Abstract
Introduction
Glutathione is a major endogenous antioxidant and its deficiency is implicated in the etiology and progression of a number of human diseases. Vitamin D is important for the prevention of osteoporosis, cardiovascular disease, diabetes, autoimmune diseases, and some cancers. Using a monocyte cell model, this study examined the hypothesis that vitamin D upregulate glutamate cysteine ligase (GCLC) and glutathione reductase (GR), which catalyzes GSH biosynthesis.
Methods
U937 monocytes were pretreated with and without 1,25 (OH) vitamin D (10-25 nM) for 24 hr and then exposed to control and high glucose (HG, 25 mM) for 4 hr. Levels of GSH determined using HPLC; GR activity by oxidation of NADPH; GCLC protein, MCP-1 and IL-8 using ELISA kits.
Results
1,25(OH)2 vitamin D supplementation significantly upregulated expression of GCLC and GR, levels of GCLC protein and GR activity, and formation of GSH in control and HG-treated monocytes. 1,25(OH)2 vitamin D caused significantly (p<0.05) lower secretion of IL-8 and MCP-1, and lower ROS levels in monocytes exposed to control and HG-treated monocytes.
Conclusions
This study demonstrates a positive link between vitamin D and GSH levels, and that some beneficial effects of vitamin D supplementation may be mediated by an improvement in the cellular GSH levels and a decrease in ROS and pro-inflammatory cytokines.
Keywords: Vitamin D, GSH, glutamate cysteine ligase, glutathione reductase, diabetes
Introduction
Glutathione (GSH) plays an important role in a multitude of cellular processes and its deficiency is implicated in the etiology and progression of a number of human diseases including cardiovascular, immune, diseases of aging, and diabetes (1-7). GSH is a cofactor of many enzymes that are involved in the detoxification of oxygen radicals and the detoxification of drugs. GSH deficiency is implicated in the progression of chronic diseases, including insulin resistance and diabetes 1, 2, 5, 6). Recently, epidemiological studies have demonstrated an association between vitamin D deficiency and the outcome of several chronic diseases, including diabetes (8-11). However, evaluation of both the basic research and clinical evidence related to the role of vitamin D supplementation in the prevention and treatment of chronic non-skeletal diseases remains to be done. The blood levels of GSH are lower in diabetes (5, 12, 13). This study demonstrates that vitamin D can upregulate GCLC and GR, and that GSH can form in cultured monocytes. In addition, this study reports that the effect of vitamin D on GSH formation was accompanied by inhibition of ROS, and IL-8 and MCP-1 secretion in monocytes treated with control and high glucose. levels. This study suggests that upregulation of cellular GSH by vitamin D provides evidence for a novel mechanism by which vitamin D supplementation may reduce oxidative stress and thereby provide lower vascular inflammation and associated complications in diabetes.
MATERIALS AND METHODS
HUMAN PRO-MONOCYTIC CELL LINE
The U937 monocyte cell line was obtained from American Type Culture Collection (ATCC, Manassas, VA). These cells were maintained at 37°C in RPMI 1640 medium containing 7 mM glucose, 10% (v/v) heat-inactivated FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 12 mM sodium carbonate, 12 mM HEPES and 2 mM glutamine in a humidified atmosphere containing 5% (v/v) CO2. For treatments, cells were washed once in plain RPMI 1640 before being suspended in fresh medium (complete) containing serum and other supplements (14, 15).
