Abstract
Background
The RASopathies are a class of human genetic syndromes caused by germline mutations in genes that encode protein components of the Ras/mitogen-activated protein kinase (MAPK) pathway. Costello syndrome (CS) is a RASopathy caused by mutations in the HRAS gene, a key regulator of signal transduction.
Objective
To quantify the specific cutaneous phenotype observed in 46 individuals with Costello syndrome with confirmed HRAS mutations
Methods
This was a cross-sectional study. Dermatologic surveys were designed by the authors and were completed by parents of mutation-positive CS individuals at the Costello Syndrome Family Network (CSFN) conferences in 2007 and 2009. Dermatologic exams were performed by the authors at the CSFN conferences.
Results
Cutaneous papillomas are reported in 33/46 (71.7%) of participants, with age of onset ranging from infancy to 22 years. Individuals with CS are more likely than patients with cardio-facio-cutaneous syndrome (CFC) to present with cutaneous papillomas (71.7% compared to 4.9%, p<0.001) and palmoplantar keratoderma (76.1% compared to 36.1%, p<0.001). Individuals with CS are less likely than individuals with CFC to present with sparse or absent eyebrows (8.7% compared to 90.2%, p<0.001) or keratosis pilaris (32.6% compared to 80.3%, p=0.001). This study also identified that loose, redundant skin on the hands and feet, “stippled” dermatoglyphs (pachydermatoglyphia) on the fingertips 8/26 (31%), and acanthosis nigricans 17/46 (37%) are frequent features of CS.
Conclusions
While there is significant phenotypic overlap among syndromes of the Ras/MAPK pathway, individuals with CS are more likely than individuals with CFC syndrome to present with cutaneous papillomas, palmoplantar keratoderma and full eyebrows, and are less likely to present with ulerythema ophryogenes, keratosis pilaris or multiple naevi. The dermatologic features of CS, a Ras dysregulation syndrome, share many features with cutaneous paraneoplastic syndromes. This may provide further insight into the role of Ras signaling in cutaneous paraneoplastic syndromes.
INTRODUCTION
Costello syndrome (CS) is a developmental disorder caused by activating mutations in the oncogene HRAS on chromosome 11p15.5(1–3). HRAS is a key regulator of the mitogen-activated protein kinase (MAPK) pathway mediated by the protein kinase Raf which is critical for cell cycle regulation, cellular differentiation, and growth. Hyperactivation of the Ras/MAPK pathway has long been implicated in cancer pathogenesis(4). Over the past several years, germline mutations have been identified in a number of Ras/MAPK genes. The term RASopathy was coined in 2009 to define this group of syndromes with Ras/MAPK germline mutations(5). The RASopathies include Costello, cardio-facio-cutaneous syndrome, neurofibromatosis 1 (NF1), Legius syndrome and Noonan syndrome(5–7). Since that time, the term RASopathy has been widely used in the literature. In fact, in July 2011, the term “RASopathy” was adopted by an international multidisciplinary group of experts at the “Genetic Syndromes of the Ras/MAPK Pathway” conference in Chicago, IL, USA (personal communication). The RASopathies have distinctive cutaneous features that, once clearly defined, may serve as valuable diagnostic markers in clinical trials (8).
In individuals with CS, HRAS gene mutations lead to constitutive activation of Ras, a small GTPase resulting in hyperactivation of the MAPK pathway(1, 3). Mutations in amino acid positions 12 and 13 of HRAS account for the vast majority of CS mutations. CS is characterized by dysmorphic facial features, feeding difficulties, congenital heart defects, hypertrophic cardiomyopathy, and neurocognitive impairment (9). Individuals with CS also have an increased risk of malignancy, most commonly solid tumors such as rhabdomyosarcoma, neuroblastoma and transitional cell cancer of the bladder(10, 11). The similarities in the cutaneous phenotypes among the RASopathies suggest that the ectodermal features manifest in response to Ras pathway activation.
In the newborn period, CS can be difficult to distinguish from NS and CFC because all three syndromes may have similar features including neonatal macrosomia, coarse facial features, failure to thrive, developmental delay and hypertrophic cardiomyopathy(12). The loose, redundant skin on the hands and feet seen in newborns with CS plays a key role in the clinical suspicion for early diagnosis(13). Later in childhood, additional distinctive cutaneous features develop. The objective of this study was to quantify the specific cutaneous phenotype observed in 46 individuals with CS with confirmed HRAS mutations. This data will serve as a useful tool for the diagnosis of CS and will help clinicians provide anticipatory guidance to CS patients.
