Abstract
Enteropathogenic E. coli (EPEC) infection is a major cause of infantile diarrhea in the developing world. Using a type-three secretion system, bacterial effector proteins are transferred to the host cell cytosol where they affect multiple physiological functions, ultimately leading to diarrheal disease. Disruption of intestinal epithelial cell tight junctions is a major consequence of EPEC infection and is mediated by multiple effector proteins, among them EspG1 and its homologue EspG2. EspG1/G2 contribute to loss of barrier function via an undefined mechanism that may be linked to their disruption of microtubule networks. Recently new investigations have identified additional roles for EspG. Sequestration of active ADP-ribosylating factor (ARF) proteins and promotion of p21-activated kinase (PAK) activity as well as inhibition of Golgi-mediated protein secretion have all been linked to EspG. In this review, we examine the functions of EspG1/G2 and discuss potential mechanisms of EspG-mediated tight junction disruption.
Keywords: EPEC, tight junctions, EspG, microtubules, ARF, PAK
Introduction
Enteropathogenic Escherichia coli (EPEC) infection is a major cause of diarrhea in the developing world. Infants are primarily affected by this pathogen and may experience repeated infections.1,2 This leads to prolonged intervals during early childhood development without the benefit of micronutrients, sufficient glucose, and other nutritive factors absorbed via the intestine.3 EPEC belongs to a family of related attaching and effacing (A/E) pathogens, including enterohemorrhagic E. coli (EHEC) and the murine pathogen Citrobacter rodentium. A/E pathogens use a type-three secretion system(TTSS) to transfer bacterial effector proteins into host epithelial cells via a needle-like structure.4–6 This “molecular syringe” spans the bacterial and host cell membranes and delivers the effectors directly into the host cytosol, working in concert with multiple chaperone proteins.
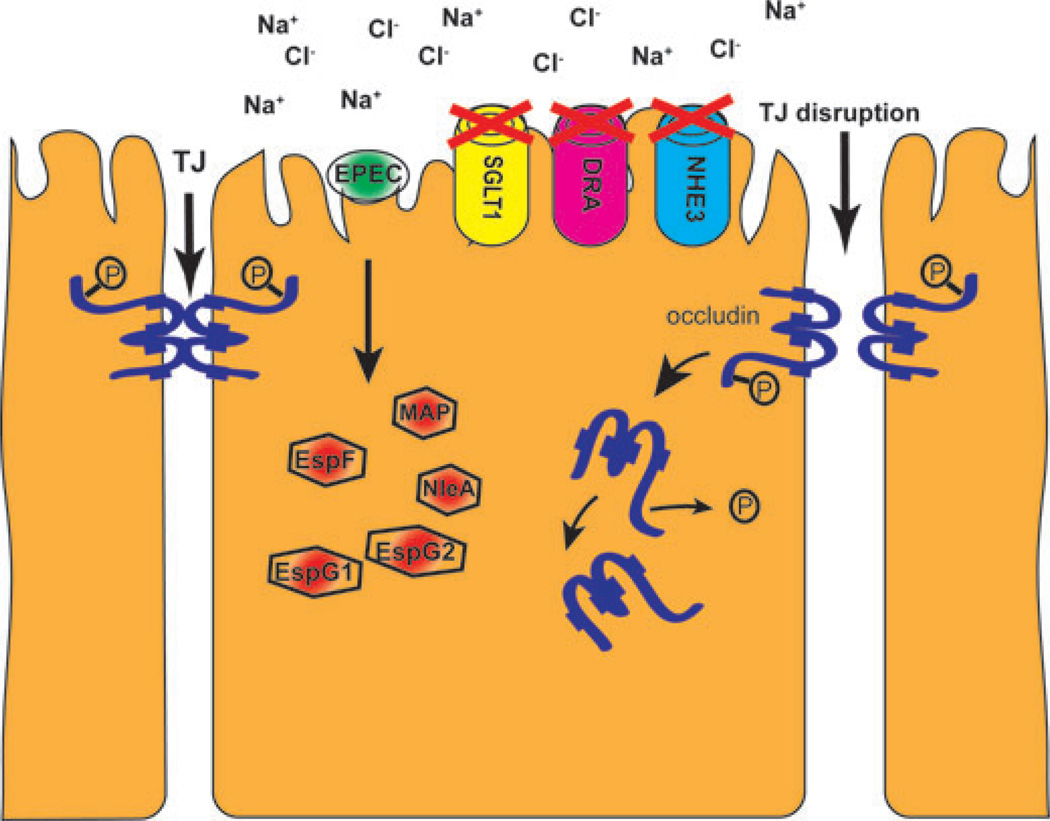
EPEC houses a 35-kb pathogenicity island called the locus of enterocyte effacement (LEE).7,8 Commensal E. coli lack this chromosomally encoded region. Some bacterial effector proteins are encoded within the LEE, while others are non-LEE encoded (Nle). EPEC effectors have multiple deleterious effects on host cells.9,10 EPEC-induced diarrhea is largely attributable to inhibition of absorption by targeting the Na+/H+ exchanger (NHE3), the Na+/glucose cotransporter (SGLT1), as well as the anion exchanger downregulated-in-adenoma (DRA) in human small intestinal epithelium.11–15 The EPEC effector EPEC-secreted protein F (EspF) inhibits NHE3 and SGLT1 activity, while EspG is responsible for decreasing the activity of DRA. In addition to the effects on intestinal transporters, multiple effectors, including EspF, MAP, NleA, and EspG, contribute to disruption of intestinal epithelial tight junctions (TJ), which enhances the diarrhea phenotype by preventing the formation of ion concentration gradients across the epithelium (Fig. 1).16–18
Figure 1.
Mechanisms of EPEC-induced diarrhea. After attachment to epithelial cells, EPEC inject effector proteins (red hexagons) into host cell cytosol. Several effectors mediate tight junction disruption by driving occludin (shown in blue) dephosphorylation and localization to the cytosol. Inhibition of the Na+/glucose cotransporter (SGLT1), the anion exchanger downregulated-in-adenoma (DRA), and Na+/H+ exchanger (NHE3) leads to an increase in luminal sodium and chloride concentrations and ensuing net water loss.
Epithelial TJs are disrupted in multiple disease states underscoring the importance of maintaining epithelial barriers. Intestinal barrier defects were first described over 25 years ago in patients with Crohn’s disease, and since then have been linked to TNF-induced expression of myosin light chain kinase MLCK and caveolin-dependent endocytosis of occludin.19,20–23 Interleukin-13 (IL-13)–induced upregulation of claudin-2 has been identified as the primary cause of barrier dysfunction in ulcerative colitis.24–26 The intestine is not the only location where barrier defects cause severe clinical consequences. The blood–testis barrier (BTB) is partially maintained via TJs. Differential expression and mislocalization of TJ proteins has been correlated with increased BTB permeability leading to increased levels of anti-sperm antibody, a possible factor in male infertility.27–30 In lung epithelial tissues, environmental factors may decrease expression of TJ proteins and play a role in the development of asthma.31–33
In general, tight junction maintenance, disruption, and assembly can be regulated at the transcriptional level, by post-translational protein modification, phosphorylation for example, and by protein half-life. Endocytosis mechanisms involving cytoskeletal changes also play a role in TJ regulation.
