Abstract
Effective and targeted delivery of cells to injured organs is critical to the development of cell therapies. However, currently available in vivo cell tracking methods still lack sufficient sensitivity and specificity. We examined, therefore, whether a highly sensitive and specific bioluminescence method is suitable to noninvasively image the organ distribution of administered mesenchymal stem cells (MSCs) in vivo. MSCs were transfected with a luciferase/neomycin phosphotransferase construct (luc/neo-MSC). Bioluminescence of these cells was measured (charge-coupled device camera) after treatment with luciferin, showing a linear increase of photon emission with rising cell numbers. To track these cells in vivo, groups of mice were injected with 1 × 105 luc/neo-MSCs/animal and imaged with bioluminescence imaging at various time points. Injection of cells in the suprarenal aorta showed diffuse distribution of cells in normal animals, whereas distinct localization to the kidneys was observed in mice with ischemia- and reperfusion-induced acute kidney injury (AKI). Intrajugular infusion of MSCs demonstrated predominant accumulation of cells in both lungs. In animals with AKI, detectable cell numbers declined over time, as assessed by bioluminescence imaging and confirmed by PCR, a process that was associated with low apoptosis levels of intrarenally located MSCs. In conclusion, the described bioluminescence technology provides a sensitive and safe tool for the repeated in vivo tracking of infused luc/neo-MSCs in all major organs. This method will be of substantial utility in the preclinical testing and design of cell therapeutic strategies in kidney and other diseases.
Keywords: luciferase, cell therapy, apoptosis, regenerative medicine
bioluminescence is a natural phenomenon that has been used in the laboratory for many different assays. Firefly luciferase is an enzyme that generates light by photon emission in an oxidation reaction with its substrate luciferin, utilizing oxygen and ATP (7). The luciferase gene can be introduced in a cell by transfection, and this cell, provided that ATP and oxygen levels are adequate, is able to emit light, which makes this reaction specific for viable, metabolically active cells. With the development of highly sensitive charged-coupled device (CCD) cameras that can detect light transmission through tissues, it has become possible to repeatedly track administered cells in vivo with bioluminescence imaging (BLI), thereby avoiding the need of killing an experimental animal (5, 8). Because of to the inherently low background bioluminescence, this method is very specific in detecting administered viable cells.
Regenerative medicine is a rapidly developing field in which bone marrow derived cells are administered for the treatment of various disorders (41). Animal studies have provided insight into the fate of administered cells, but such data largely end-point studies, thereby only providing little dynamic information regarding cell distribution and long-term engraftment (9, 21). BLI offers thus a unique possibility to repeatedly track and quantify administered cells in vivo.
Acute kidney injury (AKI) is a serious and common clinical problem with high morbidity and mortality (18). Cell therapy is a promising new experimental treatment for renal diseases, and bone marrow-derived stem cells have been shown to be promising cell therapeutic vehicles (22, 28, 35). Although there is controversy about hematopoietic stem cells (11, 12, 23, 29), multipotent mesenchymal stromal cells or MSCs have been shown to be effective in preventing injury and enhance regeneration of kidneys after AKI (2, 17, 26, 33, 39, 40). Although administered MSCs have been shown to differentiate to variable extent and late after injury into renal cells in AKI (33), their early renoprotective activity is primarily mediated by paracrine mechanisms such as immunomodulation and growth factor secretion (2, 19, 20, 33, 39, 40). MSCs have also been shown to be effective in the treatment of a rat model of glomerulonephritis and in a model of Alport's syndrome (14, 24, 25).
The current study was designed to test the utility of BLI to track MSC after intravenous or intra-arterial injection in mice with AKI as well as in normal animals, and to investigate their distribution and survival kinetics over time. Our data show that BLI is a sensitive and specific tool to track cells in vivo, demonstrating that administered MSC distribute in distinctive patterns when infused either intra-arterially or intravenously. We did not detect long-term engraftment of a significant number of cells. In conclusion, BLI is a sensitive tool that allows the in vivo definition of cellular distribution and survival data, thereby substantially aiding in the preclinical testing and optimization of various cell-based treatment protocols.
METHODS
Animals and Cells
Animal experiments were performed following approval from the University of Utah Institutional Animal Care and Use Committee. Adult C57/Bl6 mice of either sex, weighing 20–25 g, were purchased from Charles River laboratories (Wilmington, MA). Animals were housed in a temperature-controlled environment with a 12:12-h day-night cycle. All mice ate regular chow and had free access to water.