METHODS
Study Design and Setting
Approval was obtained through the Committee on Human Research at University of California San Francisco and the Institutional Review Board at Oregon Health & Science University. Consent was obtained for all individuals. The authors designed a survey to detail the dermatological phenotype of CS, with a focus on cutaneous markers associated with other Ras/MAPK pathway syndromes (Supplement 1). Variables on the surveys were chosen based on a review of the literature, with a focus on the dermatological features of other RASopathies including NF1, NS and CFC. The questionnaires were completed by the participants’ parents at the Costello Syndrome Family Network (CSFN) conferences in 2007 and 2009. An additional 15 questions were included in the 2009 version of the survey. All but two of the participants were examined in person by the study authors at the conferences.
Study participants
Participants were identified through the CSFN. All individuals included in the study had HRAS mutations confirmed by genetic testing. Individuals without identified mutations were excluded from the study.
Limitations
The study size was determined based on the number of interested participants present at the 2007 and 2009 CSFN meetings. Participants voluntarily registered to be part of the study. No individuals declined or withdrew after entering the study. The small sample size limits the power to detect differences in phenotype based on genotypes.
Statistical methods
A total of 46 individuals completed part one of the survey and 26 completed part two of the survey. In some cases participants supplied additional information not on the survey. In these cases, the number of individuals reporting that feature is identified. Qualitative data were expressed as frequency and percentage. Statistical analysis of the genotype associated difference in prevalence of characteristics utilized Statistical Package for the Social Sciences (SPSS) version 19. Analysis was done by χ2 tests with Yate’s continuity correction or by Fisher’s Exact Test.
RESULTS
Participants
Twenty-six participants (56.5%) were female and 20 (43.4%) were male. Ages of participants ranged from 11 months to 33.5 years, with a median age of 14 years. Ethnicity of the participants was reported as follows: Caucasian 28/46 (60.9%), Hispanic 2/46 (4.3%), bi-racial 13/46 (28.2%), other 2/46 (4.3%), and not noted 1/46 (2.2%). Of those that described themselves as bi-racial, two or more of the following categories were selected: African-American, Australian, British, Canadian, Caucasian, Chinese, German, Hispanic, Iranian, Japanese, Hispanic, Latina, Mexican, and Phillipino. The most common amino acid substitution was p.G12S, affecting 37/46 (80.4%) of individuals. Four participants (8.7%) had p.G12A mutations, another 4 (8.7%) had a p.G13C mutation, and 1 (2.2%) had a p.G13D mutation.
Papillomas and other neoplasia
Cutaneous papillomas were reported in 33/46 (71.7%) of individuals, with age of onset ranging from infancy to 22 years. The most commonly reported location of the papillomas was the nose, identified in 14/33 participants (42.4%). The papillomas on the nose were generally located around the alar rim and anterior nares and were small, 3–4 mm verrucous, pedunculated papules (Fig. 1). One child had papillomas on the lower eyelid margin. Three participants had larger 2–3 cm hypertrophic, verrucous plaques extending from the anterior to the posterior earlobe. Of individuals with papillomas, 21/33 (63.6%) had had them removed by a physician in the past, and many parents commented that they were an annoyance to their school-aged and older children. Histopathology was not available for review in this study. Compared to CFC, CS is more likely to present with cutaneous papillomas (71.7% compared to 4.9%, p<0.001)(14) (Table 1).
Figure 1.

Characteristic papillomas on the anterior nares.
Table 1.
Comparison of cutaneous findings in Costello syndrome vs. Cardio-facio-cutaneous syndrome
| Costello syndrome | Cardio-facio-cutaneous syndrome(14) | p (difference)a | |
|---|---|---|---|
| Curly hair | 95.7% (44/46) | 93.4% (57/61) | 0.698b |
| Hair sparse at temples | 30.4% (14/46) | 59.0% (36/61) | 0.006 |
| Eyebrow density | Sparse or absent: 8.7% (4/46) Thick/full: 47.8% (22/46) |
Sparse/absent: 90.2% (55/61) Thick/full: 1.6% (1/61) |
<0.001c |
| Keratosis Pilaris | 32.6% (15/ 46) | 80.3% (49/61) | 0.001 |
| Infantile hemangiomas | 10.9% (5/46) | 26.2% (16/61) | 0.244 |
| Papillomas | 71.7% (33/46) *14/33 (42.4%) peri-nasal |
4.9% (3/61) *none peri-nasal |
<0.001c |
| More than 50 naevi | 4% (2/46) | 23.0% (14/61) | p= 0.012 |
| Palmoplantar keratoderma | 76.1% (35/46) | 36.1% (22/61) | <0.001 |
| Unusual body odor | 73.1% (19/26) | 46.4% (13/28) | 0.25 |
| Heat intolerance | 76.1% (35/46) | 75.4% (46/61) | 0.25 |
Chi Square test with Yate’s continuity correction except where noted
Fisher’s Exact test
Chi square test
Five of 46 participants (10.9%) reported a history of an infantile hemangioma. No participants reported a skin malignancy, however 11/46 (23.9%) reported a history of skin “lump”; specific diagnoses cited by parents included ganglion cyst and ganglioma. Two individuals developed rhabdomyosarcoma, one of whom died as a result of his malignancy at the age of 6 years.
