Abstract
Dyslipidemia has been associated with an increased risk for developing cancer. However, the implicated mechanisms are largely unknown. To explore the role of dyslipidemia in breast cancer growth and metastasis, we used the apolipoprotein E (ApoE) knockout mice (ApoE−/−), which exhibit marked dyslipidemia, with elevated circulating cholesterol and triglyceride levels in the setting of normal glucose homeostasis and insulin sensitivity. Non-metastatic Met-1 and metastatic Mvt-1 mammary cancer cells derived from MMTV-PyVmT/FVB-N transgenic mice and c-Myc/vegf tumor explants respectively, were injected into the mammary fat pad of ApoE−/− and wild type (WT) females consuming a high-fat/high-cholesterol diet and tumor growth was evaluated. ApoE−/− mice exhibited increased tumor growth and displayed a greater number of spontaneous metastases to the lungs. Furthermore, intravenous injection of Mvt-1 cells resulted in a greater number of pulmonary metastases in the lungs of ApoE−/− mice compared to WT controls. To unravel the molecular mechanism involved in enhanced tumor growth in ApoE−/−mice, we studied the response of Mvt-1 cells to cholesterol in vitro. We found that cholesterol increased AktS473 phosphorylation in Mvt-1 cells as well as cellular proliferation, whereas cholesterol depletion in the cell membrane abrogated AktS473 phosphorylation induced by exogenously added cholesterol. Furthermore, in vivo administration of BKM120, a small molecule inhibitor of PI3K, alleviated dyslipidemia-induced tumor growth and metastasis in Mvt-1 model with a concomitant decrease in PI3K/Akt signaling. Collectively, we suggest that the hypercholesterolemic milieu in the ApoE−/− mice is a favorable setting for mammary tumor growth and metastasis.
Keywords: Dyslipidemia, mammary tumor, ApoE, metastasis, Akt
Introduction
There is growing evidence that metabolic disorders such as obesity and type-2 diabetes increase breast cancer risk (1-5). Moreover, it has been demonstrated that type-2 diabetes accelerates breast cancer development independent of obesity, and this effect maybe primarily mediated by hyperinsulinemia (6, 7).
Both obesity and type-2 diabetes are frequently accompanied by serum lipid abnormalities such as elevated total cholesterol, LDL-cholesterol and triglycerides and reduced HDL-cholesterol, which also increase the risk of various types of human malignancies, including breast cancer. The results of numerous epidemiological studies suggest that dysregulated cholesterol metabolism might be a key factor linking dyslipidemia and cancer (8-11). However, it remains unclear whether abnormally elevated cholesterol levels promote tumorigenesis independent of obesity and type-2 diabetes.
Cholesterol is a sterol, which serves as a metabolic precursor to other bioactive sterols and plays a major role in the structure of the plasma membrane by creating a class of detergent-resistant microdomains called lipid rafts (12). It has been shown that these lipid rafts possess a range of membrane-associated signaling proteins such as receptor tyrosine kinases (RTKs) (13). Recent studies have demonstrated an elevated level of cholesterol in the plasma membrane of prostate and breast tumor cells (14-17). Elevations of plasma cholesterol in animal models have been shown to cause accumulations of cholesterol in lipid rafts, leading to reduced apoptosis, activation of Akt, and increased prostate tumor growth (18). Thus, cholesterol may be contributing to tumor growth by modifying signal transduction through effects on lipid rafts (15, 18, 19). Indeed, recent studies provide evidence that cholesterol mediates localization of Akt molecules to lipid raft microdomains and leads to their activation (20).
Epidemiological studies have described a reduced occurrence of certain malignancies in patients consuming 3-hydroxy-3-methyl-glutaryl CoA (HMG-CoA) reductase inhibitors (statins) used for the treatment of dyslipidemia (21). Statins are inhibitors of the rate-limiting step in cholesterol biosynthesis (conversion of HMG-CoA to mevalonate). Furthermore, prostate cancer progression has recently been shown to be inhibited by a cholesterol-lowering agent (22, 23). Although the effects of statins in breast cancer are somewhat controversial, some studies report a lower risk of breast cancer in patients taking statins (24, 25).
Here we investigated the effects of hypercholesterolemia on mammary tumor growth and metastasis, using the ApoE null mice (ApoE−/−). When fed a cholesterol rich diet, ApoE−/− mice show elevated cholesterol and triglyceride levels in plasma. Interestingly, however, these mice show increased sensitivity to insulin and have normal or low glucose levels, as has been demonstrated previously (26). We found that mammary tumors in hypercholesterolemic ApoE−/−mice were larger than those of WT mice and resulted in more spontaneous pulmonary metastases.
