Abstract
We identified cancer stem cell (CSC)-enriched populations from murine melanoma D5 syngeneic to C57BL/6 mice and the squamous cancer SCC7 syngeneic to C3H mice using ALDEFLUOR/ALDH as a marker, and tested their immunogenicity using the cell lysate as a source of antigens to pulse dendritic cells (DCs). DCs pulsed with ALDHhigh CSC lysates induced significantly higher protective antitumor immunity than DCs pulsed with the lysates of unsorted whole tumor cell lysates in both models and in a lung metastasis setting and a s.c. tumor growth setting, respectively. This phenomenon was due to CSC vaccine-induced humoral as well as cellular anti-CSC responses. In particular, splenocytes isolated from the host subjected to CSC-DC vaccine produced significantly higher amount of IFNγ and GM-CSF than splenocytes isolated from the host subjected to unsorted tumor cell lysate pulsed-DC vaccine. These results support the efforts to develop an autologous CSC-based therapeutic vaccine for clinical use in an adjuvant setting.
Keywords: Cancer Biology, Issue 83, Cancer stem cell (CSC), Dendritic cells (DC), Vaccine, Cancer immunotherapy, antitumor immunity, aldehyde dehydrogenase
Introduction
Cancer stem cells are relatively resistant to conventional chemotherapy and radiotherapy1,2. On the other hand, this population of cells might be the cells responsible for the relapse and progression of cancers after traditional cancer therapies1-4. Due to the lack of expression of differentiated tumor antigens on cancer stem cells, cancer stem cells may escape current immunologic interventions of therapy for cancer, which are mostly designed to target the antigens on the differentiated tumor cells. Therefore, development of new strategies specifically targeting and destroying the cancer stem cells may hold promises to increase the therapeutic efficacy of current cancer treatment. To this end, we isolated cancer stem cell (CSC)-enriched populations from two animal tumors (melanoma D5 and squamous cell cancer SCC7), and used them as an antigen source to pulse antigen presenting cells (dendritic cells, DC) to prepare the CSC-TPDC vaccine. We then evaluated the antitumor immunity induced by the CSC-TPDC vaccine in the syngeneic immunocompetent hosts, B6 mice and C3H mice respectively. The CSC-TPDC-induced antitumor efficacy was compared with the traditional DC vaccine pulsed with lysate from unsorted heterogeneous tumor cells (H-TPDC), which has previously been used by our group5,6, as well as by other investigators7 both in preclinical studies and in clinical trials.
Protocol
1. ALDEFLUOR Staining
Cell sample preparation: Prepare single cell suspension of tumor cells, either from cultured tumor cells or from freshly harvested tumor samples. Count the cells and adjust cell suspension to a concentration of 1 x 106 cells/ml in ALDEFLUOR Assay Buffer.
Label four 12 mm x 75 mm polystyrene test tubes as control tubes as follows: #1: Unstained, #2: ALDEFLUOR, #3: ALDEFLUOR plus DEAB, and #4: 7AAD.
For these control tubes, place 1ml cell suspension into #1 and #4, but 2 ml into tube #2. Add 5 µl DEAB (ALDH inhibitor) into tube #3 and keep on ice. Then add 10 µl activated ALDEFLUOR substrate to tube #2, mix and immediately transfer 1 ml of the mixture to tube #3.
At the same time, add 2 µl activated ALDEFLUOR substrate per million cells to the rest of the cells (sample tubes) also at 1 x 106 cells/ml in ALDEFLUOR Assay Buffer.
Incubate both the 4 control tubes and the sample tubes for 30 min in 37 °C water bath.
Following incubation, add 5 µl 7AAD into control tube #4, and 1 µl 7AAD 1 x 106 cells to the sample tube. Incubate 10 min at 4 °C.
Centrifuge all tubes for 5 min at 250 x g and remove supernatant.
Resuspend cells in ALDEFLUOR Assay Buffer.
Perform flow cytometry-based sorting.
Set the sorting gates using ALDEFLUOR-stained cells treated with DEAB as negative control and the propidium iodide (PI) 7AAD- stained cells for viability control. Based on these controls, a gate was established to distinguish the ALDEFLUOR+/ALDHhigh, D5 and SCC7 cells. These gates will then be used to sort all the sample cells prepared above for the ALDEFLUOR+/ALDHhigh D5 and SCC7 cells.
2. Preparation of Cancer Stem Cell Lysate-pulse Dendritic Cell (CSC-TPDC) Vaccine
Euthanize mice (syngeneic B6 and C3H, respectively) using CO2 and isolate femur and tibia bones.