TREATMENT WITH HIGH GLUCOSE (HG) AND VITAMIN D
Cells (106/ml) were pretreated with three different concentrations of 1,25 (OH)2 vitamin D (0, 10, 25 nM) for 24 hours and followed by HG (25 mM) exposure for the next 4 h. 1,25 (OH)2 vitamin D is an active form of vitamin D. In this study control cells were exposed with media having 7 mM glucose. In the body, glucose is continuously degraded and formed to maintain a 5 mM blood glucose level. However, in cell culture studies, we observed that incubating cells with media having a 5 mM glucose concentration for 24 h caused a decrease in glucose concentration to levels lower than 2 mM. In cell culture studies, glucose gets metabolized but not replaced. For this reason, our experience shows that a 7 mM glucose concentration does not lead to a glucose deficiency at 24 h incubation. In high glucose studies, cells were exposed to a high glucose concentration of 25 mM. It is true that blood glucose levels in patients are not likely to stay as high as 25 mM for 24 h. However, tissue damage in diabetic patients occurs over many years of countless hyperglycemic episodes. Many previous studies have reported that glucose concentrations as high as 50 mM have been found in the blood of patients with uncontrolled diabetes. Thus, the glucose concentration of 25 mM used in this cell culture study does not seem unreasonable. In some experiments, cells were instead exposed to 18 mM mannitol (control) since the media contains 7 mM glucose. After treatment, cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris pH 8, 150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS) supplemented with protease and phosphatase inhibitors (1 mM PMSF, 5 μg/mL leupeptin, 2 μg/mL aprotinin, 1 mM EDTA, 10 mM NaF, and 1 mM NaVO4). Lysates were cleared by centrifugation and total protein concentrations were determined using BCA assay (Pierce/Thermo Scientific, Rockford, IL).
GSH, GR ACTIVITY AND GCLC PROTEIN ASSAYS
GSH was determined by HPLC method (16). Whole cell suspension was processed as described before (16). GSH concentration is expressed per volume of cell suspension. Glutathione reductase (GR) together with its co-factor, NADPH, catalyzes the reduction of oxidized glutathione (GSSG) to glutathione (GSH). The oxidation of NADPH to NADP is monitored as a decrease in absorbance at 340nm. The rate of decrease in (Δ A340) is directly proportional to the glutathione reductase activity in the sample because the enzyme is present in rate limiting concentrations. GR activity was determined using protocol described by Beutler (17). The GR activity was expressed as a rate of decrease in absorbance @ 340 nm per minute due to the oxidation of NADPH by GR, and was normalized per mg protein. GCLC total protein level in the cell lysate was determined by ELISA kit (Catalog #MBS 704124, MyBiosource, San Diego, CA). All appropriate controls and standards as specified by manufacturer's kit were used each time. Level of GCLC protein was expressed as total protein.
CELL VIABILITY, ROS AND CYTOKINE AND IMMUNOBLOTTING STUDIES
Cell viability was determined using the Alamar Blue reduction bioassay (Alamar Biosciences, Sacramento, CA). This method is based upon Alamar Blue dye reduction by live cells. Intracellular reactive oxygen species (ROS) levels were measured in treated cells using the fluorescent dye, H2DCFDA (2’, 7’dichlorofluorescein diacetate)(17). After treatment, cells were washed once with PBS and then loaded with 5 μM H2DCFDA in PBS with 4% FBS. The cells were incubated at 37°C for 30 min in the dark and subsequently washed with PBS, harvested in PBS with 0.5% Triton X-100, centrifuged at 12000×g for 10 min at 37°C, and the supernatant collected. The intensity of DCF fluorescence in the supernatant was read at excitation and emission wavelengths of 488 and 530 nm, respectively, using a multidetection microplate reader (Synergy HT, BIOTEK). The change in intracellular ROS level was plotted as mean fluorescence intensity (MFI). The oxidative stress sensitive dye DCFH-DA diffuses passively through the cellular membrane. Intracellular esterase activity causes the formation of DCFH, a nonfluorescent compound, which emits fluorescence when it is oxidized to DCF (18). Although ROS measurement by DCFH-DA is nonspecific, this dye has been widely used to measure the formation of overall intracellular reactive intermediates. All appropriate controls and standards as specified by each manufacturer's kit were used for IL-8 and MCP-1 assay using ELISA kits (R and D Systems, Minneapolis, MN). In the cytokine assay, control samples were analyzed each time to check the variation from plate to plate on different days of analysis. Details of immunoblotting are similar to as given in our previous publications (15, 32). The antibodies for GCLC (73KD), GCLM (31 KD) and GR (58 KD) were purchased from Abcam (Cambridge, MA). The intensity of each immunoblotting band was measured using the histogram tool of Adobe Photoshop CS5.
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise mentioned. Data were analyzed using ANOVA statistically with Sigma Stat. A p value of less than 0.05 for a statistical test was considered significant.