Results
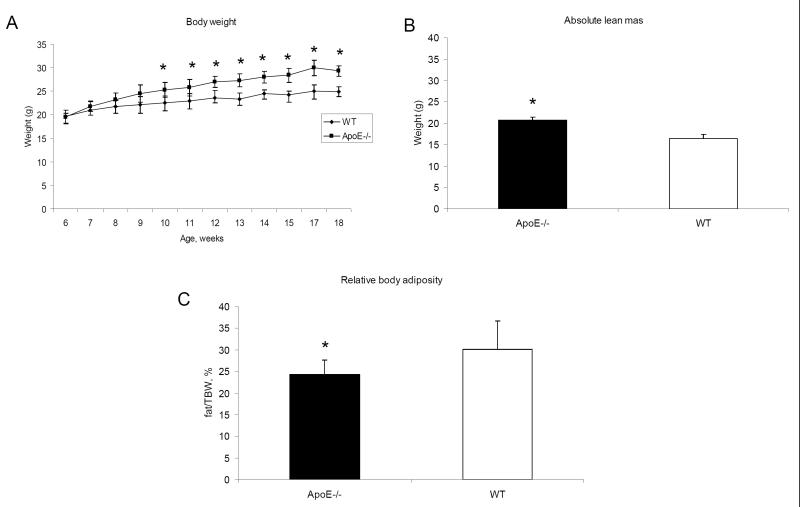
ApoE −/− mice exhibit increased body weight and decreased body adiposity when fed a diet enriched with cholesterol
During ten weeks on the high-fat/high-cholesterol (HF/HC) diet, both ApoE−/− and WT mice gained body weight. However, the ApoE−/− mice had a significantly increased body weight compared to WT mice (Fig 1A) despite the fact that food intake was similar in both groups (data not shown), an observation that has been reported previously (26). Body adiposity measurements obtained using MRI showed that ApoE−/− mice fed with high-cholesterol diet did not show increased relative fat mass (measured as per cent of body weight) despite increased body weight (Fig 1 B and 1C). Reduced body adiposity most likely results from impaired triglyceride clearance and reduced lipid uptake by adipose tissues (26). The HF/HC diet led to a larger increase in plasma cholesterol and triglyceride levels in ApoE−/− mice when compared to the WT mice (Fig 1D and 1E). Furthermore, ApoE−/− mice showed significantly lower levels of blood glucose (Fig 1F), which has previously been shown to be the consequence of improved insulin sensitivity secondary to impaired lipid uptake by adipose tissue and muscle (26).
Figure 1.
High cholesterol diet significantly increased the body weight (A) as well as the cholesterol and triglycerides levels in the plasma (D,E) of ApoE−/− mice with no increase in body adiposity (B,C). ApoE−/− mice had lower blood glucose levels (F). Statistically significant difference between groups is indicated. *, P < 0.05. Student’s t test.
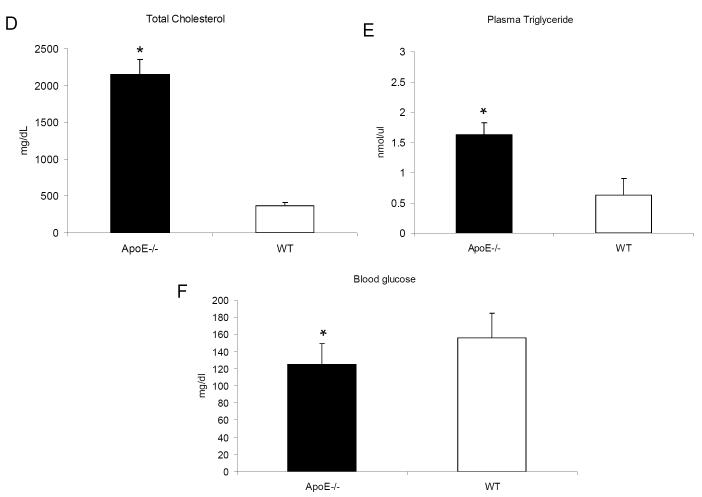
Met-1 and Mvt-1 mammary tumors demonstrate accelerated growth in the face of hyperlipidemia
To investigate the effect of elevated cholesterol/triglyceride levels on mammary tumor growth and progression, we injected mammary cancer cell lines Met-1 and Mvt-1 into the inguinal mammary fat pad of ApoE−/− and WT mice which had been fed a HF/HC diet for 10 weeks. Tumor growth was monitored over a period of 4-6 weeks, while mice were maintained on the HF/HC diet. As shown in figure 2A-D, both Met-1 and Mvt-1 tumors were significantly larger in ApoE−/−than in WT mice.
Figure 2.
Orthotopically injected mammary tumor Met-1 and Mvt-1 cells (500 000 and 50 000 cells per mammary fat pad, respectively) resulted in larger tumors in ApoE−/− mice as demonstrated by tumor volume (A,C) and tumor weight (B,D) (n= 8-10 per group). Statistically significant difference is indicated. *, P < 0.05. Student’s t test.
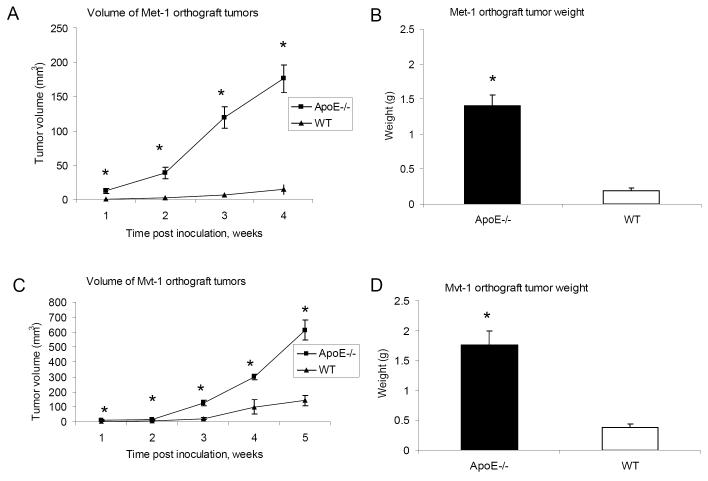
Mvt-1-induced mammary tumors in ApoE−/− mice leads to an increased number of pulmonary metastases
Tumor progression was assessed by determining the number of pulmonary metastases that occurred spontaneously after injection of the primary tumor orthografts. We found a greater number of spontaneous metastatic lesions in the lungs of ApoE−/− mice (11 ±1.6 metastases per mouse vs 5 ±1.8 metastases per mouse in controls) (Fig 3A and 3B). To address whether the higher level of pulmonary metastases observed in ApoE−/− mice is simply due to larger primary tumors or is actually a consequence of higher circulating levels of lipids, we employed intravenous injections of tumor cells to ApoE−/− and WT mice. Three weeks following intravenous injection the mice were sacrificed and the number of pulmonary metastatic lesions was analyzed. As seen in figure 3C, significantly more pulmonary metastases were detected in the lung tissue of ApoE−/− mice in comparison to the controls (9±1.3 metastases per mouse vs 3±1.5 metastases per mouse in controls). In ApoE−/− mice, the circulating blood in the lungs is enriched in cholesterol, which may contribute to proliferation of metastatic Mvt-1 cells in the lung tissue of these mice.