AKI in mice was induced by clamping both renal pedicles for 30 min. In brief, mice were anesthetized with Avertin, and kidneys were exposed after abdominal midline incision. Microvessel clamps were applied to both renal pedicles, and cessation of blood flow was confirmed by visual inspection (color change). Clamps were released after 30 min, and reflow was visually confirmed before closure of the abdominal incision.
Multipotent MSCs were kindly provided by Dr. Claudia Lange (University of Hamburg). Bone marrow from anesthetized C57/Bl6 mice was obtained by flushing of femurs with saline. Obtained cell suspensions were washed and plated in T25 flasks (Corning) containing α-minimal essential medium and preselected 10% FBS (GIBCO). MSCs were infected with pMMP-LucNeo and selected with G418. Supernatant was kindly provided by Dr. Lessnick, Huntsman Cancer Institute, University of Utah (3). Wild-type and luciferase-neomycin (luc-neo) MSCs were both subjected to differentiation protocols, showing identical differentiation patterns in adipocytes, chondrocytes, and osteocytes (data not shown). This indicates that the transfection of MSCs does not interfere with in vitro differentiation. Following selection with G418, 1 × 105 MSCs were injected in C57/Bl6 mice. Intrajugular injection was carried out after dissecting the neck with a midline incision, preparation of the internal jugular vein, and injecting 100 μl of saline containing MSCs slowly directly in the jugular vein. Carotid injection was carried out after dissection and distal ligation of the left carotid artery and introduction of a PE-10 tube in the proximal carotid artery. Thereby, MSCs were delivered directly in the descending aorta.
Mice were imaged using a Xenogen IVIS 100 imaging system, per the manufacturer's directions. For in vivo imaging of cells, mice were anesthetized with Avertin and injected intraperitoneally with 50 mg/kg d-luciferin (Caliper, Hopkintown, MA) at 5–10 min before imaging. Regions of Interest (ROIs) were drawn using Living Image 2.5 software (Caliper). In vivo biodistribution of luciferin after intraperitoneal injection is fast, with high blood levels after 5 min and high uptake in liver and kidneys according to one publication (27). We tested imaging 5, 10, and 15 min after intraperitoneal luciferin injection in preliminary experiments and found no difference in signal intensity (data not shown) at these time points; therefore, an imaging time point between 5 and 10 min after injection of luciferin was chosen. To control for background photon emission, obtained data were subjected to average background subtraction, using data from control animals that were only injected with identical doses of luciferin. Photon radiance on the surface of an experimental animal was expressed as photons per second per centimeter squared per steradian. Images shown in Figs. 1–4 are compound pictures generated by Living Image software.
Fig. 1.

A: in vitro determination of sensitivity and specificity of bioluminescence imaging (BLI) measurements of luciferase/neomycin (luc/neo)-transfected mesenchymal stem cells (MSCs). Transfected cells were incubated with luciferin, and photon emission was measured with the Xenogen IVIS system. Tubes containing 100, 1,000, and 10,000 cells were suspended in culture medium containing luciferin. The region of interest (ROI) indicates photon emission measurement of the red-encircled area (units: photons·s−1·cm−2·sr−1); 100 cells were readily detected in vitro, emitting a signal of 961,000 photons·s−1·cm−2·sr−1. Note: binning is set at 1, giving a higher spatial resolution. Red, highest photon density; blue, lowest photon density. B: regression analysis revealed significant linear increase of luminescence with cell numbers. C: in vitro placement of tubes containing 1,200 and 12,000 cells in the retroperitoneal space of a dead animal. Tubes, containing 1,200 and 12,000 cells, as shown on right, emitted 442,390 and 1,282,600 photons·s−1·cm−2·sr−1, respectively, as indicated by the corresponding ROI area measurements. After placement of the tubes in the retroperitoneal space of a dead animal (areas indicated by circles) 5,378 and 64,309 photons·s−1·cm−2·sr−1 were detected, respectively. A signal intensity corresponding to 1 and 5% of the original in vitro signal that was significantly above background levels outside the encircled areas allowed the detection of 1,200 and 12,000 cells. There is some light scattering beyond the area of tube placement. Note: binning is set at 4, thereby increasing sensitivity and the signal-to-noise ratio at the expense of spatial resolution, which results in increased pixilation of the signal.
