Abstract
Microbial poly(β, l-malic acid) was modified with either l-leucine ethyl ester (L) or l-phenylalanine methyl ester (F) to produce amphiphylic copolymers. The degradation of these copolymers in aqueous buffer took place under physiological conditions in a few weeks by hydrolysis of the side chain ester group followed by cleavage of the main chain. Spherical nanoparticles with diameters ranging between 70 and 230 nm were prepared from these copolymers by the dialysis-precipitation method. No alteration of the cell viability was observed after incubation of these nanoparticles in different cell lines. Anticancer drugs temozolomide and doxorubicin were encapsulated in the nanoparticles. Temozolomide was released within several hours whereas doxorubicin took several weeks to be completely liberated.
Keywords: anticancer, biodegradable nanoparticles, drug delivery system, polymalates, poly(malic acid)
1. Introduction
Contemporary cancer therapy is in urgent need of increasing the treatment efficiency. Although cancer cells are more vulnerable than normal cells to the effect of chemotherapy agents, drugs are nonselective and unavoidably affect normal tissues. Research is now focused on killing cancer cells using more specific targeting because toxicity of normal cells is the main constrain for dose and frequency, both being critical important factors in determining the efficiency of the cancer chemotherapy treatment.[1] In the last decades, drug delivery systems (DDS) based on biodegradable polymeric nanoparticles (NPs) have received a great attention as effective carrying devices. Nowadays, biodegradation is considered a prerequisite for any high-molecular-weight material, which is to be introduced in a living body for a limited period of time.[2]
Nanoparticles for drug delivery include numerous architectural designs in terms of size, shape, and materials. Particles differ in terms of drug-loading capacity, particle and drug stability, drug release rate, and targeted delivery ability.[1] A variety of polymers have been tested and proved to deliver the drug to a target site thus increasing the therapeutic benefit while minimizing side effects.[3] Polymer-based delivery systems are usually preferred because they are less immunogenic than protein-based ones, and they allow repetitive treatments without acute or chronic host immune response, which is a major requirement for the effective cancer treatment.[4]
Poly(β,l-malic acid) (PMLA), a biologically produced polyester, has a great potential in biomedicine because its excellent biodegradability and biocompatibility. PMLA is readily biodegraded producing easily metabolizable l-malic acid.[5] Unlike other biodegradable polymers, PMLA can be chemically modified through derivatization of the carboxylic side groups to change and modulate its properties.[6] PMLA and its derivatives have been used as platform in the synthesis of nanocarriers for drug delivery,[7–10] or as a constituent in macromolecular conjugates bearing several functionalities to treat human brain and breast tumors in mouse models.[11–13] In all of these investigations, it has been concluded that PMLA is a promising building block for the design of efficient DDS.[14]
Unmodified PMLA is highly hydrophilic and readily soluble in water due to its carboxylic polyfunctionality. Grafting of hydrophobic amino acids or peptides on hydrophilic polymers has been done to give an amphiphilic character to the polymer so it could form NPs,[15] or to introduce a membranolytic shell, which helps DDS to escape from endosomes to cytoplasm.[13] In this work, with the aim of inducing amphiphilic character, PMLA has been subjected to partial amidation with alkyl esters of l-leucine (L) (PAALM-L) and d-phenylalanine (F) (PAALM-F). The partially amidated polyesters were used for preparing self-assembled nanoparticles and the suitability of these as drug delivery systems has been examined. In the future, these copolymeric carriers could be easily vectorized, by taking benefit of the remaining free-carboxylic groups, in order to get nanoparticles with targeted properties.
2. Experimental Section
2.1. Materials
PMLA used in this work was biologically produced by cultivation of Physarum polycephalum and purified as described elsewhere.[16] Final PMLA was NMR spectroscopically pure and had a weight-averaged molecular weight of 34 kDa with a polydispersity of 1.08 as determined by gel permeation chromatography (GPC). l-Leucine ethyl ester and l-phenylalanine methyl ester were purchased from Sigma–Aldrich. Temozolomide (3,4-dihydro-3-methyl-4-oxoimidazo[5,1-d]-astetrazine-8-carboxamide) (TMZ) and doxorubicin ((7S,9S)-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione) (DOX) were supplied by AKSci (Union City, CA, USA). All organic solvents were either analytical or high-performance liquid chromatography (HPLC) grade and used without further purification.
2.2. Synthesis of Poly(β,l-malic acid)-graft-AA
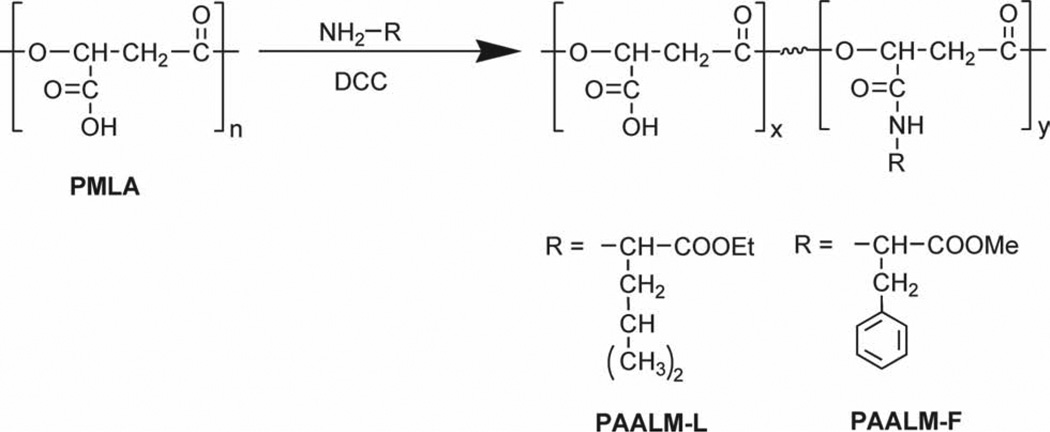
PMLA was conjugated with l-leucine ethyl ester or l-phenylalanine methyl ester, by activation of the carboxylic side groups with dicyclohexylcarbodiimide (DCC) (Scheme 1). Briefly, 1 mmol of PMLA was dissolved in 4 mL of acetone at room temperature (RT) and the solution cooled in an ice bath. The amino acid used for grafting and DCC in 1 mL of acetone were added dropwise. The amino acid and DCC amounts depended on the conversion degree that was desired (Table 1). Reaction was left to proceed under stirring for 1 h at 4 °C and then for 23 h at RT, after which the reaction mixture was cooled in the freezer and the precipitated dicyclohexylurea (DCU) removed by filtration. To remove DCU the remaining in solution, the filtrated polymer solution was dialyzed against methanol for 24 h using a cellulose membrane with a cutoff of 8 kDa. The modified PMLA was recovered from the dialyzed solution by adding water and subsequent lyophilization.
Scheme 1.
Amidation reaction of PMLA using DCC as activating agent.
Table 1.
