Abstract
Background
The RASopathies are a class of human genetic syndromes that are caused by germline mutations in genes which encode components of the Ras/MAPK pathway. Cardio-facio-cutaneous (CFC) syndrome is characterized by distinctive craniofacial features, congenital heart defects, and abnormalities of the skin and hair.
Objective
To systematically characterize the spectrum of dermatologic findings in mutation-positive individuals with cardio-facio-cutaneous (CFC) syndrome.
Methods
Dermatologic surveys were designed by the authors and distributed to the study participants through CFC International or directly by the authors (KAR and DHS) between July 2006 and August 2009. A second follow up survey was collected between December 2007 and August 2009. When available, digital images and medical records of the participants were obtained. Study participants included individuals with CFC who have a mutation in BRAF, MEK1, MEK2 or KRAS.
Results
Individuals with CFC have a variety of dermatologic manifestations caused by dysregulation of the mitogen-activated protein kinase pathway in development. Numerous acquired melanocytic nevi were one of the most striking features; greater than 50 nevi were reported by 23 % (14/61) of participants and of those, greater than 100 nevi were reported by 36% (5/14). Keratosis pilaris was reported in 80% (49/61) of cases. Ulerythema ophryogenes was common occurring in 55/61 (90%). Infantile hemangiomas occurred at a greater frequency, 26% (16/61), as compared to the general population.
Conclusions
CFC syndrome has a complex dermatologic phenotype with many cutaneous features, some of which allow it to be differentiated from the other Ras/MAPK pathway syndromes. Multiple café au lait macules and papillomata were not identified in this CFC cohort helping to distinguish CFC from other RASopathies, such as neurofibromatosis type 1 and Costello syndrome.
Keywords: BRAF, Cardio-facio-cutaneous syndrome, keratosis pilaris, melanocytic nevus, infantile hemangioma
INTRODUCTION
The mitogen-activated protein kinase (MAPK) pathway is one of the critical downstream effectors of Ras, a small GTPase that is vital in the modulation of numerous cellular functions. Ras has long been known to play an important role in cancer genetics. Recently, the Ras/MAPK pathway has been implicated in several developmental syndromes with cutaneous manifestations. The RASopathies are a class of human genetic syndromes that are caused by germline mutations in genes which encode components of the Ras/MAPK pathway 1,2. One of these syndromes, cardio-facio-cutaneous (CFC) syndrome, is caused by germline mutations in BRAF 3,4, MAP2K1 4, MAP2K2 4 and rarely KRAS 3. CFC syndrome is a rare, multiple congenital anomaly disorder characterized by distinctive craniofacial features, congenital heart defects, psychomotor delay, failure to thrive and abnormalities of the skin and hair. The ectodermal findings in CFC are key clinical features and were relied upon heavily in the diagnosis prior to the identification of the causative mutations. These features include keratosis pilaris, ulerythema ophryogenes, and brittle, sparse, curly hair. CFC syndrome has overlapping features with other syndromes caused by germline mutations in the Ras/MAPK pathway, most notably with Noonan syndrome 5–10 and Costello syndrome 11, and less so with LEOPARD syndrome 9,12,13, capillary malformation-arteriovenous malformation syndrome 14 and neurofibromatosis type-1 (NF1) (Table 1) 15–17. Prior to identification of the causative genes for the RASopathy syndromes, the clinical diagnosis was challenging. For example, Noonan syndrome, Costello syndrome and CFC have similar facial features, cardiac defects and learning disabilities. Many times, key cutaneous findings aid in the clinical diagnosis and assist in the stratification of genetic testing. To determine the key phenotypic characteristics, we performed a detailed analysis of cutaneous findings in 61 mutation-positive CFC individuals. This systematic dermatologic evaluation of mutation-positive CFC individuals aids us in further understanding the critical role of the MAPK pathway in human epidermal development.
Table 1.
The clinical features of the RASopathies.
| NF1 | Cardio-facio-cutaneous syndrome | Costello syndrome | Noonan syndrome | LEOPARD syndrome | Capillary malformation-arteriovenous malformation | |
|---|---|---|---|---|---|---|
| Gene | NF1 | BRAF, MEK1, MEK2, KRAS | HRAS | PTPN11, KRAS, SOS1, RAF1, NRAS, SHOC2, CBL* | PTPN11, RAF, BRAF | RASA1 |
| Predominant cutaneous features |
|
|
|
|
|
|
| Cardiac defects |
|
|
|
|
|
Rare |
| Cancer predisposition |
Yes
|
Rare
|
Yes
|
Rare
|
Rare
|
Rare
|
| Other |
|
|
|
|
|
|
Martinelli et al recently reported germline mutations in CBL (a tumor-suppressor gene commonly mutated in myeloid leukemias) in patients with a Noonan phenotype who had negative screening for mutations in the other genes linked with Noonan syndrome39.
