may have implications for other anti-amyloid therapies. As the original protocols for these trials included only local MRI reads, with limited neuroradiological and clinical experience with these phenomena, there was concern that some cases of ARIA-E might have been missed. A systematic, central review of the MRI from all phase 2 studies was therefore undertaken to assess the incidence of ARIA, its risk factors, and clinical characteristics in the context of bapineuzumab treatment.
Methods
A centralized MRI review was conducted on all scans performed during the completed phase 2 bapineuzumab clinical trials study 201 and study 202,1,2 and the associated ongoing open-label extension study, study 251 prior to February 1, 2009 (see Figure 1). Several procedures were adopted to assure maximal sensitivity for ARIA detection: 1) two neuroradiologists retrospectively reviewed the scans for each patient with complete access to prior and future scans within a patient’s MRI series for comparison; however, they were blinded to the participant’s assigned therapy, APOE ε4 genotype, medical history, and demographics; 2) the scans were read independently and in parallel by each reader; and 3) differences between readings were then discussed and resolved by consensus. Prior to consensus, the inter-reader kappa was 0.76 with 94% agreement on the presence or absence of ARIA-E within individual patients.
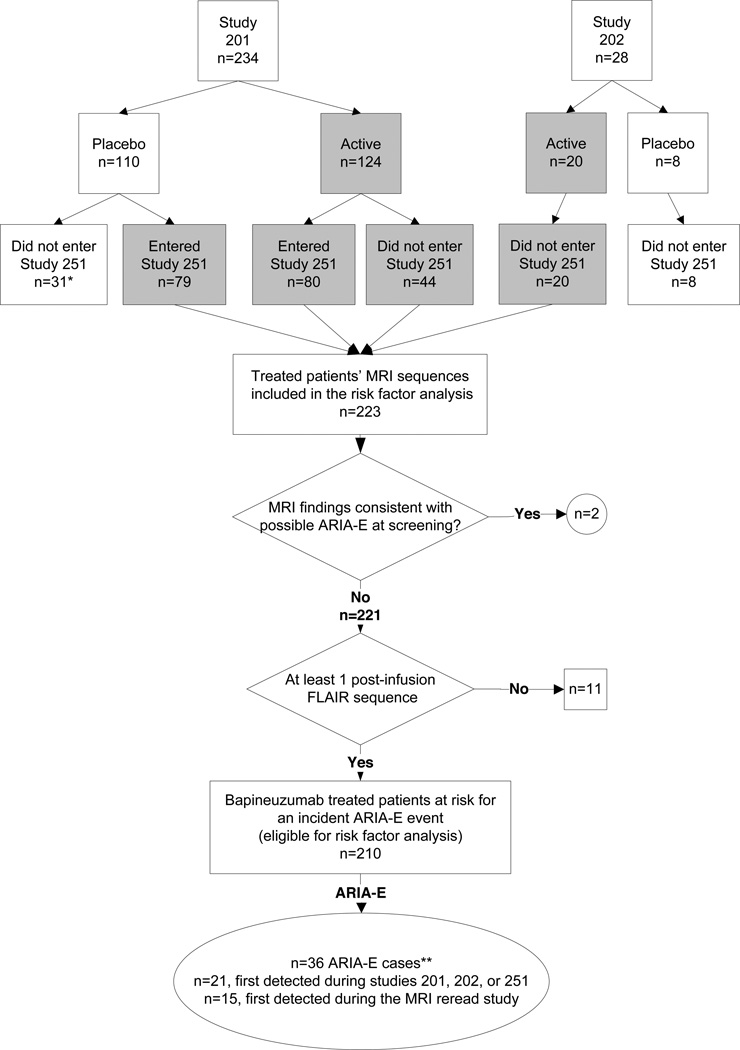
Figure 1. Flow chart of subject eligibility for risk analyses.
*One patient was detected with ARIA-E in the MRI re-read study while receiving placebo. **One patient detected with ARIA-E in the MRI re-read study had metastatic lung cancer and was not included among the ARIA-E cases in the risk factor analyses. The patient was censored at the time of ARIA-E detection. ARIA-E, amyloid-related imaging abnormality thought to represent parenchymal vasogenic edema and sulcal effusions.
Study 201 and 202 were multicenter, double-blinded, randomized, placebo-controlled, ascending-dose cohort trials.1,2 Each lasted for 18 months and included 6 infusions 13 weeks apart. Study 251 is an ongoing extension study into which eligible study 201 patients were recruited (see Supplementary Material S1 for more detail on inclusion/exclusion criteria). Patients in study 251 who previously received bapineuzumab in study 201 were assigned to the same dose of bapineuzumab in study 251 (n=80), and those who previously received placebo in study 201 (n=79) received the dose level of the cohort to which they were originally assigned. No participants from study 202 enrolled in study 251. MRI scans were performed prior to the first infusion and 6 weeks after each subsequent infusion in all studies.
Subjects were identified with incident ARIA-E if they developed new parenchymal or sulcal hyperintensities on FLAIR MRI, consistent with extravascular fluid in the absence of other pathologies. Parenchymal hyperintensities were recorded as present or absent for the posterior fossa and for the left and right side in each of 5 regions (frontal, temporal, parietal, occipital and basal ganglia); in addition, the overall presence or absence of sulcal hyperintensities was assessed. The total number of regions with ARIA-E was calculated. Microhemorrhages (ARIA-H) were also noted as present or absent in the same regions on the T2* images. White matter hyperintensities (WMH) were assessed using an adapted 4-point scale:6 0 (none), 1 (focal lesions), 2 (beginning of confluent lesions), and 3 (diffuse involvement). For the risk analyses, WMH levels 0 and 1 were combined, as were levels 2 and 3, in order to determine whether higher-grade white matter hyperintensity (ie, more severe vascular pathology)7 was associated with ARIA-E.
The sample for the risk analyses included patients treated with bapineuzumab in studies 201, 202 or 251, with a post-infusion FLAIR-MRI, and no other diagnosed conditions deemed to be responsible for the observed imaging abnormalities (see Results section and Figure 1). The patients’ bapineuzumab dose was defined as the highest dose received during the study. Subjects identified with ARIA-E were classified as to whether ARIA was first identified during the course of the trial or during the MRI re-read study, and as either symptomatic or asymptomatic. A subject was considered symptomatic if one or more symptoms were reported as either related or possibly related to study drug in the ARIA safety report from the study investigator.
Kaplan-Meier survival analysis was used to examine the distribution of incident ARIA-E from start of treatment among different groups. Cox proportional hazards (PH) models were used to explore risk factors. SAS release 9.1 was used for all statistical analyses. The PH assumption was evaluated by examining log, negative log plots, with no observed violations. Sensitivity analyses of the PH models performed with a truncated follow-up time found similar results (Supplementary Material S2). P-value significance thresholds were not pre-specified nor adjusted for multiplicity.
Role of Funding Source
Employees of both sponsor companies (Janssen Alzheimer Immunotherapy Research & Development, LLC. and Pfizer Inc.) were involved in the study design, collection, analysis, and interpretation of data, as well as in the development and submission of this manuscript. All authors had access to the study data and shared responsibility for submitting the manuscript for publication.