Figure 3.
Orthotopic inoculation of Mvt-1 cells resulted in more numerous pulmonary macrometastases in ApoE−/− mice compared to WT as demonstrated by H & E staining of lung sections (A) (arrows indicate metastases) and the number of macrometastatic lesions detected in the lungs (B) (n= 8-10 per group). Injection of Mvt-1 cells intravenously (12 000 cells) led to a greater level of pulmonary metastases in the lungs of ApoE −/− mice in comparison to WT (n= 10-17 per group) (C). Statistically significant difference is indicated. *, P < 0.05. Student’s t test.
Cholesterol potentiates Akt activation in Mvt-1 cells
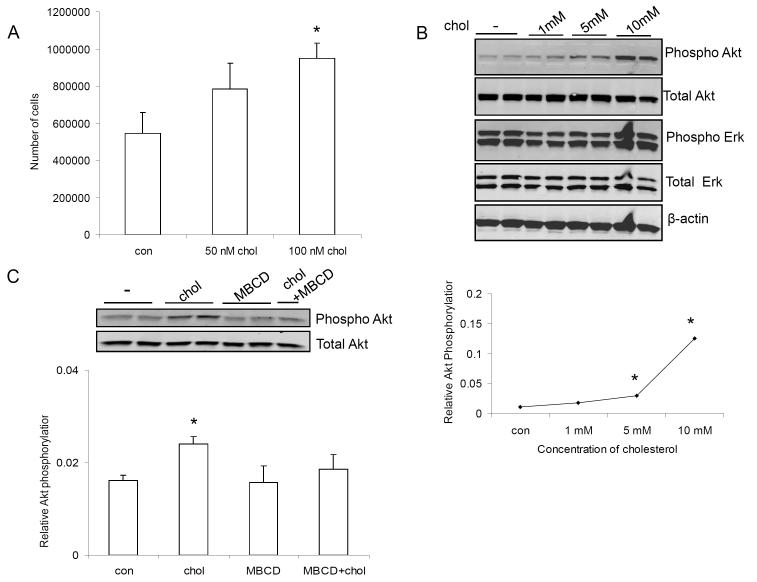
To evaluate the effect of cholesterol on cell proliferation, 10 000 Mvt-1 cells/ml were cultured in 24-well plates. The following day, cells were starved and exposed to 50 nM and 100 nM (equivalent to 2 μg/dl and 4 μg/dl) cholesterol for 72 hours. As shown in figure 4A, 100 nM of cholesterol significantly increased the number of Mvt-1 cells in culture, with 50 nM also showing a trend to increase the proliferation rate.
Figure 4.
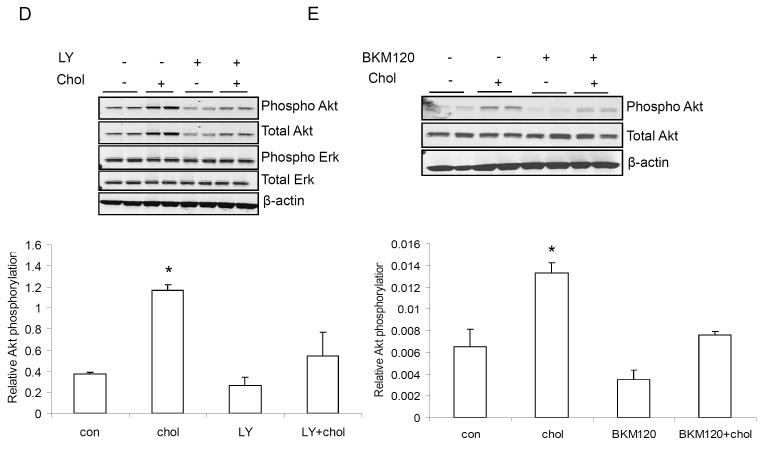
100 nM (equivalent to 4 μg/dl) cholesterol significantly increased the number of Mvt-1 cells in vitro (A). Cholesterol stimulated AktS473 phosphorylation in Mvt-1 cells in a dose dependent manner (1-10 mM equivalent to 40-400 mg/dl) (B). 1mM of cholesterol activated Akt during a shorter time period (20 min and 40 min) (C). MBCD is known to deplete cholesterol from plasma membrane leading to disruption of lipid rafts. The presence of MBCD (5 mM) inhibited cholesterol-induced AktS473 phosphorylation (D). Densitometric analysis of Akt phosphorylation is presented as levels of phosphorylation compared to total Akt protein. Statistically significant differenced is indicated as *, P<0.05, Student’s t test. (*, P<0.05 untreated vs cholesterol treated Mvt-1 cells and/or cholesterol treated vs cholesterol and inhibitor treated Mvt-1 cells). Statistical analyses were determined using the student’s t test where 2 groups were present and ANOVA for comparing more than 2 groups.
