Abstract
Objective
Nicotinic acid (a.k.a. niacin or vitamin B3), widely used to treat dyslipidemias, represents an effective and safe means to reduce the risk of mortality from cardiovascular disease. Nonetheless, a substantial fraction of patients discontinue treatment due to a strong side effect of cutaneous vasodilation, commonly termed flushing. In the present study we tested the hypothesis that nicotinic acid causes flushing partially by activating the capsaicin receptor TRPV1, a polymodal cellular sensor that mediates the flushing response upon consumption of spicy food.
Approach and Results
We observed that the nicotinic acid-induced increase in blood flow was substantially reduced in Trpv1−/− knockout mice, indicating involvement of the channel in flushing response. Using exogenously expressed TRPV1, we confirmed that nicotinic acid at sub-millimolar to millimolar concentrations directly and potently activates TRPV1 from the intracellular side. Binding of nicotinic acid to TRPV1 lowers its activation threshold for heat, causing channel opening at physiological temperatures. Activation of TRPV1 by voltage or ligands (capsaicin and 2-APB) is also potentiated by nicotinic acid. We further demonstrated that nicotinic acid does not compete directly with capsaicin but may activate TRPV1 through the 2-APB activation pathway. Using live-cell fluorescence imaging, we observed that nicotinic acid can quickly enter the cell through a transporter-mediated pathway to activate TRPV1.
Conclusions
Direct activation of TRPV1 by nicotinic acid may lead to cutaneous vasodilation that contributes to flushing, suggesting a potential novel pathway to inhibit flushing and improve compliance.
Keywords: vasodilation, ion channels, lipoproteins, cardiovascular disease
Introduction
Nicotinic acid (also commonly called niacin or vitamin B3) is a water-soluble small molecule that is converted in vivo to nicotinamide adenine dinucleotide, a coenzyme involved in the catabolism of fat. As one of the oldest lipid lowering medications 1, nicotinic acid has been prescribed for over 50 years. At a daily dosage of gram quantities, nicotinic acid (but not its derivative nicotinamide) lowers the serum concentrations of total cholesterol as well as low-density lipoprotein while raising that of high-density lipoprotein, reducing the risk of mortality from cardiovascular disease 2. This beneficial effect is thought to be mediated in part by activation of hydroxy-carboxylic acid receptor 2 (HCA2) expressed in adipocytes, causing a drop in the intracellular cAMP level and inhibition of lipolysis 3-5.
Despite its well-known anti-dyslipidemic effects, clinical use of nicotinic acid has been significantly hindered by a very unpleasant side effect called flushing, which is characterized by cutaneous vasodilation and symptoms of hot flashes and burning. A dose of 0.05-to-0.1 g of nicotinic acid is sufficient to elicit flushing of the face and upper body, whereas the rest of the body may be affected when higher doses (0.5-to-1.0 g) are used 6. Occurring in up to 90% of patients, flushing usually lasts for 30-90 min and is associated with intense erythema, tingling, itching, and elevation in skin temperature. Some patients have more severe skin reactions, such as urticaria, periorbital edema, conjunctivitis, or nasal congestion 6. Flushing was thought to be mediated by nicotinic acid-induced HCA2 activation in Langerhans cells and keratinocytes of the skin. The resulted activation of arrestin beta 1 and the downstream effector ERK 1/2 MAP kinase 7 in turn leads to activation of cyclooxygenase and release of vasodilatory prostaglandin D2 and E2. The flushing response (but not the antidyslipidemic effects) is subject to tolerance 8-10; it markedly decreases after continuous treatment (a property called tachyphylaxis). Nonetheless, up to one-third of patients refused to continue treatment mainly due to intolerable flushing 11, 12.
To fully take advantage of the beneficial effects of nicotinic acid and reduce the drop-off rate, a better understanding of the molecular events underlying flushing and potential treatments is of great practical importance. Interestingly, recent studies discovered that pharmacological blockade of cyclooxygenase (by aspirin) and prostaglandin D2 receptor 1 (by laropiprant) does not fully inhibit flushing 13, 14. Meanwhile, research in both humans and animal models showed that nicotinic acid–induced flushing is a biphasic process15, 16. These findings, together with the selective tachyphylaxis behavior, indicate that flushing may be mediated by target(s) outside the beneficial HCA2 pathway, raising hope that flushing can be inhibited while preserving the clinic efficacy of nicotinic acid.
Intriguingly, capsaicin (the active compound of spicy chili peppers) also causes flushing symptoms closely resembling that caused by nicotinic acid 17. The capsaicin receptor TRPV1 is a heat-sensing ion channel that responds to many physical and chemical stimuli 18-20. Activation of TRPV1 causes hot and pain sensations and thermoregulatory responses such as sweating and vasodilation21. Noticeably, Langerhans cells and keratinocytes, the critical carriers for flushing reaction, respond to nicotinic acid with an increase in intracellular Ca2+ 22, 23, whereas TRPV1 is a non-selective Ca2+-permeable cation channel richly expressed in keratinocytes and Langerhans cells 24, 25. Furthermore, repetitive administration of capsaicin results in tachyphylaxis, similar to that seen with nicotinic acid 26. Taken together, these findings point to the possibility that TRPV1 may play a role in nicotinic acid-induced flushing. We were further drawn to this possibility by observations that a TRPV1 specific antagonist, AMG9810 [(E)-3-(4-t-Butylphenyl)-N-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)acrylamide], can selectively inhibit the early phase of nicotinic acid-induced flushing response (unpublished data by Schaefer et al). Hence in the present study we tested TRPV1 for nicotinic acid response.