Results
Among 262 study participants in studies 201, 202, or 251 (Figure 1), 223 patients were treated with bapineuzumab and 2572 MRI scans were reviewed. Two ARIA-E cases were detected among the 262 patients (0.8%) at screening, both of which were subsequently treated with bapineuzumab, and 11 patients lacking a post-infusion FLAIR MRI were excluded. The analyzable sample for bapineuzumab-associated ARIA-E risk factors was thus restricted to 210 participants. One patient identified with possible ARIA-E during the MRI review was subsequently diagnosed with brain metastases and considered to not have bapineuzumabassociated ARIA. Another ARIA-E case was detected among 118 patients (0.8%) treated with placebo.
The incidence rate of ARIA-E was 17.1% (36/210) among bapineuzumab-treated subjects. Figure 2 illustrates the range of ARIA observed in this study. ARIA-E manifested as increased signal in the white or gray matter parenchyma and/or within sulcal spaces on the FLAIR-MRI sequence, whereas ARIA-H was detected on GRE/T2* weighted sequences. The baseline prevalence rate of microhemorrhage (ARIA-H) was 9.2% (19/207; Table 1). Incident ARIA-H was observed in 17/36 (47.2%) of the ARIA-E cases compared with 7/177 (4.0%) subjects without ARIA-E. Among the 17/36 ARIA-E patients with incident ARIA-H, 14 had ARIA-H noted either on the scan immediately prior (2), coincident with (6) or immediately following (6) the scan in which ARIA-E was first noted. Eight of these 14 had ARIA-H detected in the same region as ARIA-E.
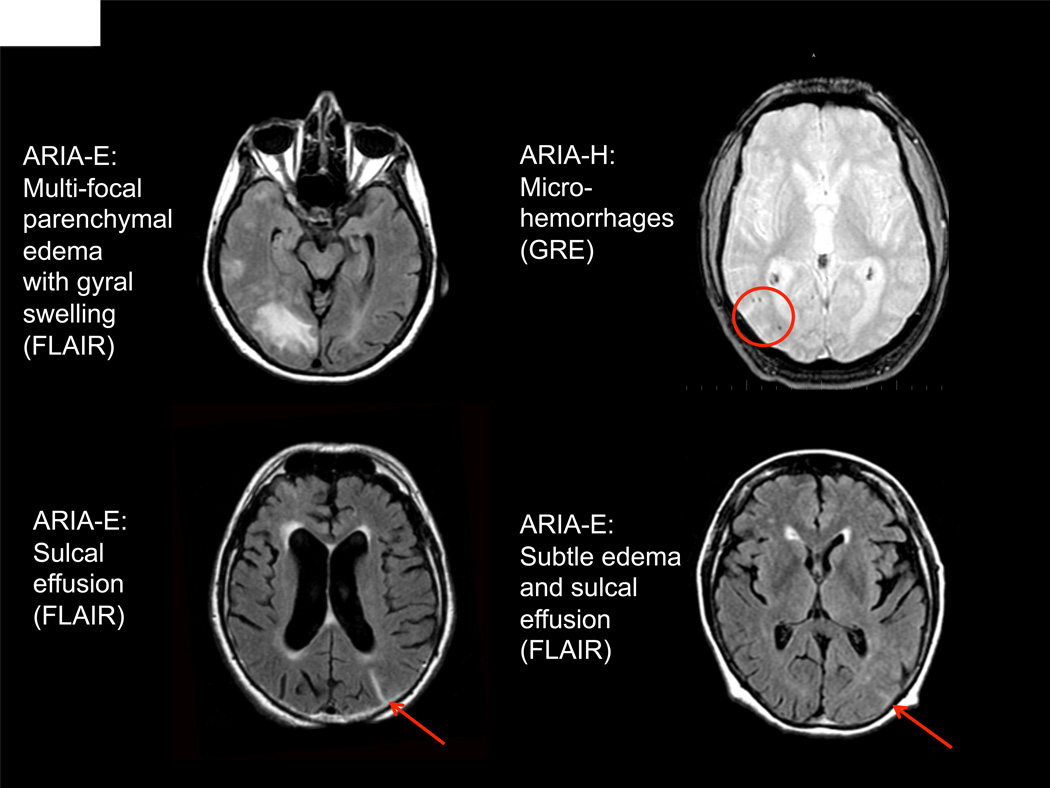
Figure 2. MRI findings in four ARIA cases.
(Upper Left) ARIA-E: Multifocal parenchymal signal abnormalities involving white and gray matter with evidence of gyral swelling (FLAIR Sequence) (Upper Right): ARIA-H: Foci of microhemorrhage in right parietal lobe (GRE-T2* sequence). (Lower left) ARIA-E: Sulcal FLAIR hyperintensity localized to the left parietal surface, without appreciable parenchymal involvement (FLAIR Sequence). (Lower right) ARIA-E: Subtle gyral swelling with faint sulcal hyperintensity (FLAIR Sequence).
Table 1.
Demographics and potential risk factors for ARIA-E
| All Subjects n=210 |
Subjects who did not experience ARIA-E n=174 (82·9%) |
Subjects who did experience ARIA-E n=36 (17·1%) |
Proportional Hazards Model HR (95% CI), P value |
|||
|---|---|---|---|---|---|---|
| Age (in years) | mean (std) | 69·6 (8·9) | 69·6 (9·0) | 69·3 (8·8) | 1·00 (0·49, 2·04), 0·99* | |
| Gender | ||||||
| Women | n | 107 | 87 (81·3%) | 20 (18·7%) | ||
| Men | n | 103 | 87 (84·5%) | 16 (15·5%) | 0·75 (0·39, 1·44), 0·38 | |
| MMSE score | mean (std) | 19·7 (5·2) | 19·7 (5·3) | 20·0 (5·2) | 0·77 (0·39, 1·53), 0·46† | |
| Hypertensive | ||||||
| No | n | 93 | 76 (81·7%) | 17 (18·3%) | ||
| Yes | n | 113 | 95 (84·1%) | 18 (15·9%) | 1·07 (0·55, 2·09), 0·85 | |
| Missing | n | 4 | 3 (75·0%)` | 1 (25·0%) | ||
| White matter hyperintensities | ||||||
| none or focal | n | 186 | 156 (83·9%) | 30 (16·1%) | ||
| confluent or diffuse | n | 22 | 16 (72·7%) | 6 (27·3%) | 1·63 (0.68, 3.92), 0·28 | |
| missing | n | 2 | 2 (100·0%) | |||
| Small hemosiderin deposits | ||||||
| None | n | 188 | 157 (83·5%) | 31 (16·5%) | ||
| present | n | 19 | 15 (78·9%) | 4 (21·1%) | 1·25 (0·44, 3·54), 0·68 | |
| missing | n | 3 | 2 (66·7%) | 1 (33·3%) | ||
| APOE ε4 allele frequency | 2·55 (1·57, 4·12), 0·0001‡ | |||||
| Non-carriers | n | 73 | 68 (93·2%) | 5 (6·8%) | ||
| Heterozygote | n | 102 | 84 (82·4%) | 18 (17·6%) | ||
| Homozygote | n | 33 | 21 (63·6%) | 12 (36·4%) | ||
| missing | n | 2 | 1 (50·0%) | 1 (50·0%) | ||
| Bapineuzumab dose | 2·25 (1·40, 3·62), 0·0008¶ | |||||
| 0·15 mg/kg | n | 42 | 39 (92·7%) | 3 (7·1%) | ||
| 0·5 mg/kg | n | 61 | 55 (90·2%) | 6 (9·8%) | ||
| 1·0 mg/kg | n | 68 | 53 (77·9%) | 15 (22·1%) | ||
| 2·0 mg/kg | n | 39 | 27 (69·2%) | 12 (30·8%) | ||
ARIA-E, amyloid-related imaging abnormalities thought to represent parenchymal vasogenic edema and sulcal effusions; ApoE ε4, apolipoprotein E epsilon 4 allele frequency; CI, confidence interval; HR, hazard ratio; MMSE, Mini-Mental State Examination, std, standard deviation; Row percentages are based on total number of patients in the corresponding category.