METHODS
Study participants
The authors designed a survey to definitively delineate dermatologic manifestations, with a focus on phenotypic markers associated with other Ras/MAPK pathway syndromes (Supplementary material). The surveys were distributed to the participants with confirmed mutations via CFC International; surveys were returned by 55 participants. The surveys were coded with a CFC number prior to release to the authors. Six additional participants were enrolled in person at the CFC 2009 family conference. After receiving the initial surveys, an additional questionnaire consisting of follow-up questions was distributed by CFC International and returned by 28 individuals. When available, photographs were obtained in digital format via CFC International (20/61 participants). Dermatology clinic notes were reviewed when available. The rationale for the survey-based approach was that CFC is a very rare condition and therefore it is not possible to gather significant numbers of participants in a clinic-based setting. Approval was obtained through the Committee on Human Research at University of California San Francisco, the Institutional Review Board at Oregon Health & Science University and the ethics board for CFC International. Consent was obtained for all individuals. Only individuals with known mutations were included in this study. CFC international is an incorporated family support network that has created a clinical biobank (database) with an aim to support research on the CFC syndrome.
Caucasian ethnicity was reported by 85% (53/61) of participants. Of the remainder, Hispanic ethnicity was reported by four participants, Caucasian and Hispanic ethnicity was reported by 3 participants and one individual reported Caucasian and African-Caribbean ethnicity. The age of the participants ranged from 16 months to 31 years old, and there were 35 female and 26 male participants. The average age is 104 months and the median age is 92.5 months. The number of participants with a mutation in each gene is as follows: BRAF (47), MEK1 (11), MEK2 (2), KRAS (1) (The spectrum of mutations are listed in the supplementary material).
Limitations
The surveys were filled out in most cases by the parents of the participants rather than by a physician. Many of the participants have not been evaluated by a dermatologist. There may have been selection bias based on the subject enrollment through CFC International which may have skewing toward more severely affected individuals. The small sample size limits the power to detect differences in phenotype based on genotypes.
Statistical analysis
Statistical analysis was done using SPSS 16.0. The presence of specific findings was correlated with specific loci using a Fisher’s exact test (2-sided) and was compared to published population values using a Chi Square goodness of fit test.
RESULTS
Melanocytic nevi
Of the respondents, 23% (14/61) reported having greater than 50 melanocytic nevi and of those, 36% (5/14) reported having greater than 100 nevi (Table 2). The number of melanocytic nevi in the cohort increased with age. Respondents who were aged 7 or older were significantly more likely to be in higher nevi categories (39.4% had 50 or more nevi compared to 3.8% of respondents less than 7 years old, p=0.001 by Mann-Whitney U test). The age range for participants with greater than 50 nevi was 8 years to 17 years (n = 9) and the range for those with greater than 100 nevi was 12 years to 25 (n = 5) years.
Table 2.
Frequency of melanocytic nevi in mutation-positive CFC individuals.
| Number of Nevi |
BRAF (n =47) No. (%) |
MEK1 (n = 11) No. (%) |
MEK2 (n = 2) No. (%) |
KRAS (n=1) No. (%) |
Total (n = 61) No. (%) |
|---|---|---|---|---|---|
| 0–9 nevi | 18 (38%) | 4 (36%) | 0 (0%) | 1 (100%) | 23 (36%) |
| 10–50 nevi | 20 (42%) | 4 (36%) | 0 (0%) | 0 (0%) | 24 (40%) |
| 50+ nevi | 5 (10%) | 3 (27%) | 1 (50%) | 0 (0%) | 9 (15%) |
| 100+ nevi | 4 (8%) | 0 (0%) | 1 (50%) | 0 (0%) | 5 (8%) |
| Progressive nevi | 31 (65%) | 5 (45%) | 1 (50%) | 0 (0%) | 37 (61%) |
Of the participants with greater than 100 nevi, only one had a family history of multiple nevi (an individual with a MEK2 F57C mutation). In this family, the mother and maternal grandmother both had numerous melanocytic nevi, and the mother had a history of a melanoma. The family history of multiple nevi was positive in only two of the participants with greater than 50 nevi (one with a BRAF gene deletion of exon 11 and one with a MEK2 Y134C mutation).