TJs are highly dynamic structures that undergo continual maintenance through a process that requires actin.34–36 Cells transfected to stably express fluorescent-tagged occludin, claudin-1, and ZO-1 were treated with the actin-perturbing drug latrunculin A and imaged and transepithelial electrical resistance (TER) was measured simultaneously in real time. Actin depolymerization induced the relocalization of TJ proteins to the cytosol with a corresponding drop in TER, suggesting that actin is required for TJ maintenance. Evidence suggests that microtubules also contribute to TJ maintenance as their disruption by nocodazole impedes significantly the movement of occludin-containing vesi-cles.37 Real-time tracking of GFP-occludin revealed that the fraction of mobile cytosolic occludin was significantly reduced in the presence of nocodazole. Colchicine, an inhibitor of microtubule assembly, also disrupts epithelial cell barrier function although it is not clear if this is due to the mislocalization of TJ proteins or another mechanism.38
These studies indicate that cytoskeletal components play an important role in TJ homeostasis, making EPEC effectors that interfere with the cytoskeleton of high interest with regard to their effects on TJs. The EPEC effector EspG1 and its homologue EspG2 have previously been demonstrated to disrupt microtubules. Recent investigations have identified another role for EspG, that of a GTPase pathway regulator with potential downstream effects on the actin cytoskeleton and on inhibition of vesicle trafficking. Effects of EspG on the Golgi apparatus include the inhibition of protein secretion and possible interference with proper membrane trafficking. Although these studies did not specifically investigate how the various roles ascribed to EspG impact TJs, several mechanisms can be envisioned. In this review, we examine the current literature regarding the identified activities of EspG and discuss the potential mechanisms of EspG-mediated TJ disruption.
EPEC infection disrupts TJ structure and function
EPEC infection has been demonstrated to perturb TJs in both in vitro and in vivo models. Simonovic et al. demonstrated that EPEC infection of intestinal epithelial cells (IEC) shifts occludin localization from the plasma membrane to an intracellular compartment.39 There was a strong correlation between decreased occludin phosphorylation and movement into the cytosol. There was also a corresponding drop in TER, suggesting interdependence of TJ structure and barrier function. Both the structural and functional alterations normalized following eradication of infection with gentamicin, highlighting the restorative nature of TJs. Infection of IEC with an EPEC mutant lacking the effector EspF reduced the shift in occludin localization and the decrease in TER. Claudin-1 and ZO-1 localization are also altered in EPEC-infected IEC.40
Occludin localization and TER are similarly affected in the mouse model of EPEC infection.41 After one day of EPEC infection, ileal, and colonic tissues exhibited substantial relocalization of occludin to the cytosol as well as a significant drop in TER. Neither of these phenomena was observed following infection with an EspF mutant. In contrast to studies done in IEC, ZO-1 localization was not altered in the infected murine intestine.41 In a separate study, claudin-1 was shown to move from the membrane to the cytosol as well.42
EPEC infection also induces a number of transcriptional and post-translational changes that are important for pathogenesis.39,43–46
EspG plays a role in host colonization and disrupts intestinal epithelial TJ function
While EspF and MAP have been implicated in disruption of TJs, EspG has also been shown to contribute to loss of TJ function. 16–18 In EPEC, EspG1 is LEE-encoded and its homolog EspG2 is encoded in the espC pathogenicity island.18,47–49 EspG1 was first described as a type-three secreted homolog of the Shigella flexneri effector VirA harboring 21% identity and 40% similarity.47 EspG is also highly conserved across other attaching and effacing pathogens, namely EHEC O157:H7 and the murine pathogen Citrobacter rodentium. EspG2 is 42% identical and 62% similar to EspG and 20% identical and 38% similar to VirA.47 Functionally, EspG1 can complement an S. flexneri VirA mutant.47 An EPEC EspG1/G2 double mutant can be complemented by EspG1, EspG2, or VirA, indicating a high level of functional conservation.18,50 Structurally EspG and VirA are similar. Both contain a centrally placed six-stranded sheet, a four-stranded sheet forming the amino terminal domain, and both also contain a helical domain.51,52
Relatively few studies have investigated the in vivo function of EspG, but in a rabbit model of infection, deletion of espG from rabbit EPEC greatly reduced colonization compared to wild type. A study performed in mice demonstrated similar findings.47,53 While neither study examined TJ structure or function in vivo, reduced colonization suggests that EspG plays an important role in EPEC pathogenesis.