Age is dichotomized at <65 and >= 65 years.
MMSE is dichotomized at <=21 and >21.
APOE ε4 is by allele frequency, 0, 1, or 2 as a continuous variable
Bapineuzumab dose is in mg/kg as a continuous variable.
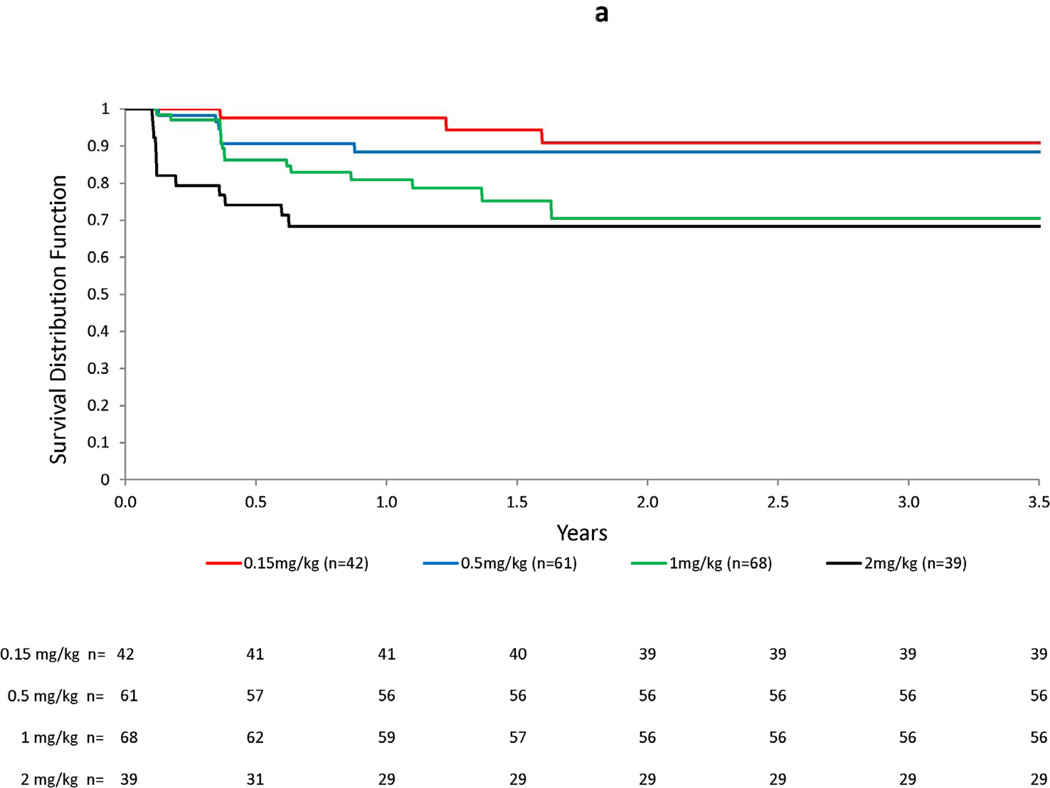
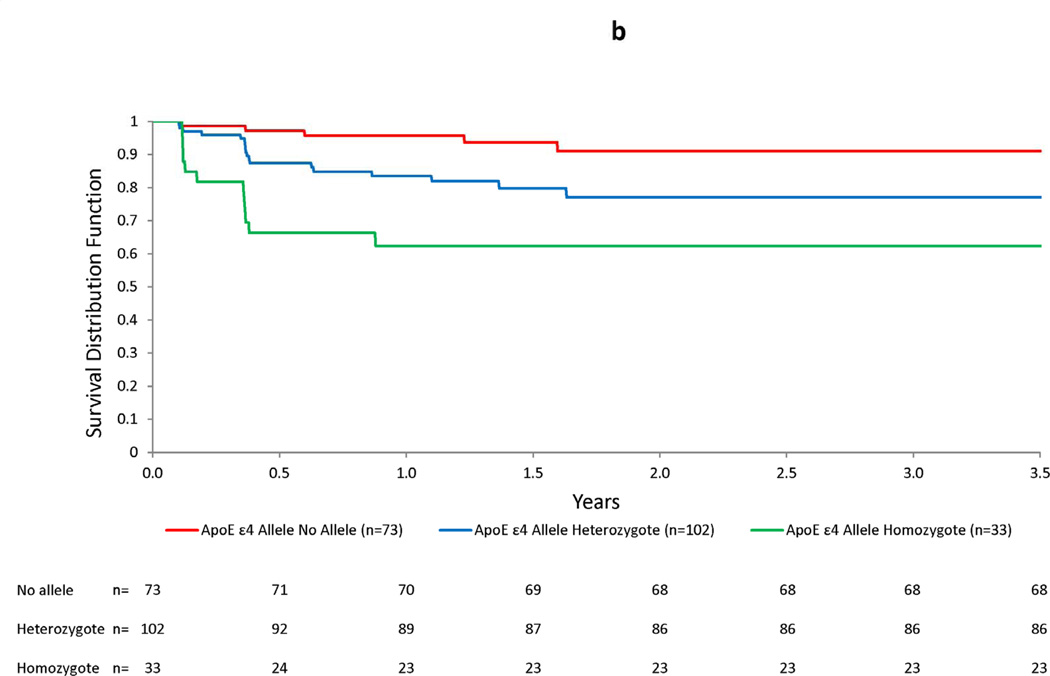
Table 1 presents selected risk factors and baseline MRI findings for subjects included in the risk factor analysis. Baseline characteristics were similar for subjects with ARIA-E versus those without, with the exception that the incidence rate of ARIA increased with bapineuzumab dose and number of APOE ε4 alleles. The respective HRs were 2.24 per mg/kg increase in dose and 2.55 per additional APOE ε4 allele. Additional PH models predictive of ARIA-E are shown in Table 2. For bapineuzumab dose, the HRs exceeded 3.0 for the 2 highest doses when compared with the lowest dose. Similarly, the HRs were approximately 3-fold higher in ε4 heterozygotes and 7-fold higher in ε4 homozygotes compared with ε4 non-carriers. There was no increase in HR for small hemosiderin deposits or white matter hyperintensities on baseline MRI. Models for age, gender, baseline MMSE, Hamilton Depression rating scale score, Rosen-Modified Hachinski score, history of cerebrovascular disease, and hypertension were also not predictive of ARIA-E. Figure 3 presents Kaplan-Meier plots for ARIA-E by dose, APOE ε4 allele frequency, and the presence of small hemosiderin deposits at baseline. A dose-related increase in ARIA-E risk was observed with an increased risk of ARIA-E for the 2 mg/kg dose, particularly notable after the first dose. APOE ε4 allele number showed a clear separation early that persisted with continued follow-up.