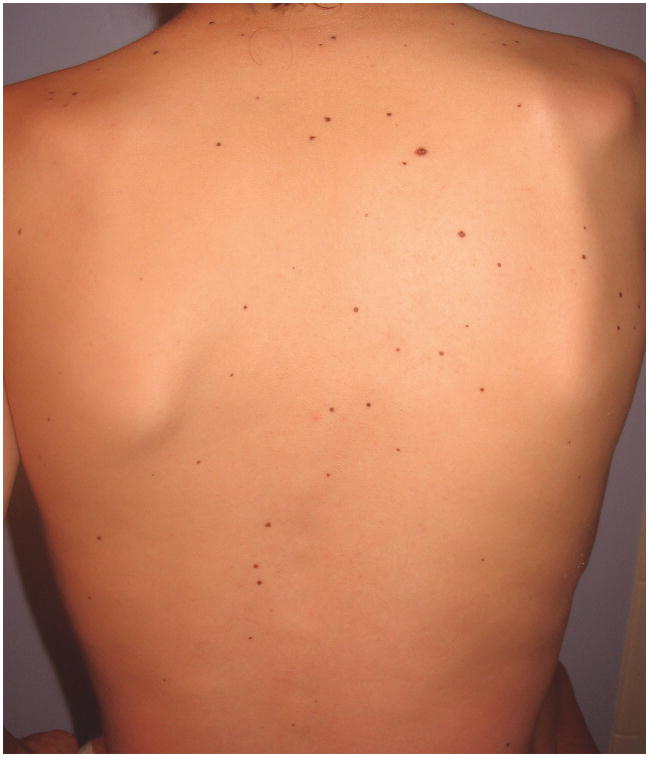
Photographic review or clinical exams of nevi were performed in 19 of the participants. The nevi appear in an even distribution over all body surface areas, including the face, torso, buttocks, extremities and palms and soles in many cases (Fig. 1). The nevi did not follow a photo-distributed pattern. The majority of nevi range in size from 2 to 6 mm and are evenly pigmented, monomorphous, medium to dark brown nevi. One individual with a BRAF Q257R mutation reported having halo nevi.
Figure 1.
Multiple melanocytic nevi which are widely distributed on the back of a 13 year old boy with a MEK2 mutation.
Keratosis pilaris
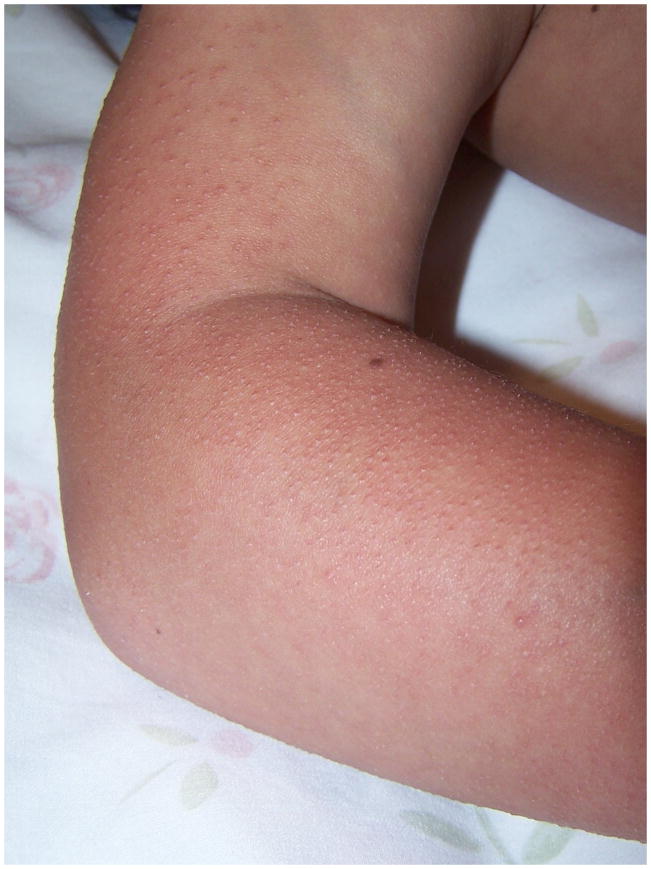
Keratosis pilaris was reported in 80% (49/61) of participants, a significantly higher frequency than the reported population average of 34% (p=0.018) 18. When analyzed specifically by gene, 12/13 (96%) with MEK1 or MEK2 mutations reported keratosis pilaris, compared with 77% (36/47) in the participants with BRAF mutations. The differences in frequency between genotypes are not statistically significant (p=0.433, Fisher’s Exact test). The location was on the face in 51% (31/61) and dorsal arms and legs in 72% (44/61) (Fig. 2). Respondents frequently mentioned involvement of the ears, back and torso.
Figure 2.
Keratosis pilaris and sparse hair on the arm of a 9 year old girl with a MEK1 mutation.
Hair
Wavy or curly hair was reported in 93% (57/61) of participants (Fig. 3a, b). Sparse hair at the temples was reported in 59% (36/61) participants. Poor hair growth on the scalp was reported in 43% (12/28), while only 5% (3/61) reported thick hair. Ulerythema ophryogenes, characterized by erythema of the brow with loss of follicles, occurred in a majority of participants, 55/61 (90%) (Fig. 4a, b). The eyebrows were sparse in 59% (36/61) and absent in 31% (19/61). Normal eyebrows were reported by 8% (5/61) of the participants and one reported thick eyebrows.