Material and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Trpv1−/− Knockout Mice Exhibited Much Reduced Response to Nicotinic Acid
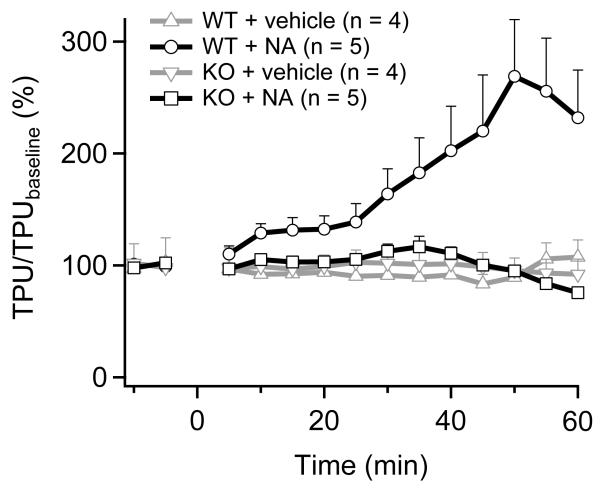
To develop an animal model of nicotinic acid-induced vasodilation, we used laser Doppler perfusion imaging (LDPI) to examine the cutaneous perfusion increase (vasodilation) in mouse. Nicotinic acid (dissolved in physiological saline, pH 7.4) was administered subcutaneously to anesthetized mice at a dosage of 120 mg/kg. Change in the ear blood flow was measured with a laser Doppler flowmeter. As shown in Fig. 1, wildtype mice responded to nicotinic acid treatment with a substantial increase in blood flow 27, which is similar to the nicotinic acid-induced flushing in human 28, 29. Noticeably, the nicotinic acid response was substantially reduced in Trpv1−/− mice. In addition, neither wildtype or knockout mice exhibited detectable response to vehicle treatment. These observations are fully consistent with the hypothesis that TRPV1 serves as a target for nicotinic acid.
Figure 1.
Nicotinic acid induced cutaneous vasodilation was largely attenuated in Trpv1−/− mice. Ear blood flow (expressed as a percentage of baseline flow) in wildtype (WT) and TRPV1 knockout (KO) mice given either nicotinic acid (solved in physiological saline, pH 7.4) or vehicle (physiological saline, with osmolarity adjusted to the same level as nicotinic acid solution using glucose, pH 7.4) at 0 min is plotted over 1 hour. The normal flushing response induced by nicotinic acid was characterized by a more than 100% increase in ear blood flow, which was substantially attenuated in the Trpv1−/− knockout mice (p < 0.05, mixed-model ANOVA). Neither wildtype nor knockout mice developed a detectable response to vehicle treatment. TPU (%) denotes percentage increase in ear blood flow (tissue perfusion units) over the baseline level.
Nicotinic Acid Directly and Potently Activates TRPV1
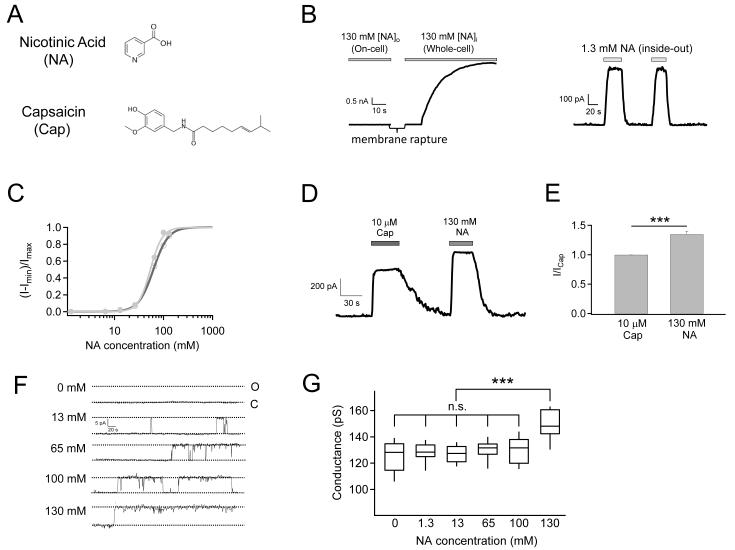
The molecular structure of nicotinic acid loosely resembles the head-group of capsaicin (Fig. 2A). To test whether nicotinic acid acts as a TRPV1 agonist, we first conducted patch-clamp recordings in either the cell-attached or the whole-cell configuration from mTRPV1-expressing HEK293 cells. When applied from the extracellular side, no channel activation was observed acutely (Fig. 2B, left). However, when applied to the intracellular side of inside-out patches, nicotinic acid strongly potentiated the channel, eliciting large currents at room temperature (Fig. 2B, right). We measured the dose-response relationship for nicotinic acid, which yielded an estimated EC50 value of 62.34 ± 0.75 mM (n = 4) (Fig. 2C). Since the EC50 value for capsaicin under the same conditions was 157.71 ± 16.87 nM (n = 4), nicotinic acid is a much less potent agonist for TRPV1. The Hill coefficient of nicotinic acid response was estimated to be 2.87 ± 0.09 (n = 4), suggesting the binding of at least three nicotinic acid molecules that promote channel activation with positive cooperativity (Fig. 2C).
Figure 2.
Nicotinic acid activates mTRPV1 at room temperature. (A) Molecular structures of nicotinic acid and capsaicin. (B) Nicotinic acid (pH 7.4) directly activates TRPV1 from the intracellular side but not from the extracellular side. Representative recordings with nicotinic acid in the pipette solution (left) or in perfusion solution (right) using the indicated patch configurations. (C) Nicotinic acid dose-response curves before (open circle) and after (filled circle) correction for the change in single-channel conductance. Superimposed are fits of a Hill equation with (before correction, black) EC50 = 62.34 ± 0.75 mM, slope factor = 2.87 ± 0.09 (n = 4), and (after correction, gray) EC50 = 53.9 ± 1.81 mM, slope factor = 3.53 ± 0.32 (n = 4). (D) Representative currents showing the relative efficacy of nicotinic acid and capsaicin as agonists. (E) Quantitative summary of the efficacy of 130 mM Nicotinic acid relative to 10 μM capsaicin on TRPV1 activation (n = 4). (F) Representative single-channel current traces activated by nicotinic acid at the indicated concentrations. (G) Box-and-whisker plot of conductance measurements. The whisker top, box top, line inside the box, box bottom, and whisker bottom represent the maximum, 75th percentile, median, 25th percentile, and minimum value of each pool of conductance measurements, respectively. n = 4. ***, p < 0.001; n.s., not significant.
While the apparent binding affinity for nicotinic acid is low, we found surprisingly that its efficacy is even higher than capsaicin. At 130 mM, nicotinic acid elicited a current ~35% higher than 10 μM capsaicin (a saturating concentration, Fig. 2 D&E). Given that capsaicin at saturating concentrations activates TRPV1 to an open probability of ~80%, the observation indicates that the larger current elicited by nicotinic acid could not simply be due to a higher maximum open probability; instead, there has to be an increase in single-channel conductance, or both open probability and conductance. Single-channel recordings confirmed that indeed nicotinic acid activates TRPV1 dose-dependently, reaching a very high open probability at 130 mM (Fig. 2F). In addition, while the single-channel conductance at most nicotinic acid concentrations was similar to that of capsaicin-induced currents, at 130 mM the conductance increased significantly (Fig. 2 F&G). Correction for the change in single-channel conductance slightly shifted the dose-response curve (Fig. 2C). Since the concentration required to exhibit a permeation effect was much higher than that for gating, nicotinic acid must bind to a distinct site (or sites, most likely close to the pore) to affect conductance.