Table 2.
Proportional hazards regression models for ARIA-E associated with bapineuzumab
| Factor | Level | Univariate Models HR (95% CI) (P value) |
Full Model HR (95% CI) (P value) |
Reduced Model HR (95% CI) (P value) |
|---|---|---|---|---|
| Dose | ||||
| 0·15 mg/kg | 1·00 (referent) | 1·00 (referent) | 1·00 (referent) | |
| 0·5 mg/kg | 1·76 (0·44, 7·03) (0·43) | 1·76 (0·43, 7·23) (0·43) | 2·00 (0·50, 8·04) (0·33) | |
| 1·0 m/kg | 3·40 (0.97, 11·85) (0·06) | 3·13 (0·89, 10·95) (0·07) | 3·13 (0·89, 10·94) (0·07) | |
| 2·0 mg/kg | 5·60 (1·58, 19·88) (<0·01) | 6·83 (1·89, 24·62) (<0·01) | 6·80 (1·90, 24·33) (<0·01) | |
| APOE ε4 genotype | ||||
| No alleles | 1·00 (referent) | 1·00 (referent) | 1·00 (referent) | |
| Heterozygote | 3·07 (1·14, 8·27) (0·03) | 3·62 (1·30, 10·08) (0·01) | 3·80 (1·38, 10·51) (0·01) | |
| Homozygote | 6·87 (2·42, 19·53) (<0·01) | 7·28 (2·53, 20·95) (<0·01) | 7·15 (2·50, 20·48) (<0·01) | |
| White matter hyperintensities | ||||
| confluent or diffusion lesions vs. none or punctuate |
1·65 (0·68, 3·97 (0·27) | 1·75 (0·69, 4·43) (0·24) | ||
| Small hemosiderin deposits | ||||
| present (one or more) vs.
absent (none) |
1·24 (0·44, 3·50) (0·69) | 1·32 (0·45, 3·88) (0·61) | ||
All factors are analyzed as categorical variables. Univariate Models: each factor is alone in the model. Full Model: all factors are in the model; thus, each factor may be considered adjusted for the other factors. Reduced Model: only the factors in which the lower 95% CI exceeded 10 in at least one stratum, specifically dose and APOE ε4 genotype. ARIA-E, amyloid-related imaging abnormalities thought to represent parenchymal vasogenic edema and sulcal effusions; ApoE ε4, apolipoprotein E epsilon 4 allele frequency; CI, confidence interval; HR, hazard ratio; APOE ε4 = apolipoprotein E epsilon 4 allele frequency. These analyses were performed on a subset of data with complete information on all four factors, total subjects n= 204, ARIA-E events n = 35; hence the number of subjects differs slightly from that in the descriptive summary found in Table 1.
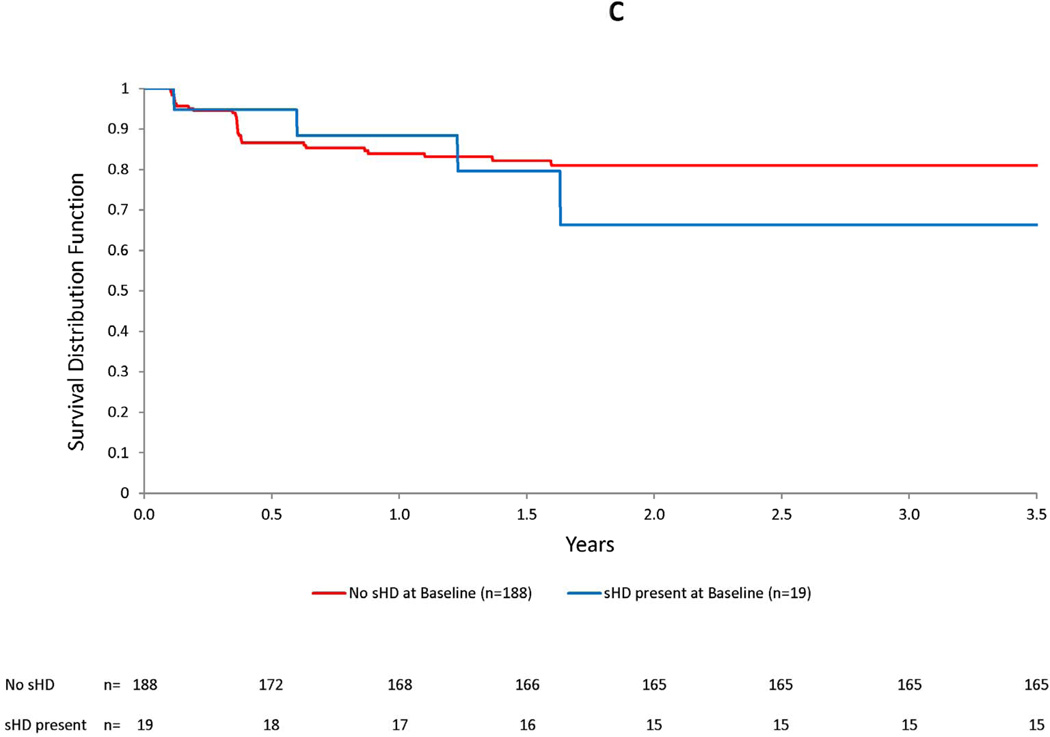
Figure 3. Kaplan-Meier plots for ARIA-E by bapineuzumab dose (a), number of APOE ε4 alleles (b), and presence of small hemosiderin deposits at baseline (c).
Increasing dose and number of APOE ε4 alleles were associated with an increased risk of ARIA-E over time. In the accompanying graphs, an increased risk of ARIA-E may be readily visualized by the decrease in the Kaplan-Meier survivor function after the first two doses, based on MRI readings performed 6 weeks after the baseline and month 3 infusions, in both subjects treated with the highest dose (2 mg/kg) and in APOE ε4 homozygotes. No increased risk was apparent for the presence small hemosiderin deposits at baseline.
The mean number of infusions prior to identification of ARIA-E was 2.4 (S.D.=1.7; range 1 to 7). The majority of ARIA-E cases (25/36; 69%) were identified after the first or second infusion. ARIA-E was not observed beyond 2 years after the first exposure to bapineuzumab. The median duration of ARIA-E for the 31/36 subjects in whom resolution was demonstrable on follow-up scans was 113 days (range 17 to 534). Eight patients with ARIA-E identified during the trial were re-dosed at a lower dose after ARIA-E resolved; ARIA-E recurred in one subject who remained asymptomatic.