EspG also contributes to loss of TJ function during EPEC infection. Tomson et al. reported that infection of either T84 or Caco-2 IEC with ΔespG1/G2 attenuated the drop in TER, compared to wild-type EPEC.18 Five hours after infection with wild-type EPEC, TER dropped approximately 70%, while infection with ΔespG1/G2 reduced TER by only 30%. Complementation of the mutant with espG restored the reduction in TER to levels comparable to wild type. A separate study reported the decrease in TER to be comparable between wild type and ΔespG1/G2 although the different results are possibly attributable to the use of a nonintestinal cell line.54 While ectopic expression of EspG1 in Madin-Darby canine kidney epithelial cells in the absence of other EPEC effectors failed to decrease TER, para-cellular permeability to 4 kDa dextran was increased significantly, indicating that EspG may have an impact on the mechanisms that regulate the selective permeability of TJs.54 The phenotypes attributable to EspG are specific as this effector was shown to play no role in induction of the inflammatory cascade or pedestal formation.18,55
EspG disrupts microtubule networks
EspG was first identified as a homolog of the Shigella flexneri effector VirA.47 VirA disrupts microtubule networks and extensive literature has been published regarding a similar function for EspG1/G2 (Fig. 2).47,56 It was first established that IEC microtubule networks were absent under attached EPEC microcolonies and that this phenomenon was dependent on a functional TTSS.48 Infection of IEC with various EPEC mutants identified EspG1/G2 as the relevant effector proteins. EspG1 was also spatially associated with microtubule loss as tagged bacterial protein localized directly under microcolonies in areas devoid of microtubules.48
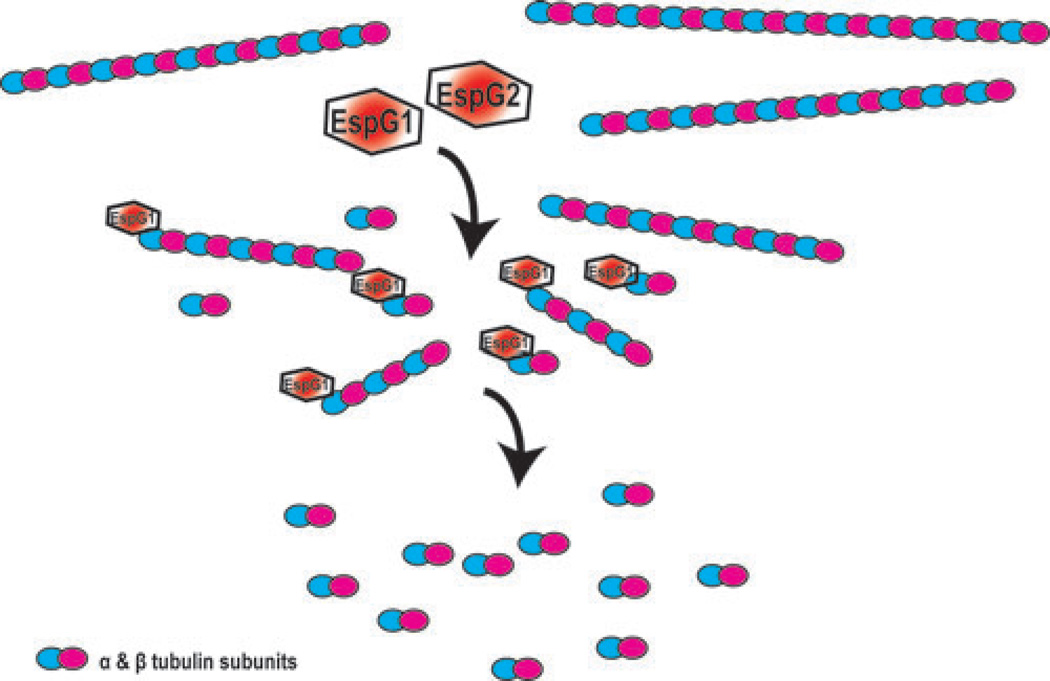
Figure 2.