Put the bones in 75% ethanol for 1 min at room temperature. Then wash the bones using HBSS.
Cut head off bones and use a 21 G needle and 10 ml syringe to push cells into a dish.
Aspirate and blow the cells using the syringe to make the single cell suspension.
After centrifugation in 1,500 rpm for 5 min, discard the supernatant. Then add 5 ml RBC lysis buffer to lysis the RBC for 1 min in 37 °C water bath.
Count cell number and adjust cell suspension to a concentration of 1 million cells/ml in complete medium (CM) containing 10 ng/ml GM-CSF and 10 ng/ml IL-4.
Culture the cells and compensate CM plus IL-4 and GM-CSF 3 days later.
At the 5th day, harvest the immature dendritic cells (DCs) and prepare the DC isolation medium containing 4.2 ml solution C plus 1 ml OptiPrep density gradient medium.
Suspend the cell pellets in 5 ml CM. Add the cell suspension onto the isolation medium slowly. Centrifuge at 2,000 rpm at room temperature.
Collect the DCs between the CM and isolation medium. Count cell number and adjust cell suspension to a concentration of 1 million cells/ml in culture medium containing 10 ng/ml GM-CSF and 10 ng/ml IL-4.
Prepare tumor lysates by suspending ALDHhigh or unsorted D5 or SCC7 cells in culture medium and subject to rapid freeze-thaw exposures four times followed by spin at ∼100 x g for 5 min to collect the membrane portion of the lysates.
To prepare CSC-TPDC, pulse DCs with the lysate of autologous ALDHhigh cells. To prepare H-TPDC, pulse DCs with unsorted heterogeneous tumor cell lysate. The ratio of DC to tumor cell lysate is the same.
3. Vaccination and Evaluation of the Efficacy
Vaccinate normal B6 mice respectively with 2,500 D5 CSC-TPDC or D5 H-TPDC tumor cells subcutaneously (s.c.). Vaccinate normal C3H animals s.c. with 5,000 SCC7 CSC-TPDC or SCC7 H-TPDC tumor cells, respectively.
After vaccination, challenge the B6 mice with the heterogeneous D5 tumor cells i.v. Euthanize the mice using CO2 20 days later. Harvest the lungs and enumerate lung metastases.
In SCC7 model, challenge the C3H mice with unsorted SCC7 tumor cells s.c on the opposite side of the DC vaccine. Monitor the tumor size.
At the end of the experiments euthanize the B6 and C3H mice using CO2. At Day 34 after first vaccine, euthanize mice with CO2, and at the same time collect the spleens of each group with an aseptic procedure.
Activate spleen T and/or B cells with immobilized anti-CD3 plus anti-CD28 mAbs in CM containing hrIL-2 or LPS plus anti-CD40 (FGK45) mAb ascites.
After activation, collect supernatants and use for ELISA assay.
For ELISA assay, coat plates with antibodies for IFNγ, GM-CSF and IgG at 4 °C O/N.
After the application of blocking buffer, add samples and standards and incubate at room temperature.
Wash the plate and add HRP detection antibody and TMB substrate for incubation.
Measure the absorbance on an ELISA plate reader at 450 nm within 30 min after stopping the reaction.
Representative Results
ALDEFLUOR/ALDH has been used as a single marker to isolate stem cells in multiple malignancies8-11. We identified cancer stem cell-enriched populations in two tumor models D5 and SCC7 by using ALDEFLUOR as a marker. We detected ALDEFLUOR+ cells in murine melanoma B16-D5 and squamous cell cancer SCC7. We found that ALDEFLUOR+ cells contribute approximately 0.5% and 5.2% in cultured D5 and SCC7 tumor cell lines, respectively (Figure 1). Freshly harvested tumor cells from established tumors have been analyzed to confirm the existence of ALDEFLUOR+ cells. As also shown in Figure 1, there were 2.5% and 4.2% of the ALDEFLUOR+ cells from in vivo established D5 and SCC7 tumors, respectively. The tumorigenicity and the self-renewal capacity of these sorted D5 and SCC7 ALDEFLUOR+/ALDHhigh populations were evaluated in the syngeneic immunocompetent host, the C57BL/6 and C3H mice, respectively8.
We have used heterogeneous unsorted tumor cell lysate to pulse DCs (H-TPDC) both in animal studies and in clinical trials5,6. To examine the immunogenicity of CSCs, we isolated ALDHhigh CSC and pulsed DC with the lysate of the CSCs to generated CSC-TPDC (Figure 2) and used H-TPDC as a conventional cancer vaccine control to test if CSC-TPDC has any beneficial effect in preventing tumor growth.