Results of the PMLA modification reaction.
| Copolymer | AAa) | PMLA:DCC:AAb) | Reaction results | GPC | Solubilityc) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conversion [%] |
Yield [%] |
M̅w | PD | A | B | C | D | E | F | G | |||
| PAALM-L30 | L-Leu | 1:0.5:1 | 28 | 57 | 34 400 | 2.2 | + | + | + | + | − | − | − |
| PAALM-L60 | 1:1:1 | 58 | 56 | 33 600 | 1.8 | + | + | + | + | − | − | − | |
| PAALM-L90 | 1:1.5:2 | 88 | 65 | 33 700 | 1.9 | + | + | + | + | + | − | − | |
| PAALM-F30 | L-Phe | 1:0.5:1 | 27 | 56 | 32 900 | 2.3 | + | + | + | + | − | − | − |
| PAALM-F60 | 1:1:1 | 55 | 63 | 31 800 | 1.9 | + | + | + | + | − | − | − | |
| PAALM-F90 | 1:1.5:2 | 89 | 60 | 34 500 | 2.1 | + | − | + | + | + | − | − | |
| PMLA | − | − | − | 30,000 | 1.2 | + | + | + | − | − | − | + | |
Amino acid;
Reagent molar ratios used in the amidation reaction;
A: DMSO, B: methanol, C: acetone, D: HFIP, E: chloroform, F: diethyl ether, G: water.
2.3. Hydrolytic Degradation
The hydrolytic degradation of amino acid-modified PMLA was studied by GPC and NMR. For GPC, degradation assays were carried out in vials with 2 mg of polymer immersed in 1 mL of phosphate buffer at pH 7.4 and 37 °C; vials collected at scheduled times and freeze-dried were later subjected to chromatography analysis. For the NMR analysis, 10 mg of polymer was placed in NMR tubes containing 1 mL of deuterated water and incubated at 37 °C, and1H NMR spectra were recorded from supernatant at scheduled times.
2.4. Nanoparticles Formation and Drug Encapsulation
The precipitation-dialysis method was applied to produce nanoparticles of PAALM-L and PAALM-F. Briefly, to a solution of 5–10 mg of polymer in 1 mL of DMSO, acetone or methanol, 1 mL of water was added dropwise under magnetic stirring, and the mixture was dialyzed against distilled water for 24 h. NPs were recovered by freeze-drying. Particle morphology was characterized by scanning electron microscopy (SEM) and their average hydrodynamic diameters were determined by light scattering. The same procedure was used for drug encapsulation with TMZ and DOX using methanol as solvent for both polymer and drugs and the drug added at a concentration of 30% (w/w) with respect to polymer. Methanol was in this case removed by rotaevaporation to avoid drug losses during dialysis.
2.5. Cell Lines and Culture Media
All the cell lines U87MG, CRL-5904, and MDA-MB-468 were obtained from American Type Culture Collection (ATCC, USA). U87MG cells were cultured in MEM supplemented with final concentrations of 10% fetal bovine serum, 1% MEM NEAA, 1 × 10−3 m sodium pyruvate, and 2 × 10−3 m l-glutamine. For MDA-MB-468, Leibovitz’s L-15 medium with 10% final concentration fetal bovine serum was used and for CRL-5904, RPMI 1640 (ATCC) medium was used. Cells were seeded at 10 000 cells per well (0.1 mL) in 96-well flat-bottomed plates and incubated overnight at 37 °C in humid atmosphere containing 5% of CO2.
2.6. Cytotoxicity Test
Nanospheres were dispersed in appropriate culture media, at a maximum concentration of 1 mg mL−1, and serial dilutions were done to achieve several concentrations. Cells were exposed to NPs for 24 h. Cell viability was measured using the CellTiter 96 AQueous One Solution Cell Proliferation Assay kit (Promega Co.). Protocols supplied by the company were exactly followed. This test is based in a yellow tetrazolium compound (MTS) that is reduced to formazan only by living and metabolically active cell mitochondria, and phenazine methosulfate an electron-coupling reagent. Absorbance of formazan product (490 nm) can be measured directly from 96-well assay plates without additional processing and is directly proportional to the number of living cells in the culture.[17] The viability of the untreated cells was regarded as 100%. The results shown are the means s.d. of three independent experiments.
2.7. In Vitro Drug Release Studies
In vitro TMZ and DOX release was assessed by dialysis method. Briefly, 10 mg of freeze-dried drug-loaded nanoparticles was resuspended in 1 mL of PBS pH 7.4 and transferred into a dialysis tube with 8 kDa molecular cutoff (Spectrum Laboratories, CA, USA). For drug release, the tube was immersed into 15 mL of PBS and incubated at 37 °C. For measuring, aliquots of 0.5 mL of the releasing media were taken at scheduled times for analysis, and the volume replaced by fresh medium every time. Drug concentration was determined by HPLC at 330 and 480 nm for TMZ and DOX, respectively. Since TMZ is hydrolytically labile its degradation product 5-aminoimidazole-4-carboxamide (AIC) absorbing at 254 nm was also measured.
2.8. Measurements
SEM images were taken with a field-emission JEOL JSM-7001F instrument (JEOL, Japan) from uncoated samples. Particle size measurements, based on intensity, were performed with a ZetaSizer NS, (Malvern Instruments, UK) with the particles suspended in deionized water. NMR spectra were recorded on a Bruker AMX-300 instrument. 1H NMR spectra where recorded from samples immersed in D2O or deuterated DMSO at 25 °C operating at 300.1 MHz. 128 scans were acquired with 32-K data points and relaxation delays of 2 s. 13C NMR spectra were taken from deuterated acetone solutions with 64-K data points and 5000–10000 scans, and relaxation delays of 2 s.
GPC was done using a Waters 515 HPLC pump with a Waters 410 differential refractometer detector and a Waters Styragel HR 5E column (7.8 × 300 mm) (Waters, Massachusetts, USA). Samples were chromatographied using 0.05 m sodium trifluoroacetate in hexafluoroisopropanol (HFIP) at 0.5 mL min−1 flow rate. Chromatograms were calibrated against poly(methyl methacrylate) standards (Varian, California, USA). HPLC was carried out on a Waters 600 system with a Waters 996 photodiode array detector provided with a GL Sciences (Torrance, California, USA) Inertsil ODS-3V column (5 µm, 4.6 × 250 mm). The mobile phase used for TMZ and DOX was methanol in 0.5% aqueous acetic acid (10:90) and a mixture of 0.02 m sodium hydrogen phosphate and acetonitrile (60:40), respectively, pumped at a rate of 1 mL min−1 in both cases.