Figure 3.
a. Characteristic facial features with dark curly hair, sparse eyebrows and multiple melanocytic nevi in a 9 year old with a BRAF mutation. b. Dark curly hair, sparse eyebrows, melanocytic nevi, and characteristic facial features in a 21 year old with a BRAF mutation.
Figure 4.

a. Ulerythema ophryogenes with sparse eyebrows and erythema of the brow line in a 9 year old with a MEK1 mutation. b. Ulerythema ophryogenes of the eyebrows with keratosis pilaris rubra on the cheeks in a 3 year old with a MEK1 mutation.
Hyperkeratosis
Hyperkeratosis of the palms and soles was present in 36% (22/61) and it was noted to occur in areas of pressure (age range: 18 months–31 years) (Fig. 5).
Figure 5.
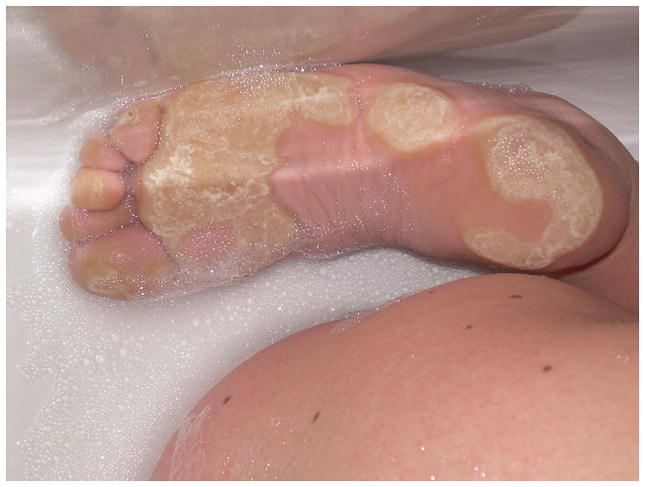
Thick hyperkeratosis on the plantar surface of the feet in a 20 year old female with a BRAF mutation. The appearance of the plantar hyperkeratosis is prominent in this image because she was soaking in the bathtub at the time photograph was taken. In addition, multiple melanocytic nevi are present on the lower back.
Infantile hemangiomas
The incidence of infantile hemangiomas was 26% (16/61). This is significantly higher than the estimated population average of 5% (p<0.001) 19. If the respondent reported having an infantile hemangioma, the diagnosis was confirmed via photographs, dermatology notes and/or a history of growth in the first several months of life followed by an involution phase. One individual with a BRAF Q257R mutation reported having greater than 100 infantile hemangiomas on his skin which were beginning to involute at the time the survey was completed (age 5 years old). That subject had a normal liver ultrasound in infancy, which was done to rule out liver hemangiomas. A large, ulcerated perianal hemangioma was reported in an individual with a KRAS D153V mutation.
Additional cutaneous features
Based on the information gained in the original survey, additional information regarding skin findings such as quantity of body hair, rate of nail growth, ear lobe creases, and acanthosis nigricans was gathered and are presented in Table 3. Ear lobe creases were reported in 43% (12/28) of participants. One subject with a MEK1 G128V mutation reported that her ears appeared “pierced” due to the crease, another individual with a BRAF Q257R mutation reported small indentations behind the ears. Ear pits were reported in an individual with a BRAF Q257R mutation.
Table 3.
Additional dermatologic features in 28 mutation positive CFC individuals.
| Total | Percentage | |
|---|---|---|
| Fast growth of nails | 23 | 82% |
| Sparse arm and leg hair | 18 | 64% |
| Prepubertal axillary odor | 13 | 46% |
| Poor hair growth on scalp | 12 | 43% |
| Ear lobe creases (now or when younger) | 12 | 43% |
| Acanthosis nigricans | 6 | 21% |
| Hyperplastic nipples | 3 | 11% |
| Creases on the fingertips | 3 | 11% |
Papillomas
The inquiry into the presence of papillomas revealed a heterogeneous group of results. Only one subject reported having multiple perianal papillomata which appeared around 4 years of age (mutation in MEK1, exon 2). Photo review of these lesions demonstrated a chronic irritant dermatitis with pseudoverrucous papules, but no true papillomas. One subject with a BRAF F595L mutation reported a solitary papilloma below the knee which presented at age 6 years. A subject with a BRAF K499N mutation reported a solitary papilloma on the dorsal surface of the nose at age 10 years, which spontaneously resolved. One subject with a BRAF Q257R mutation reported a papillomatous growth on the glabella, which in photographs had the clinical appearance of a midline fusion defect resembling a dermoid or a nasal glioma. One subject with a BRAF G464V mutation reported thick warty growths on the elbows since birth which are increasing in number. A 22 year old with a BRAF G469E mutation reported rough, hyperkeratotic skin on the back. None of the participants reported the classic nasal and perianal papillomata typically seen in Costello syndrome.