Fig. 4.
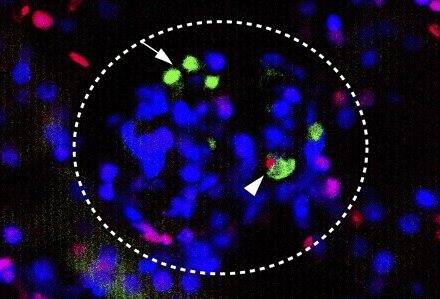
TdT-dUTP nick end-labeling (TUNEL) staining (red fluorescence) of a representative kidney section containing CFDA-stained MSCs (green) in a glomerulus (encircled). Nuclei were counterstained with Hoechst 33342 dye (blue). MSCs are readily identified by bright green staining (arrow). An apoptotic MSC (arrowhead) 24 h after injection is depicted in red.
Binning increases the pixel size on the CCD, which delivers higher sensitivity at the expense of spatial resolution. Binning a luminescent image results in a significant improvement in the signal-to-noise ratio. Although spatial resolution is degraded at high binning, this is often acceptable for in vivo images where light emission is rather diffuse: binning 1 = 16 CCD pixels/field; binning 2 = 4 CCD pixels/field (4 pixel summed together); binning 4 = 1 pixel/CCD field (16 pixels together).
Real-Time RT-PCR
Total RNA for real-time PCR was extracted with an RNeasy kit (Qiagen, Valencia, CA), including a DNase digestion step to exclude contaminating DNA. Reverse transcription was performed using Moloney murine leukemia virus RT (Invitrogen, Carlsbad, CA) for 60 min at 42°C. Real-time PCR with relative quantification of target gene copy numbers in relation to β-actin transcripts was carried out using the following primers: luciferase forward: tgaagagatacgccctggtt; reverse: ctacggtaggctgcgaaatg. β-actin forward: cactgtgttggcatagaggtc; reverse: agagggaaatcgtgcgtgaca. The Smart-Cycler system (Cepheid, Sunnyvale, CA) was used to monitor real-time PCR amplification using SYBR Green I (Invitrogen) and as previously reported (9).
TdT-dUTP Nick End-Labeling
Kidneys from mice with AKI, killed at 2 and 24 h after trans-carotid, intra-aortic infusion of carboxyfluorescein diacetate (CFDA; Invitrogen)-labeled MSCs were cryopreserved in Tissue-Tek Cryo-OCT (Fisher Scientific, Pittsburgh, PA). Sections (8 μm) were prepared on a cryotome and fixed with ethanol. Tdt-dUTP nick end-labeling (TUNEL) staining was carried out with the In-situ Cell Death Detection kit (Roche, Mannheim, Germany), following the manufacturer's instructions. The percentage of TUNEL-positive MSCs (red-stained nuclei) was calculated based on the total number of MSCs per kidney section. In a similar way, the percentage of apoptotic kidney cells was determined by counting the total cell numbers of 10 high-power fields/section, and from this the percentage of red-stained apoptotic cells was determined.
Statistical Analysis
Data are expressed as means ± SD. Primary data collection used Excel (Microsoft, Redmond, WA), and statistical analyses were carried out using Prism (GraphPad, San Diego, CA). ANOVA and t-tests were used to assess differences between data means as appropriate. A P value of <0.05 between data means was considered significant.
RESULTS
In Vitro Determination of Bioluminescence Sensitivity
Imaging of dilution series containing 10,000–100 luc/neo MSCs in vitro showed an excellent linear correlation between photon emission intensity and cell numbers (P = 0.0016, Fig. 1). As few as 100 cells were easily detected by this in vitro system. Photon emission was neither detected in transfected cells that were not treated with luciferin substrate nor in untransfected cells (data not shown), together demonstrating the sensitivity and specificity of BLI. In vivo sensitivity was tested in a model system whereby 1,200 and 12,000 cells in an Eppendorf tube were placed in the retroperitoneal space of a dead mouse. Twelve hundred cells were readily detected (Fig. 1C). There was some scattering of the signal, but the location corresponded well to the placement location, and 1–5% of the in vitro signal intensity for both cell numbers was detected after retroperitoneal placement (Fig. 1C).
In Vivo Studies
Intra-arterial injection.