RESULTS
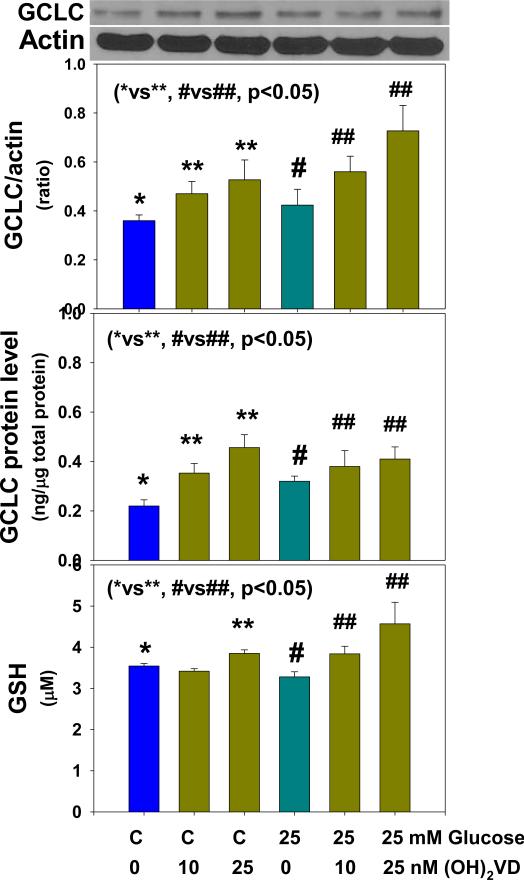
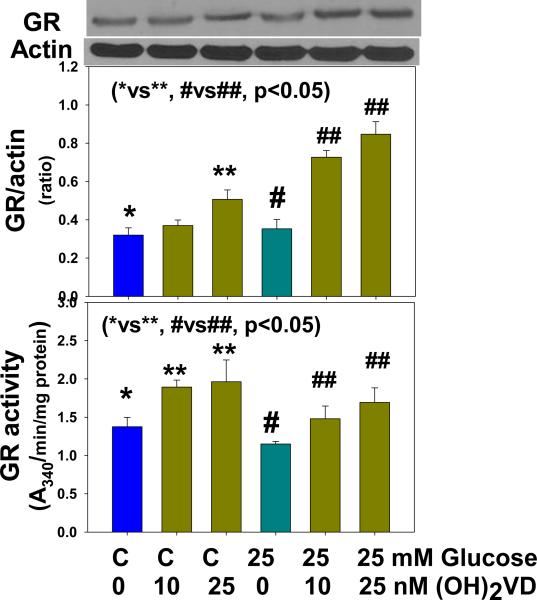
Figure 1 illustrates the effect of 1,25(OH)2 vitamin D supplementation on the GCLC expression and GCLC protein levels and GSH formation in monocytes treated with control and high glucose. 1,25(OH)2 vitamin D supplementation significantly upregulated GCLC expression in monocytes exposed to control and high glucose. The effect of vitamin D on upregulation of GCLC was also seen when GCLC protein level determined in monocytes exposed to control and high glucose levels. Similarly, there was significantly more GSH formation in vitamin D supplemented monocytes. 1,25(OH)2 vitamin D supplementation had no effect on GCLM expression (data not given here). Figure 2 shows that vitamin D supplementation upregulated GR expression and GR activity in both control and HG-treated monocytes. The differences between control and high-glucose treated cells were not significant for changes in GCLC, GR or GSH levels. GCLC catalyzes first step in the GSH biosynthesis from L-cysteine and glutamate. Whereas, GR catalyzes the recycling of GSSG to GSH. These results demonstrate that vitamin D supplementation can increase cellular GSH by upregulating GCLC and GR.
Figure 1.
Effect of 1,25 (OH)2 vitamin D supplementation on up-regulation of glutamate-cysteine ligase (GCLC) expression, GCLC protein and GSH levels in U937 monocytes cultured without and with high glucose. Values are Mean ±SE (n=4).
Figure 2.
Effect of 1,25 (OH)2 vitamin D supplementation on expression of glutathione reductase (GR) and GR activity levels in U937 monocytes cultured without and with high glucose. Values are Mean ±SE (n=3).