We evaluated the immunogenicity of CSCs by examining protective antitumor immunity induced by CSC-TPDC. In D5 model, naïve immunocompetent mice were vaccinated s.c with CSC-TPDC or H-TPDC (at the same lysate: DC ratio). Control groups received saline (PBS). One week after the last vaccine, the mice were challenged with unsorted D5 tumor cells intravenously (i.v.), and the lungs were harvested 3 weeks later to enumerate lung metastases. As shown in Table 1, compared with PBS group, mice treated with the H-TPDC developed less lung metastases. Importantly, mice treated with CSC-TPDC had significantly fewer lung metastases than H-TPDC vaccine group in both experiments performed. In SCC7 model, normal C3H animals were vaccinated s.c with SCC7 CSC-TPDC or H-TPDC respectively on the right flank, followed by challenging with unsorted SCC7 tumor cells s.c into the left flank. Compared with PBS group, H-TPDC induced modest anti-tumor immunity against tumor growth. However, there was significant inhibition of tumor growth in mice that were treated with CSC-TPDC when compared with both the control group and H-TPDC group (p<0.05, Figure 3). These results indicate that CSCs could be used as an more effective antigen source to load DCs than traditional unsorted tumor cells in inducing protective immunity against the challenge of tumor cells.
To further understand the mechanisms underlying the observed CSC-induced protective antitumor immunity, we harvested the spleens from the animals subjected to DC vaccinations at the end of the experiments. The spleen cells were then activated by anti-CD3/CD28/IL-2 or anti-CD3/CD28/IL-2 + LPS/anti-CD40. Then the culture supernatants were collected to detect the expression of cytokines and antibody. There were significantly higher productions of IFNγ and GM-CSF by the spleen cells from the animals vaccinated with D5 CSC-TPDC or SCC7 CSC-TPDC (Figure 4). Furthermore, there was significantly (p<0.05) higher IgG production by LPS/anti-CD40 activated spleen cells collected from the animals vaccinated with D5 CSC-TPDC or SCC7 CSC-TPDC compared with D5 H-TPDC or SCC7 H-TPDC. These antibodies were found to bind to D5 and SCC7 CSCs respectively, and such binding could result in the CSC lysis in the presence of complement8.
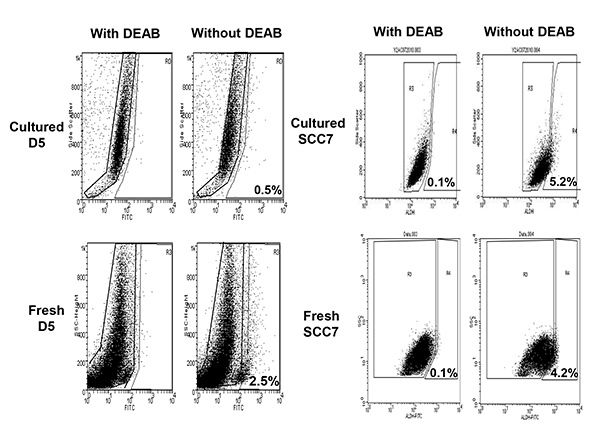 Figure 1. ALDHFLUOR+/ALDHhigh populations were detected in cultured as well as newly harvested fresh murine D5 melanoma and SCC7 squamous cell tumors. Tumor cells treated with 50 mmol/L DEAB, a specific ALDH inhibitor, were used as the negative control. Click here to view larger image.
Figure 1. ALDHFLUOR+/ALDHhigh populations were detected in cultured as well as newly harvested fresh murine D5 melanoma and SCC7 squamous cell tumors. Tumor cells treated with 50 mmol/L DEAB, a specific ALDH inhibitor, were used as the negative control. Click here to view larger image.