To determine the distribution of luc/neo MSCs infused in the aorta via the left carotid artery, in normal and AKI animals, BLI measurements were always performed after prior intraperitoneal administration of luciferin. Measurements were carried out immediately after the single administration of luc/neo MSC and repeated at 24 and 72 h and 7 days. At 10–15 min after cell infusion, total body luminescence was 681,400 in AKI (n = 5, SD 415,000) and 622,500 photons·s−1·cm−2·sr−1 in normal animals (n = 4, SD 388,000) (P = 0.83). AKI animals showed distinct accumulation of infused cells in the areas corresponding to the location of the kidneys (Fig. 2A), whereas normal animals showed a diffuse, whole body distribution with greater accumulation in the lungs in some animals (Fig. 2B). Renal location of injected cells in AKI animals was further confirmed by lateral imaging as well as by imaging excised organs directly at 24 h after injection (Fig. 2C). After cell infusion (24 h), reassessment of total animal luminescence showed a significant decline to 203,000 (SD 72,500) in AKI animals and to 156,000 (SD 41,000) photons·s−1·cm−2·sr−1 in normal animals. Total animal bioluminescence decreased further at 72 h to 64,300 (SD 2,500) in AKI and 44,000 (SD 35,000) photons·s−1·cm−2·sr−1 in normal animals. There was no statistically significant difference between the 24- and 72-h time points as determined by ANOVA. At 7 days after injection, total photon emission fell to 25,000 (SD 2,200) and 26,000 (SD 28,500) photons·s−1·cm−2·sr−1 in AKI and normal mice, indicating that most of the cells had vanished. RT-PCR for luciferase expression was used to detect cells in RNA extracts from kidneys 7 days after injection. This was done to determine whether there were cells remaining that were below the detection limit of the BLI method. After luc-neo MSC administration (7 days), mRNA transcript numbers of luciferase, determined by quantitative RT-PCR, in lungs, kidneys, liver, and spleen were at least nine log fold lower than in 10,000 luc/neo MSC cells, indicating the virtual absence of residual cell numbers in the examined organs.
Fig. 2.
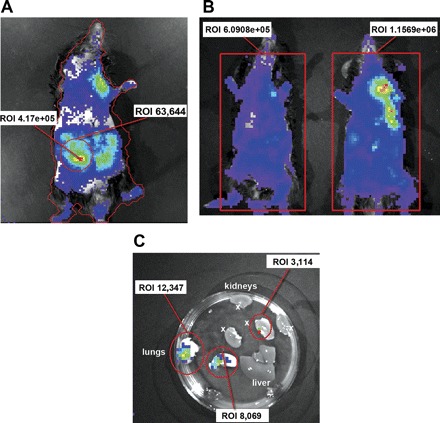
A: immediately after intra-arterial infusion, acute kidney injury (AKI) animals showed distinct accumulation of cells in kidneys (as shown by green/red areas). Whole animal photon emission ROI (red line around animal) is 4.1705 photons·s−1·cm−2·sr−1 and the right kidney emits 63,644 photons·s−1·cm−2·sr−1 (encircled red). B: normal animals show diffuse whole body distribution with eventual accumulation in the lungs in some animals. Representative animals of each group are shown. C: BLI of organs (in a petri dish) after an animal is killed demonstrating organ distribution at 24 h after iv MSC injection. Imaging of harvested organs at 24 h after injection shows the accumulation in the lungs (12,347 and 8,069 photons·s−1·cm−2·sr−1) and in the kidney (3,114 photons·s−1·cm−2·sr−1; kidney halves marked with x). The liver showed no distinct accumulation of cells. Red, highest photon emission density; blue, lowest photon emission density. Measurement areas (ROI) are encircled in red. Binning is set at 4, giving an increased sensitivity and signal-to-noise-ratio at the expense of spatial resolution (bigger pixel size).
Intravenous injection.
Administration of luc/neo MSCs in the jugular vein revealed almost exclusive accumulation of cells in the lungs of normal animals (Fig. 3A), whereas only one AKI animal showed renal signal immediately after injection (Fig. 3B). Positive signals from administered cells gradually decreased over the next 3 days, and 7 days after injection no positive signal was detectable above background level in normal animals. In one animal in the AKI group, a midabdominal signal of 1,287,000 photons·s−1·cm−2·sr−1 was seen, corresponding to a poorly healing abdominal wound (data not shown).
Fig. 3.