In order to investigate the pathway involved in the accelerated mammary tumor growth and metastasis in the hypercholesterolemic ApoE−/− mice, cultured Mvt-1 cells were exposed to stimulation with 1, 5 and 10 mM (equivalent to 40, 200 and 400 mg/dl) cholesterol respectively, for 1 hour and with 1 mM cholesterol for a shorter time frame (20 min and 40 min) (Fig 4C). Mvt-1 cells responded to cholesterol addition with activation of Akt in a dose-dependent manner (Fig 4B). Depleting cholesterol from the plasma membrane using methyl-ß-cyclodextrin (MBCD) prevented cholesterol-induced activation of Akt, indicating that cholesterol-rich lipid rafts might be involved in activating Akt (Fig 4D).
Inhibition of PI3K/Akt pathway suppresses tumor growth in ApoE−/− mice
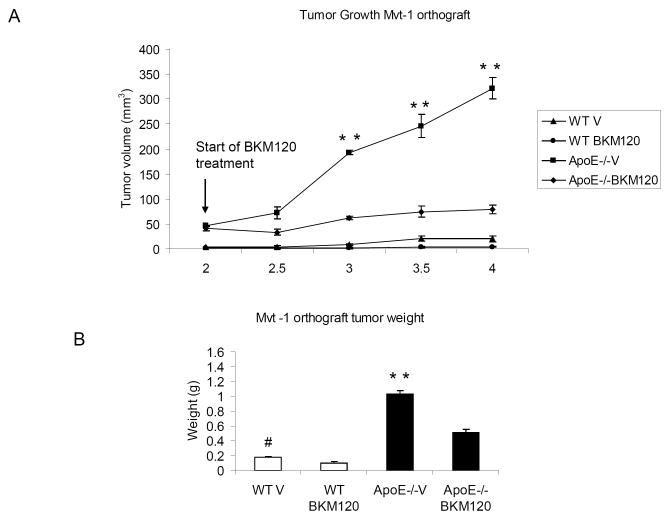
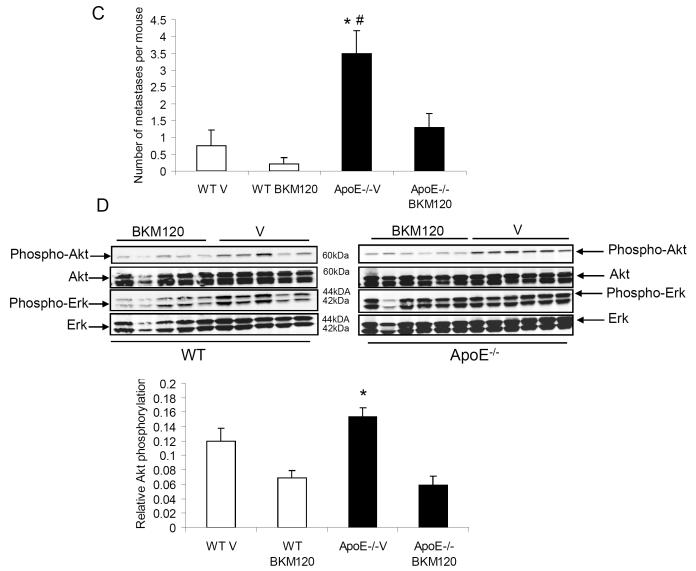
To examine the effect of PI3K/Akt pathway inhibition in vivo, both ApoE−/− and WT mice (fed with HF/HC diet for 10 weeks) were orthotopically injected with Mvt-1 cells into the inguinal mammary fat pad, as described above. Two weeks after cell inoculation, the mice were treated with BKM120 (50mg/kg/d) or vehicle (10% NMP, 90% PEG300), orally by gavage for two weeks. The treatment with BKM120 resulted in a significantly attenuated tumor growth in ApoE−/− mice (Fig 5A and 5B), indicating that the PI3K/Akt pathway is one mediator of the tumor-promoting activity of hypercholesterolemia. Furthermore, PI3K blockade resulted in a significant inhibition of metastasis in BKM120-treated ApoE−/− mice compared to vehicle-treated mice (1.5± 0.5 spontaneous metastases per mouse in BKM120-treated ApoE−/− vs 3.5±1.7 metastases per mouse in vehicle-treated ApoE−/−) (Fig 5C). Additionally, tumor tissue extracted from ApoE−/− mice displayed an increase in AktS473 phosphorylation which was downregulated by BKM120 treatment (Fig 5D).
Figure 5.
Inhibition of the PI3K/Akt pathway by BKM120 significantly reduced growth of Mvt-1 orthograft tumors (A) (**, P<0.001 for ApoE−/− -BKM120-treated vs ApoE−/− vehicle) as well as tumor weight (B) in ApoE−/− mice (**, P<0.001 for ApoE−/− -BKM120-treated vs ApoE−/− vehicle, #, P<0.05 WT-BKM120-treated vs WT vehicle). Two weeks after tumor inoculation (50 000 Mvt-1 cells), the ApoE−/− and WT were treated with the PI3K inhibitor, BKM120 (50mg/kg/d) or vehicle (10% NMP, 90% PEG300) by oral gavage for 13 days. The number of metastases was significantly decreased in BKM120-treated ApoE−/− mice in comparison to vehicle-treated mice (C) (*, P<0.05 for ApoE−/− -BKM120-treated vs ApoE−/− vehicle and #, P<0.05 ApoE−/− vehicle vs WT vehicle) (n=5-7 per group). A reduced level of phosphorylated AktS473 was found in the BKM120-treated ApoE−/−and WT mice in comparison to vehicle-treated controls. Densitometric analysis of Akt phosphorylation is presented as levels of phosphorylation compared to total Akt protein (D) (*, P<0.05 for ApoE−/− -BKM120-treated vs ApoE−/− vehicle). Statistical analyses were determined using the student’s t test where 2 groups were present and ANOVA for comparing more than 2 groups.