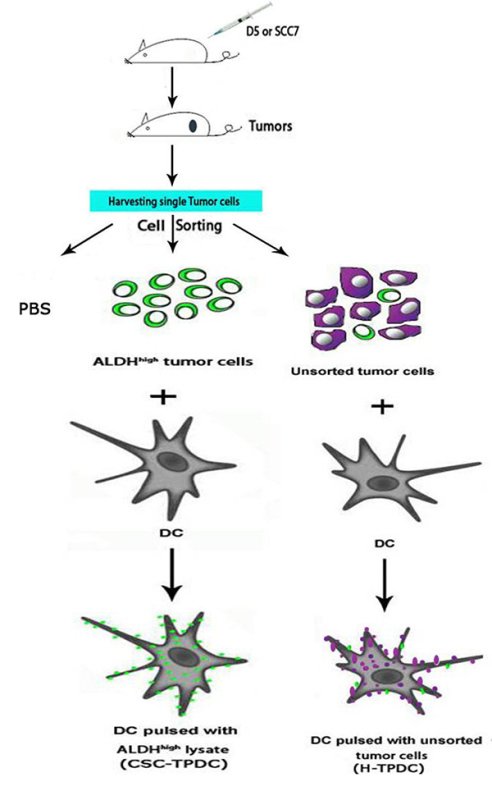 Figure 2. Generation of dendritic cell-based cancer stem cell vaccines. For the preparation of CSC-TPDC and H-TPDC, bone marrow-derived dendritic cells were pulsed with ALDHhigh or unsorted tumor cell lysates, respectively. Click here to view larger image.
Figure 2. Generation of dendritic cell-based cancer stem cell vaccines. For the preparation of CSC-TPDC and H-TPDC, bone marrow-derived dendritic cells were pulsed with ALDHhigh or unsorted tumor cell lysates, respectively. Click here to view larger image.
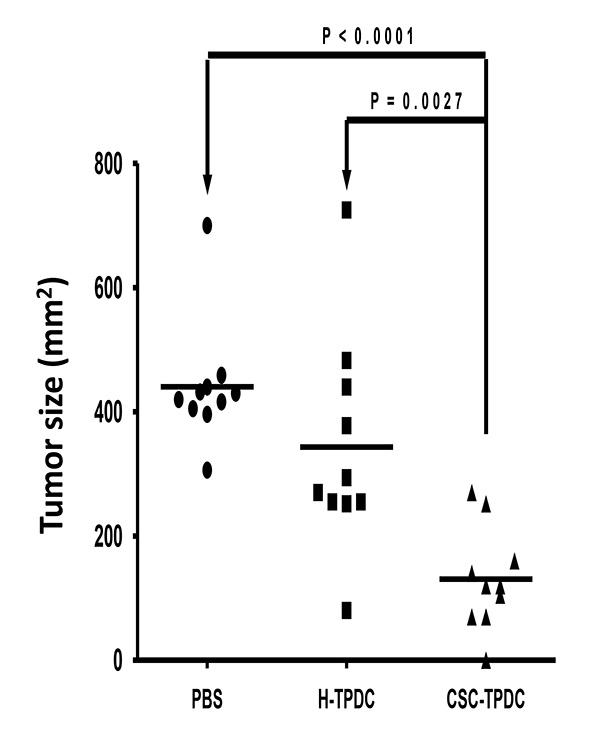 Figure 3. CSC lysate pulsed DC (CSC-TPDC) vaccine could induce more effective protective antitumor immunity in the s.c. SCC7 tumor model.
Click here to view larger image.
Figure 3. CSC lysate pulsed DC (CSC-TPDC) vaccine could induce more effective protective antitumor immunity in the s.c. SCC7 tumor model.
Click here to view larger image.
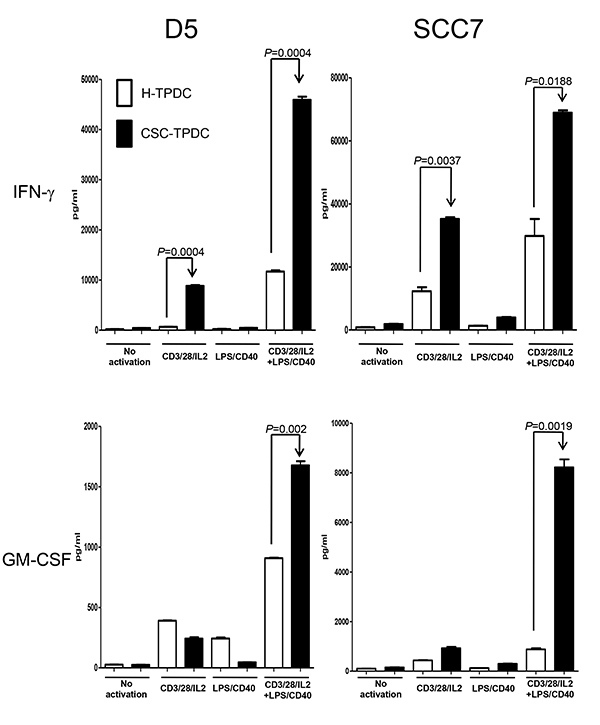 Figure 4. More potent systemic cellular responses in immunocompetent host vaccinated with CSC-TPDC. Splenocytes were harvested from the animals subjected to H-TPDC or CSC-TPDC vaccination, and were activated as indicated. The culture supernatants were then collected for cytokine detection using ELISA. Click here to view larger image.