Eight of the 36 ARIA-E patients had symptoms (adverse events [AEs]) that were considered related or possibly related to study drug. The categories for these AEs were general (n=7, with headache in 4), neurologic (n=16, with confusion in 5), psychiatric (n=3), and gastrointestinal (n=3) (See Supplementary Material S3 for additional details). Among the 8 symptomatic patients, 7 were APOE ε4 carriers (5 homozygotes and 2 heterozygotes). Six of the 8 symptomatic cases had been dosed at 2 mg/kg dose (4), or 1 mg/kg dose (2). In all these cases bapineuzumab was discontinued, and a follow-up MRI subsequently showed resolution of ARIA-E. One symptomatic patient in the 251 study developed a left frontal stroke temporally associated with ARIA-E but did not have focal symptoms clearly referable to either the location of the stroke or ARIA-E.8 Although the ARIA-E resolved, the patient eventually expired ∼8 weeks later due to complications of the stroke. Seven patients had incident microhemorrhage (ARIA-H) without ARIA-E; none of these cases manifested clinical symptoms.
Fifteen patients were identified with ARIA-E only during the MRI re-read study. All were asymptomatic and had fewer brain regions involved (1·3+/− 0·5) versus those identified during the clinical studies (2·6+/−2·4; p< 0·02). Thirteen of these 15 received additional infusions of bapineuzumab after ARIA-E first appeared, as the ARIA was not detected during the trial, and remained asymptomatic. In 2 of these 13, ARIA-E recurred with additional infusions, but the patients remained asymptomatic.
Eight out of 36 subjects with ARIA had cerebrospinal fluid (CSF) collected. Five had a normal CSF profile; 3 had elevated protein (259, 264, 337 mg/dL) and red cell count (360, 1280, 108 cells/ mm3). Two had an increased number of white cells (8, 19 cells/mm3), both of whom were symptomatic and APOE ε4 carriers.
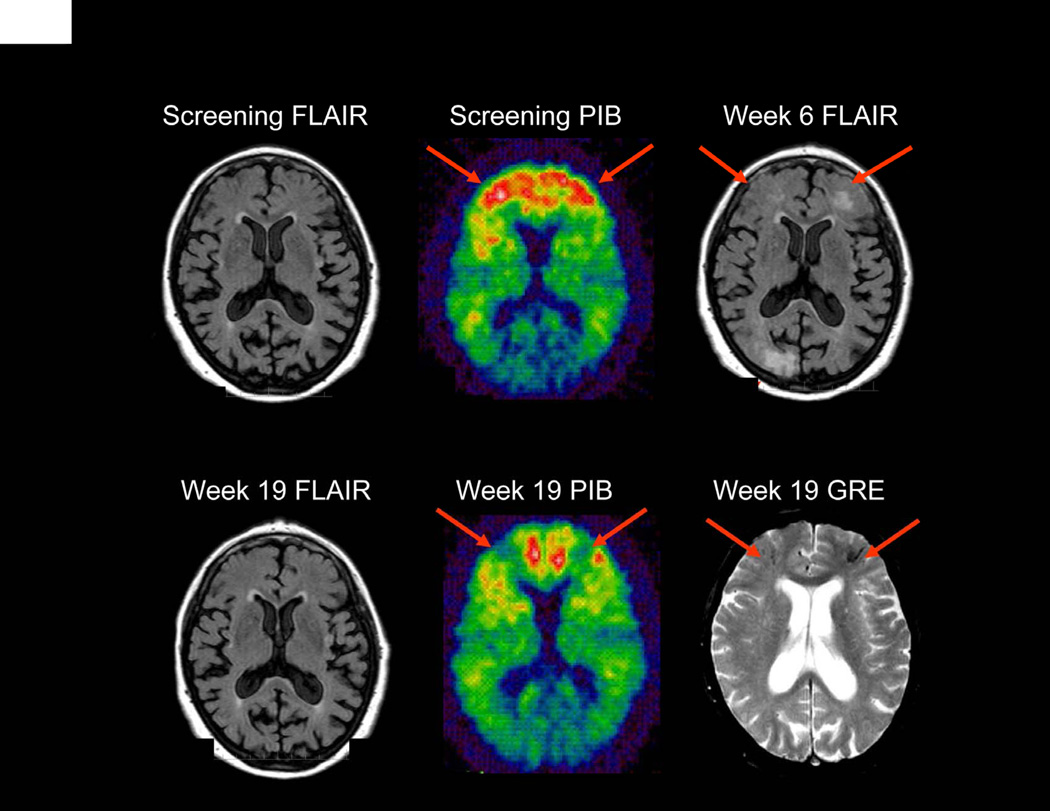
Figure 4 shows a bapineuzumab-treated subject who developed ARIA at the 2 mg/kg dose in the 202 study. This subject underwent 11-C Pittsburgh Compound B – position emission tomography (11-C PIB-PET) amyloid imaging pre- and post-ARIA. The baseline PIB retention was elevated (consistent with high fibrillar Aβ burden) in several regions in which ARIA-E and ARIA-H subsequently developed. PiB retention was reduced in these regions at the follow-up scan after ARIA-E had resolved.
Figure 4. APOE ε4 carrier (3, 4) dosed with bapineuzumab (2·0 mg/kg).
Week 6 FLAIR sequence reveals bi-frontal parenchymal hyperintensity (arrows) (ARIA-E) which resolves by Week 19. Additionally Week 19 GRE-T2* sequence reveals development of bifrontal microhemeorrhages (arrows) (ARIA-H) not present on prior imaging studies. Corresponding Week 19 PIB scan reveals reduced PIB uptake (arrows) when compared to baseline in regions of ARIA-E and ARIA-H.
Discussion
This study involved a centralized, blinded, retrospective assessment of the incidence of ARIA in bapineuzumab-treated subjects from a series of phase 2 studies. ARIA was detected in 17% of bapineuzumab-treated subjects who met the inclusion criteria for the risk analyses. The majority of cases were clinically silent. Approximately 40% of ARIA cases were first detected in this retrospective central review, all of whom were asymptomatic and continued to be dosed while having ARIA-E. In some patients, ARIA recurred, but they remained asymptomatic. These newly identified ARIA-E cases appeared to have milder FLAIR signal changes with fewer involved brain regions. When the phase 2 studies started, little was known about the range of ARIA changes on MRI. With greater awareness of these phenomena, it seems likely that local radiologists will miss fewer ARIA cases in the future.
The exact mechanisms giving rise to ARIA remain to be fully elucidated, but the risk factors identified in this study, as well as recent data from animal models, give rise to a number of potential theories (see Figure 5). The risk analyses provide quantitative evidence for increased risk of ARIA-E with increasing APOE ε4 allele number, extending a preliminary report suggesting that the risk of ARIA-E is increased among APOE ε4 carriers.1 APOE ε4 has been linked to development of cerebral amyloid angiopathy (CAA) in transgenic mice. 9,10 Postmortem human studies indicate that APOE ε4 is a risk factor for CAA11–13 and spontaneous ARIA-E-like phenomena have been reported in CAA patients.14,15 APOE ε4 carrier status is also a risk factor for microhemorrhage in the general population16,17 and among patients in memory clinics.11,18,19 ARIA-E cases were too few to fully explore an interaction between APOE ε4 allele and bapineuzumab dose, although the observations that 7/8 symptomatic cases were ε4 carriers and 6/8 were treated at the two highest doses, is suggestive of a potential interrelationship that remains to be fully explored in larger studies. The risk factor analyses, together with the latter findings, suggest that it may be possible to reduce bapineuzumab-related ARIA-E and associated symptoms by careful dosing with attention to APOE ε4 status.