3. Results and Discussion
3.1. Amino Acid Grafting on PMLA
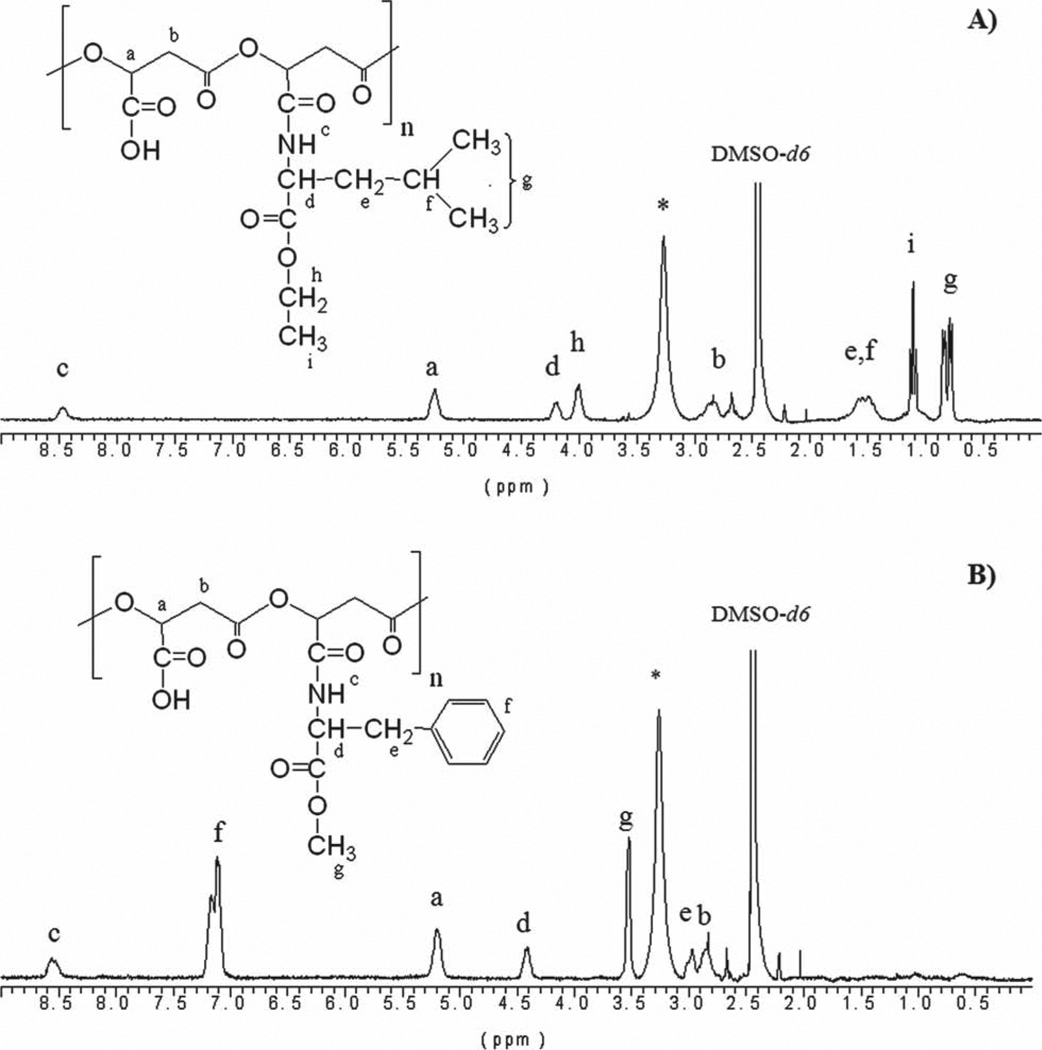
PAALM-L and PAALM-F copolymers nominally containing 30%, 60%, and 90% of amidated units were obtained at yields in the 55%–65% range by reaction of PMLA with the esterified amino acids l-leucine and l-phenylalanine using DCC as carboxylic group activator (Table 1). Conversions attained were precisely assessed by 1H NMR (Figure 1) and the GPC analysis showed that the initial polymer did not undergo significant reduction in the molecular weight. The yields of all the copolymers are rather low. Probably, some amount of PMLA was trapped by DCU when this compound was precipitated form the frozen acetone solution. All copolymers are soluble in dimethylsulfoxide, acetone, and HFIP but non-soluble in diethyl ether and water. Only the copolymers amidated at 90% were soluble in chloroform.
Figure 1.
1H NMR spectra of: A) PAALM-L60 and B) PAALM-F60. (*) Peak of water.
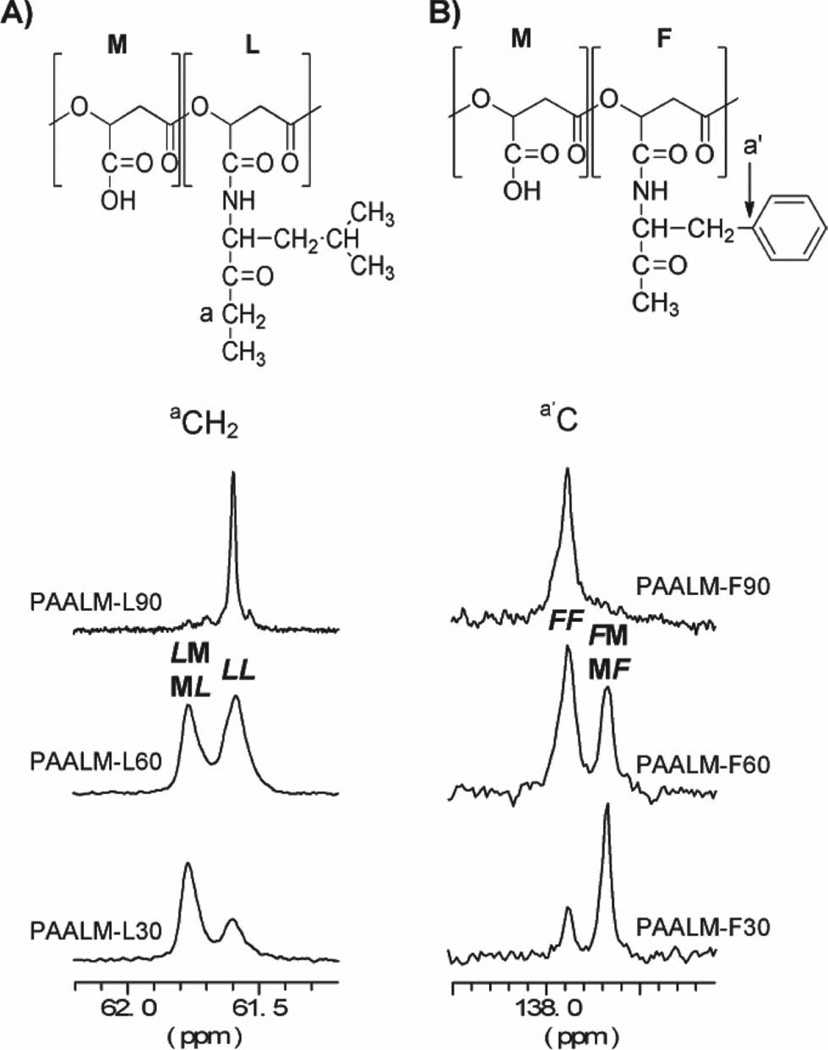
Unfortunately, the 13C NMR spectra recorded from these copolymers did not provide the information required to carry out a statistical analysis of the chain microstructure. Nevertheless, the evolution of the signal arising from different carbons of the amino acid moiety units (a, a ′) with changes in composition revealed that the distribution of amidated and free-carboxylic units along the copolymer chain must be essentially at random. As it is illustrated in Figure 2, this signal evolves from an essentially single peak for the 90% amidated PMLA to a doublet for both the 60% and 30% amidated copolymers with relative peak intensities changing according to composition. The observed splitting is interpreted as due to the presence of dyads made of amidated-amidated (LL or FF) and amidated-non-amidated (L M/M L or M F / F M) units, which is an indication of a random microstructure.
Figure 2.
13C NMR spectra of the amino acid region corresponding to aCH2 of PAALM-L (A) and non protonated aromatic carbon of PAALM-F (B) of copolymers with different conversion degrees.