Figure 4. More potent systemic cellular responses in immunocompetent host vaccinated with CSC-TPDC. Splenocytes were harvested from the animals subjected to H-TPDC or CSC-TPDC vaccination, and were activated as indicated. The culture supernatants were then collected for cytokine detection using ELISA. Click here to view larger image.
Discussion
The immunocompromised hosts, such as SCID mice, preclude immunological assessments of CSCs due to the lack of adaptive immunity within the hosts. In this study, we evaluated the immunogenicity of CSCs in immunocompetent hosts, which could more closely mimic patient settings. Enriched CSCs are immunogenic and could induce more effective tumor protective immunity when their lysates are loaded to DCs as a vaccine compared with unselected tumor cell lysate-pulsed DCs. Mechanistically, the protection was conferred by selective induction of CSC-reactive antibodies and T cells8 as well as the production of type 1 cytokine, e.g. IFNγ and GM-CSF.
Most of the current immunotherapies, including dendritic cell-based vaccines and the adoptive T cell transfer, are designed to target tumor- differentiated antigens. CSCs, which may not express these differentiated antigens may therefore escape these immunological targeting. In contrast, CSC vaccine designed to specifically target cancer stem cells may destroy this special population of the cancer cells, and thus improve the therapeutic efficacy of the vaccine by preventing tumor relapse and metastasis.
In both cultured tumor cells and freshly harvested tumors, we identified CSC-enriched population by flow cytometry based on high aldehyde dehydrogenase activity. Such ALDHhigh cells could be isolated by flow sorting to be used as an antigen source to pulse DC to generate CSC-TPDCs. Comparison using ALDHhigh cells isolated from cultured tumor cells vs from freshly harvested tumors demonstrated no significantly difference in term of the induction of anti-CSC immunity8. These results revealed the potential to use CSCs isolated either from cultured tumor cells or from freshly harvested tumors for clinical application.
To be clinically relevant, a vaccine needs to be examined in a therapeutic setting. These experiments are now being performed in our laboratory.
Disclosures
STEMCELL Technologies has supported Open Access publication fees.
Acknowledgments
This work was supported by The Will and Jeanne Caldwell Endowed Research Fund of the University of Michigan Comprehensive Cancer Center and in part by NIH grant CA82529 and the Gillson Longenbaugh Foundation.
References
- Shafee N, Smith CR, et al. Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors. Cancer Res. 2008;68:3243–3250. doi: 10.1158/0008-5472.CAN-07-5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi S, Ulasov IV, et al. Low-dose radiation enhances survivin-mediated virotherapy against malignant glioma stem cells. Cancer Res. 2008;68:5778–5784. doi: 10.1158/0008-5472.CAN-07-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas NA, Xia L, et al. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009;69:1951–1957. doi: 10.1158/0008-5472.CAN-08-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K, Yang M, et al. Induction of cancer metastasis by cyclophosphamide pretreatment of host mice: an opposite effect of chemotherapy. Cancer Res. 2008;68:516–520. doi: 10.1158/0008-5472.CAN-07-3063. [DOI] [PubMed] [Google Scholar]
- Chang AE, Redman BG, et al. A phase I trial of tumor lysate-pulsed dendritic cells in the treatment of advanced cancer. Clin. Cancer Res. 2002;8:1021–1032. [PubMed] [Google Scholar]
- Ito F, Li Q, Shreiner AB, et al. Anti-CD137 monoclonal antibody administration augments the antitumor efficacy of dendritic cell-based vaccines. Cancer Res. 2004;64:8411–8419. doi: 10.1158/0008-5472.CAN-04-0590. [DOI] [PubMed] [Google Scholar]
- Kirk CJ, Hartigan-O'Connor D, et al. T cell-dependent antitumor immunity mediated by secondary lymphoid tissue chemokine: augmentation of dendritic cell-based immunotherapy. Cancer Res. 2001;61:2062–2070. [PubMed] [Google Scholar]
- Ning N, Pan Q, et al. Cancer stem cell vaccination confers significant antitumor immunity. Cancer Res. 2012;72:1853–1864. doi: 10.1158/0008-5472.CAN-11-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestier C, Liu S, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J. Clin. Invest. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentino JE, Hynes MJ, et al. Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer Res. 2009;69:8208–8215. doi: 10.1158/0008-5472.CAN-09-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charafe-Jauffret E, Ginestier C, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]