Discussion
Here we present that hypercholesterolemia (and/or hypertriglyceridemia) without concomitant hyperglycemia or hyperinsulinemia affects mammary tumor growth and metastasis.
The ApoE glycoprotein functions as a regulator of plasma lipid levels and participates in the uptake of lipids into different tissues by binding to lipoprotein receptors (LDLr) and thereby delivering both cholesterol and triglycerides into cells. The absence of ApoE leads to the accumulation of cholesterol and triglycerides in plasma (27). Therefore, mice lacking the ApoE gene fed high-cholesterol diet represent a well-known model for studying atherosclerosis (27). In addition, deletion of the ApoE gene results in significantly lower levels of blood glucose and insulin, which is most likely the consequence of an impaired lipid uptake by adipose tissue and muscle, leading to an improvement of insulin sensitivity (26). The hypercholesterolemic phenotype found in ApoE−/− mice also makes them a useful tool for investigating the effect of elevated cholesterol levels on different types of malignancies. The effect of up-regulated triglycerides on mammary tumor growth in this model cannot be excluded, since elevated triglyceride levels have been demonstrated in patients with different types of malignancies including breast cancer patients (28-30). Furthermore, triglyceride-rich lipoproteins have been shown to promote proliferation of PC-3 prostate cancer cells, potentially by influencing the regulation of LDLr expression (31). In addition, the role of dietary energy intake and its contribution to tumor growth in our model cannot be excluded since obesity and high total energy intake has been reported to be associated with progression of prostate cancer (24, 32)
We used two orthotopic mammary tumor models; Met-1 (derived from MMTV-PyVmT/FVB/N transgenic mice) and Mvt-1 (derived from c-Myc/vegf tumor explants), in ApoE−/− and WT female mice. When fed regular chow, tumor growth in ApoE−/− and WT mice did not show significant differences (data not shown). However, when fed HF/HC diet, tumors in ApoE−/− mice were more aggressive, evident by increased primary tumor size and metastatic lesions. In concordance with our data, a recent study examined the role of cholesterol in tumor progression using the MMTV-PyMT transgenic mice. In this study, a diet induced elevation in plasma cholesterol levels enhanced mammary tumor development (33). Additionally, in our study we show that the number of pulmonary metastases is increased in the hyperlipidemic ApoE−/− mouse. Likewise, growth of prostate tumor xenografts was significantly increased in SCID mice with elevated levels of circulating cholesterol. This growth was associated with increased cholesterol content in lipid rafts isolated from the tumor xenografts, and was accompanied by activation of membrane-localized Akt (18).
The lung is a susceptible organ to colonization by circulating tumor cells due to its large surface area and rich blood supply (34). Upon examination of WT and ApoE−/− mice harboring metastatic Mvt-1 tumors, we detected significantly elevated numbers of spontaneous pulmonary metastases in the ApoE−/− mice. However, it has been proposed that larger tumors may distribute an enhanced amount of metastasizing cells (34, 35). To investigate whether increased metastases in ApoE−/− mice is independent of primary tumor size, we intravenously injected an identical number of Mvt-1 cells into ApoE−/− and WT mice and quantified metastatic lesions in the lungs after three weeks. We found significantly more macro-metastases in ApoE−/− mice, suggesting that elevated cholesterol levels promoted the proliferation of Mvt-1 cells in the lungs. The ability of cancer cells to metastasize to a greater degree in the hypercholesterolemic environment may be due to the formation of cholesterol-rich cell extensions called invadopodia, which are believed to have a role in migration of cancer cells, essential for invasion and metastasis (36).
To begin investigating the molecular mechanism involved in enhanced tumor growth in the ApoE−/− mice, we studied the response of Mvt-1 cells to cholesterol in vitro. Acute cell response to cholesterol was studied using 40-200 mg/dl cholesterol to reproduce the physiological range of blood cholesterol in normal and hypercholesterolemic states, respectively. Using these concentrations, we demonstrated that cholesterol enhanced Akt activation in Mvt-1 cells. These results are in agreement with a previous study of human epidermoid carcinoma cells treated with 40 mg/dl cholesterol leading to activation of Akt (14). Furthermore, cholesterol depletion in the cell membrane in the presence of MBCD abrogated Akt phosphorylation induced by exogenously added cholesterol. Statistical analysis was determined using ANOVA. Similar to our observation in vivo, we also found that cholesterol increased cellular proliferation of Mvt-1 cells in vitro. We found that the concentration of cholesterol required to activate the Akt pathway was much higher than that required to induce cellular proliferation. The reason for this difference is as yet undefined, but one possibility is that to mediate the aggregation of a significant amount of lipid raft-mediated membrane receptor signaling complexes over a short time period (20-60 min) much higher levels of cholesterol are required than to induce cell proliferation over an extended time period of 72 hours. We also found the same effect in MC-38 colon cancer cell line (data not shown).