Ectodermal features
Hands and feet
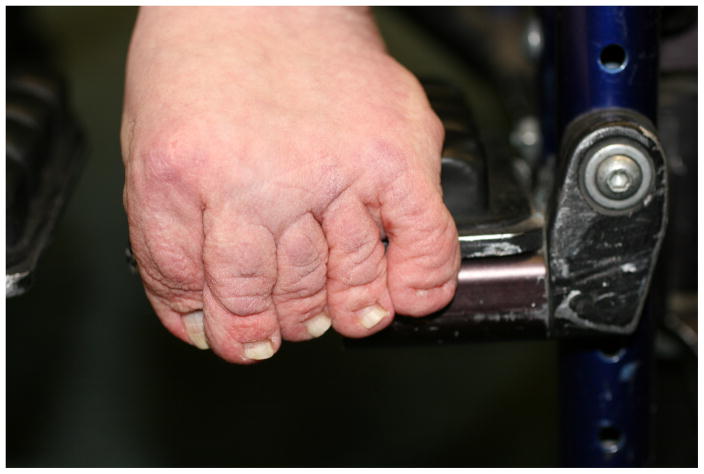
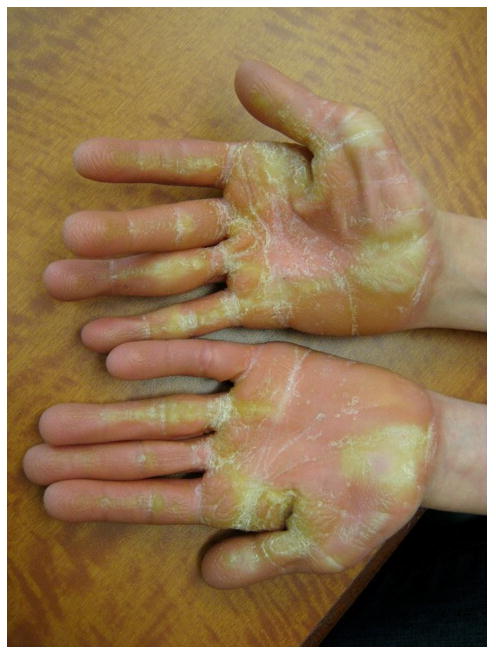
Deep creases on the hands and feet were reported in 39/46 individuals (84.8%). These findings result in the characteristic appearance of loose, redundant skin on the hands and feet (Fig. 2). Soft or velvety skin texture was found in 28/46 (60.8%) of participants. Palmoplantar keratoderma was very common and was reported among 35/46 (76.1%) of the individuals with CS (Fig. 3). The palmoplantar keratoderma in CS is more common than in CFC (76.1% compared to 36.1%, p<0.001)(14) (Table 1). The study authors observed abnormal “stippled” dermatoglyphs (pachydermatoglyphia) in 8/26 (31%) of individuals examined at the 2009 conference (Fig. 4 a,b). Fingertip creases were present among 42.3% (11/26) of individuals examined at the 2009 CSFN conference.
Figure 2.
Loose, redundant skin on the feet.
Figure 3.
Hyperkeratosis of the palms is most prominent in areas of pressure.
Figure 4.
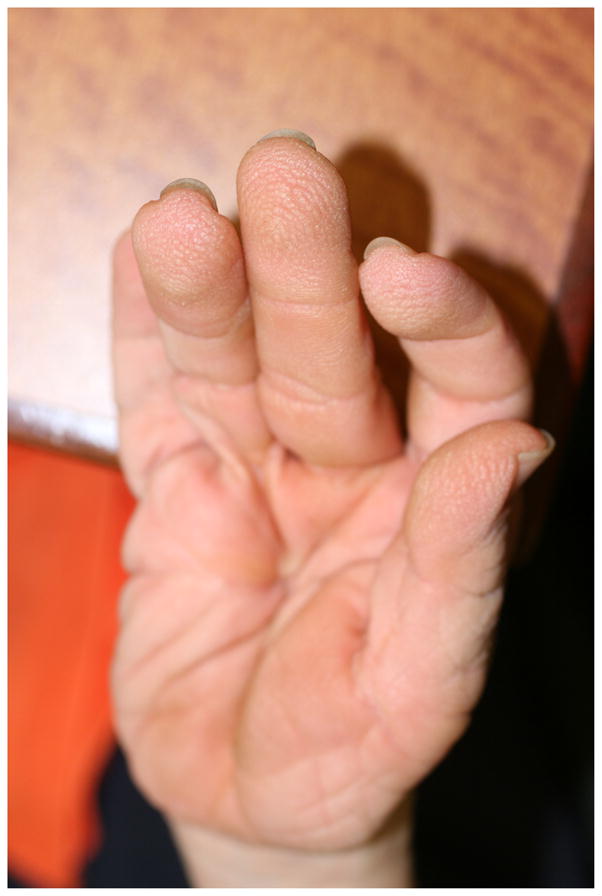
(a) Pachydermatoglyphia with stippled dermatoglyphs on the fingertips, (b) A close-up of the accentuated fingertip markings.