3.2. Hydrolytic Degradation
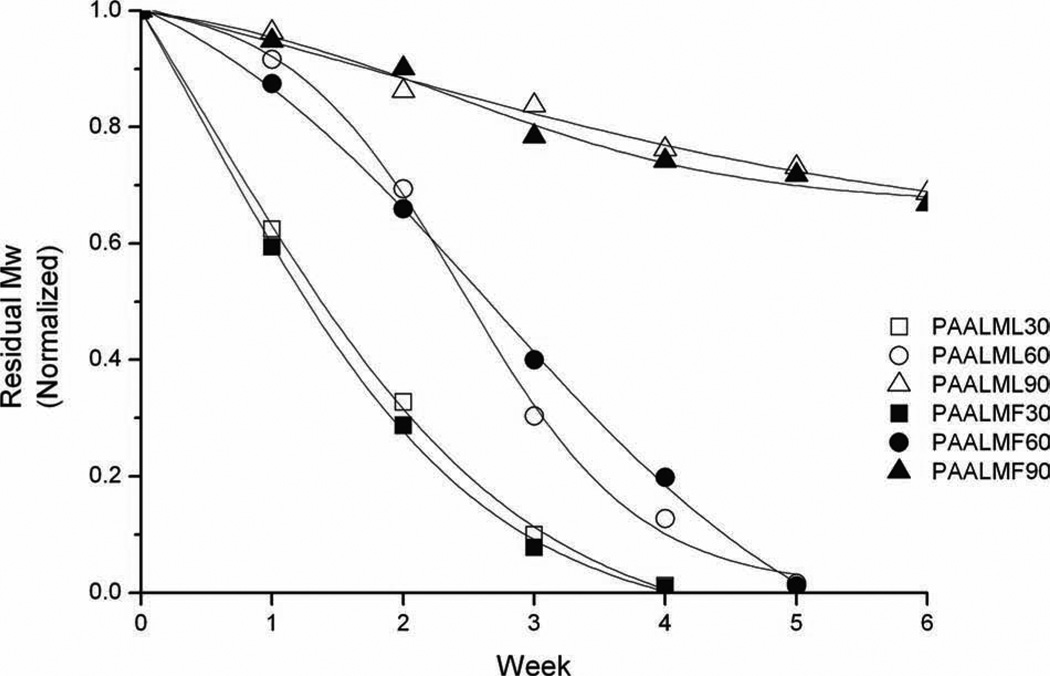
The hydrolytic degradation of the copolyesters was performed under physiological conditions (pH 7.4, 37 °C), and the process was followed by GPC of the residue and by 1H NMR of the released products. According to expectations, GPC results showed that degradation rate decreased with the increasing amidation degree of the copolyester and results were similar for both Leu and Phe derivatives. As it is shown in Figure 3, the 30% amidated copolyesters become fully degraded after four weeks of incubation whereas for the 90% amidated copolymers, the reduction in molecular weight was less than 30% after six weeks of treatment.
Figure 3.
Molecular weight reduction of PAALM-L and PAALM-F copolyesters as a function of incubation time in PBS, pH 7.4 at 37 °C.°C.
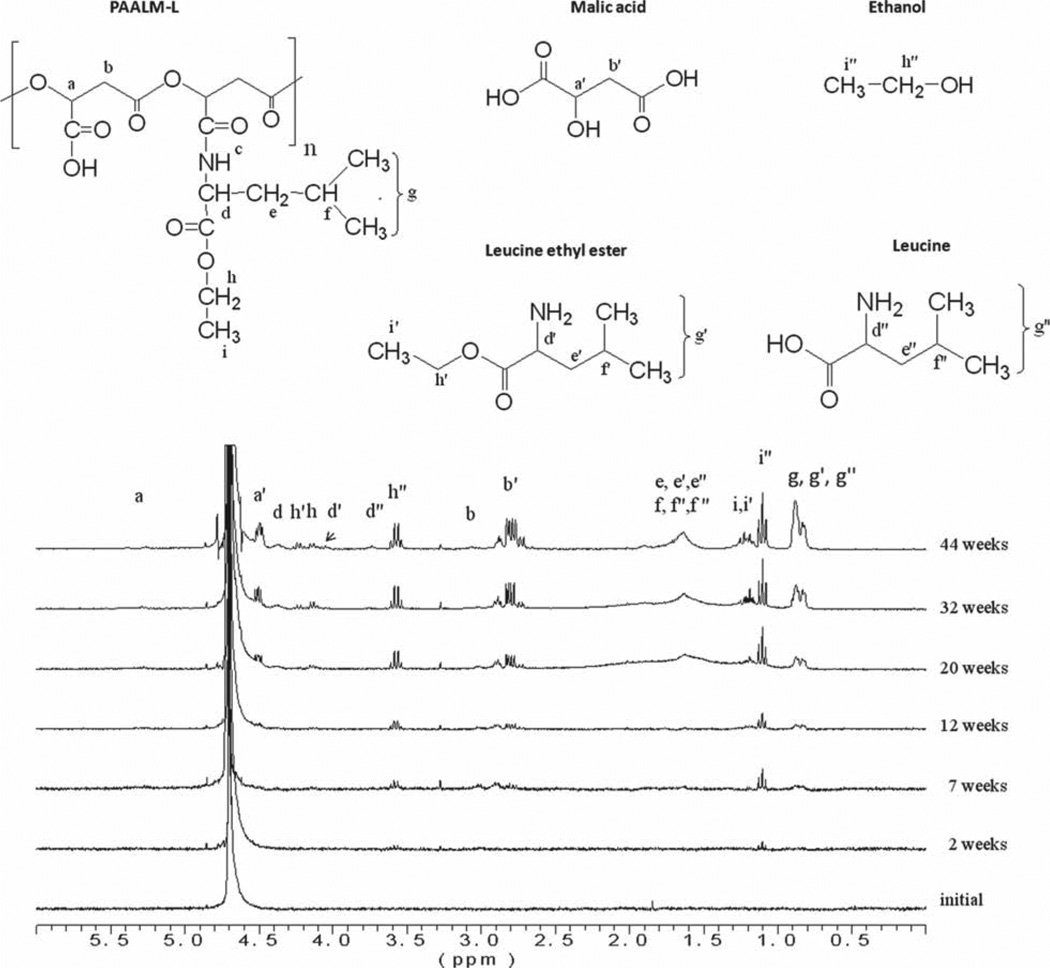
The copolyesters PAALM-L60 and PAALM-F60, which are those with a more equilibrated compositions, were used to carry out the analysis of the released products by NMR and results are shown in Figure 4. Samples were incubated at 37 °C in deuterated water, and 1H NMR signals arising from degradation soluble products were monitored. In the case of PAALM-L, the spectra clearly revealed the presence of ethanol in the solution just after two weeks of incubation as well as of free malic acid at the seventh week. In addition, signals from leucine methyl groups and from the polyester main chain were also detectable suggesting either oligomer solubilization or partial polymer solubilization. As expected, all signals increase in intensity at longer incubation times except those arising from oligomers, which show a progressive diminution. For PAALM-F the hydrolysis proceeded similarly with methanol being the first product detected in the mother solution, and with signals arising from the degraded main chain products coming out after 20 weeks of incubation.
Figure 4.
Evolution of the 1H NMR spectra recorded from the water mother solution of the incubation of PAALM-L60 at 37 °C with time.