EspG disrupts microtubules via an unknown mechanism. EspG disrupts microtubules via an unknown mechanism. EPEC EspG has been demonstrated to bind directly to α-tubulin. The presence of EspG alone in the absence of other effectors induces depolymerization of microtubules into tubulin subunits.
A separate study examined the state of microtubules during EPEC infection of IEC revealing extensive loss of microtubule networks at five hours after wild-type infection but no change in cells infected with ΔespG1.18 Eradication of infection with gentamicin allowed for recovery of microtubules, which was dependent on new protein synthesis as treatment with cycloheximide blocked microtubule formation. This finding is consistent with the reported degradation of α-tubulin by EspG.18
Transient expression of EspG in mammalian cells, which allows for assessment of EspG effects in the absence of other bacterial effectors, confirmed that this protein alone is sufficient to depolymerize microtubules in the absence of other EPEC effectors.18 Furthermore, gel overlay assays using purified His-tagged EHEC EspG demonstrated that it complexes directly with tubulin heterodimers in the absence of additional cofactors.53 The same study used a bacterial two-hybrid assay to determine that EspG binds directly to the α-tubulin subunit. In another reductionist system, purified EspG depolymerized microtubules in solution, underscoring the effect of this protein on the cytoskeleton.57
While the role of microtubules in TJ maintenance has been only superficially investigated, two studies suggest that these structures are required for TJ homeostasis. Subramanian et al. transiently expressed GFP-occludin in epithelial cells and tracked its movement.37 Individual vesicle tracks were aligned and the distance traveled was measured over time. Approximately 75 ±10% of vesicles traveled >6 µm/min. Microtubule disruption with nocodazole reduced the percentage of mobile vesicles to 13%. Although this study did not examine directionality, it suggests that occludin traffics on microtubules, supporting the speculation that EspG-mediated microtubule disruption impedes the movement of TJ proteins.
Calcium chelation studies investigated the requirement for microtubules in TJ disassembly.58 Chelation of calcium from epithelial monolayers rapidly disrupts TJs, driving TJ proteins into the cytosol. Microtubule disruption by nocodazole prevented the mislocalization of TJ proteins following calcium chelation in two different intestinal epithelial cell lines; (β-catenin, occludin, and ZO-1 all remained junction-associated even after 60 min of chelation. Cells harboring intact microtubule networks, however, exhibited an increase in cytosolic TJ proteins. While this study investigated only the movement of TJ proteins from the plasma membrane to the cytosol, it supports the theory that microtubules participate in the movement of TJ proteins. We speculate that EspG-mediated microtubule disruption may interfere with TJ protein movement, thus preventing TJ restoration and contributing to EPEC-induced barrier loss.
Additional data link EspG-induced microtubule disruption to remodeling of the actin cytoskeleton. A microtubule-bound RhoA-specific guanine nucleotide exchange factor, GEF-H1, was shown to be released and activated following infection with wild-type EPEC, but not after infection with an EspG1/G2 mutant.57 This suggests that EspG1/G2-induced microtubule depolymerization activates GEF-H1 and induces the ensuing downstream effects on the actin cytoskeleton.54,57 As predicted, RhoA was found to be active after wild-type EPEC infection, but not after infection with an EspG1/G2 mutant.54 RhoA mediates its downstream effects via Rho-associated kinase (ROCK). ROCK phosphorylates multiple targets including the myosin phosphatase target subunit (MYPT1) of myosin light chain phosphatase.59–62 Phosphorylation of MYPT1 inactivates the phosphatase resulting in increased phospho-rylated myosin light chain (MLC). MLC activity is regulated by the opposing actions of MLCK and MYPT1. EPEC induces phosphorylation of MLC causing contraction of the perijunctional acto-myosin ring and a decrease in TER, which is attenuated by inhibition of MLCK.39,63–66 The mechanism by which MLCK is activated by EPEC is not fully defined however. We speculate that EspG-mediated microtubule disruption may lead to GEF-H1 release, Rho/ROCK activation and inhibition of MYPT1. Inhibition of MYPT1 combined with activation of MLCK might contribute to greatly increased levels of phospho-MLC and ensuing TJ disruption.