Hair, eyebrows and eyelashes
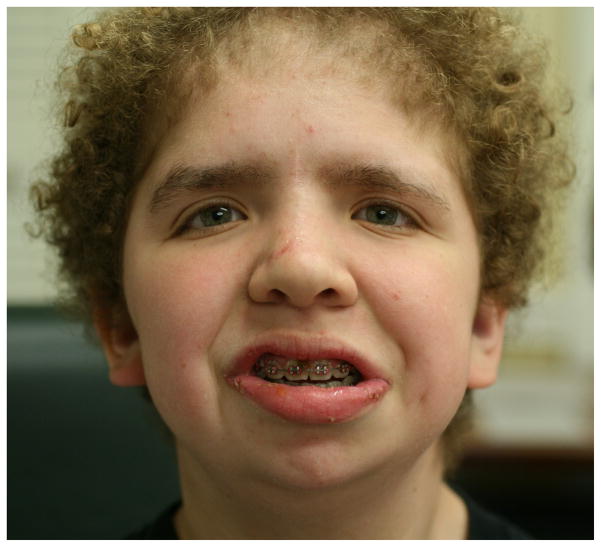
Notably, 44/46 (95.7%) of individuals had curly or wavy hair (Fig. 5). Sparse or thin hair density was reported by 30/46 participants (65.2%), and 20/46 (43.5%) reported brittle hair. Temporal alopecia was noted by 14/46 (30.4%) of participants with CS, which is significantly less than the 59% of CFC individuals (p=0.006)(14) (Table 1). Hair type was described as different from that of other family members by 29/46 (63%) of participants’ parents. Seventy-seven percent (20/26) of individuals examined in 2009 had poor hair growth, with one 6 year-old having never had a haircut and one 15-year-old having only had a single haircut in her life.
Figure 5.
The characteristic facial appearance with sparse, curly hair, thick eyebrows and full lips in a 13 year old female.
Thick, full, or “unruly” eyebrows were reported in 22/46 (47.8%) of individuals (Fig. 5), while 4 individuals (8.7%) had sparse eyebrows and 3 individuals (6.5%) had the lateral aspect of eyebrows missing. In contrast to CFC, no participants with CS had ulerythema ophryogenes. Individuals with CS are less likely to present with sparse or absent eyebrows (8.7% compared to 90%, p<0.001)(14). Three individuals reported eyelashes that grew so long that they intermittently had to be trimmed by a parent.
Pigmentation and melanocytic naevi
Acanthosis nigricans
Acanthosis nigricans on the neck and axilla reported by 17/46 (37%) of participants. On exam this was characterized by hyperpigmentation, a velvety texture, accentuation of the skin lines and in some cases papillomas. Hyperpigmented, velvety plaques were noted on the posterior earlobes of many younger children. Older children were noted to have thick, verrucous plaques in the same areas.
Generalized hyperpigmentation
Eighteen out of 46 individuals (39.1%) reported fair or medium-to-fair complexion, 16/46 (34.8%) reported medium complexion, and 10/46 (21.7%) reported medium-to-dark or dark complexion. Sixteen out of 46 (34.8%) of parents reported that their child’s skin color was darker than that of other family members.
Melanocytic naevi and melanoma
Thirty-four of 46 participants (74%) reported 0–10 naevi, and 10/46 (22%) had between 10–50 naevi. Two individuals reported more than 50 naevi. This is significantly different than the high rates of individuals with CFC having greater than 50 naevi (4% compared to 23%)(14). These two individuals were 15 and 17 years old. There were 25 individuals in the adolescent and adult age group (range 13 years to 31 years, mean 20.3 years, median 20 years). No participants had a history of melanoma.
Hyperpigmented patches
Fourteen out of 46 individuals (30.4%) reported hyperpigmented patches although none had greater than five. No individuals reported intertriginous freckling.