Café-au-lait macules
No participants reported greater than two café au lait macules. The percentage of participants reporting 1–2 café au lait macules was 31% (19/61).
DISCUSSION
CFC is one of the RASopathies characterized by distinctive craniofacial features, cardiac defects, neurocognitive delay and ectodermal abnormalities 2. CFC is most commonly caused by heterogeneous, heterozygous activating germline mutations in BRAF, MEK1 (MAP2K1) or MEK2 (MAP2K2) and KRAS, with approximately 75% of mutation positive individuals having BRAF mutations and 25% with either MEK1 or MEK2 mutations. KRAS mutations have been reported in less than 1% of the cases 20. In our cohort 77% had BRAF mutations while 22% had either MEK1 or MEK2 mutations and <1% (1/61) had a KRAS mutation. Although the numbers are too few to reach statistical significance, a higher percentage of the participants with MEK1 or MEK2 mutations reported dermatologic issues, such as greater than 50 nevi, keratosis pilaris and infantile hemangiomas (Table 4).
Table 4.
Genotype-phenotype comparisons.
| Gene | Keratosis pilaris | Callouses on hands or feet | Hemangioma | Hair - Curly | Eyebrows- sparse or absent | Skin darker than family | Seen by a dermatologist |
|---|---|---|---|---|---|---|---|
| BRAF | 36/47 (77%) | 18/47 (38%) | 9/47 (19%) | 45/47 (96%) | 41/47 (87%) | 13/47 (28%) | 29/47 (62%) |
| MEK1 | 10/11 (91%) | 3/11 (27%) | 5/11 (45%) | 9/11 (82%) | 11/11 (100%) | 0/11 (0%) | 9/11 (82%) |
| MEK2 | 2/2 (100%) | 1/2 (50%) | 1/2 (50%) | 2/2 (100%) | 2/2 (100%) | 1/2 (50%) | 1/2 (50%) |
| Fisher’s Exact p | 0.6358 | 0.8722 | 0.1303 | 0.2684 | 0.6630 | 0.0647 | 0.3808 |
All probabilities are 2-sided tests of the table probability.
Dermatologic findings provide key signs in distinguishing CFC from the other RASopathies. We found cutaneous abnormalities to be universally present in individuals with CFC. The development of melanocytic nevi throughout childhood is expected in the general population, but this increase was striking in the CFC cohort. In a recent longitudinal study of children from the general population in Colorado, the median number of nevi in 7 year olds was 25 and in 8 years olds was 29 21. We could not determine a median nevus count for this study due to the data collection method, however four out of six of the children aged 7–8 years old in this study reported greater than 50 nevi. One of the most striking features was the number of melanocytic nevi that develop in late adolescence in a portion of the cohort. The percentage of CFC adolescents and adults in this study with over 100 nevi was higher than expected in the general population 22. A population study in Hungary evaluating the prevalence of melanocytic nevi on a full body exam in 14–18 year olds demonstrated that 5.4% of the subjects had greater than 100 nevi. Of the age matched CFC individuals in this study, 36 % (4/11) had greater than 100 nevi 23. The age range for individuals with greater than 100 nevi was 12 years to 25 years. Only 10/61 respondents were over 12 years old, therefore it is likely that the number with 50–100 nevi will continue to increase over time. The nevi were widely distributed on all body surface areas, and were not accentuated on photo-exposed skin. The pathogenesis of the increased melanocytic nevi is not yet established, but 75% of these individuals report intolerance to the sun and sun avoidance. Therefore it is unlikely that sun exposure plays a role in the development of the nevi and it is much more likely to be a consequence of aberrant Ras/MAPK signaling.
The MAPK pathway has been studied extensively in the context of oncogenesis since its dysregulation is one of the primary causes of cancer. Ras is found to be somatically mutated in approximately 20% of malignancies 24 and hyperactivated ERK is found in approximately 30% of human cancers 25. In a recent report, increased ERK phosphorylation was demonstrated in fibroblasts from skin biopsies from a MEK2 mutation-positive individual with CFC 26. Somatic mutations in BRAF have been reported at a high frequency in melanoma and other cancers. One mutation, BRAF V600E, results in increased protein kinase activity. Somatic BRAF V600E mutations are found in benign skin lesions and premalignant colon polyps 27–29. The majority of benign nevi and primary and metastatic melanoma have BRAF V600E mutations. This suggests that MAPK activation is important in melanocytic neoplasia, but in isolation, is insufficient for tumorigenesis 29. The BRAF mutations in CFC, Noonan and LEOPARD have been shown to be less activating than the V600E mutations13. Like BRAF, mutations in MEK have been identified in cancer including ovarian 30 and lung 31, and it is unknown if MEK mutations are found in melanoma or other skin cancers. To date, there are no known reported cases of melanoma in CFC, but the cohort of patients is still relatively young.