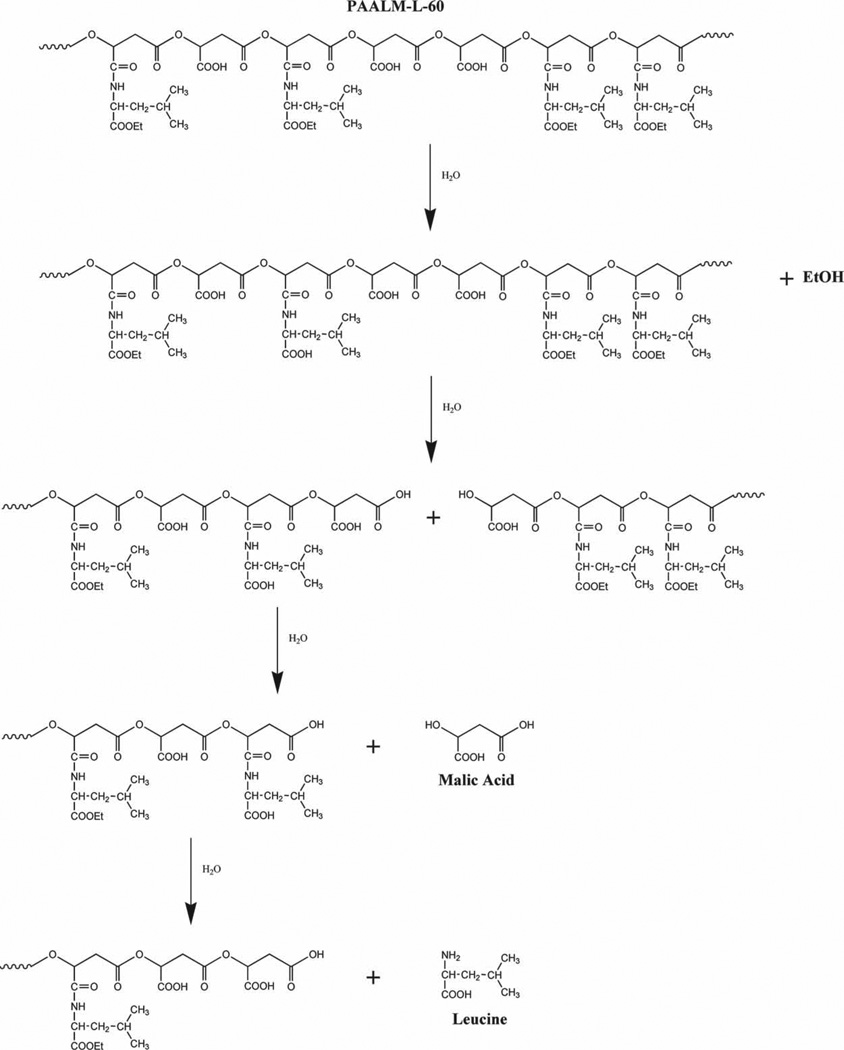
These NMR results supported by others previously obtained by us in the hydrolytic degradation of alkyl esters of PMLA suggest the hydrolytic mechanism for the copolyesters PAALM-L and PAALM-P depicted in Scheme 2. Hydrolysis starts with the cleavage of the amino acid ester groups with releasing of the corresponding alcohol, and continues with the splitting of the main chain at the ester linkages between non-amidated units with generation of oligomeric fragments and malic acid. Oligomers become solubilized or not depending on their amidation degree, and finally the hydrolysis of the main chain is completed with releasing of malic acid and free l-leucine or l-phenylalanine.
Scheme 2.
Hydrolytic degradation mechanism of PAALM-L.
3.3. Nanospheres
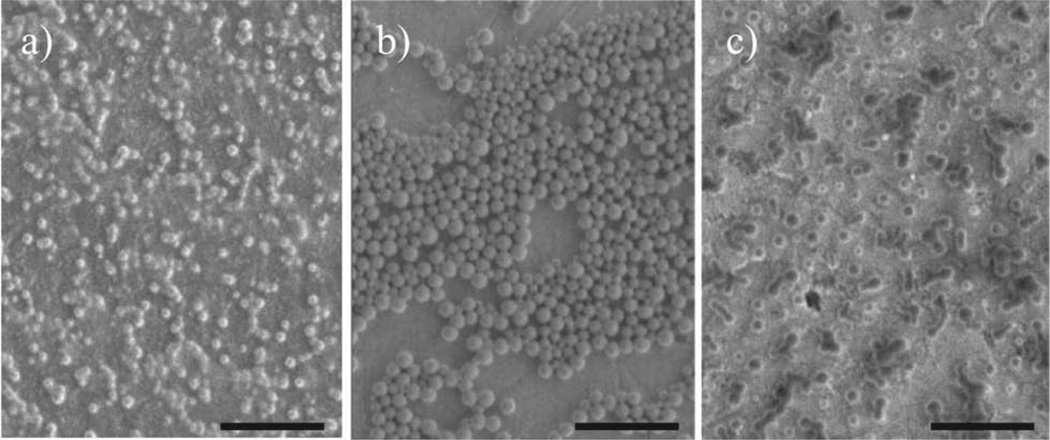
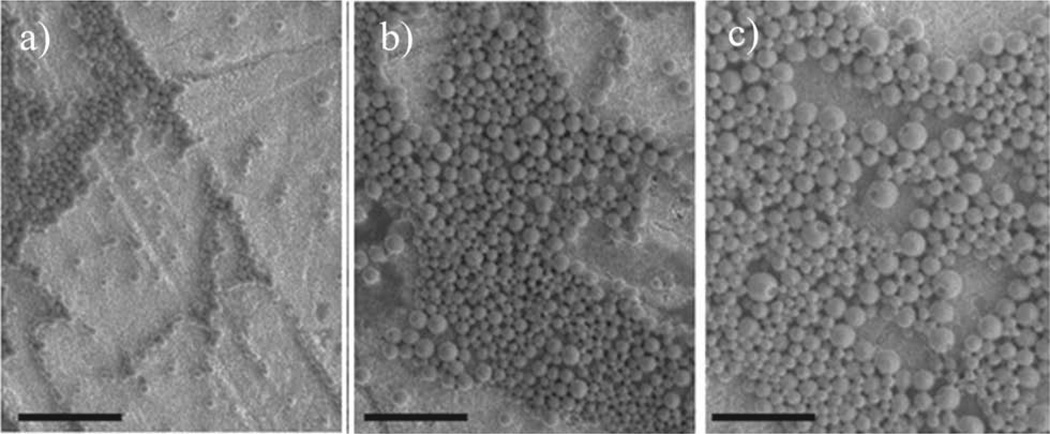
Spheric nanoparticles were prepared from copolymers PAALM-L and PAALM-F by applying the precipitationdialysis method. An exploratory study of the influence of preparation conditions and copolymer conversion on the characteristics of the resulting nanoparticles was performed. Solutions at two different polymer concentrations (0.5% and 1.0% w/v) in acetone, DMSO, and methanol were tested. It was found that NPs were formed under all assayed conditions with a mean hydrodynamic diameter ranging between 70 and 230 nm (Table 2) depending on both the procedure applied and copolymer chosen. First, the nanoparticle-forming capability of copolymers with varying composition was examined, and results obtained with the PAALM-F series are illustrated in Figure 5. As it could be anticipated,[15] the morphology of the formed NPs varied with the ratio of hydrophobic to hydrophilic counterparts.
Table 2.