Other dermatological issues
Thermoregulation
By their parents’ report, 27/46 participants (58.7%) either did not like the sun or were extremely photosensitive. Thirty-five of 46 (76.1%) of parents reported that their children did not tolerate heat well. Ten of 46 parents (21.7%) noted that their children sweated excessively. Parents reported an unusual body odor in 73.1% (19/26) of participants. Among parents who reported that their child had an unusual body odor, 6/19 (31.6%) noted that the odor had been present since infancy. Adjectives that parents used to describe the odor included “sour”, “ripe”, and “vinegary”.
Atopy
Eczema was reported by 11/46 (23.9%) of individuals and asthma was reported by 2/46 (4.3%). A family history of eczema was reported among 14/46 (30.4%) of individuals and a family history of asthma was reported among 13/46 (28.2%) of individuals.
DISCUSSION
Prior to the availability of genetic testing, the diagnosis of CS was made based on clinical features including coarse facies, curly hair, low set ears, depressed nasal bridge, full lips, loose skin, and cardiac abnormalities. Because causative genes for RASopathies now number greater than ten, it is helpful to use the dermatological features to help guide genetic testing (5, 15–18). Other authors have cited various dermatological features of CS such as thick, loose skin on the dorsal hands and feet, deep creases on the hands and feet, perinasal and perianal papillomas, focal palmoplantar hyperkeratosis, and sparse, curly hair (13, 19, 20), but no previous case series have specifically described and quantified the dermatological phenotype in individuals with CS with identified mutations. We sought to expand on these prior case reports and smaller series by describing and quantifying the cutaneous attributes of 46 individuals with CS with confirmed mutations in HRAS from a dermatologist’s perspective and to specifically compare these findings with those in CFC syndrome. Limitations of this study include the descriptive nature and the relatively small sample size.
A comparison of Costello Syndrome and Cardio-facio-cutaneous Syndrome
While a number of cutaneous features occur in both CS and CFC, we have determined several unique dermatological findings in Costello individuals that allow the two syndromes to be distinguished from each other (Table 1). Eyebrows were most often full and thick among CS individuals and in contrast, sparse or absent eyebrows were seen in the majority of individuals with CFC (14). Loose, redundant palmar skin and deep palmoplantar creases are common features in CS which are not seen in CFC. Stippled dermatoglyphs were observed in 31% of individuals with CS, and were not observed in any CFC individuals. Large numbers of melanocytic naevi were uncommon among individuals with CS (2/46 participants reported greater than 50), whereas among individuals with CFC, 23% had greater than 50 naevi. Keratosis pilaris was seen in a minority of individuals with CS (32.6%), while the majority of CFC individuals have keratosis pilaris and ulerythema ophryogenes. The number of individuals with KP and CS is similar to that seen in the general population(21).
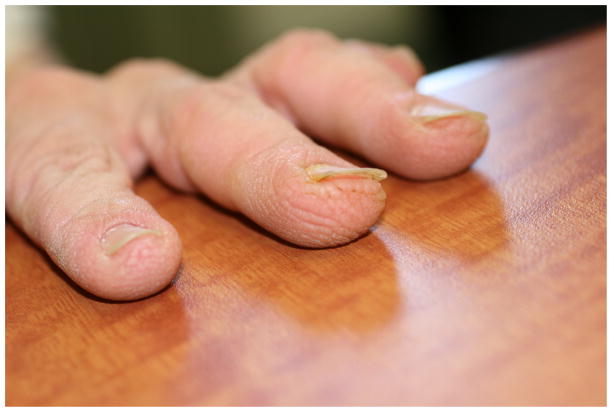
Similarities between CFC and CS included brown, curly, brittle, and sparse hair, with some CS individuals demonstrating temporal alopecia. Nails among individuals with both CFC and CS are typically thin and with koilonychia. Over 73% of individuals with CS in our cohort and 80% of previously described CFC individuals have rapid nail growth, necessitating frequent trimming. Earlobe creases are common in both syndromes.
Ectodermal features of Ras dysregulation
In our study, cutaneous papillomas were observed in 71.7% of patients with CS. Importantly, these neoplasms are not seen in other RASopathies, including CFC. Papillomas were most commonly located around the nose in this cohort, but were also reported on the eyelids, ears, neck, arms, fingers and perianal region. Because of their noticeable location on the face, nearly half of parents had brought their children to see a physician for removal.
There has been debate among experts as to whether the papillomas seen among individuals with CS are induced by human papillomavirus (HPV), or whether they are non-viral neoplasms caused by hyperactivation of the Ras/MAPK pathway. Mouse models provide strong evidence that papillomas in CS are due to Ras pathway activation and not HPV. Well-studied mouse models of skin cell tumorigenesis have demonstrated that alkylating agents initiate skin tumors by causing the specific nt 35G→A transition in codon 12 of Hras(22). The initial primary tumors are benign papillomas in which a subset goes on to become squamous carcinoma. The mechanism of tumor progression results from nonrandom duplication of the chromosomal region spanning the Hras locus resulting in an increased copy number of activated Ras, followed by loss of the normal Hras allele.