Keratosis pilaris on the extremities and face is one of the hallmark features of CFC and was seen in 80% of subjects. This is slightly higher than the previously reported rate of 73%32. Ulerythema ophryogenes (erythema of the brow with scarring of the follicle) occurred in a majority of participants. In keratosis pilaris histopathologic features include follicular dilatation and keratin plugs with a non-specific inflammatory cell infiltrate around the follicle. In ulerythema ophryogenes, there is more intense chronic inflammation in the skin with scarring and subsequent loss of follicles 33. The rate of keratosis pilaris in the participants with MEK mutations was even higher than in the participants with BRAF mutations, occurring in 96% vs. 77% of participants. One subject, a 23 month old female, reported a KRAS mutation. The dermatologic features in this subject included curly hair, sparse eyebrows and keratosis pilaris on the arms, as well as a large infantile hemangioma on the buttocks and genital area. The subject had no nevi, but was less than two years old at the time of the survey. This finding is in contrast to the previously reported cases with KRAS mutations showing a less prominent ectodermal phenotype.
In the children in this study, hyperkeratosis of the palms and soles typically developed in pressure areas, with the feet of toddlers often being the first location noted. Hyperkeratosis in pressure areas is also commonly reported in Costello syndrome, but not in the other Ras/MAPK pathway syndromes 34.
The incidence of infantile hemangiomas in this cohort is much higher than the estimated 5% in the general population 19. Infantile hemangiomas that occur in CFC individuals have a similar natural history to those in general population, characterized by a growth phase over the first several months of life, followed by a slow involution phase. Infantile hemangiomas have not been reported to occur with increased frequency in the other RASopathies making this another distinguishing feature. GLUT1 has been used as a histological marker in the diagnosis of infantile hemangiomas; it is almost universally present in infantile hemangiomas, but absent in most other vascular tumors and malformations 35,36. GLUT1 expression has been reported as an important factor in the development of BRAF and KRAS positive tumors 37. It is possible an interaction between GLUT1 expression and abnormal Ras/MAPK signaling leads to the increased incidence of infantile hemangiomas in this cohort.
Multiple small wart-like lesions (papillomata) of unclear etiology are a characteristic finding in Costello syndrome. A majority of HRAS-mutation positive individuals with Costello syndrome develop papillomata 2. In Costello syndrome, these papillomas have consistent phenotypic characteristics and most frequently occur on the face and anus (at the junction of the mucosal and squamous epithelium), although they have also been reported elsewhere on the body. The histopathologic features of biopsies from the papillomas from individuals with Costello syndrome have been more consistent with a verrucous acanthoma than human papilloma virus 38. While rare papillomas were reported in this CFC cohort, the lesions were quite heterogeneous and do not appear to be similar to those commonly seen in Costello syndrome. Three individuals had solitary lesions, which were located on the legs in two participants and on the dorsal nose in one subject and most likely represented classic verruca vulgaris. Only one individual reported perianal papillomas, but photographic review revealed that the lesions were consistent with pseudo-verrucous papules due to diaper dermatitis, distinct from the type of papillomata seen in Costello syndrome. Therefore, papillomata remain a useful feature to help distinguish Costello syndrome from CFC syndrome.
There are several cutaneous features in the diagnostic criteria for NF1 including 6 or more café au lait macules that are 1.5 cm or greater in diameter in adults, intertriginous freckling and cutaneous neurofibromas. Multiple café au lait macules may also be seen in individuals with Noonan syndrome. In this study, 31% of individuals had 1–2 café au lait macules, but none had more than two. Multiple café au lait macules, axillary freckling and neurofibromas were absent in the CFC individuals in this study suggesting these features are useful in distinguishing CFC from NF1.
In summary, we systematically report on the spectrum of dermatologic features in mutation-positive CFC individuals. We found high rates of keratosis pilaris and ulerythema ophryogenes. Over 20% of participants had greater than 50 nevi and the majority noted nevi to be increasing with age. Infantile hemangiomas are present at higher rates than the general population, a characteristic not seen in the other RASopathies. A strong body odor and rapid nail growth were unique features that were noted in close to half of our patients. Papillomata and multiple café au lait macules were not features among these participants helping to differentiate CFC from CS, NF1 and LEOPARD syndrome. Overall, these cutaneous features further define the clinic spectrum seen in mutation-positive CFC participants and assist in the differentiation from other Ras/MAPK pathway syndromes.
Bulleted statements.
What is already known about this topic?
CFC individuals have dermatologic issues including keratosis pilaris and sparse, brittle hair with scant eyebrows and eyelashes. These skin findings have been used to differentiate CFC from other RASopathies.
What does this study add?
This is the first systematic study to define the cutaneous phenotype in mutation-positive individuals with CFC syndrome. This study confirms the high rates of keratosis pilaris and ulerythema ophryogenes and characterizes the additional dermatologic findings, such as progressive melanocytic nevi and infantile hemangiomas.