Mean hydrodynamic diameter of PAALM-L and PAALM-F nanoparticles obtained by the precipitation-dialysis method under different conditions.
| PAALM-L | PAALM-F | |||||||
|---|---|---|---|---|---|---|---|---|
| Conv [%]a) | 30 | 60 | 90 | 30 | 60 | 90c) | ||
| Conc [%w/v]b) | 1.0 | 0.5 | 1.0 | 1.0 | 1.0 | 0.5 | 1.0 | 1.0 |
| DMSO | 110 ± 35 | 66 ± 29 | 141 ± 24 | 95 ± 13 | 123 ± 29 | 113 ± 34 | 159 ± 54 | 104 ± 18 |
| Methanol | 126 ± 27 | 76 ± 38 | 199 ± 36 | 109 ± 31 | 145 ± 18 | 137 ± 37 | 222 ± 42 | − |
| Acetone | 173 ± 20 | 156 ± 33 | 227 ± 19 | 139 ± 12 | 180 ± 24 | 180 ± 41 | 231 ± 22 | 147 ± 35 |
Degree of amidation;
Initial concentration of the polymer solution;
No measured in MeOH due to nonsolubility of the copolymer in this solvent.
Figure 5.
Nanoparticles of PAALM-F copolymers with different amidation degrees obtained from a 1.0% (w/w) DMSO solution: a) PAALM-F30, b) PAALM-F60, and c) PAALM-F90. Scale bar: 1 µm.
NPs prepared from 60% amidated copolymers produced well-defined nanospheres essentially exempted of amorphous material. On the other hand, solvent was the factor mainly deciding the nanoparticle size, the minimum values and dispersities being obtained with DMSO. This effect is well illustrated in Figure 6 for the case of PAALM-L copolymers. Results were very similar for the two series although particle sizes were much smaller when they were made from leucine amidated copolymers.
Figure 6.
Nanospheres of PAALM-L60 prepared from 1% (w/w) solution in different solvents: a) DMSO, b) methanol, and c) acetone. Scale bar: 1 µm.
3.4. Nanoparticle Cytotoxicity
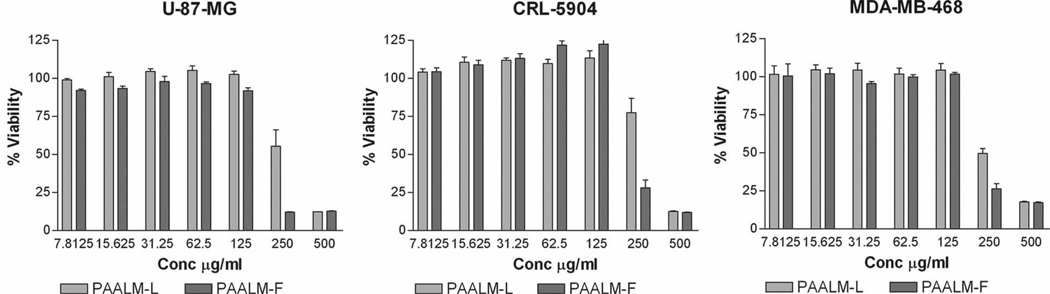
For its potential use as a biomaterial it was mandatory to evaluate the toxicity of nanoparticles. Thus, an in vitro study of PAALM-L60 and PAALM-F60 nanoparticles cytotoxicity on primary human glioma cell lines U-87MG, human non-small cell lung cancer, (metastatic in brain), CRL-5904, and invasive human breast carcinoma cell line MDA-MB-468, was performed. All cell lines presented a similar pattern with cell viability remaining near 100% after 24 h of exposure to NPs in concentrations lower than 125 µg mL−1. At higher concentrations, there is a significant reduction in cell viability (Figure 7). Such concentration-dependent cytotoxicity is related to the binding of poly mer to membrane, followed by copolymer intrusion and irreversible membrane reorganization followed by a lytic event. Interactions between the copolymer hydrophobic microdomains and lipid bilayer membranes would explain membrane binding of the copolymers with subsequent membrane disruption.[18]
Figure 7.
Cell viability of U-87-MG, CRL-5904, and MDA-MB-468 after 24 h of contact between polymer NPs of PAALM-L60 and PAALM-F60 and cells as a function of polymer concentration.
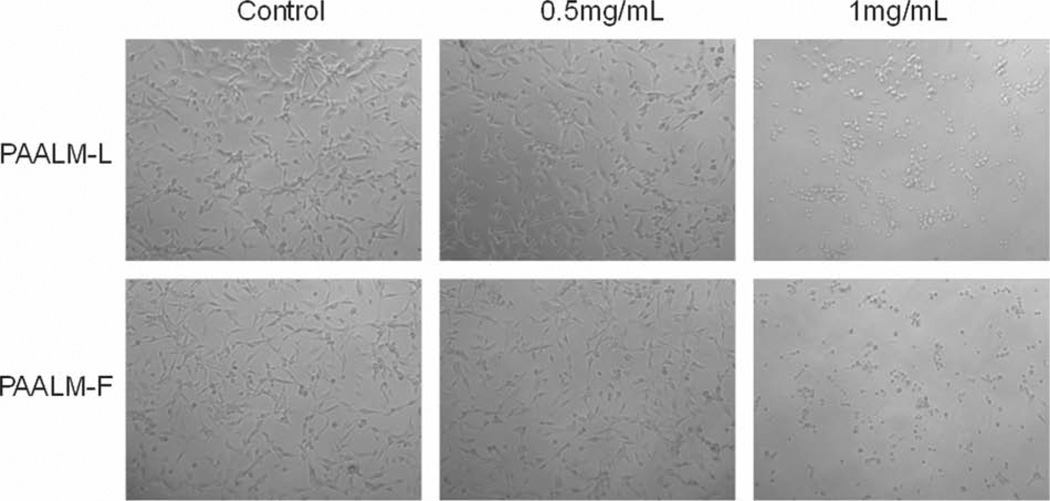
Ding and co-workers[13] observed that an increase in hydrophobicity and/or elimination of negative charges resulted in membranolytic activity of PMLA copolymers. They also found that the density of substituents on the polymer chain has an effect on membranolytic activity, observing a maximum efficacy at 40%–60% of substituents. The viability drop of U87MG after treatment with 0.5% (w/v) of PAALM-L and PAALM-F60, could be also observed by optical microscopy and related with a significant change in cell morphology, which is more drastic after a treatment with 1% (w/v) of NPs (Figure 8).
Figure 8.
Cell morphology of cultured cells after 24 h incubation at 37 °C for U-87MG cell line in the presence of nanoparticles of of PAALM-L60 (top) and PAALM-F60 (bottom).
3.5. Drug Encapsulation and In Vitro Release
PAALM-L60 and PAALM-F60 nanoparticles were chosen for studying the temozolomide and doxorubicin encapsulation and releasing. Drugs were encapsulated by the precipitation-dialysis method, using methanol as the common solvent for both drugs and polymer. The two copolymers showed a very similar behavior, with an entrapment around 5% (w/w) of TMZ and 8% (w/w) for DOX. Although drug contents in the NPs could be considered acceptable, the encapsulation efficiency was low; DOX was entrapped about 28% of its initial concentration and TMZ only around the half of such value (Table 3). The higher encapsulation efficiency for DOX can be explained by the fact that this compound could form ionic complexes with anionic olyelectrolytes.[19] Compared to our results obtained in the encapsulation of these drugs in poly(methyl malate) (PAALM-1) nanoparticles, encapsulated contents were doubled in the present case, which is likely due to the possibility of using a common solvent for both drug and polymer.
Table 3.