Further evidence that papillomas develop from Ras activation comes from CS mouse models. Similar to that seen in patients with CS, one conditional HRAS p.G12V mouse model develops skin papillomas between 40–58 weeks in 88% of mice(23). The papillomas were frequently found in the skull, face, and external auditory canal; epidermal and sebaceous hyperplasia are also noted. Additionally, older mice develop multiple papillomas in the forestomach with complete penetrance. A RASopathy mouse model harboring a Kras p.G12D mutation produces multiple cutaneous features that resemble CS including redundant skin and papillomas. Spontaneous papillomas were observed in 78% of mice as early as 2 weeks of age with papilloma formation observed mostly in the perianal area, forepaw and hindpaw, and on the head(24).
Interestingly, many of the ectodermal features noted in CS, including papillomas, pachydermatoglyphia, palmoplantar keratoderma and acanthosis nigricans, have been reported as signs of cutaneous paraneoplastic syndromes (25, 26). Pachydermatoglyphia has also been referred to as “tripe palms” based on the similarity to the rugose appearance of the cow’s stomach(27). Tripe palms are characterized clinically by an exaggerated ridging pattern on the palms and fingertips and histologically by marked epidermal hyperkeratosis, acanthosis, papillomatosis and hypergranulosis. The pachydermatoglyphia noted in the individuals with CS is shown in Fig. 4a and 4b. Malignant acanthosis nigricans is characterized by velvety, brown papillomatous plaques on the neck, axillae, and groin. Several case reports have described tripe palms, malignant acanthosis nigricans and florid cutaneous papillomatosis occurring in the same patient, suggesting dysregulation of transforming growth factor alpha or epidermal growth factor may play a role(28–30). These cutaneous paraneoplastic signs are most commonly associated with adenocarcinoma, squamous cell carcinoma and lymphoma, but tripe palms and malignant acanthosis nigricans have also been reported in association with bladder cancer(31). The most common malignancies in Costello syndrome include rhabdomyosarcoma, bladder cancer, and neuroblastoma. We hypothesize the dysregulation of the Ras pathway in CS has an effect on keratinocyte biology which mimics that of the cutaneous paraneoplastic syndromes.
Summary of Cutaneous Features Unique to Costello Syndrome
In summary, we detail a wide range of dermatological findings as determined by a systematic evaluation of mutation-positive CS individuals. Features which are distinctive in CS as compared to CFC include: 1) cutaneous papillomas, most often located on or around the nose, 2) full, thick eyebrows, 3) loose, redundant skin and deep creases on the hands and feet, 4) stippled dermatoglyphs on the fingertips, and 5 generalized hyperpigmentation. Additionally, most participants had curly, sparse, and brittle hair. Nails were thin and grew rapidly, while scalp hair growth was typically very slow. In contrast, keratosis pilaris, ulerythema ophryogenes, large numbers of melanocytic naevi were uncommon among participants, helping to differentiate CS from CFC. Overall, these unique dermatological characteristics further define the phenotype seen in mutation-positive individuals with CS and may serve as excellent biomarkers in clinical trials. Many of the ectodermal features noted in Costello syndrome: papillomas, acanthosis nigricans and pachydermatoglyphia are also features of cutaneous paraneoplastic syndromes’ suggesting that dysregulation of the Ras pathway contributes to this epidermal phenotype.
Table 2.
Additional Dermatological Features in Individuals with Costello Syndrome
| Number of patients | Percentage | |
|---|---|---|
| Deep creases on the hands and feet | 39/46 | 84.80% |
| Nail abnormalities (thin, pliable, upturned) | 22/26 | 84.60% |
| Poor scalp hair growth | 20/26 | 76.90% |
| Soft, velvety skin texture | 28/46 | 60.80% |
| Fingertip creases | 11/26 | 42.30% |
| Earlobe creases | 11/26 | 42.30% |
| Acanthosis nigricans | 17/46 | 37.00% |
| Skin color darker than other family members | 16/46 | 34.80% |
What is already known about the topic?
Distinctive cutaneous features such as papillomas and loose, redundant skin on the hands and feet have been observed in individuals with Costello syndrome. These characteristic features can provide clues to the diagnosis and help direct gene testing.
What does this study add?
This is the first systematic study to define the cutaneous phenotype in individuals with Costello syndrome with confirmed mutations in HRAS. This study quantifies the prevalence of papillomas, palmoplantar hyperkeratosis, hair and nail changes, and other dermatological features among these individuals and discusses how these features can be used to distinguish Costello syndrome from the other RASopathies, focusing primarily on cardio-facio-cutaneous syndrome.