In summary, our results confirmed that nicotinic acid dose-dependently activates TRPV1. Compared to capsaicin, nicotinic acid exhibits a much lower apparent affinity but higher efficacy, due to a combination of gating (higher open probability) and permeation (higher conductance) effects.
Human TRPV1 Exhibits High Nicotinic Acid Sensitivity
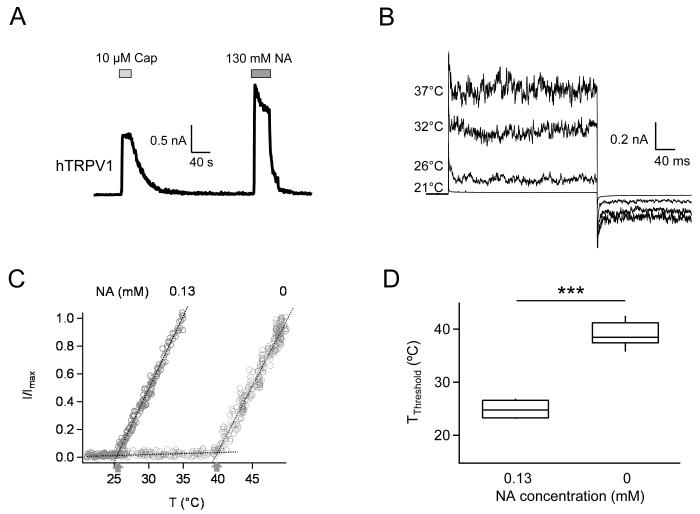
TRPV1 channels from different species exhibit distinct properties 20, 30, 31. In order to make sure that knock-out mice and the mouse TRPV1 channel are suitable models for the study of nicotinic acid effects in human, we repeated patch recordings from cells expressing human TRPV1 channels. We found that nicotinic acid could also potently activate hTRPV1 from the intracellular side (Fig. 3A). Most properties tested, for example, the relative amplitudes between capsaicin and nicotinic acid-induced currents, were similar to those of mTRPV1. To test the sensitivity of hTRPV1 to nicotinic acid, the 0.13 mM concentration (a clinically attainable concentration in the plasma during niacin treatment) was used while the recording temperature was raised to 37°C (near normal human body temperature). We found that indeed even at this low concentration nicotinic acid could already activate hTRPV1 when the recording temperature was higher than room temperature; at 37°C robust currents were observed (Fig. 3B). As expected, in the absence of nicotinic acid, hTRPV1 was not activated at 37°C but could be heat-activated at higher temperatures (38.92 ± 0.68°C, n = 12; Fig. 3C). In the presence of 0.13 mM nicotinic acid, the channel started to be heat-activated at significantly lower temperatures (24.88 ± 0.86°C, n = 4; p < 0.001; Fig. 3C&D). Since we did not observe detectable activity from the mouse TRPV1 at this concentration even at 37°C (data not shown), it appears that the human TRPV1 is more sensitive to nicotinic acid. However, since hTRPV1 current in inside-out patches inactivated rapidly (see, for example, Fig. 3A), biophysical analyses were carried out using the mouse TRPV1.
Figure 3.
Nicotinic acid activates human TRPV1 with higher sensitivity. (A) Representative current trace recorded from an inside-out patch exposed to capsaicin and nicotinic acid. (B) Current traces recorded at different temperatures in the presence of 0.13 mM nicotinic acid. (C) Heat-dependent activation in the absence and presence of 0.13 mM nicotinic acid. Dotted lines indicate the baseline current and the channel current. Arrows indicate the activation threshold temperature. (D) Comparison of activation threshold temperatures. n = 4 for 0.13 mM nicotinic acid application and 12 for control condition, respectively. ***, p < 0.001.
Nicotinic Acid and Capsaicin Activate TRPV1 Synergistically
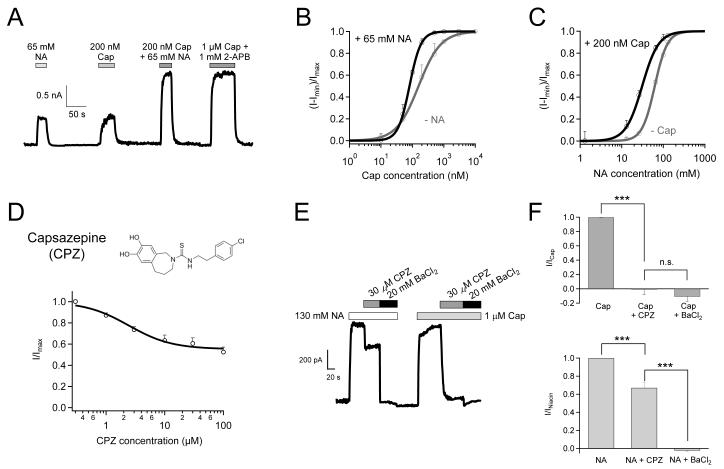
TRPV1 is a well-known molecular integrator of many chemical and physical stimuli that elicit pain. In order to understand how nicotinic acid, a new TRPV1 agonist, interacts with the channel, we first investigated the relationship between nicotinic acid and capsaicin. We observed that a combined application of low concentrations of nicotinic acid and capsaicin potentiated TRPV1 to a greater extent than either of them applied independently (Fig. 4A). Due to this synergistic effect, 65 mM nicotinic acid (a near-EC50 concentration) left-shifted the capsaicin dose-response curve appreciably, reducing the EC50 value for capsaicin by about one-half (from 157.71 ± 16.87 nM to 81.04 ± 16.4 nM; p < 0.05) (Fig. 4B). This change makes capsaicin a more potent agonist in the presence of nicotinic acid. Interestingly, the Hill coefficient was also doubled in the presence of nicotinic acid, indicating that nicotinic acid promotes the cooperativity of capsaicin-induced activation gating.
Figure 4.