Drug content and encapsulation efficiency of temozolomide and doxorubicin in PAALM-L60 and PAALM-F60 nanoparticles.
| TMZ | DOX | |||
|---|---|---|---|---|
| Percentage of Conta) | Percentage of E.E.b) | Percentage of Cont.a) | Percentage of E.E.b) | |
| PAALM-L60 | 4.9 ± 1.5 | 16.4 ± 5.1 | 8.5 ± 1.2 | 28.5 ± 4.2 |
| PAALM-F60 | 4.2 ± 0.8 | 14.2 ± 6.2 | 8.4 ± 0.6 | 28.0 ± 2.1 |
Percentage (w/w) of drug contained in the nanoparticles upon encapsulation;
Percentage of initial drug that is encapsulated.
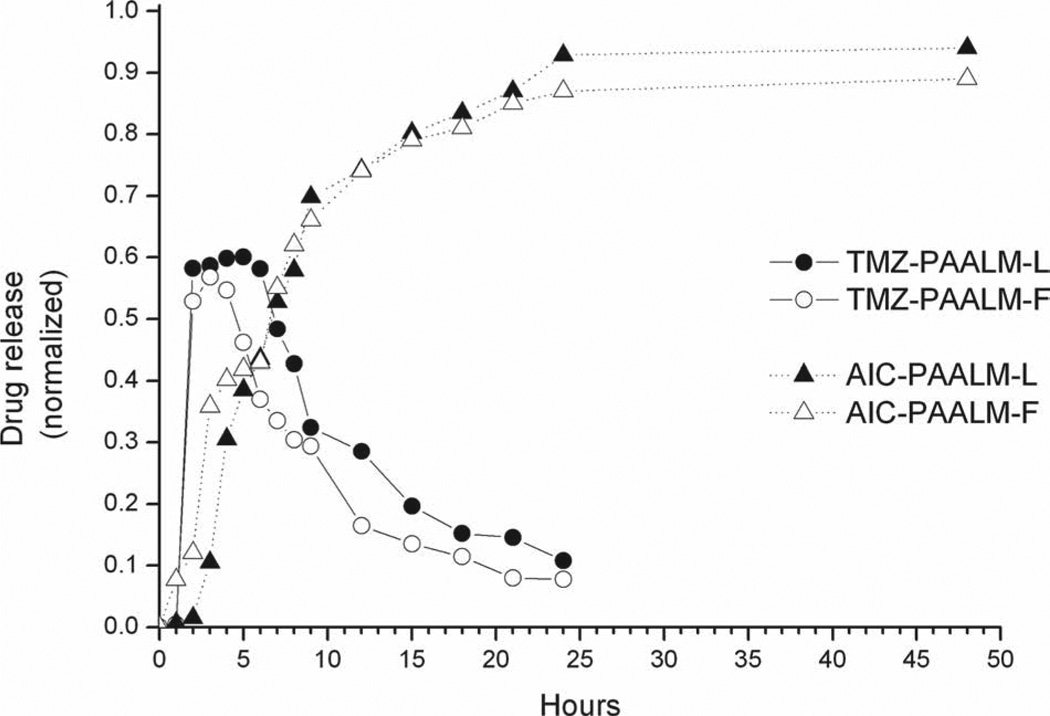
Cumulative temozolomide release profiles from PAALM-L60 and PAALM-F60 nanospheres are presented in Figure 9. Since TMZ undergoes fast hydrolysis above pH 7.0 yielding AIC together with the methyldiazonium ion, which is the chemotherapeutically active molecule,[20, 21] the analysis of the release profile under physiological conditions is complex. Thus, for a correct evaluation of in vitro TMZ release, it will be therefore necessary to quantify the delivery of both compounds TMZ and AIC. The releasing profiles are very similar for the two copolymers, reaching almost 60% of TMZ release after 3 h of incubation and displaying a progressive decay concomitant to its hydrolytic degradation, after which TMZ was not longer detectable. Simultaneously, AIC concentration increased rapidly from the third hour to the ninth, and more slowly afterwards. After 24 h of incubation, released TMZ was completely degraded. These results suggest that TMZ was firstly released form nanoparticles and then decomposed in the medium forming AIC with the consequent release of the methyldiazonium ion. A similar pattern of behavior was observed for the TMZ releasing from poly(methyl malate) nanoparticles.[10]
Figure 9.
Profiles of TMZ in vitro release from PAALM-L60 and PAALM-F60 nanoparticles and formation of AIC from released TMZ at pH 7.4.
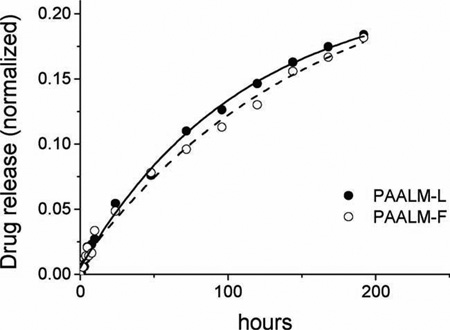
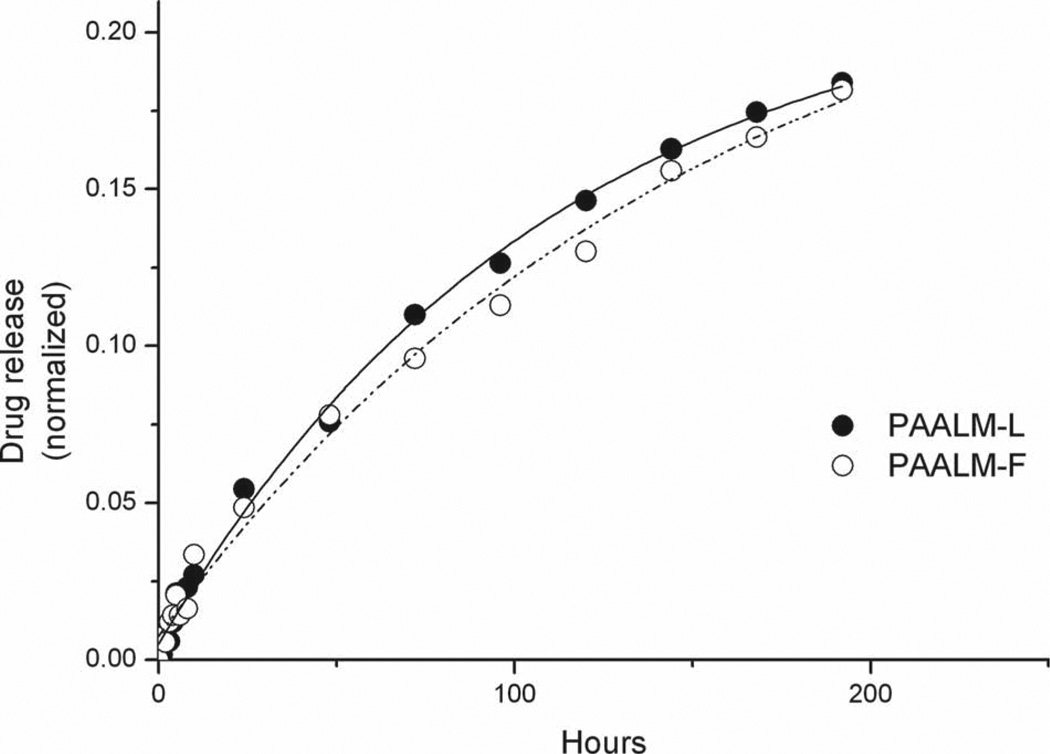
The release of DOX from PAALM-L60 and PAALM-F60 followed a similar profile in the two systems (Figure 10). The profiles were much simpler and revealed a much slower delivery rate than for TMZ; while only a few hours were required for the complete liberation of TMZ, the complete release of DOX took more than ten days. Such a comparative delay is likely to be due to the ionic interaction taking place between DOX and the free carboxylic groups present in the copolymers. Recently, a similar behavior for the release of DOX from poly(γ-glutamic acid) particles has been reported,[19] and it has been shown that DOX release was highly pH dependant; they found that at pH 2.2, which is below PGA pKa, the release reaches 60% after 180 h of incubation whereas at pH 7.4 it was below 20% after such time, a behavior very close to that observed in this work.