Supplementary Material
Acknowledgments
Funding: This work was supported in part by NIH grant HD048502 (K.A.R.). Statistical analysis was provided by the Oregon Clinical and Translational Research Institute, grant number UL1 RR024140 01 from the National Center for Research Resources.
We are grateful to the patients and the families who participated in this study. We would like to thank CFC International for making this study possible, especially Molly Santa Cruz and Brenda Conger for their efforts in gathering the data. This work was supported in part by NIH grant HD048502 (K.A.R.). Statistical analysis was provided by the Oregon Clinical and Translational Research Institute, grant number UL1 RR024140 01 from the National Center for Research Resources.
Footnotes
Conflict of interest: None reported.
References
- 1.Tidyman WE, Rauen KA. Noonan, Costello and cardio-facio-cutaneous syndromes: dysregulation of the Ras-MAPK pathway. Expert Rev Mol Med. 2008;10:e37. doi: 10.1017/S1462399408000902. [DOI] [PubMed] [Google Scholar]
- 2.Tidyman WE, Rauen KA. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr Opin Genet Dev. 2009;19:230–6. doi: 10.1016/j.gde.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niihori T, Aoki Y, Narumi Y, et al. Germline KRAS and BRAF mutations in cardio-facio-cutaneous syndrome. Nat Genet. 2006;38:294–6. doi: 10.1038/ng1749. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Viciana P, Tetsu O, Tidyman WE, et al. Germline mutations in genes within the MAPK pathway cause cardio-facio-cutaneous syndrome. Science. 2006;311:1287–90. doi: 10.1126/science.1124642. [DOI] [PubMed] [Google Scholar]
- 5.Tartaglia M, Mehler EL, Goldberg R, et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29:465–8. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- 6.Roberts AE, Araki T, Swanson KD, et al. Germline gain-of-function mutations in SOS1 cause Noonan syndrome. Nat Genet. 2007;39:70–4. doi: 10.1038/ng1926. [DOI] [PubMed] [Google Scholar]
- 7.Tartaglia M, Pennacchio LA, Zhao C, et al. Gain-of-function SOS1 mutations cause a distinctive form of Noonan syndrome. Nat Genet. 2007;39:75–9. doi: 10.1038/ng1939. [DOI] [PubMed] [Google Scholar]
- 8.Razzaque MA, Nishizawa T, Komoike Y, et al. Germline gain-of-function mutations in RAF1 cause Noonan syndrome. Nat Genet. 2007;39:1013–7. doi: 10.1038/ng2078. [DOI] [PubMed] [Google Scholar]
- 9.Pandit B, Sarkozy A, Pennacchio LA, et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 2007;39:1007–12. doi: 10.1038/ng2073. [DOI] [PubMed] [Google Scholar]
- 10.Schubbert S, Zenker M, Rowe SL, et al. Germline KRAS mutations cause Noonan syndrome. Nat Genet. 2006;38:331–6. doi: 10.1038/ng1748. [DOI] [PubMed] [Google Scholar]
- 11.Aoki Y, Niihori T, Kawame H, et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005;37:1038–40. doi: 10.1038/ng1641. [DOI] [PubMed] [Google Scholar]
- 12.Digilio MC, Conti E, Sarkozy A, et al. Grouping of multiple-lentigines/LEOPARD and Noonan syndromes on the PTPN11 gene. Am J Hum Genet. 2002;71:389–94. doi: 10.1086/341528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarkozy A, Carta C, Moretti S, et al. Germline BRAF mutations in Noonan, LEOPARD, and cardiofaciocutaneous syndromes: molecular diversity and associated phenotypic spectrum. Hum Mutat. 2009;30:695–702. doi: 10.1002/humu.20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eerola I, Boon LM, Mulliken JB, et al. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003;73:1240–9. doi: 10.1086/379793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cawthon RM, Weiss R, Xu GF, et al. A major segment of the neurofibromatosis type 1 gene: cDNA sequence, genomic structure, and point mutations. Cell. 1990;62:193–201. doi: 10.1016/0092-8674(90)90253-b. [DOI] [PubMed] [Google Scholar]
- 16.Viskochil D, Buchberg AM, Xu G, et al. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell. 1990;62:187–92. doi: 10.1016/0092-8674(90)90252-a. [DOI] [PubMed] [Google Scholar]
- 17.Wallace MR, Marchuk DA, Andersen LB, et al. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990;249:181–6. doi: 10.1126/science.2134734. [DOI] [PubMed] [Google Scholar]