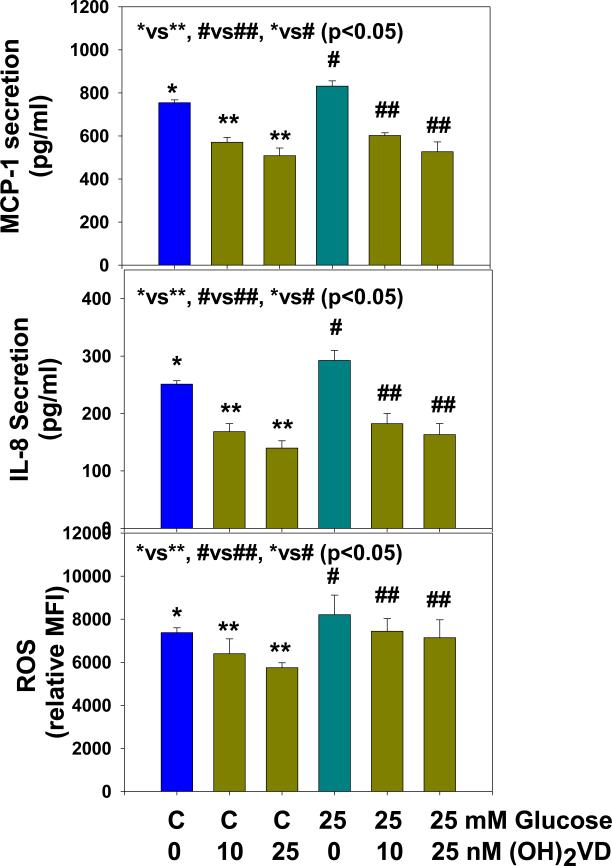
Figure 3 shows that 1,25(OH)2 vitamin D supplementation significantly inhibited ROS and secretion of the pro-inflammatory cytokines MCP-1 and IL-8 in control and HG-treated U937 monocytes. Mannitol treatment has no effect on GCLC, GR, GSH, ROS or cytokine secretion (data not given here). There was no change in cell viability in any of the treatments.
Figure 3.
Effect of 1,25 (OH)2 vitamin D supplementation on inhibition of ROS, IL-8 and MCP-1 secretion in high glucose treated U937 monocytes. Values are Mean ±SE (n=4).
DISCUSSION
Mounting evidence suggests that higher rates of vitamin D (VD) deficiency could be linked to several chronic diseases, including diabetes (8-11). However, evaluation of both the basic research and clinical evidence related to the role of vitamin D supplementation in the prevention and treatment of chronic non-skeletal diseases, such as diabetes, remains to be determined. GSH is formed from L-cysteine by the enzymatic action of glutamate cysteine ligase (19-21). Glutamate cysteine ligase consist of two subunits, glycine-cysteine ligase catalytic subunit (GCLC) and another modulatory subunit (GCLM). GSH is a potent physiological antioxidant, and needed for its ability to scavenge reactive oxygen species in the body (1,19). Current studies in the literature indicate that impaired GSH may contribute to the pathogenesis of disease (1,19). Hyperglycemia and diabetes is associated with increased oxidative stress and blood levels of GSH are lower in diabetic patients (5, 22-24). A number of studies have shown that supplementation with L-cysteine, a precursor of GSH, can lower oxidative stress, insulin resistance, and markers of vascular inflammation (15, 25-27). Other studies have reported that intravenous GSH infusion significantly increased the intraerythrocytic GSH/GSSH ratio and total glucose uptake in diabetic patients (27). The insulin releasing capacity of tolbutamide drugs was potentiated by GSH in isolated perfused pancreas studies (28). Similarly, co-adminstration of GSH inhibited the insulin resistance in skeletal muscle caused by lipid infusion in mice (29) and in Wistar rats (30). These studies and others indicate that an increase in GSH levels can lower insulin resistance and has a positive effect on glucose metabolism.
The present study demonstrates that 1,25(OH)2 vitamin D can upregulate GCLC and GR and increases cellular GSH formation in cultured monocytes. The upregulation in GCLC and GR, and increase in GSH formation was seen both in high glucose treated as well as in control glucose treated cells. High concentration of actin may compromise precise quantitation of actin and the data on expression of GCLC and GR. Higher amount of total protein loading during western blotting studies gives good band for GCLC and GR but brighter band for actin. Because the same amount of total protein was loaded for each treatment and that similar results were obtained after repetition, we believe that the reported results are relative but reasonable. In addition, conclusion obtained with western blotting studies was also validated using other approaches. GCLC protein level and GR activity were also determined in treated cells. The additional approach also suggests that vitamin D up regulate the GCLC and GR. The third approach that actual GSH levels (determined using HPLC) are increased in 1,25(OH)2 vitamin D-treated cells further support that 1,25(OH)2 vitamin D upregulated GSH formation in monocytes.