A: iv injection in a normal animal shows accumulation of MSCs in the lungs. Total body photon emission, as indicated by the red square, is shown as ROI measurement (photons·s−1·cm−2·sr−1). B: iv injection in 3 different AKI animals shows accumulation of cells in the lungs immediately after injection, and only one animal with AKI showed retroperitoneal uptake indicating cell localization in the kidneys. C: control animal for background subtraction (BKG = background; photon emission that is automatically subtracted by the imaging program from the measurement area). Binning is set at 4.
Intrarenal apoptosis of MSC.
To assess whether administered CFDA-labeled luc/neo MSC undergo intrarenal apoptosis, kidney sections from mice killed at 2 and 24 h after injection were examined by TUNEL assay. MSCs were easily detectable by their bright green fluorescence and were localized mainly in glomerular and less commonly in peritubular capillaries. Consistently, ∼1% of identified MSCs were TUNEL positive, indicating low-level apoptosis of intrarenal MSCs (Fig. 4). This is in contrast to an apoptosis rate of ∼10% of tubular cells (counting total and apoptotic cells in 10 high-power fields/cortical section; n = 3) at each time point (2 and 24 h), a response that is expected in an ischemic model of AKI.
DISCUSSION
The aim of the current study was twofold: first to determine if BLI is a sufficiently sensitive and specific method for in vivo cell tracking of administered single cell suspensions and second, to determine the distribution kinetics over time of cells infused by different routes (ia or iv) to normal and AKI animals. We demonstrate here that BLI is a suitable tool for sensitive and specific in vivo cell tracking. Despite expected scattering and signal attenuation through overlaying tissues, noninvasive in vivo organ localization of injected stem cells is possible for small cell numbers. The distribution and residence kinetics of MSCs vary considerably, dependent on the route of administration and underlying renal function. This information will likely be important for the design of clinical trials using cell therapy.
Various methods of in vivo cell tracking have been tested, but all of them have certain disadvantages. Magnetic resonance imaging (MRI) of iron-loaded cells or fluorescence-based imaging systems lack sensitivity in vivo, and signals may be lost or may become false positive due to shedding of iron particles with MRI or tissue background fluorescence with the latter (4, 16). We have previously used MRI to track cells in vivo and were able to detect them in the kidney with reasonable sensitivity (21, 26). The disadvantage of this method is the potential spilling or release of iron particles from MSCs and subsequent imaging of non-MSC cell types that have taken up iron as well as the imaging of nonviable cells. A recent publication stressed the possibility of overestimating stem cell survival after administration when using ferumoxide-labeled cells in conjunction with MRI (38). Positron emission tomography currently lacks suitable tracing compounds that allow cell tracking for longer periods (10). BLI has the distinct advantage of good signal intensity due to stable transfection of cells with a light-producing enzyme and continuous expression of the reporter gene; therefore, only viable and surviving cells are detected. Normal tissue does not produce light and is therefore naturally void of background signal. BLI has been successfully used for detection of tumor cells, tumor growth, and spread in mouse models of cancer (1, 5, 8). In contrast, in cell therapy, a single cell suspension is administered, and cells are distributed throughout the whole animal, i.e., in the normal animal without any distinct cell accumulation or engraftment site. Localization and cell engraftment in disease models depends on the type of organ injury and the studied cell type and commonly is diffuse rather than localized, making cell detection a challenge (8, 30). Our data show that BLI is sensitive and specific for monitoring the distribution and numbers of injected cells over time, information that is critical in the design of cell therapies.
The method, site, and route of cell administration are important determinants in the development of effective, disease- or organ-specific cell therapies. This is illustrated by MSCs that were administered in the present study. This cell type requires attachment to extracellular matrix for survival and growth and is thus not naturally found in the circulation. Accordingly, when such cells are no longer attached to an extracellular matrix or vascular endothelial cells, they may undergo anoikis, a form of apoptosis. We hypothesize that anoikis is important in this context because MSCs are by their nature not circulating cells and require extracellular matrix-derived signals for survival, and deprivation of these signals in the vasculature might induce apoptosis/anoikis of MSCs. The extent to which this phenomenon applies to the here-demonstrated disappearance of administered MSC suspensions was elucidated. Because MSCs gradually disappear from injured kidneys, we assessed whether they undergo apoptosis (13, 32). We found that ∼1% of MSCs within glomerular and peritubular capillaries were TUNEL positive, indicating ongoing apoptosis and suggesting that this process may contribute to the declining number of intrarenal MSCs after administration. Although this number might seem low at first, continuing attrition of small numbers of cells eventually leads to the disappearance of all cells if there is no substrate supporting permanent attachment. Accordingly, strategies that reduce apoptosis might improve effectiveness of cell therapy as has been shown by Mangi et al. (31). Strategies to genetically engineer MSCs to withstand apoptosis, such as transduction with protein kinase B or incubation with lysophosphatidic acid, might prove valuable in the future to enhance effectiveness of MSC therapy (6, 34).