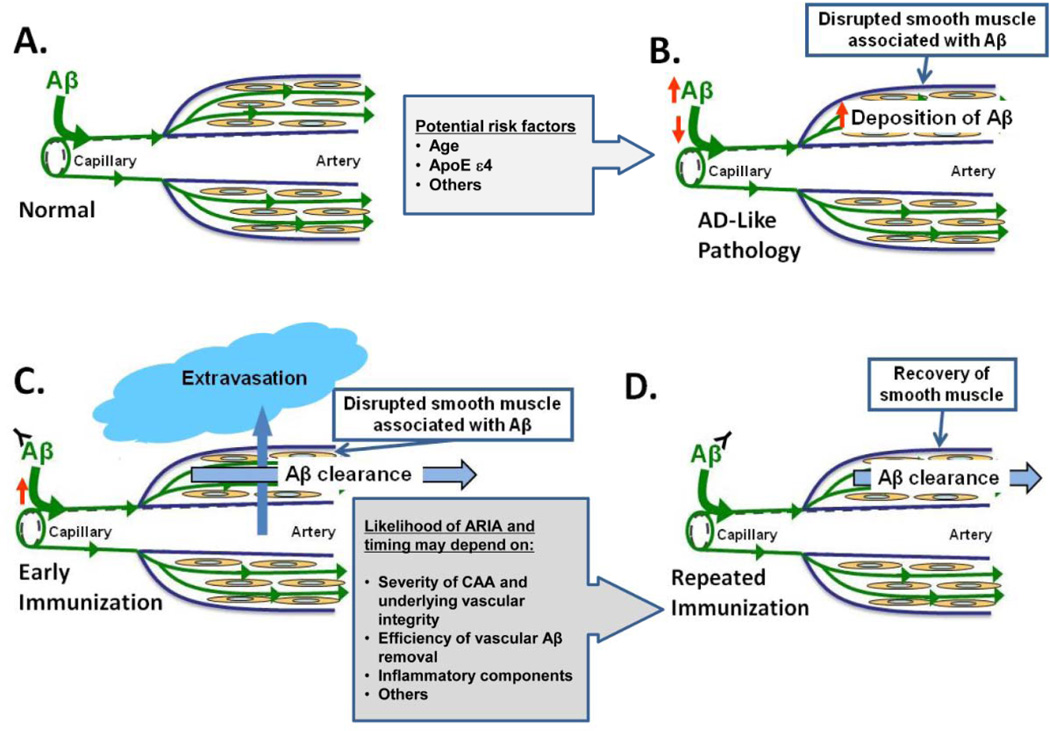
Figure 5. A–D: Vascular beta-amyloid clearance model of ARIA generation with anti-beta-amyloid treatments (22).
In the figure, a cerebral vessel evolves over the course of AD from a normal state (A) to one with AD-like vascular pathology (B) associated with vascular amyloid deposition, disrupted vascular integrity, and impaired perivascular pathways. Age and APOE ε4 genotype may contribute to these changes. After initiation of a treatment predicted to remove beta amyloid from the cerebral vasculature such as immunotherapy, a period of time may exist in some patients when vessels with pre-existing amyloid vascular pathology are transiently more susceptible to vascular extravasation events as beta amyloid is removed from the vessel wall. These events are visualized on MRI as ARIA when fluid, protein, or red cells leak into surrounding tissues (C). The likelihood and timing of such events may depend, in part, on the degree to which the vascular structural integrity was previously disrupted by beta-amyloid deposition, the efficiency of vascular beta-amyloid clearance, and local inflammation. Mobilization of parenchymal beta amyloid to perivascular drainage pathways that are impaired could also give rise to a transient paradoxical increase in vascular beta amyloid following anti-beta-amyloid immunotherapy. With maintained vascular beta-amyloid clearance and recovery of vascular structural integrity, the risk of such extravasation events would be predicted to decrease (D). Portions of this figure were adapted with permission from Weller RO et al. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer's disease.
The presence of baseline ARIA-H was not found to be a significant risk factor for ARIA-E in this study. However, subjects with multiple baseline microhemorrhages were excluded from participation in the 251 study due to concern about a potential link between risk factors for CAA and ARIA-E. Approximately half of the bapineuzumab-treated subjects who developed ARIA-E also developed incident ARIA-H, with the majority of these subjects demonstrating evidence of temporal and spatial co-occurrence. These findings suggest that ARIA-E and ARIA-H are related phenomena, perhaps both related to increases in vascular permeability.5 Depending on the location (intraparenchymal or meningeal) of the vessel, leakage of proteinaceous fluid could give rise to an increased signal detected on FLAIR images (ARIA-E) in the brain parenchyma (vasogenic edema) and leptomeningeal spaces (sulcal effusions), while leakage of red cells would result in ARIA-H, seen on T2*GRE MRI as cerebral microhemorrhages and hemosiderosis.5
The increased risk of ARIA associated with higher doses of bapineuzumab, as well as the illustrative case with PET amyloid imaging, also suggest a potential relationship of ARIA to amyloid clearance. The reduction of PiB retention, presumably representing clearance of fibrillar amyloid from both plaque and cerebral vessels, appeared to be both temporally and regionally associated with vasogenic edema and microhemorrhage. A recent report in a small number of subjects treated with gantenerumab, a conformationally specific monoclonal antibody with high affinity for fibrillar forms of amyloid-β, also demonstrated evidence of reduced amyloid burden on PiB-PET imaging in two patients who developed ARIA-E.4 It is also possible that ARIA may be related to clearance of amyloid from parenchymal plaques with a transient increase in vascular or perivascular amyloid burden20,21 or to inflammation.22 However, similar to reports in transgenic animals,23–25 eventual normalization of cerebral blood vessel integrity in humans may be possible. In a post-mortem study of subjects included in an earlier active amyloid immunization trial (AN1792), two of the longest treated participants treated showed a virtually complete absence of CAA.26 The observation that the majority of ARIA-E occurred within the first few doses may also support the hypothesis that vascular remodeling after amyloid clearance may reduce the risk of ARIA over time.
It appears that ARIA-E can rarely occur spontaneously, as one case was identified in the placebo group of the 201 study and 2 cases were noted at screening (Figure 1). A recent report identified two spontaneous ARIA-E cases in large cohorts screened for AD clinical trials with other agents.27 Given reports of similar ARIA findings in CAA patients,13,14 it is possible that some of these spontaneous ARIA-E cases represent AD patients with concomitant CAA.
Several potential limitations to our study should be considered. The MRI review was retrospective, and the results might have been different if scans had been read prospectively without access to other scan timepoints for comparison. Our primary intent in having two neuroradiologists assess the scans was to increase the likelihood of detecting any potential MRI findings consistent with ARIA; hence, we did not provide extensive training to enhance interreader reliability. Nevertheless, the inter-reader reliability was reasonably high. As we asked the readers to then discuss any cases with discrepant reads, we were not able to assess intra-rater reliability. A more detailed rating scale for both ARIA-E and ARIA-H is under development, and will be used in future projects with the phase 3 data. We did not find evidence of associated clinical symptoms in the majority of ARIA cases, but it is possible that subtle symptoms could be missed in patients with mild to moderate dementia. Although this study represents the largest collection of treatment-related ARIA cases to date, given the relatively small numbers of subjects, we are not able to definitively explore all potential risk factors, which will require further study in larger trials.