Acknowledgments
We are grateful to the patients and the families who participated in this study. We would like to thank Ilona Frieden, MD for her critical review of the manuscript. We would like to thank the Costello Syndrome Family Network. This work was supported in part by NIH grant HD048502 (K.A.R.). Statistical analysis was provided by Oregon Clinical and Translational Research Institute, grant number UL1 RR024140 01 from the National Center for Research Resources.
Footnotes
Conflicts of interest: None
References
- 1.Estep AL, Tidyman WE, Teitell MA, Cotter PD, Rauen KA. HRAS mutations in Costello syndrome: detection of constitutional activating mutations in codon 12 and 13 and loss of wild-type allele in malignancy. Am J Med Genet A. 2006 Jan 1;140(1):8–16. doi: 10.1002/ajmg.a.31078. [DOI] [PubMed] [Google Scholar]
- 2.Gripp KW, Lin AE, Stabley DL, Nicholson L, Scott CI, Jr, Doyle D, et al. HRAS mutation analysis in Costello syndrome: Genotype and phenotype correlation. Am J Med Genet A. 2006 Jan 1;140(1):1–7. doi: 10.1002/ajmg.a.31047. [DOI] [PubMed] [Google Scholar]
- 3.Aoki Y, Niihori T, Kawame H, Kurosawa K, Ohashi H, Tanaka Y, et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005 Oct;37(10):1038–40. doi: 10.1038/ng1641. [DOI] [PubMed] [Google Scholar]
- 4.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999 Jul 29;400(6743):464–8. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 5.Tidyman WE, Rauen KA. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr Opin Genet Dev. 2009 Jun;19(3):230–6. doi: 10.1016/j.gde.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tidyman WE, Rauen KA. Mutational and functional analysis in human Ras/MAP kinase genetic syndromes. Methods Mol Biol. 661:433–47. doi: 10.1007/978-1-60761-795-2_27. [DOI] [PubMed] [Google Scholar]
- 7.Rauen KA, Schoyer L, McCormick F, Lin AE, Allanson JE, Stevenson DA, et al. Proceedings from the 2009 genetic syndromes of the Ras/MAPK pathway: From bedside to bench and back. Am J Med Genet A. Jan;152A(1):4–24. doi: 10.1002/ajmg.a.33183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rauen KA, Banerjee A, Bishop WR, Lauchle JO, McCormick F, McMahon M, et al. Costello and cardio-facio-cutaneous syndromes: Moving toward clinical trials in RASopathies. Am J Med Genet C Semin Med Genet. 2011 May 15;157(2):136–46. doi: 10.1002/ajmg.c.30294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rauen KA. HRAS and the Costello syndrome. Clin Genet. 2007 Feb;71(2):101–8. doi: 10.1111/j.1399-0004.2007.00743.x. [DOI] [PubMed] [Google Scholar]
- 10.Gripp KW. Tumor predisposition in Costello syndrome. Am J Med Genet C Semin Med Genet. 2005 Aug 15;137C(1):72–7. doi: 10.1002/ajmg.c.30065. [DOI] [PubMed] [Google Scholar]
- 11.Karalis A, Tischkowitz M, Millington GW. Dermatological manifestations of inherited cancer syndromes in children. Br J Dermatol. 2011 Feb;164(2):245–56. doi: 10.1111/j.1365-2133.2010.10100.x. [DOI] [PubMed] [Google Scholar]
- 12.Digilio MC, Sarkozy A, Capolino R, Chiarini Testa MB, Esposito G, de Zorzi A, et al. Costello syndrome: clinical diagnosis in the first year of life. Eur J Pediatr. 2008 Jun;167(6):621–8. doi: 10.1007/s00431-007-0558-0. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen V, Buka RL, Roberts BJ, Eichenfield LF. Cutaneous manifestations of Costello syndrome. Int J Dermatol. 2007 Jan;46(1):72–6. doi: 10.1111/j.1365-4632.2007.02920.x. [DOI] [PubMed] [Google Scholar]
- 14.Siegel DH, McKenzie J, Frieden IJ, Rauen KA. Dermatological findings in 61 mutation-positive individuals with cardiofaciocutaneous syndrome. Br J Dermatol. 2011 Mar;164(3):521–9. doi: 10.1111/j.1365-2133.2010.10122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tidyman WE, Rauen KA. Noonan, Costello and cardio-facio-cutaneous syndromes: dysregulation of the Ras-MAPK pathway. Expert Rev Mol Med. 2008;10:e37. doi: 10.1017/S1462399408000902. [DOI] [PubMed] [Google Scholar]