Furthermore, there is concern of causing capillary clogging when larger cell types such as MSCs are infused, a complication that could result in hemodynamic compromise, interference with pulmonary gas exchange, and respiratory distress (15). Schrepfer et al. (37) have shown that intravenously injected MSCs localize mainly to the pulmonary capillary bed, which can, however, be prevented by the administration of vasodilators. Our data confirm this observation, showing pulmonary localization of intravenously administered MSC, a pattern not seen with intra-aortic administration. Specifically, our data demonstrate prompt homing of MSCs to the injured kidney after intra-arterial administration. However, the robust renal signal started to decline already within 24 h of administration, indicating either redistribution and dilution or death of administered cells. Using highly sensitive PCR analysis, we demonstrated that this decline in renal signal intensity was the result of cell loss from the kidneys and, at least in part, due to apoptotic cell loss. Although we and others showed that the therapeutic effectiveness of MSCs in AKI is primarily mediated by paracrine mechanisms, prolonged presence of MSCs at sites of injury might improve their effectiveness (2, 20, 40). Strategies, therefore, that enhance attachment, engraftment, and that improve MSC recruitment to specific organs or compartments are being developed and include genetic engineering of surface properties (36).
In summary, our data demonstrate that BLI is a sensitive and specific in vivo method to monitor the distribution and fate of administered cells over time. We conclude that its application in preclinical studies provides a new tool for the development and testing of optimal organ- and disease-specific treatment protocols.
GRANTS
This work was supported in part by funds from the Veterans Administration, the National Institutes of Health, the American Heart Association, and the National Kidney Foundation (Utah/Idaho).
Acknowledgments
We thank Dr. Steve Lessnick for providing the luc/neo construct, for helpful advise, and for giving us access to the Xenogen IVIS 100 system at the Huntsman Cancer Institute, University of Utah.
Part of this work was presented in poster form at the 2006 American Society of Nephrology meeting (J Am Soc Nephrol 17: 663A, 2006), San Diego, CA, November 2006.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland CB, Herman EI, Baker EM, Cao YA, Contag CH, Negrin RS. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood 106: 1113–1122, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol 18: 2486–2496, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Braunreiter CL, Hancock JD, Coffin CM, Boucher KM, Lessnick SL. Expression of EWS-ETS fusions in NIH3T3 cells reveals significant differences to Ewing's sarcoma. Cell Cycle 5: 2753–2759, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bulte JW, Duncan ID, Frank JA. In vivo magnetic resonance tracking of magnetically labeled cells after transplantation. J Cereb Blood Flow Metab 22: 899–907, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Cao YA, Bachmann MH, Beilhack A, Yang Y, Tanaka M, Swijnenburg RJ, Reeves R, Taylor-Edwards C, Schulz S, Doyle TC, Fathman CG, Robbins RC, Herzenberg LA, Negrin RS, Contag CH. Molecular imaging using labeled donor tissues reveals patterns of engraftment, rejection, and survival in transplantation. Transplantation 80: 134–139, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Baydoun AR, Xu R, Deng L, Liu X, Zhu W, Shi L, Cong X, Hu S, Chen X. Lysophosphatidic acid protects mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis. Stem Cells 26: 135–145, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Contag CH, Bachmann MH. Advances in in vivo bioluminescence imaging of gene expression. Ann Rev Biomed Eng 4: 235–260, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Contag CH, Ross BD. It's not just about anatomy: in vivo bioluminescence imaging as an eyepiece into biology. J Magn Reson Imaging 16: 378–387, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood 101: 2999–3001, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Doyle B, Kemp BJ, Chareonthaitawee P, Reed C, Schmeckpeper J, Sorajja P, Russell S, Araoz P, Riederer SJ, Caplice NM. Dynamic tracking during intracoronary injection of 18F-FDG-labeled progenitor cell therapy for acute myocardial infarction. J Nucl Med 48: 1708–1714, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Duffield JS, Bonventre JV. Kidney tubular epithelium is restored without replacement with bone marrow-derived cells during repair after ischemic injury. Kidney Int 68: 1956–1961, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 115: 1743–1755, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng J, Yang S, Xu L, Tian H, Sun L, Tang X. Role of caspase-3 inhibitor in induced anoikis of mesenchymal stem cells in vitro. J Huazhong Univ Sci Techn Med Sc 27: 183–185, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Floege J, Kunter U, Weber M, Gross O. Bone marrow transplantation rescues Alport mice. Nephrol Dial Transplant 21: 2721–2723, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cell Tissue Organ 169: 12–20, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Graves EE, Weissleder R, Ntziachristos V. Fluorescence molecular imaging of small animal tumor models. Curr Mol Med 4: 419–430, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med 14: 1035–1041, 2004 [PubMed] [Google Scholar]
- 18.Hoste EA, Kellum JA. Incidence, classification, and outcomes of acute kidney injury. Contr Nephrol 156: 32–38, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med 59: 311–325, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Imberti B, Morigi M, Tomasoni S, Rota C, Corna D, Longaretti L, Rottoli D, Valsecchi F, Benigni A, Wang J, Abbate M, Zoja C, Remuzzi G. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol 18: 2921–2928, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Ittrich H, Lange C, Togel F, Zander AR, Dahnke H, Westenfelder C, Adam G, Nolte-Ernsting C. In vivo magnetic resonance imaging of iron oxide-labeled, arterially-injected mesenchymal stem cells in kidneys of rats with acute ischemic kidney injury: detection and monitoring at 3T. J Magn Reson Imaging 25: 1179–1191, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest 112: 42–49, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krause D, Cantley LG. Bone marrow plasticity revisited: protection or differentiation in the kidney tubule? J Clin Invest 115: 1705–1708, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunter U, Rong S, Boor P, Eitner F, Muller-Newen G, Djuric Z, van Roeyen CR, Konieczny A, Ostendorf T, Villa L, Milovanceva-Popovska M, Kerjaschki D, Floege J. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol 18: 1754–1764, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Kunter U, Rong S, Djuric Z, Boor P, Muller-Newen G, Yu D, Floege J. Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J Am Soc Nephrol 17: 2202–2212, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Lange C, Togel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int 68: 1613–1617, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Lee KH, Byun SS, Paik JY, Lee SY, Song SH, Choe YS, Kim BT. Cell uptake and tissue distribution of radioiodine labelled d-luciferin: implications for luciferase based gene imaging. Nucl Med Commun 24: 1003–1009, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Lin F, Cordes K, Li L, Hood L, Couser WG, Shankland SJ, Igarashi P. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol 14: 1188–1199, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Lin F, Moran A, Igarashi P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest 115: 1756–1764, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandl S, Schimmelpfennig C, Edinger M, Negrin RS, Contag CH. Understanding immune cell trafficking patterns via in vivo bioluminescence imaging. J Cell Biochem 39: 239–248, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nature medicine 9: 1195–1201, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Michel JB. Anoikis in the cardiovascular system: known and unknown extracellular mediators. Arteriosclerosis Thrombosis Vasc Biol 23: 2146–2154, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N, Alison M, Remuzzi G. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol 15: 1794–1804, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther 14: 840–850, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Poulsom R, Alison MR, Cook T, Jeffery R, Ryan E, Forbes SJ, Hunt T, Wyles S, Wright NA. Bone marrow stem cells contribute to healing of the kidney. J Am Soc Nephrol 14, Suppl 1: S48–S54, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, Wohlgemuth R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med 14: 181–187, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplanta Proc 39: 573–576, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Terrovitis J, Stuber M, Youssef A, Preece S, Leppo M, Kizana E, Schar M, Gerstenblith G, Weiss RG, Marban E, Abraham MR. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation 117: 1555–1562, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 289: F31–F42, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol 292: F1626–F1635, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Togel F, Westenfelder C. Adult bone marrow-derived stem cells for organ regeneration and repair. Dev Dyn 236: 3321–3331, 2007 [DOI] [PubMed] [Google Scholar]