Nicotinic acid and capsaicin synergistically activate TRPV1 and have different binding sites. (A) Representative currents activated by low concentrations of nicotinic acid and capsaicin independently or jointly, compared to the fully activated current by a combination of 1 μM capsaicin and 1 mM 2-APB. (B) Capsaicin dose-response curves with (green) or without (grey) 65 mM nicotinic acid. (C) Nicotinic acid dose-response curves with (green) or without (grey) 200 nM capsaicin. Parameters (EC50 and slope factor) for the Hill fits are: capsaicin without nicotinic acid, 157.71 ± 16.87 nM and 1.23 ± 0.13; capsaicin with nicotinic acid, 81.04 ± 16.4 nM and 2.4 ± 0.37; nicotinic acid without capsaicin, as in Fig. 2D; nicotinic acid with capsaicin, 31.04 ± 2.59 mM and 2.2 ± 0.47. n = 4 each. (D) The molecular structure of capsazepine and Dose-response curve of capsazepine inhibition of 130 mM nicotinic acid activated TRPV1 current. (E) Representative currents sequentially stimulated by 130 mM nicotinic acid or 1 μM capsaicin in the absence or presence of 30 μM capsazepine or 20 mM BaCl2. (F) Currents normalized to the response to 1 μM capsaicin (top) or 130 mM nicotinic acid (bottom) as in the experiment in (E). n = 4 each. ***, p < 0.001; n.s., not significant.
Conversely, capsaicin at 200 nM (a near-EC50 concentration) also shifted the nicotinic acid dose-response curve to the left, reducing its EC50 value by about 50% (Fig. 4C). This was achieved without a statistically significant change in the Hill coefficient value. In summary, nicotinic acid and capsaicin mutually potentiate each other in activating TRPV1.
Nicotinic Acid and Capsaicin Bind to Different Channel Sites
To test whether nicotinic acid binds to the same binding site for capsaicin, which is located at a pocket formed by S2-S3 linker, S3, S4, S4-S5 linker, S5 and S631, 32, we used a TRPV1 antagonist, capsazepine (CPZ) (Fig. 4D, top). As a capsaicin analog, CPZ is known to compete for the same binding sites in TRPV1; however, being an antagonist, binding of CPZ inhibits TRPV1 activity induced by capsaicin with an IC50 of 420 nM 33, whereas inhibits nicotinic acid-induced TRPV1 currents with an IC50 of 2.42 ± 0.66 μM (n = 3; Fig. 4D, bottom). As expected, we observed that CPZ at 30 μM (a saturating concentration) almost completely inhibited capsaicin-evoked currents (Fig. 4E and 4F top panel). However, at this high concentration, CPZ could only partially block channel activation triggered by 130 mM nicotinic acid (Fig. 4E and 4F bottom panel). As a control, 20 mM intracellular Ba2+ (a pore blocker 34) fully inhibited TRPV1 currents induced by either capsaicin or nicotinic acid (Fig. 4E&4F). These results confirmed that nicotinic acid and capsaicin bind to distinct sites in TRPV1.
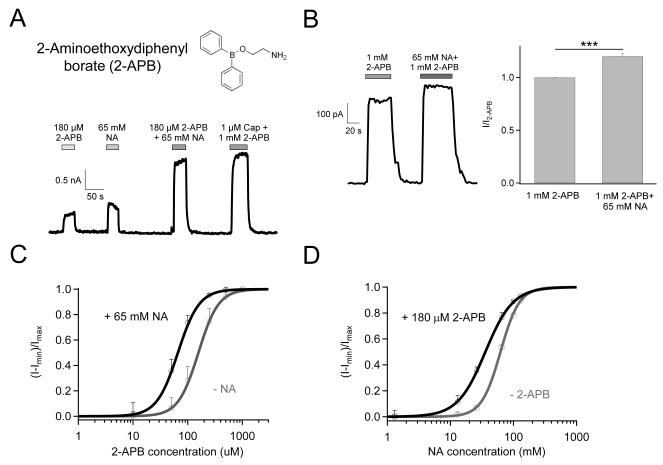
Nicotinic Acid and 2-Aminoethoxydiphenyl borate (2-APB) Also Activate TRPV1 Synergistically
2-APB (Fig. 5A, top) is a common activator for TRPV1-3 channels 35. For TRPV1, 2-APB exhibited an EC50 value of 157.2 ± 17.9 μM (n = 4), making 1 mM a saturating concentration (Fig. 5C). We observed that nicotinic acid and 2-APB also exhibited synergistic effects in promoting TRPV1 activation. The current amplitude induced by 180 μM 2-APB was significantly increased in the presence of 65 mM nicotinic acid (Fig. 5A, bottom). Co-application of 65 mM nicotinic acid also further promoted open probability of the channel activated by 2-APB at saturating concentration (Fig. 5B). As further evidence of synergistic effects, 65 mM nicotinic acid left-shifted the 2-APB dose-response curve without changing the Hill slope factor (Fig. 5C), while 180 μM 2-APB left-shifted the nicotinic acid dose-response curve but also significantly reduced the Hill slope factor (Fig. 5D).
Figure 5.
Nicotinic acid and 2-APB synergistically activate TRPV1. (A) Molecular structure of 2-APB (top) and representative currents elicited by 65 mM nicotinic acid and 180 μM 2-APB independently or jointly (bottom). (B) Additive effect of nicotinic acid on 2-APB-induced channel activation shown as representative recording (left) and statistical analysis (right, n = 11. ***, p < 0.001). (C) 2-APB dose-response curves with (green) or without (grey) 65 mM nicotinic acid. (D) Nicotinic acid dose-response curves with (green) or without (grey) 180 μM 2-APB. Superimposed are fits of a Hill equation with the following parameters (EC50 and slope factor): 2-APB without nicotinic acid, 157.2 ± 17.9 μM and 2.35 ± 0.15; 2-APB with nicotinic acid, 66.28 ± 7.69 μM and 2.24 ± 0.31; Nicotinic acid without 2-APB, as in Fig. 2D; Nicotinic acid with 2-APB, 35.81 ± 2.7 mM and 1.98 ± 0.29. n = 4 each.