GCLC catalyzes first step in the GSH biosynthesis from L-cysteine and glutamate. Whereas, GR catalyzes the recycling of GSSG to GSH. Our study also suggest that an increase in GSH formation may be mediated by the effect of vitamin D on upregulation of GCLC as well as GR, which recycle oxidized GSSG to GSH. GSH is a water phase sulfhydryl antioxidant Accumulation of GSH may scavenge reactive oxygen species and that explain a decreased in cellular ROS in vitamin D supplemented cells observed in the present study. A decrease in oxidative stress can in turn result in the inhibition of pro-inflammatory cytokines MCP-1 and IL-8 secretion by the monocytes (24, 31).
Many cross-sectional studies in healthy and diabetic subjects support the hypothesis of a link between low vitamin D status, hyperglycemia, insulin resistance and the impaired β-cell function (8-11, 32-34). Recent studies support that vitamin D supplementation can lower hyperglycemia in diabetic patients (9-11). Many studies report that VD has important health benefits through paracrine and autocrine mechanisms and that higher blood 25-OH-VD levels are associated with better health outcomes (8-11); however, a scientific explanation for the beneficial role of vitamin D supplementation and its validation has not been found. This study reports a novel link between vitamin D supplementation and improvement in cellular GSH levels in cell culture studies. Various studies report lower levels of GSH and vitamin D in the blood of African-Americans in comparison to European-Americans (13, 34-37). There is no previous study in the literature that has examined the potential role/benefit of vitamin D supplementation in boosting cellular GSH levels. Thus, a clinical trial examining the ability of vitamin D supplementation to boost circulating GSH levels and lower inflammatory markers is warranted to validate this finding in a patient population. If such trial leads to tangible clinical results, it would provide the basic mechanism that explains the beneficial role of vitamin D in reducing the adverse effect of diabetes.
Highlights.
Glutathione is a major endogenous antioxidant.
Vitamin D is important for the prevention of metabolic diseases.
This study demonstrates that vitamin D upregulate GSH biosynthesis.
Vitamin D upregulate glutamate cysteine ligase and glutathione reductase.
Beneficial effects of vitamin D may be mediated by increase in the cellular GSH.
Acknowledgements
The authors are supported by grants from NIDDK and the ODS (RO1 DK072433) and the Malcolm Feist Chair in Diabetes. The authors thank Ms Morgan for excellent editing.
Abbreviations
- GSH
glutathione
- ROS
reactive oxygen species
- GCLC
glutamate cysteine ligase catalytic unit
- GCLM
glutamate cysteine ligase modulatory unit
- GR
glutathione reductase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors declare no conflict of interest.
REFERENCES
- 1.Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomedicine Pharmacotherapy. 2003;57:145–155. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qin L, Bartlett H, Griffiths HR, et al. Macular pigment optical density is related to blood glutathione levels in healthy individuals. Investigative Ophthalmology and Visual Sci. 2011;52:5029–5033. doi: 10.1167/iovs.11-7240. [DOI] [PubMed] [Google Scholar]
- 3.Zampagni M, Wright D, Cascella R, et al. Novel S-acyl glutathione derivatives prevent amyloid oxidative stress and cholinergic dysfunction in Alzheimer disease models. Free Rad Biol Med. 2012;52:1362–1371. doi: 10.1016/j.freeradbiomed.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Dröge W. Oxidative stress and ageing: is ageing a cysteine deficiency syndrome? Philos Trans R Soc Lond B Biol Sci. 2005;360:2355–2372. doi: 10.1098/rstb.2005.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sekhar RV, McKay SV, Patel SG, et al. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care. 2011;34:162–167. doi: 10.2337/dc10-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris D, Khurasany M, Nguyen T, Kim J, Guilford F, Mehta R, Gray D, Saviola B, Venketaraman V. Glutathione and infection. Biochim Biophys Acta. 2013;1830:3329–49. doi: 10.1016/j.bbagen.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Darmaun D, Smith SD, Sweeten S, Hartman BK, Welch C, Mauras N. Poorly controlled type 1 diabetes is associated with altered glutathione homeostasis in adolescents: apparent resistance to N-acetylcysteine supplementation. Pediatr Diabetes. 2008;9:577–582. doi: 10.1111/j.1399-5448.2008.00436.x. [DOI] [PubMed] [Google Scholar]