In summary, our results suggest that ARIA represents a spectrum of imaging findings with variable clinical correlates, including a substantial number of subtle cases that went undetected during the trials and remained asymptomatic despite continued treatment. The frequent co-occurrence of ARIA-E and ARIA-H suggests a common pathophysiological mechanism that may be related to transient increases in vascular permeability. The increased risk of ARIA in APOE ε4 carriers further supports a key role of vascular amyloid, whereas the relationship to bapineuzumab dosing and the PiB-PET amyloid results suggests that ARIA may be related to amyloid clearance. Ongoing studies with bapineuzumab and other potential amyloid-modifying therapies should shed additional light on the long term implications of these phenomena.
Panel: Research in Context
Systematic review
We searched PubMed for articles published in English between Jan 1, 1980 and December 1, 2011 using the search terms “vasogenic edema”, “amyloid-related imaging abnormalities (ARIA)”, and “Alzheimer’s disease.” This search yielded only the three published reports from the bapineuzumab phase 1 and 2 trials; one report from another monoclonal antibody, ganteneremab; one recent report of ARIA detected in screening cohorts for Alzheimer’s disease clinical trials; and several recent review articles on drug development. A recent workgroup report also cites published abstracts from other classes of amyloid-modifying agents, but these data are not yet published. As amyloid-related imaging abnormalities occurring in Alzheimer’s disease clinical trials represent a relatively new concept, we broadened our search to include imaging reports from cerebral amyloid angiopathy cases, which also appear relevant to our findings.
Interpretation
This is the first systematic study of amyloid-related imaging abnormalities (ARIA) in the context of amyloid-modifying therapies; in particular, abnormalities related to MR-signal alterations thought to represent vasogenic edema (ARIA-E). We identified a number of ARIA-E cases that were initially missed during the previously reported studies with bapineuzumab, and reported the clinical symptomatology or lack thereof in all identified ARIA-E cases. In addition, by combining cases across three Phase 2 studies, we were able to examine a number of risk factors for developing ARIA-E. The increased risk of incident ARIA-E with increased number of APOE ε4 alleles, as well as previous case reports in the literature of similar phenomema occurring spontaneously in cerebral amyloid angiopathy, suggest a potential relationship with vascular amyloid burden. The increased risk of ARIA with a higher bapineuzumab dose and the illustrative case with PET-amyloid imaging, as well as a similar report in a small number of subjects treated with another monoclonal antibody, suggest a possible relationship of ARIA-E to beta-amyloid clearance. Our findings have important implications for elucidating the mechanisms underlying ARIA, and the continued need for close monitoring of ARIA in the development of anti-amyloid therapies for Alzheimer’s disease.
Supplementary Material
Acknowledgements
This study was sponsored by Elan Corporation, plc. (Janssen Alzheimer Immunotherapy acquired the Alzheimer Immunotherapy Program from Elan Corporation, plc. in September 2009) and Wyeth Pharmaceuticals (which was acquired by Pfizer Inc. in October 2009). The authors would like to thank Lorna Fang for her contributions to the analytic programming, and Selena Scaglione and Mark McGovern for their contributions to study operation and management. Editorial support was provided by Elizabeth Yepez at Phase Five Communications Inc., and was funded by Janssen Alzheimer Immunotherapy Research & Development, LLC. Her involvement consisted solely of manuscript formatting, and no contribution was made to editorial content.
Funding: This study was sponsored by Elan Corporation, plc. (Janssen Alzheimer Immunotherapy acquired the Alzheimer Immunotherapy Program from Elan Corporation, plc. in September 2009) and Wyeth Pharmaceuticals (which was acquired by Pfizer Inc. in October 2009).
Footnotes
Contributions of Authors
RS served as first author on this manuscript contributing to the study conception and design, data analysis and interpretation, with additional contributions including literature searches, primary writing of the introduction and discussion sections, critical revision of the manuscript, and figure preparation. SS contributed to the study design, data analysis and interpretation, and writing of this manuscript. DB contributed in planning this study and in reviewing and editing this manuscript. DT contributed to the study design, and reviewed both the MRI images and the manuscript. JB participated in the collection of some of the data and also in writing this manuscript. NF contributed to the drafting, editing, and review of this manuscript. MR contributed to the study conceptualization as a study investigator, and also in manuscript preparation. MS was involved in data collection and performed a critical review of the manuscript; he also served on the steering committee for this study. LH contributed to data collection, data analysis, and the writing of this manuscript. AP participated in the study design, data collection, data interpretation, and writing of this manuscript. IL was involved with the study design, collection, analysis, and interpretation of data, and also in the development of the manuscript. MA contributed to the study design and execution, and the analysis and interpretation of data; he was also involved in the development of the manuscript. KM contributed to some writing and reviewing of the manuscript. YL contributed to the study design, study execution, and data analysis. EL contributed to the study design, implementation, review of analyses, and manuscript generation and review. KG provided statistical input and review. RHB was involved in the study design, data collection, interpretation and analysis, and also in the development and submission of the manuscript. GK contributed to writing, figure preparation, literature review, and manuscript preparation. RB contributed to the study design, analysis, interpretation of data, and review of the manuscript. MG contributed to the study design, study conduct, data analysis, and manuscript writing.
Conflicts of Interest
RS has served as a study investigator and a consultant for Janssen Alzheimer Immunotherapy Research & Development, LLC, and for Pfizer Inc., and has received honoraria for participation in symposiums. She has also served as a consultant and/or site investigator for Bristol-Myers-Squibb, Roche, Elan, Biogen-IDEC, Avid, and Bayer. SS has served as a consultant and study investigator for Janssen Alzheimer Immunotherapy Research & Development, LLC., Pfizer Inc., and Elan Corporation, plc. phase 2 and 3 studies of bapineuzumab. DB reports no conflicts of interest. DT provides review of MRI images for Janssen Alzheimer Immunotherapy Research & Development, LLC. JB serves as a neuroradiological consultant to SYNARC Inc., an imaging contract research organization contracted by both sponsor companies (Janssen Alzheimer Immunotherapy Research & Development, LLC. and Pfizer Inc.); he also serves as a consultant to Janssen Alzheimer Immunotherapy Research & Development, LLC. for non-clinical research activities. NF has provided consulting and/or image analysis services to Elan Corporation, plc., Janssen Alzheimer Immunotherapy Research & Development, LLC., Pfizer Inc., and Wyeth Pharmaceuticals as well as to AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, GE Healthcare, Lundbeck A/S, and IXICO. MR serves as consultant to Janssen Alzheimer Immunotherapy Research & Development, LLC. MS participates in a consulting/advisory capacity for Eli Lilly and Company, Amerisciences, Takeda Pharmaceuticals Inc., Eisai Co., Ltd., Pfizer Inc., and GlaxoSmithKline plc. and receives royalties from Wiley and AmeriSciences, LP. He receives contracting fees/grants from Celgene Corporation, Ceregene, Inc., Bayer AG, Baxter International Inc., Bristol-Myers Squibb, Eli Lilly and Company, Pfizer Inc., Janssen Alzheimer Immunotherapy Research & Development, LLC., Avid Radiopharmaceuticals, Inc., Genentech, Inc. and Eisai Co., Ltd. LH serves on the study steering committee and has acted as a consultant for Janssen Alzheimer Immunotherapy Research & Development, LLC., but receives less than $10,000 annually for such consulting activities. AP has received grant/research support from Baxter International Inc., Bristol-Myers Squibb, Eisai Co., Ltd., Elan Corporation, plc., Genentech, Inc./ Hoffmann-La Roche Inc., Janssen Alzheimer Immunotherapy Research & Development, LLC., Medivation, Inc., Pfizer Inc., and Toyama Chemical Co., Ltd. He has also served as a consultant/participated on advisory boards for Elan Corporation, plc., Janssen Alzheimer Immunotherapy Research & Development, LLC., Medivation, Inc., Pfizer Inc., Transition Therapeutics Inc., and Toyama Chemical Co., Ltd.. He is also a member of the speakers’ bureau for Forest Laboratories, Inc. IL is a stockholder in Elan Corporation, plc. RB is an employee of and receives stock and stock options from Pfizer Inc. MA, KM, YL, EL, KG, RHB, and GK are employees of Janssen Alzheimer Immunotherapy Research & Development, LLC. MG is a consultant to Janssen Alzheimer Immunotherapy Research & Development, LLC. and is a stockholder in Elan Corporation, plc.