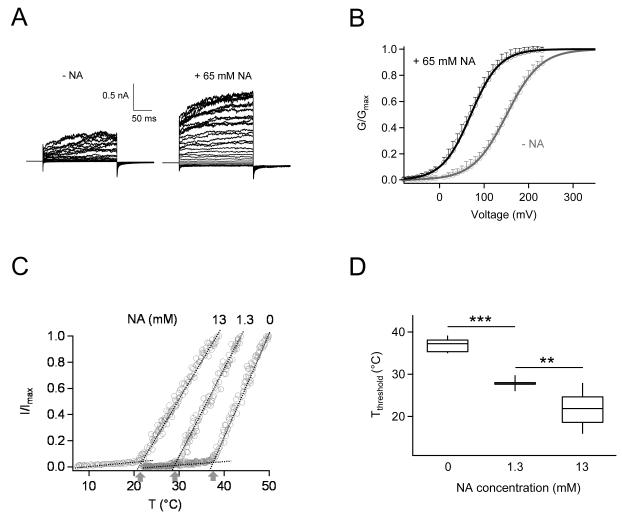
Nicotinic Acid Promotes Voltage- and Heat-Dependent TRPV1 Activation
Results so far demonstrated that nicotinic acid both directly activates TRPV1 and facilitates channel activation by other agonists. Since TRPV1 is a polymodal sensor for both chemical and physical stimuli, in order to fully understand how nicotinic acid promotes TRPV1 activity under physiological conditions, we investigated effects of nicotinic acid on voltage- and heat-dependent channel activation. As shown in Fig. 6A, voltage-dependent activation was clearly boosted in the presence of 65 mM nicotinic acid. As a result, there is a significant left-shift of the G-V curve, with half-activation voltage changing from 150.9 ± 5.34 mV to 70.21 ± 6.55 mV (Fig. 6B). This shift would not only make depolarization more effective in activating TRPV1, but also confer a higher open probability at the resting membrane potential (−20 mV to −30 mV in keratinocytes; comparing to −60 mV to −70 mV in neurons) 36, 37. However, it seems that at clinically attainable concentrations, nicotinic acid cannot appreciably activate TRPV1 by shifting voltage-dependent activation towards the resting membrane potential range.
Figure 6.
Effects of nicotinic acid on voltage- and temperature-dependent TRPV1 activation. (A) Representative current traces in response to a family of voltage steps in the absence (left) or presence (right) of 65 mM nicotinic acid. Voltage steps were applied from a holding potential of 0 mV to various membrane potentials from −80 mV to +230 mV, in 10 mV steps. (B) Normalized G-V relationships in the presence (green) or absence (grey) of nicotinic acid fitted to a Boltzmann function with the following Vhalf and k values: without nicotinic acid, 150.9 ± 5.34 mV, 37.98 ± 1.69; with nicotinic acid, 70.21 ± 6.55 mV, 32.17 ± 1.35. n = 4 each. (C) Nicotinic acid strongly potentiates the heat response of TRPV1. The takeoff temperature at the intersection of a pair of dotted lines (representing the leak current and the heat-activated TRPV1 current) is taken as the activation threshold temperature, as indicated by arrows. (D) Nicotinic acid dose-dependently lowered the activation threshold temperature of TRPV1. n = 12 (0 mM), 6 (1.3 mM), 10 (13 mM). **, p < 0.01, ***, p < 0.001.
Importantly, we found that nicotinic acid can strongly affect the heat activation of TRPV1, as demonstrated by results shown in Fig. 3. Gating of TRPV1 is very strongly temperature-dependent. The sharply defined temperature activation threshold is characteristic of the channel and can be modulated by factors such as chemical ligands and the phosphorylation state of the channel 20. This plasticity potentially confers a broader range of temperature sensitivity on TRPV1-expressing cells. We observed that, similar to other TRPV1 activators such as capsaicin, proton and Mg2+ 20, nicotinic acid substantially left-shifted the temperature dependence of TRPV1, making it easier to open at lower temperatures (Fig.6 C&D). In the absence of nicotinic acid, the heat activation threshold of TRPV1 was estimated to be 36.57 ± 0.54 °C (n = 12). With a very low concentration of 1.3 mM, nicotinic acid lowered the threshold temperature significantly to 27.92 ± 0.65 °C (n = 6; p < 0.001). Further increasing the nicotinic acid concentration to 13 mM shifted the threshold temperature to the room temperature range (22.47 ± 1.5 °C, n = 10; p < 0.005 compared to 1.3 mM nicotinic acid). Therefore, at clinically attainable concentrations nicotinic acid will cause a substantial fraction of TRPV1 channel to be heat-activated. Combining this strong sensitization effect on heat activation with potentiation effects on other activators, it is conceivable that nicotinic acid can substantially activate TRPV1 in vivo, leading to the flushing response.
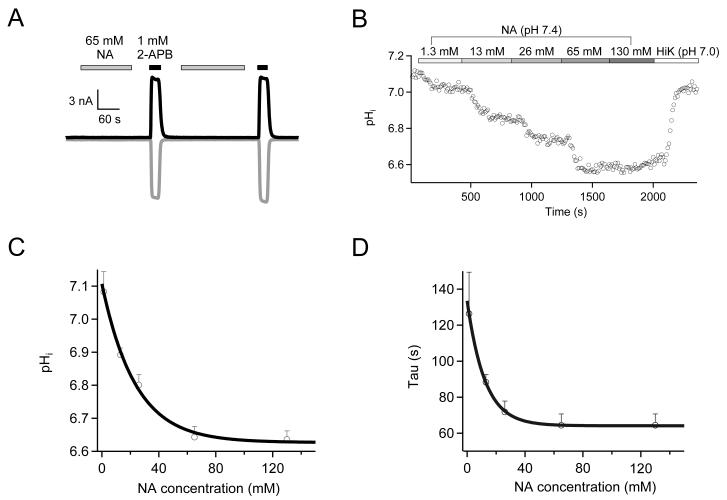
Nicotinic Acid Activates TRPV1 from the Intracellular Side
Our data showed that nicotinic acid only activates TRPV1 from the intracellular side but not from the extracellular side (Fig. 2B, Fig. 7A). This observation raised an important question, that is, could nicotinic acid from extracellular sources (blood supply) get in the cell to activate TRPV1? To address this question, we used live-cell fluorescence imaging to monitor intracellular pH (pHi) level upon extracellular application of nicotinic acid, which would drop if nicotinic acid enters the cell and acidifies the cytoplasm. We observed that, with increasing concentrations of extracellular nicotinic acid (all titrated to pH 7.4), pHi dropped exponentially over time in a concentration-dependent manner, and plateaued at 6.63 ± 0.02 (n = 3) with 130 mM nicotinic acid (Fig. 7 B&C). The rate of pHi reduction also increased exponentially when the nicotinic acid concentration was increased, reaching a saturated level of 64.4 ± 6.3 s−1 (n = 3) at room temperature when nicotinic acid concentration was higher than 60 mM (Fig. 7D). The fact that nicotinic acid translocation saturates at high concentrations points to a transporter-mediated mechanism, which is consistent with previous reports in intestinal epithelia cells 38, 39 and liver cells 40.