- 8.Lamendola CA, Ariel D, Feldman D, Reaven GM. Relation between obesity, insulin resistance and 25 hydroxyvitamin D. Amer J Clin Nutr. 2012;95:1055–9. doi: 10.3945/ajcn.111.032060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM. Vitamin D and cardiometabolic outcomes: A systematic review. Ann Intern Med. 2010;152(5):307–314. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, Murad MH, Kovacs CS. The nonskeletal effects of vitamin D: an endocrine society scientific statement. Endocrine Reviews. 2012;33(3):456–492. doi: 10.1210/er.2012-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabadi SM, Lee BK, Liu L. Joint effects of obesity and vitamin D insufficiency on insulin resistance and type 2 diabetes: results from the NHANES 2001-2006. Diabetes Care. 2012;35:2048–54. doi: 10.2337/dc12-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain SK, McVie R. Hyperketonemia can increase lipid peroxidation and lower glutathione levels in human erythrocytes in vitro and in Type-I diabetic patients. Diabetes. 1999;48:1850–55. doi: 10.2337/diabetes.48.9.1850. [DOI] [PubMed] [Google Scholar]
- 13.Jain SK, McVie R. Effect of glycemic control, race (white versus black), and duration of diabetes on reduced glutathione content in erythrocytes of diabetic patients. Metabolism. 1994;43:306–309. doi: 10.1016/0026-0495(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 14.Jain SK, Kannan K, Lim G, McVie R, Bocchini JA. Hyperketonemia increases TNF-α secretion in cultured U937 monocytes and Type-1 diabetic patients and is apparently mediated by oxidative stress and cAMP-deficiency. Diabetes. 2002;51:2287–2293. doi: 10.2337/diabetes.51.7.2287. [DOI] [PubMed] [Google Scholar]
- 15.Manna P, Jain SK. Hydrogen sulfide and L-cysteine increase phosphatidylinositol 3,4,5-trisphosphate (PIP3) and glucose utilization by inhibiting phosphatase and tensin homolog (PTEN) protein and activating phosphoinositide 3-kinase (PI3K)/serine/threonine protein kinase (AKT)/protein kinase Cζ/λ (PKCζ/λ) in 3T3l1 adipocytes. J Biol Chem. 2011;18286:39848–59. doi: 10.1074/jbc.M111.270884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfeiffer CM, Huff DL, Gunter EW. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem. 1999;45:290–292. [PubMed] [Google Scholar]
- 17.Beutler E. A Manual of Biochemical Methods. Grune & Stratton; Orlando: 1984. Red Cell Metabolism. [Google Scholar]
- 18.Rubinstein R, Genaro AM, Motta A, Cremaschi G, Wald MR. Impaired immune responses in streptozotocin-induced type I diabetes in mice. Involvement of high glucose. Clin Exp Immunol. 2008;154:235–246. doi: 10.1111/j.1365-2249.2008.03742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franklin CC, Backos DS, Mohar I, White CC, Forman HJ, Kavanagh TJ. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects of Med. 2009;30:86–98. doi: 10.1016/j.mam.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langton W, Circu ML, Aw TY. Insulin stimulation of gamma-glutamylcysteine ligase catalytic subunit expression increases endothelial GSH during oxidative stress: influence of low glucose. Free Rad Biol Med. 2008;45:1591–9. doi: 10.1016/j.freeradbiomed.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SK, Woodcroft KJ, Khodadadeh SS, Novak RF. Insulin signaling regulates gamma-glutamylcysteine ligase catalytic subunit expression in primary cultured hepatocytes. J Pharmacol Exp Ther. 2004;311:99–108. doi: 10.1124/jpet.104.070375. [DOI] [PubMed] [Google Scholar]