The PI3K/Akt pathway plays an important role in many cancers, contributing to apoptosis, cell differentiation and cell proliferation (37). Blocking the PI3K/Akt pathway in vivo by treating ApoE−/− mice with the BKM120 compound significantly alleviated the accelerated tumor growth and metastases to the lung in comparison to vehicle treated mice; however we did not observe a complete cessation of tumor growth in the BKM120-treated mice. Therefore, these data suggest that additional signaling pathways may be involved in dyslipidemia-induced tumor development and progression. We also see an effect of BKM120 in WT mice, likely due to inhibition of the PI3/Akt pathway and its effect on cell proliferation.
In conclusion, we demonstrate that dyslipidemia markedly accelerates primary mammary tumor growth and metastasis. Increased cholesterol levels are associated with enhanced phosphorylation of Akt and mammary cancer cell growth in vitro. Furthermore, the mammary tumor-promoting effect of dyslipidemia in ApoE−/− mice is abrogated by BKM120, a specific inhibitor of the catalytic subunit of PI3K, the upstream activator of Akt. To our knowledge, the PI3K/Akt pathway has only been shown to be cholesterol-sensitive in prostate cancer. Our investigations have indicated one potential mechanism whereby cholesterol can promote tumor growth and metastasis in breast cancer. Our data link dyslipidemia and the PI3K/Akt pathway in mammary tumor growth mechanistically and thus suggest that reducing total cholesterol levels may be an important therapeutic modality in the prevention and treatment of breast cancer.
Material and methods
Animals
All the mice used in this study were on an FVB/N background and were maintained in an animal facility at the Mount Sinai School of Medicine, with a 12 hour light/dark cycle and free access to water. The study was approved by the Animal Care and Use Committee of Mount Sinai School of Medicine. Both ApoE−/− (kind gift of Jan Breslow, Rockefeller, NY) (38) and FVB/N control mice (WT) were fed a high-fat/high-cholesterol (HF/HC) diet containing 1.25% cholesterol and 20% fat for 10 weeks (D12108: Research Diets, New Brunswick, NJ, USA) starting from 6 weeks of age.
The body weight was monitored weekly and whole-body fat and lean mass assessed using the quantitative magnetic resonance method (Echo, MRI 3-in-1, Echo Medical Systems, Houston, TX, USA). Total cholesterol levels were measured using ELISA (BioVision, Mountain View CA, USA). Triglyceride levels were determined using Triglyceride (GPO Liquid) Reagent Set (Pointe Scientific, INC, Canton, MI). Blood glucose was determined using a glucose monitoring system (Bayer HealthCare LLC, Mishawaka, IN, USA).
Orthotopic tumor models
Two mammary cancer cell lines, Met-1 (500 000 cells), derived from MMTV-PyVmT/FVB/N transgenic mice (39), and the metastatic Mvt-1 cells (50 000 cells), derived from c-Myc/vegf tumor explants (40), were inoculated (in 100 μl sterile PBS) into the fourth mammary fat pads of ApoE−/− and WT mice after 10 weeks on the HF/HC diet. Tumor volume was measured twice a week using calipers. The volume of tumors was determined using the following formula corresponding to the three dimensional volume of a spheroid: 4/3 × π × (lateral width/2) × (cranio-caudal length/2) × (antero-posterior depth/2), until tumors reached 1.5 cm in one dimension. At dissection, the lungs were inflated and removed for determination of macro-metastatic lesions. Lungs were then fixed in 4% paraformaldehyde, embedded in paraffin, sectioned and stained using H&E staining.
In vivo metastases were achieved by Intravenous cell injection of 12 000 Mvt-1 cells in 100 μl sterile PBS.
The effect of PI3K/Akt pathway inhibition on tumor growth and progression in vivo was examined by injecting both ApoE−/− and WT mice (fed with HF/HC diet for 10 weeks) with Mvt-1 cells into the inguinal mammary fat pad, as described above. Two weeks after cell inoculation, the mice were treated with BKM120 (Novartis, Basel, Switzerland) (50mg/kg/d) or vehicle (10% NMP, 90% PEG300), orally by gavage for two weeks.
The Met-1 cells were donated by S.D Hurstings (Department of Nutritional Science, University of Texas, Austin, TX and Department of Carcinogenesis, University of Texas M. D. Anderson Cancer Center, Smithville, TX) and N.P. Nunez (Department of Nutritional Science, University of Texas, Austin, TX). The Mvt-1 cells were donated by Ken Hunter from (Laboratory of Cancer Biology and Genetics, National Cancer Institute).
Cell culture
Mvt-1 cells were cultured in DMEM complete (Cellgro, Manassas, VA) with 10% fetal bovine serum (Invitrogen/GIBCO, Carlsband, CA) and 1% penicillin-streptomycin (Cellgro, Manassas, VA). Cells were grown to approximately 70% confluence followed by starvation (in serum free medium) for 4h prior to treatment. The starved cells were incubated with water soluble cholesterol (1 mM, 5 mM and 10 mM equivalent to 40 mg/dl, 200 mg/dl and 400 mg/dl) (Sigma-Aldrich, Saint Louis, USA) for 1 hour. To investigate the effect of cholesterol depletion from the plasma membrane, the starved cells were treated with 5 mM of methyl-ß-cyclodextrin (MBCD) (Sigma-Aldrich, Saint Louis, USA), a cholesterol depleting agent, in the presence and absence of cholesterol.