- 18.Brown SJ, Relton CL, Liao H, et al. Filaggrin haploinsufficiency is highly penetrant and is associated with increased severity of eczema: further delineation of the skin phenotype in a prospective epidemiological study of 792 school children. Br J Dermatol. 2009;161:884–9. doi: 10.1111/j.1365-2133.2009.09339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilcline C, Frieden IJ. Infantile hemangiomas: how common are they? A systematic review of the medical literature. Pediatr Dermatol. 2008;25:168–73. doi: 10.1111/j.1525-1470.2008.00626.x. [DOI] [PubMed] [Google Scholar]
- 20.Tidyman WERKA. Molecular Cauese of Cardio-Facio-Cutaneous Syndrome. In: MZ, editor. Monographs in Human Genetics: Noonan Syndrome and Related Disorders -A matter of deregulated Ras signaling. Vol. 17. Bassel, Switzerland: Karger; 2009. pp. 73–82. [Google Scholar]
- 21.Crane LA, Mokrohisky ST, Dellavalle RP, et al. Melanocytic nevus development in Colorado children born in 1998: a longitudinal study. Arch Dermatol. 2009;145:148–56. doi: 10.1001/archdermatol.2008.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bataille V, Bishop JA, Sasieni P, et al. Risk of cutaneous melanoma in relation to the numbers, types and sites of naevi: a case-control study. Br J Cancer. 1996;73:1605–11. doi: 10.1038/bjc.1996.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Csoma Z, Erdei Z, Bartusek D, et al. The prevalence of melanocytic naevi among teenagers. Orv Hetil. 2008;149:2173–82. doi: 10.1556/OH.2008.28446. [DOI] [PubMed] [Google Scholar]
- 24.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–9. [PubMed] [Google Scholar]
- 25.Hoshino R, Chatani Y, Yamori T, et al. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene. 1999;18:813–22. doi: 10.1038/sj.onc.1202367. [DOI] [PubMed] [Google Scholar]
- 26.Terao M, Sakai N, Higashiyama S, et al. Cutaneous symptoms in a patient with cardio-facio-cutaneous syndrome and increased ERK phosphorylation in skin fibroblasts. Br J Dermatol. doi: 10.1111/j.1365-2133.2010.09875.x. [DOI] [PubMed] [Google Scholar]
- 27.Yuen ST, Davies H, Chan TL, et al. Similarity of the phenotypic patterns associated with BRAF and KRAS mutations in colorectal neoplasia. Cancer Res. 2002;62:6451–5. [PubMed] [Google Scholar]
- 28.Rajagopalan H, Bardelli A, Lengauer C, et al. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature. 2002;418:934. doi: 10.1038/418934a. [DOI] [PubMed] [Google Scholar]
- 29.Pollock PM, Harper UL, Hansen KS, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 30.Estep AL, Palmer C, McCormick F, et al. Mutation Analysis of BRAF, MEK1 and MEK2 in 15 Ovarian Cancer Cell Lines: Implications for Therapy. PLoS ONE. 2007;2:e1279. doi: 10.1371/journal.pone.0001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marks JL, Gong Y, Chitale D, et al. Novel MEK1 mutation identified by mutational analysis of epidermal growth factor receptor signaling pathway genes in lung adenocarcinoma. Cancer Res. 2008;68:5524–8. doi: 10.1158/0008-5472.CAN-08-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armour CM, Allanson JE. Further delineation of cardio-facio-cutaneous syndrome: clinical features of 38 individuals with proven mutations. J Med Genet. 2008;45:249–54. doi: 10.1136/jmg.2007.054460. [DOI] [PubMed] [Google Scholar]
- 33.McKee P, editor. Pathology of the Skin. Vol. 1. Elsevier; 2005. [Google Scholar]
- 34.Nguyen V, Buka RL, Roberts BJ, et al. Cutaneous manifestations of Costello syndrome. Int J Dermatol. 2007;46:72–6. doi: 10.1111/j.1365-4632.2007.02920.x. [DOI] [PubMed] [Google Scholar]
- 35.North PE, Mizeracki A, Mihm MC, Jr, et al. GLUT1 immunoreaction patterns reliably distinguish hemangioblastoma from metastatic renal cell carcinoma. Clin Neuropathol. 2000;19:131–7. [PubMed] [Google Scholar]
- 36.North PE, Waner M, Mizeracki A, et al. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31:11–22. doi: 10.1016/s0046-8177(00)80192-6. [DOI] [PubMed] [Google Scholar]
- 37.Yun J, Rago C, Cheong I, et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325:1555–9. doi: 10.1126/science.1174229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez ME, Blanco FP, Garzon MC. Verrucous papules and plaques in a pediatric patient: cutaneous papillomas associated with Costello syndrome. Arch Dermatol. 2007;143:1201–6. doi: 10.1001/archderm.143.9.1201-b. [DOI] [PubMed] [Google Scholar]
- 39.Martinelli S, De Luca A, Stellacci E, et al. Heterozygous germline mutations in the CBL tumor-suppressor gene cause a Noonan syndrome-like phenotype. Am J Hum Genet. 87:250–7. doi: 10.1016/j.ajhg.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.