Figure 7.
Nicotinic acid rapidly permeates to the intracellular side. (A) No TRPV1 activation by extracellular nicotinic acid in whole-cell recordings. n = 4. (B) Representative pH imaging recording with a nicotinic acid (pH 7.4) concentration ladder applied in sequence, followed by a high potassium solution with nigericin (pH 7.0). (C&D) Amplitude (C) or time constant (D) of intracellular pH change (pHi) is plotted against the nicotinic acid concentration and fitted to an exponential function. n = 3 each.
Discussion
Nicotinic acid remains an underutilized therapy for dyslipidemias and cardiovascular disease due to the strong unwanted “niacin flush” side effect. While nicotinic acid binds to HCA2, resulting in catabolism of arachidonic acid and release of prostaglandins, there are data suggesting that nicotinic acid may cause vasodilation by other mechanisms. Results presented in the present study on knockout animal physiology, electrophysiology and pharmacology, as well as live-cell fluorescence imaging collectively demonstrate that nicotinic acid directly and potently activates TRPV1. The concentrations needed to elicit an effect are within the range attainable in patient plasma 41. Our findings present a likely additional pathway for the “niacin flushing” response that substantially hinders the highly beneficial nicotinic acid treatment. As a member of the B family vitamins, nicotinic acid at normal physiological concentrations regulates blood cholesterol and fat in part by interacting with HCA2 in adipocytes.
Since under clinical settings the blood concentration of nicotinic acid is substantially raised 41, unintended targets such as the polymodal cellular sensor TRPV1 are activated. The noticeable low apparent binding affinity of nicotinic acid to TRPV1 is consistent with this view. Indeed, while TRPV1 in nerve terminals under the skin is thought to serve as a primary temperature sensor, it is also abundantly expressed in internal organs where temperature variation is minimal. It is thought that TRPV1 serves its physiological role in these organs as a nociceptor through activation induced by endogenous ligands or extracellular H+ (for example, under inflammatory or ischemic conditions) 42. Nicotinic acid appears to be yet another chemical compound that TRPV1 can sense. The flushing response to nicotinic acid is a multi-component complex biological event that, in blood flow profiles, is presented as a biphasic increase in dermal blood flow 16. In a mouse model with repetitive capsaicin application-induced tachyphylaxis, acute exposure to nicotinic acid resulted in a much diminished initial flushing response compared to control, thus significantly blunted the vasodilatory response of nicotinic acid (unpublished data by Schaefer et al). The observation further underpins the proposed process of TRPV1-mediated flushing response.
Our results further suggest that nicotinic acid activates TRPV1 under physiological conditions predominantly through a shift of the heat activation threshold. While the voltage-dependent activation and ligand-dependent activation are also affected, it appears that these processes are not capable of independently bringing the activity of TRPV1 to a level sufficient to produce a physiological response. Nonetheless, it is possible that these processes may contribute to the physiological effects of nicotinic acid through a multi-allosteric mechanism 43. Further investigations under more physiological conditions are needed to elucidate the details on how nicotinic acid alters cellular physiology.
The emerging picture from the present study is as follows. When a patient undergoes treatment, the blood nicotinic acid concentration elevates significantly. Nicotinic acid is transported into Langerhans cells and keratinocytes by a transporter yet to be identified. Transporters for nicotinic acid have been functionally identified, however, their molecular identify remain unknown 38-40. As has been previously proposed, the ubiquitous H+-coupled monocarboxylate transporters and Na+-coupled monocarboxylate transporters are very interesting potential candidates underlying nicotinic acid transportation 39, 44-46. Monocarboxylate transporters are known to transport L-lactate, pyruvate, the ketone bodies and many other monocarboxylates across the plasma membrane 47. Once entered into the cytosol, nicotinic acid binds to TRPV1 and causes the channel to be heat-activated at physiological temperatures. Potentiation of ligandand voltage-dependent activations may contribute to this process. Activation of TRPV1 leads to Ca2+ influx into Langerhans cells and keratinocytes, causing downstream vasodilation and nerve sensation. Studies with Trpv1−/− knockout mice suggested that missing the TRPV1 channel leads to vasoconstriction 48, supporting the notion that up-regulation of TRPV1 activity by nicotinic acid or other means may lead to vasodilation.
Conclusions
Identification of TRPV1 activation as a likely candidate mediating nicotinic acid-induced flushing side-effects opens up new ways to improve patient compliance of this beneficial treatment. Indeed, since TRPV1 is a polymodal receptor, its activity can be regulated by numerous physical and chemical methods. The channel thus presents ample opportunities to reduce the flushing response.
Supplementary Material
Significance.
Nicotinic acid (a.k.a. niacin or vitamin B3) is widely used for treating dyslipidemias to reduce the risk of mortality from cardiovascular disease. Nonetheless, a substantial fraction of patients discontinue the treatment due to a strong side effect of cutaneous vasodilation, commonly termed flushing. In the present study we identified the polymodal capsaicin/heat receptor TRPV1 ion channel as a molecular target of nicotinic acid at the clinical dosage. We demonstrated that nicotinic acid directly and strongly activates TRPV1, by interacting with the intracellular side of the channel and lowering the channel’s heat activation threshold. Our observations suggest that TRPV1 is a potential target mediating nicotinic acid’s vasodilation side effect, pointing to a novel pathway to inhibit flushing and improve compliance.
Acknowledgments
We thank Dr. Manuel Navedo for sharing the tsA201 strain of HEK cells, Dr. Natalie Yuen for assistance in fluorescence imaging, Drs. Donald Bers and Manuel Navedo for comments on the manuscript, and our lab members for assistance and helpful discussion.
Sources of Funding This work was supported in part by National Institutes of Health grant R01NS072377 (to J.Z.), and an Australian National Health and Medical Research Council fellowship (to L.M.).
Abbreviations
- NA
nicotinic acid
- mTRPV1
mouse TRPV1
- hTRPV1
human TRPV1
- CPZ
capsazepine
- HCA2
hydroxy-carboxylic acid receptor 2
- 2-APB
2-Aminoethoxydiphenyl borate
- pHi
intracellular pH
Footnotes
Disclosures None.