Isolation of proteins and Western blot analysis
Tumor tissue and treated cells were lysed in protein extraction buffer (pH 7.4) containing 50 mM Tris, 150 mM NaCl, 1 mM EDTA, 1.25% CHAPS (Roche, Indianapolis, IN), 1 mM sodium orthovanadate, 2 mM sodium fluoride, 10 mM sodium pyrophosphate (Sigma-Aldrich, Saint Louis, USA), 8 mM beta-glycerophosphate (VWR, West Chester, PA) and Complete Protease Cocktail (Roche). 20 μg of the protein content were analyzed on SDS-PAGE (8% Tris-Glycine gel) (Invitrogen, CA) followed by transfer to nitrocellulose membrane (Bio-Rad, CA). The membrane was thereafter blocked with 5% milk followed by probing with primary and secondary antibodies. The membrane was analyzed by a direct infrared Imaging System (Li-cor Bio-science, Lincoln, NE). The primary antibodies used in the study were: Anti-Akt, phospho-Akt (Ser 473), anti-p44/42MAP kinase and anti-phospho p44/42MAP kinase (Thr202/Tyr204) (Cell Signaling, Boston, MA) and beta-actin (Sigma-Aldrich, Saint Louis, USA).
Cell proliferation assay
10 000 Mvt-1 cells/ml were plated in 24-well plates for 24 hours followed by incubation in serum-free medium for 2 hours before addition of various concentration of cholesterol (50 nM and 100 nM equivalent to 2 μg/dl and 4 μg/dl). Cells were incubated with cholesterol for 72 hours, after which the cells were trypsinised, diluted in PBS-trypan blue in at a 1:1 ratio and the live cells counted.
Densitometric and statistical analysis
Densitometric analyses of the western blots were carried out using MacBAS V2.52 software. Results are expressed as mean +/− SEM. Statistical analyses were determined using the student’s t-test where 2 groups were present and ANOVA for comparing more than 2 groups. P<0.05 was used to define statistical significance.
Acknowledgments
This work was supported by ADA mentor based postdoctoral fellowship award 7-08-MN-33 and National Cancer Institute grant RO1CA128799, both to DLR. We thank Novartis for providing us with BKM120 compound. In addition, partial support was from the Israel Cancer Association to DLR.
Financial support: This work was supported by ADA mentor based postdoctoral fellowship award 7-08-MN-33, National Cancer Institute grant RO1CA128799 and Israeli Cancer Association
Footnotes
Authors’ Disclosure of Potential Conflicts of Interest: The authors declare that there are no conflicts of interest regarding this work.
References
- 1.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002 Sep;3(9):565–74. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 2.Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. Am J Clin Nutr. 2007 Sep;86(3):s823–35. doi: 10.1093/ajcn/86.3.823S. [DOI] [PubMed] [Google Scholar]
- 3.Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008 Dec 17;300(23):2754–64. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallagher EJ, LeRoith D. Insulin, insulin resistance, obesity, and cancer. Curr Diab Rep. 2010 Apr;10(2):93–100. doi: 10.1007/s11892-010-0101-y. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher EJ, Leroith D. Minireview: IGF, Insulin, and Cancer. Endocrinology. 2011 May 3; doi: 10.1210/en.2011-0231. [DOI] [PubMed] [Google Scholar]
- 6.Novosyadlyy R, Lann DE, Vijayakumar A, Rowzee A, Lazzarino DA, Fierz Y, et al. Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res. 2010 Jan 15;70(2):741–51. doi: 10.1158/0008-5472.CAN-09-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fierz Y, Novosyadlyy R, Vijayakumar A, Yakar S, LeRoith D. Insulin-sensitizing therapy attenuates type 2 diabetes-mediated mammary tumor progression. Diabetes. 2010 Mar;59(3):686–93. doi: 10.2337/db09-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Stefani E, Mendilaharsu M, Deneo-Pellegrini H, Ronco A. Influence of dietary levels of fat, cholesterol, and calcium on colorectal cancer. Nutr Cancer. 1997;29(1):83–9. doi: 10.1080/01635589709514606. [DOI] [PubMed] [Google Scholar]
- 9.Platz EA, Clinton SK, Giovannucci E. Association between plasma cholesterol and prostate cancer in the PSA era. Int J Cancer. Oct 1;123(7):1693–8. doi: 10.1002/ijc.23715. 20 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zielinski CC, Stuller I, Rausch P, Muller C. Increased serum concentrations of cholesterol and triglycerides in the progression of breast cancer. J Cancer Res Clin Oncol. 1988;114(5):514–8. doi: 10.1007/BF00391503. [DOI] [PubMed] [Google Scholar]
- 11.Chae YK, Valsecchi ME, Kim J, Bianchi AL, Khemasuwan D, Desai A, et al. Reduced risk of breast cancer recurrence in patients using ACE inhibitors, ARBs, and/or statins. Cancer Invest. 2011 Nov;29(9):585–93. doi: 10.3109/07357907.2011.616252. [DOI] [PubMed] [Google Scholar]
- 12.Edidin M. Lipids on the frontier: a century of cell-membrane bilayers. Nat Rev Mol Cell Biol. 2003 May;4(5):414–8. doi: 10.1038/nrm1102. [DOI] [PubMed] [Google Scholar]
- 13.Patra SK. Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim Biophys Acta. 2008 Apr;1785(2):182–206. doi: 10.1016/j.bbcan.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Li YC, Park MJ, Ye SK, Kim CW, Kim YN. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol. 2006 Apr;168(4):1107–18. doi: 10.2353/ajpath.2006.050959. quiz 404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman MR, Solomon KR. Cholesterol and prostate cancer. J Cell Biochem. 2004 Jan 1;91(1):54–69. doi: 10.1002/jcb.10724. [DOI] [PubMed] [Google Scholar]