References
- 1.Salloway S, Sperling R, Gilman S, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73(24):2061–70. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rinne JO, Brooks DJ, Rossor MN, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9(4):363–72. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- 3.Black RS, Sperling RA, Safirstein B, et al. A single ascending dose study of bapineuzumab in patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;24(2):198–203. doi: 10.1097/WAD.0b013e3181c53b00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostrowitzki S, Deptula D, Thurfjell L, et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol. 2011 doi: 10.1001/archneurol.2011.1538. [DOI] [PubMed] [Google Scholar]
- 5.Sperling RA, Jack CR, Black SE, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: Recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7(4):367–85. doi: 10.1016/j.jalz.2011.05.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32(6):1318–22. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- 7.Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43(9):1683–9. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 8.Roher AE, Maarouf CL, et al. Neuropathology and amyloid-β spectrum in a bapineuzumab immunotherapy recipient. J Alzheimers Dis. 2011;24(2):315–25. doi: 10.3233/JAD-2011-101809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fryer JD, Simmons K, Parsadanian M, et al. Human apolipoprotein E4 alters the amyloid-beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J Neurosci. 2005;25(11):2803–10. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan PM, Mase BE, Estrada JC, Schmechel DE, Alberts MJ. Human apolipoprotein E4 targeted replacement mice show increased prevalence of intracerebral hemorrhage associated with vascular amyloid deposition. J Stroke Cerebrovasc Dis. 2008;17(5):303–11. doi: 10.1016/j.jstrokecerebrovasdis.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg SM, Rebeck GW, Vonsattel JPG, Gomez-Isla T, Hyman BT. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol. 1995;38(2):254–9. doi: 10.1002/ana.410380219. [DOI] [PubMed] [Google Scholar]
- 12.Chalmers K, Wilcock GK, Love S. APOE epsilon 4 influences the pathological phenotype of Alzheimer's disease by favouring cerebrovascular over parenchymal accumulation of A beta protein. Neuropathol Appl Neurobiol. 2003;29(3):231–8. doi: 10.1046/j.1365-2990.2003.00457.x. [DOI] [PubMed] [Google Scholar]
- 13.Caselli RJ, Walker D, Sue L, Sabbagh M, Beach T. Amyloid load in nondemented brains correlates with APOE e4. Neurosci Lett. 2010;473(3):168–71. doi: 10.1016/j.neulet.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinnecom C, Lev MH, Wendell L, et al. Course of cerebral amyloid angiopathy-related inflammation. Neurology. 2007;68(17):1411–6. doi: 10.1212/01.wnl.0000260066.98681.2e. [DOI] [PubMed] [Google Scholar]
- 15.Oh U, Gupta R, Krakauer JW, Kahandji AG, Chin SS, Elkind MSV. Reversible leukoencephalopathy associated with cerebral amyloid angiopathy. Neurology. 2004;62(3):494–7. doi: 10.1212/01.wnl.0000106951.94624.df. [DOI] [PubMed] [Google Scholar]
- 16.Poels MMF, Ikram MA, van der Lugt A, et al. Incidence of cerebral microbleeds in the general population: the Rotterdam Scan Study. Stroke. 2011;42(3):656–61. doi: 10.1161/STROKEAHA.110.607184. [DOI] [PubMed] [Google Scholar]
- 17.Vernooij MW, van der Lugt A, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70(14):1208–14. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 18.Goos JD, Henneman WJP, Sluimer JD, et al. Incidence of cerebral microbleeds: a longitudinal study in a memory clinic population. Neurology. 2010;74(24):1954–60. doi: 10.1212/WNL.0b013e3181e396ea. [DOI] [PubMed] [Google Scholar]
- 19.Goos JDC, Kester MI, Barkhof F, et al. Patients with Alzheimer disease with multiple microbleeds: relation with cerebrospinal fluid biomarkers and cognition. Stroke. 2009;40(11):3455–60. doi: 10.1161/STROKEAHA.109.558197. [DOI] [PubMed] [Google Scholar]
- 20.Boche D, Zotova E, Wller RO, et al. Consequence of Abeta immunization on the vasculature of human Alzheimer's disease brain. Brain. 2008;131(Pt 12):3299–310. doi: 10.1093/brain/awn261. [DOI] [PubMed] [Google Scholar]
- 21.Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer's disease. Brain Pathol. 2008;18(2):253–66. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eng JA, Frosch MP, Choi K, Rebeck W, Greenberg SM. Clinical manifestations of cerebral amyloid angiopathy-related inflammation. Ann Neurol. 2004;55(2):250–6. doi: 10.1002/ana.10810. [DOI] [PubMed] [Google Scholar]
- 23.Christie R, Yamada M, Moskowitz M, Hyman B. Structural and functional disruption of vascular smooth muscle cells in a transgenic mouse model of amyloid angiopathy. Am J Pathol. 2001;158(3):1065–71. doi: 10.1016/S0002-9440(10)64053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroeter S, Khan K, Barbour R, et al. Immunotherapy reduces vascular amyloid-beta in PDAPP mice. J Neurosci. 2008;28(27):6787–93. doi: 10.1523/JNEUROSCI.2377-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zago W, Schroeter S, Khan K, et al. Microvascular changes associated with passive immunotherapy in PDAPP mice - Potential implication for the etiology of vasogenic edema; Presented at: Alzheimer's Association International Conference on Alzheimer's Disease; July, 16–21, 2011; Paris, France. P3-052. [Google Scholar]
- 26.Boche D, Denham N, Holmes C, Nicoll JAR. Neuropathology after active Abeta42 immunotherapy: implications for Alzheimer's disease pathogenesis. Acta Neuropathol. 2010;120(3):369–84. doi: 10.1007/s00401-010-0719-5. [DOI] [PubMed] [Google Scholar]
- 27.Carlson C, Estergard W, Oh J, et al. Prevalence of asymptomatic vasogenic edema in pretreatment Alzheimer's disease study cohorts from phase 3 trials of semagacestat and solanezumab. Alzheimers Dement. 2011;7(4):396–401. doi: 10.1016/j.jalz.2011.05.2353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.