References
- 1.Altschul R, Hoffer A, Stephen JD. Influence of nicotinic acid on serum cholesterol in man. Archives of biochemistry and biophysics. 1955;54:558–559. doi: 10.1016/0003-9861(55)90070-9. [DOI] [PubMed] [Google Scholar]
- 2.Vosper H. Niacin: A re-emerging pharmaceutical for the treatment of dyslipidaemia. Br J Pharmacol. 2009;158:429–441. doi: 10.1111/j.1476-5381.2009.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K, Offermanns S. Puma-g and hm74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nature medicine. 2003;9:352–355. doi: 10.1038/nm824. [DOI] [PubMed] [Google Scholar]
- 4.Wise A, Foord SM, Fraser NJ, et al. Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem. 2003;278:9869–9874. doi: 10.1074/jbc.M210695200. [DOI] [PubMed] [Google Scholar]
- 5.Richman JG, Kanemitsu-Parks M, Gaidarov I, et al. Nicotinic acid receptor agonists differentially activate downstream effectors. J Biol Chem. 2007;282:18028–18036. doi: 10.1074/jbc.M701866200. [DOI] [PubMed] [Google Scholar]
- 6.Gille A, Bodor ET, Ahmed K, Offermanns S. Nicotinic acid: Pharmacological effects and mechanisms of action. Annual review of pharmacology and toxicology. 2008;48:79–106. doi: 10.1146/annurev.pharmtox.48.113006.094746. [DOI] [PubMed] [Google Scholar]
- 7.Walters RW, Shukla AK, Kovacs JJ, Violin JD, DeWire SM, Lam CM, Chen JR, Muehlbauer MJ, Whalen EJ, Lefkowitz RJ. Beta-arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. The Journal of clinical investigation. 2009;119:1312–1321. doi: 10.1172/JCI36806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JM, Robson MD, Yu LM, Shirodaria CC, Cunnington C, Kylintireas I, Digby JE, Bannister T, Handa A, Wiesmann F, Durrington PN, Channon KM, Neubauer S, Choudhury RP. Effects of high-dose modified-release nicotinic acid on atherosclerosis and vascular function: A randomized, placebo-controlled, magnetic resonance imaging study. Journal of the American College of Cardiology. 2009;54:1787–1794. doi: 10.1016/j.jacc.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 9.Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: Arbiter 3. Current medical research and opinion. 2006;22:2243–2250. doi: 10.1185/030079906x148508. [DOI] [PubMed] [Google Scholar]
- 10.Karas RH, Kashyap ML, Knopp RH, Keller LH, Bajorunas DR, Davidson MH. Long-term safety and efficacy of a combination of niacin extended release and simvastatin in patients with dyslipidemia: The oceans study. American journal of cardiovascular drugs : drugs, devices, and other interventions. 2008;8:69–81. doi: 10.2165/00129784-200808020-00001. [DOI] [PubMed] [Google Scholar]
- 11.Kamal-Bahl S, Watson DJ, Ambegaonkar BM. Patients’ experiences of niacin-induced flushing in clinical practice: A structured telephone interview. Clinical therapeutics. 2009;31:130–140. doi: 10.1016/j.clinthera.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson TA. A “hot” topic in dyslipidemia management--“how to beat a flush”: Optimizing niacin tolerability to promote long-term treatment adherence and coronary disease prevention. Mayo Clinic proceedings. Mayo Clinic. 2010;85:365–379. doi: 10.4065/mcp.2009.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cefali EA, Simmons PD, Stanek EJ, McGovern ME, Kissling CJ. Aspirin reduces cutaneous flushing after administration of an optimized extended-release niacin formulation. International journal of clinical pharmacology and therapeutics. 2007;45:78–88. doi: 10.5414/cpp45078. [DOI] [PubMed] [Google Scholar]
- 14.McKenney J, Bays H, Koren M, Ballantyne CM, Paolini JF, Mitchel Y, Betteridge A, Kuznetsova O, Sapre A, Sisk CM, Maccubbin D. Safety of extended-release niacin/laropiprant in patients with dyslipidemia. Journal of clinical lipidology. 2010;4:105–112. e101. doi: 10.1016/j.jacl.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Benyo Z, Gille A, Kero J, Csiky M, Suchankova MC, Nusing RM, Moers A, Pfeffer K. Offermanns S. Gpr109a (puma-g/hm74a) mediates nicotinic acid-induced flushing. The Journal of clinical investigation. 2005;115:3634–3640. doi: 10.1172/JCI23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanson J, Gille A, Zwykiel S, Lukasova M, Clausen BE, Ahmed K, Tunaru S, Wirth A, Offermanns S. Nicotinic acid- and monomethyl fumarate-induced flushing involves gpr109a expressed by keratinocytes and cox-2-dependent prostanoid formation in mice. The Journal of clinical investigation. 2010;120:2910–2919. doi: 10.1172/JCI42273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell LC, Burchiel KJ. Neurophysiological effects of capsaicin. Brain research. 1984;320:165–176. doi: 10.1016/0165-0173(84)90005-5. [DOI] [PubMed] [Google Scholar]
- 18.Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R64–76. doi: 10.1152/ajpregu.00446.2006. [DOI] [PubMed] [Google Scholar]
- 19.Clapham DE. Trp channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 20.Zheng J. Molecular mechanism of trp channels. Comprehensive Physiology. 2013:221–242. doi: 10.1002/cphy.c120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 22.Benyo Z, Gille A, Bennett CL, Clausen BE, Offermanns S. Nicotinic acid-induced flushing is mediated by activation of epidermal langerhans cells. Molecular pharmacology. 2006;70:1844–1849. doi: 10.1124/mol.106.030833. [DOI] [PubMed] [Google Scholar]
- 23.Maciejewski-Lenoir D, Richman JG, Hakak Y, Gaidarov I, Behan DP, Connolly DT. Langerhans cells release prostaglandin d2 in response to nicotinic acid. The Journal of investigative dermatology. 2006;126:2637–2646. doi: 10.1038/sj.jid.5700586. [DOI] [PubMed] [Google Scholar]
- 24.Bodo E, Kovacs I, Telek A, Varga A, Paus R, Kovacs L, Biro T. Vanilloid receptor-1 (vr1) is widely expressed on various epithelial and mesenchymal cell types of human skin. The Journal of investigative dermatology. 2004;123:410–413. doi: 10.1111/j.0022-202X.2004.23209.x. [DOI] [PubMed] [Google Scholar]