- 16.Graziani SR, Igreja FA, Hegg R, Meneghetti C, Brandizzi LI, Barboza R, et al. Uptake of a cholesterol-rich emulsion by breast cancer. Gynecol Oncol. 2002 Jun;85(3):493–7. doi: 10.1006/gyno.2002.6654. [DOI] [PubMed] [Google Scholar]
- 17.Freeman MR, Di Vizio D, Solomon KR. The Rafts of the Medusa: cholesterol targeting in cancer therapy. Oncogene. 2010 Jul 1;29(26):3745–7. doi: 10.1038/onc.2010.132. [DOI] [PubMed] [Google Scholar]
- 18.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005 Apr;115(4):959–68. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002 Apr 15;62(8):2227–31. [PubMed] [Google Scholar]
- 20.Adam RM, Mukhopadhyay NK, Kim J, Di Vizio D, Cinar B, Boucher K, et al. Cholesterol sensitivity of endogenous and myristoylated Akt. Cancer Res. 2007 Jul 1;67(13):6238–46. doi: 10.1158/0008-5472.CAN-07-0288. [DOI] [PubMed] [Google Scholar]
- 21.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005 Dec;5(12):930–42. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 22.Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, et al. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006 Dec 20;98(24):1819–25. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 23.Mondul AM, Caffo B, Platz EA. Minimal detection bias in the inverse association between statin drug use and advanced prostate cancer risk: a simulation study. Cancer Epidemiol. 2011 Aug;35(4):e6–11. doi: 10.1016/j.canep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007 Oct 1;121(7):1571–8. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cauley JA, Zmuda JM, Lui LY, Hillier TA, Ness RB, Stone KL, et al. Lipid-lowering drug use and breast cancer in older women: a prospective study. J Womens Health (Larchmt) 2003 Oct;12(8):749–56. doi: 10.1089/154099903322447710. [DOI] [PubMed] [Google Scholar]
- 26.Kawashima Y, Chen J, Sun H, Lann D, Hajjar RJ, Yakar S, et al. Apolipoprotein E deficiency abrogates insulin resistance in a mouse model of type 2 diabetes mellitus. Diabetologia. 2009 Jul;52(7):1434–41. doi: 10.1007/s00125-009-1378-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992 Oct 16;258(5081):468–71. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 28.Alexopoulos CG, Blatsios B, Avgerinos A. Serum lipids and lipoprotein disorders in cancer patients. Cancer. 1987 Dec 15;60(12):3065–70. doi: 10.1002/1097-0142(19871215)60:12<3065::aid-cncr2820601234>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 29.Favrot MC, Dellamonica C, Souillet G. Study of blood lipids in 30 children with a malignant hematological disease or carcinoma. Biomed Pharmacother. 1984;38(1):55–9. [PubMed] [Google Scholar]
- 30.Halton JM, Nazir DJ, McQueen MJ, Barr RD. Blood lipid profiles in children with acute lymphoblastic leukemia. Cancer. 1998 Jul 15;83(2):379–84. [PubMed] [Google Scholar]
- 31.Sekine Y, Koike H, Nakano T, Nakajima K, Takahashi S, Suzuki K. Remnant lipoproteins induced proliferation of human prostate cancer cell, PC-3 but not LNCaP, via low density lipoprotein receptor. Cancer Epidemiol. 2009 Jul;33(1):16–23. doi: 10.1016/j.canep.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Mandal CC, Ghosh-Choudhury N, Yoneda T, Choudhury GG. Simvastatin prevents skeletal metastasis of breast cancer by an antagonistic interplay between p53 and CD44. J Biol Chem. 2011 Apr 1;286(13):11314–27. doi: 10.1074/jbc.M110.193714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu J, La Vecchia C, de Groh M, Negri E, Morrison H, Mery L. Dietary cholesterol intake and cancer. Ann Oncol. 2011 May 4; doi: 10.1093/annonc/mdr155. [DOI] [PubMed] [Google Scholar]
- 34.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003 Jun;3(6):453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 35.Minn AJ, Gupta GP, Padua D, Bos P, Nguyen DX, Nuyten D, et al. Lung metastasis genes couple breast tumor size and metastatic spread. Proc Natl Acad Sci U S A. 2007 Apr 17;104(16):6740–5. doi: 10.1073/pnas.0701138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caldieri G, Buccione R. Aiming for invadopodia: organizing polarized delivery at sites of invasion. Trends Cell Biol. 2010 Feb;20(2):64–70. doi: 10.1016/j.tcb.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006 Mar;6(3):184–92. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 38.Dansky HM, Charlton SA, Sikes JL, Heath SC, Simantov R, Levin LF, et al. Genetic background determines the extent of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 1999 Aug;19(8):1960–8. doi: 10.1161/01.atv.19.8.1960. [DOI] [PubMed] [Google Scholar]
- 39.Borowsky AD, Namba R, Young LJ, Hunter KW, Hodgson JG, Tepper CG, et al. Syngeneic mouse mammary carcinoma cell lines: two closely related cell lines with divergent metastatic behavior. Clin Exp Metastasis. 2005;22(1):47–59. doi: 10.1007/s10585-005-2908-5. [DOI] [PubMed] [Google Scholar]
- 40.Pei XF, Noble MS, Davoli MA, Rosfjord E, Tilli MT, Furth PA, et al. Explant-cell culture of primary mammary tumors from MMTV-c-Myc transgenic mice. In Vitro Cell Dev Biol Anim. 2004 Jan-Feb;40(1-2):14–21. doi: 10.1290/1543-706X(2004)40<14:ECOPMT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]