Figure 10.
DOX in vitro release from PAALM-L60 and PAALM-F60 nanoparticles at pH 7.4.
4. Conclusions
Microbial polymalic acid partially amidated (30%–90%) with hydrophobic l-leucine (L) and l-phenylalanine (F) amino acid esters can be used to produce nanospheres with an average diameter ranging between 70 and 230 nm, depending on the kind of amino acid and the solvent used for preparation. The amino acid ester-grafted copolyesters are readily hydrolyzed in water at physiological conditions in times of weeks at a rate that decreases with the increasing degree of amidation. DOX and TMZ can be encapsulated in these nanoparticles and released upon incubation under physiological conditions. TMZ was released within a few hours with subsequent hydrolytic pH-dependent conversion into AIC, while DOX was steadily released in a time scale of days. The particles described did not show a sign of toxicity during the 24 h of administration provided that NPs concentration is kept below 0.125 mg mL−1.
Acknowledgements
This work was funded by MICINN (Spain) with project MAT2009-14053-CO2-01. CONACyT (México) and Programa AlBan (Spain) granted ALL for postgraduate. Grants from NIH (R01 CA123495, R01 CA 136841 and U01 CA151815 to JYL), and Winnick Family Foundation clinical grant (to JYL). To A. Rekechenetskiy for his help with cytotoxicity studies.
Contributor Information
Alberto Lanz-Landázuri, Departament d’Enginyeria Química, Universitat Politècnica de Catalunya, ETSEIB, Diagonal 647, 08028, Barcelona, Spain.
Montserrat García-Alvarez, Email: montserrat.garcia@upc.edu, Departament d’Enginyeria Química, Universitat Politècnica de Catalunya, ETSEIB, Diagonal 647, 08028, Barcelona, Spain.
José Portilla-Arias, Department of Neurosurgery, Cedars-Sinai Medical Center, 8631 W Third Street, Suite 800E, Los Angeles, CA 90048, USA.
Antxon Martínez de Ilarduya, Departament d’Enginyeria Química, Universitat Politècnica de Catalunya, ETSEIB, Diagonal 647, 08028, Barcelona, Spain.
Eggehard Holler, Department of Neurosurgery, Cedars-Sinai Medical Center, 8631 W Third Street, Suite 800E, Los Angeles, CA 90048, USA.
Julia Ljubimova, Department of Neurosurgery, Cedars-Sinai Medical Center, 8631 W Third Street, Suite 800E, Los Angeles, CA 90048, USA.
Sebastián Muñoz-Guerra, Departament d’Enginyeria Química, Universitat Politècnica de Catalunya, ETSEIB, Diagonal 647, 08028, Barcelona, Spain.
References
- 1.Haley B, Frenkel E. Urology Oncology. 2008;26:57. doi: 10.1016/j.urolonc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Abdellaoui K, Boustta M, Vert M, Morjani H, Manfait M. Eur. J. Pharm. Sci. 1998;6:61. doi: 10.1016/s0928-0987(97)00069-9. [DOI] [PubMed] [Google Scholar]
- 3.Soppimath KS, Aminabhavi TM, Kulkarni AR. J. Control Release. 2001;70:1. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 4.Patil R, Portilla-Arias J, Ding H, Inoue S, Konda B, Hu J, Wawrowsky KA, Shin PK, Black KL, Holler E, Ljubimova J. Pharm. Res. 2010;272:2317. doi: 10.1007/s11095-010-0091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holler E, Lee BS. Recent Res. Develop. Anal. Chem. 2002;2:177. [Google Scholar]
- 6.Portilla-Arias JA, García-Alvarez M, Galbis JA, Muñoz-Guerra S. Macromol. Biosci. 2008;8:551. doi: 10.1002/mabi.200700249. [DOI] [PubMed] [Google Scholar]
- 7.Osanai S, Nakamura K. Biomaterials. 2000;21:867. doi: 10.1016/s0142-9612(99)00210-0. [DOI] [PubMed] [Google Scholar]
- 8.Cammas S, Béar MM, Harada A, Guérin Ph, Kataoka K. Macromol. Chem. Phys. 2000;201:355. [Google Scholar]
- 9.Martínez-Barbosa ME, Cammas S, Appel M, Ponchel G. Biomacromolecules. 2004;5:137. doi: 10.1021/bm0300608. [DOI] [PubMed] [Google Scholar]
- 10.Lanz-Landázuri A, García-Alvarez M, Portilla-Arias JA, Martínez de Ilarduya A, Patil R, Holler E, Ljubimova JY, Muñoz-Guerra S. Macromol. Biosci. 2011;11:1370. doi: 10.1002/mabi.201100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee BS, Fujita M, Khazenzon NM, Wawrowsky KA, Wachsmann-Hogiu S, Farkas DL, Black KL, Ljubimova JY, Holler E. Bioconjugate Chem. 2006;17:317. doi: 10.1021/bc0502457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ljubimova JY, Fujita M, Khazenzon NM, Lee BS, Wachsmann-Hogiu S, Farkas DL, Black KL, Holler E. Chem. Biol. Interact. 2008;171:195. doi: 10.1016/j.cbi.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding H, Inoue S, Ljubimova LV, Patil R, Portilla-Arias JA, Hu J, Konda B, Wawrowsky KA, Fujita M, Karabalin N, Sasaki T, Black KL, Holler E, Ljubimova JY. Proc. Natl. Acad. Sci. 2010;107:18143. doi: 10.1073/pnas.1003919107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang ZW, Laurent V, Chetouania G, Ljubimova JY, Holler E, Benvegnua T, Loyer P, Cammas-Mariona S. Int. J. Pharm. 2012;423:84. doi: 10.1016/j.ijpharm.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akagi T, Higashi M, Kaneko T, Kida T, Akashi M. Biomacromolecules. 2006;7:297. doi: 10.1021/bm050657i. [DOI] [PubMed] [Google Scholar]
- 16.Holler E. In: Handbook of Engineering Polymeric Materials. Cheremisinoff NP, editor. Marcel Dekker, NY: p. 93. [Google Scholar]
- 17.Mosmann T. J. Immun. Meth. 1983;65:55. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 18.Chen R, Khormaee S, Eccleston ME, Slater NKH. Biomaterials. 2009;30:1954. doi: 10.1016/j.biomaterials.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manocha B, Margaritis A. J. Nanomat. 2010:780171. [Google Scholar]
- 20.Arrowsmith J, Jennings SA, Clark AS, Stevens MFG. J. Med. Chem. 2002;45:5458. doi: 10.1021/jm020936d. [DOI] [PubMed] [Google Scholar]
- 21.Baker SD, Wirth M, Statkevich P, Reidenberg P, Alton K, Sartorius SE, Dugan M, Cutler D, Batra V, Grochow LB, Donehower RC, Rowinsky EK. Clin. Cancer Res. 1999;5:309. [PubMed] [Google Scholar]