- 16.Tartaglia M, Zampino G, Gelb BD. Noonan syndrome: clinical aspects and molecular pathogenesis. Mol Syndromol. 2010 Feb;1(1):2–26. doi: 10.1159/000276766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brems H, Chmara M, Sahbatou M, Denayer E, Taniguchi K, Kato R, et al. Germline loss-of-function mutations in SPRED1 cause a neurofibromatosis 1-like phenotype. Nat Genet. 2007 Sep;39(9):1120–6. doi: 10.1038/ng2113. [DOI] [PubMed] [Google Scholar]
- 18.Revencu N, Boon LM, Mulliken JB, Enjolras O, Cordisco MR, Burrows PE, et al. Parkes Weber syndrome, vein of Galenaneurysmal malformation, and other fast-flow vascular anomalies are caused by RASA1 mutations. Hum Mutat. 2008 Jul;29(7):959–65. doi: 10.1002/humu.20746. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez ME, Blanco FP, Garzon MC. Verrucous papules and plaques in a pediatric patient: cutaneous papillomas associated with Costello syndrome. Arch Dermatol. 2007 Sep;143(9):1201–6. doi: 10.1001/archderm.143.9.1201-b. [DOI] [PubMed] [Google Scholar]
- 20.Orstavik KH, Tangeraas T, Molven A, Prescott TE. Distal phalangeal creases--a distinctive dysmorphic feature in disorders of the RAS signalling pathway? Eur J Med Genet. 2007 Mar-Apr;50(2):155–8. doi: 10.1016/j.ejmg.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Brown SJ, Relton CL, Liao H, Zhao Y, Sandilands A, McLean WH, et al. Filaggrin haploinsufficiency is highly penetrant and is associated with increased severity of eczema: further delineation of the skin phenotype in a prospective epidemiological study of 792 school children. Br J Dermatol. 2009 Oct;161(4):884–9. doi: 10.1111/j.1365-2133.2009.09339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown K, Quintanilla M, Ramsden M, Kerr IB, Young S, Balmain A. v-ras genes from Harvey and BALB murine sarcoma viruses can act as initiators of two-stage mouse skin carcinogenesis. Cell. 1986 Aug 1;46(3):447–56. doi: 10.1016/0092-8674(86)90665-3. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Mitsutake N, LaPerle K, Akeno N, Zanzonico P, Longo VA, et al. Endogenous expression of Hras(G12V) induces developmental defects and neoplasms with copy number imbalances of the oncogene. Proc Natl Acad Sci U S A. 2009 May 12;106(19):7979–84. doi: 10.1073/pnas.0900343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukhopadhyay A, Krishnaswami SR, Yu BD. Activated Kras alters epidermal homeostasis of mouse skin, resulting in redundant skin and defective hair cycling. J Invest Dermatol. 2011 Feb;131(2):311–9. doi: 10.1038/jid.2010.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen PR, Grossman ME, Silvers DN, Kurzrock R. Tripe palms and cancer. Clin Dermatol. 1993 Jan-Mar;11(1):165–73. doi: 10.1016/0738-081x(93)90114-r. [DOI] [PubMed] [Google Scholar]
- 26.Moore RL, Devere TS. Epidermal manifestations of internal malignancy. Dermatol Clin. 2008 Jan;26(1):17–29. vii. doi: 10.1016/j.det.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Cohen PR, Grossman ME, Almeida L, Kurzrock R. Tripe palms and malignancy. J Clin Oncol. 1989 May;7(5):669–78. doi: 10.1200/JCO.1989.7.5.669. [DOI] [PubMed] [Google Scholar]
- 28.Douglas F, McHenry PM, Dagg JH, MacBeth FM, Morley WN. Elevated levels of epidermal growth factor in a patient with tripe palms. Br J Dermatol. 1994 May;130(5):686–7. doi: 10.1111/j.1365-2133.1994.tb13128.x. [DOI] [PubMed] [Google Scholar]
- 29.Gorisek B, Krajnc I, Rems D, Kuhelj J. Malignant acanthosis nigricans and tripe palms in a patient with endometrial adenocarcinoma--a case report and review of literature. Gynecol Oncol. 1997 Jun;65(3):539–42. doi: 10.1006/gyno.1997.4674. [DOI] [PubMed] [Google Scholar]
- 30.Kebria MM, Belinson J, Kim R, Mekhail TM. Malignant acanthosis nigricans, tripe palms and the sign of Leser-Tre'lat, a hint to the diagnosis of early stage ovarian cancer: a case report and review of the literature. Gynecol Oncol. 2006 May;101(2):353–5. doi: 10.1016/j.ygyno.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 31.Mohrenschlager M, Vocks E, Wessner DB, Nahrig J, Ring J. Tripe palms and malignant acanthosis nigricans: cutaneous signs of imminent metastasis in bladder cancer? J Urol. 2001 May;165(5):1629–30. doi: 10.1016/s0022-5347(05)66369-0. [DOI] [PubMed] [Google Scholar]