- 22.Jain SK. Hyperglycemia can cause membrane lipid peroxidation and osmotic fragility in human red blood cells. J Biol Chem. 1989;264:21340–5. [PubMed] [Google Scholar]
- 23.Jain SK, McVie R, Duett J, Herbst JJ. Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes. 1989;38:1539–43. doi: 10.2337/diab.38.12.1539. [DOI] [PubMed] [Google Scholar]
- 24.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blouet CB, Mariotti F, Azzout-Marniche D, et al. Dietary cysteine alleviates sucrose-induced oxidative stress and insulin resistance. Free Radic Biol Med. 2007;42:1089–1097. doi: 10.1016/j.freeradbiomed.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Jain SK, Velusamy T, Croad JL, Rains JL, Bull R. L-cysteine supplementation lowers blood glucose, glycated hemoglobin, CRP, MCP-1, oxidative stress and inhibits NFkB activation in the livers of Zucker diabetic rats. Free Radic Biol Med. 2009;46:1633–1638. doi: 10.1016/j.freeradbiomed.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Mattia G, Bravi MC, Laurenti O, Cassone-Faldetta M, Armiento A, Ferri C, Balsano F. Influence of reduced glutathione infusion on glucose metabolism in patients with non-insulin-dependent diabetes mellitus. Metabolism. 1998;47:993–997. doi: 10.1016/s0026-0495(98)90357-2. [DOI] [PubMed] [Google Scholar]
- 28.Ammon HP, Abdel-hamid M. Potentiation of the insulin-releasing capacity of tolbutamide by thiols: studies on the isolated perfused pancreas. Naunyn Schmiedebergs Arch Pharmacol. 1981;317:262–267. doi: 10.1007/BF00503828. [DOI] [PubMed] [Google Scholar]
- 29.Kim BS, Cha HN, Kim YW, et al. Inhibition of lipid infusion-induced skeletal muscle insulin resistance by cotreatment with tempol and glutathione in mice. J Pharmacol Sci. 2009;110:370–80. doi: 10.1254/jphs.09046fp. [DOI] [PubMed] [Google Scholar]
- 30.Guarino MP, Macedo MP. Co-administration of glutathione and nitric oxide enhances insulin sensitivity in Wistar rats. Br J Pharmacol. 2006;147:959–965. doi: 10.1038/sj.bjp.0706691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egashira K. Molecular mechanisms mediating inflammation in vascular disease: special reference to monocyte chemoattractant protein-1. Hypertension. 2003;41:834–841. doi: 10.1161/01.HYP.0000051642.65283.36. [DOI] [PubMed] [Google Scholar]
- 32.Pittas AG, Sun Q, Manson JE, Dawson-Hughes B, Hu FB. Plasma 25-hydroxyvitamin D concentration and risk of incident type 2 diabetes in women. Diabetes Care. 2010;33:2021–2023. doi: 10.2337/dc10-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manna P, Jain SK. Vitamin D up-regulates glucose transporter 4 (GLUT4) translocation and glucose utilization mediated by cystathionine-γ-lyase (CSE) activation and H2S formation in 3T3L1 adipocytes. J Biol Chem. 2012;287:42324–32. doi: 10.1074/jbc.M112.407833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain SK, Manna P, Micinski D, Lieblong BJ, Kahlon G, Morehead L, Hoeldtke R, Bass PF, 3rd, Levine SN. In African American type 2 diabetic patients, is vitamin D deficiency associated with lower blood levels of hydrogen sulfide and cyclic adenosine monophosphate, and elevated oxidative stress? Antioxid Redox Signal. 2013;18:1154–8. doi: 10.1089/ars.2012.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reis JP, Michos ED, von Mühlen D, Miller ER., III Differences in vitamin D status as a possible contributor to the racial disparity in peripheral arterial disease. Am J Clin Nutr. 2008;88:1469–1477. doi: 10.3945/ajcn.2008.26447. [DOI] [PubMed] [Google Scholar]
- 36.Richie JP, Jr., Muscat JE, Ellison I, Calcagnotto A, Kleinman W, El-Bayoumy K. Association of selenium status and blood glutathione concentrations in blacks and whites. Nutr Cancer. 2011;63:367–375. doi: 10.1080/01635581.2011.535967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jain SK. Effect of Glucose-6-phosphate dehydrogenase deficiency on reduced and oxidized glutathione and lipid peroxide levels in the blood. Clin Chim Acta. 1996;253:181–183. doi: 10.1016/0009-8981(96)06371-1. [DOI] [PubMed] [Google Scholar]