- 25.Denda M, Fuziwara S, Inoue K, Denda S, Akamatsu H, Tomitaka A, Matsunaga K. Immunoreactivity of vr1 on epidermal keratinocyte of human skin. Biochem Biophys Res Commun. 2001;285:1250–1252. doi: 10.1006/bbrc.2001.5299. [DOI] [PubMed] [Google Scholar]
- 26.Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inceoglu AB, Clifton HL, Yang J, Hegedus C, Hammock BD, Schaefer S. Inhibition of soluble epoxide hydrolase limits niacin-induced vasodilation in mice. Journal of cardiovascular pharmacology. 2012;60:70–75. doi: 10.1097/FJC.0b013e3182580a5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng K, Wu TJ, Wu KK, Sturino C, Metters K, Gottesdiener K, Wright SD, Wang Z, O’Neill G, Lai E, Waters MG. Antagonism of the prostaglandin d2 receptor 1 suppresses nicotinic acid-induced vasodilation in mice and humans. Proc Natl Acad Sci U S A. 2006;103:6682–6687. doi: 10.1073/pnas.0601574103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai E, De Lepeleire I, Crumley TM, Liu F, Wenning LA, Michiels N, Vets E, O’Neill G, Wagner JA, Gottesdiener K. Suppression of niacin-induced vasodilation with an antagonist to prostaglandin d2 receptor subtype 1. Clinical pharmacology and therapeutics. 2007;81:849–857. doi: 10.1038/sj.clpt.6100180. [DOI] [PubMed] [Google Scholar]
- 30.Gavva NR, Klionsky L, Qu Y, et al. Molecular determinants of vanilloid sensitivity in trpv1. J Biol Chem. 2004;279:20283–20295. doi: 10.1074/jbc.M312577200. [DOI] [PubMed] [Google Scholar]
- 31.Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- 32.Cao E, Liao M, Cheng Y, Julius D. Trpv1 structures in distinct conformations reveal activation mechanisms. Nature. 2013;504:113–118. doi: 10.1038/nature12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bevan S, Hothi S, Hughes G, James IF, Rang HP, Shah K, Walpole CS, Yeats JC. Capsazepine: A competitive antagonist of the sensory neurone excitant capsaicin. Br J Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang F, Cui Y, Wang K, Zheng J. Thermosensitive trp channel pore turret is part of the temperature activation pathway. Proc Natl Acad Sci U S A. 2010;107:7083–7088. doi: 10.1073/pnas.1000357107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clapham DE. Snapshot: Mammalian trp channels. Cell. 2007;129:220. doi: 10.1016/j.cell.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 36.Wohlrab D, Wohlrab J, Markwardt F. Electrophysiological characterization of human keratinocytes using the patch-clamp technique. Experimental dermatology. 2000;9:219–223. doi: 10.1034/j.1600-0625.2000.009003219.x. [DOI] [PubMed] [Google Scholar]
- 37.Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia AS, McNamara JO, SM W. Neuroscience. Sinauer Associates, Inc.; Sunderland (MA): 2001. [Google Scholar]
- 38.Kumar JS, Subramanian VS, Kapadia R, Kashyap ML, Said HM. Mammalian colonocytes possess a carrier-mediated mechanism for uptake of vitamin b3 (niacin): Studies utilizing human and mouse colonic preparations. American journal of physiology. Gastrointestinal and liver physiology. 2013;305:G207–213. doi: 10.1152/ajpgi.00148.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takanaga H, Maeda H, Yabuuchi H, Tamai I, Higashida H, Tsuji A. Nicotinic acid transport mediated by ph-dependent anion antiporter and proton cotransporter in rabbit intestinal brush-border membrane. The Journal of pharmacy and pharmacology. 1996;48:1073–1077. doi: 10.1111/j.2042-7158.1996.tb05902.x. [DOI] [PubMed] [Google Scholar]
- 40.Said HM, Nabokina SM, Balamurugan K, Mohammed ZM, Urbina C, Kashyap ML. Mechanism of nicotinic acid transport in human liver cells: Experiments with hepg2 cells and primary hepatocytes. American journal of physiology. Cell physiology. 2007;293:C1773–1778. doi: 10.1152/ajpcell.00409.2007. [DOI] [PubMed] [Google Scholar]
- 41.Menon RM, Adams MH, Gonzalez MA, Tolbert DS, Leu JH, Cefali EA. Plasma and urine pharmacokinetics of niacin and its metabolites from an extended-release niacin formulation. International journal of clinical pharmacology and therapeutics. 2007;45:448–454. doi: 10.5414/cpp45448. [DOI] [PubMed] [Google Scholar]
- 42.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 43.Cao X, Ma L, Yang F, Wang K, Zheng J. Divalent cations potentiate trpv1 channel by lowering the heat activation threshold. The Journal of general physiology. 2014;143:75–90. doi: 10.1085/jgp.201311025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gopal E, Fei YJ, Miyauchi S, Zhuang L, Prasad PD, Ganapathy V. Sodium-coupled and electrogenic transport of b-complex vitamin nicotinic acid by slc5a8, a member of the na/glucose co-transporter gene family. The Biochemical journal. 2005;388:309–316. doi: 10.1042/BJ20041916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simanjuntak MT, Tamai I, Terasaki T, Tsuji A. Carrier-mediated uptake of nicotinic acid by rat intestinal brush-border membrane vesicles and relation to monocarboxylic acid transport. Journal of pharmacobio-dynamics. 1990;13:301–309. doi: 10.1248/bpb1978.13.301. [DOI] [PubMed] [Google Scholar]
- 46.Tachikawa M, Murakami K, Martin PM, Hosoya K, Ganapathy V. Retinal transfer of nicotinate by h+ -monocarboxylate transporter at the inner blood-retinal barrier. Microvascular research. 2011;82:385–390. doi: 10.1016/j.mvr.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halestrap AP. The monocarboxylate transporter family--structure and functional characterization. IUBMB life. 2012;64:1–9. doi: 10.1002/iub.573. [DOI] [PubMed] [Google Scholar]
- 48.Garami A, Pakai E, Oliveira DL, Steiner AA, Wanner SP, Almeida MC, Lesnikov VA, Gavva NR, Romanovsky AA. Thermoregulatory phenotype of the trpv1 knockout mouse: Thermoeffector dysbalance with hyperkinesis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:1721–1733. doi: 10.1523/JNEUROSCI.4671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.