We note here that one study used transient trans-fection of GFP-GEF-H1 to investigate its association with microtubules.57 This raises the possibility that protein overexpression is responsible for the association with microtubules and that endogenous GEF-H1 is not as strongly associated with microtubules, as a separate study showed that native GEF-H1 is associated with TJs and not microtubules in polarized epithelia.67 If indeed GEF-H1 is not associated with microtubules, EspG-mediated microtubule disruption may affect the TJ simply by preventing the trafficking of TJ proteins.37 TJ homeostasis requires the correct targeting of recycled and newly synthesized proteins to these structures. Interruption of TJ protein delivery could have substantial effects on both TJ structure and function. Further work is needed to clarify the effect of EspG-mediated microtubule disruption on the TJ.
EspG acts as a regulator of GTPase signaling
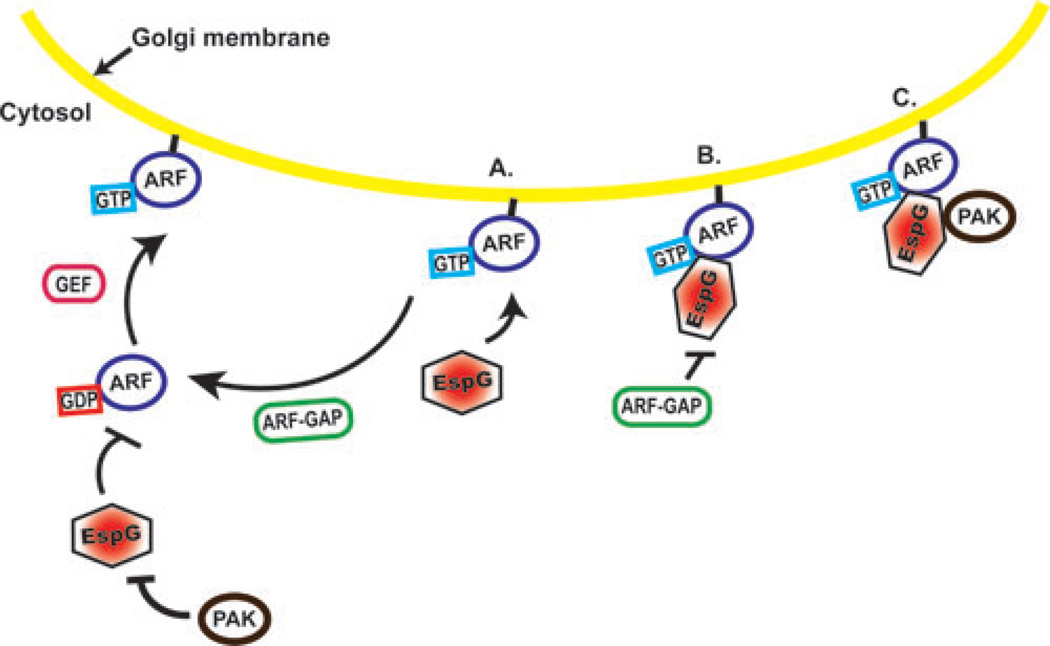
Two recent investigations revealed previously unrecognized activities for EspG. Selyunin et al. demonstrated that EHEC EspG binds to both and p21-activated kinase (PAK) proteins.52 Structural analysis of crystallized EspG in complex with ARF6 revealed that upon binding, ARF6 adopts a conformation nearly identical to that of the protein in its active GTP-bound state. In GST-pulldown assays, EspG selectively bound to GTP-loaded ARF1 and ARF6. In the GDP-bound (inactive) conformation, the EspG–binding region of ARF is inaccessible to EspG. The EspG–ARF interaction also blocked the access of GTPase activating proteins (GAPs), thus preventing hydrolysis of the ARF-GTP γ -phosphate and disrupting the normal guanine nucleotide cycle. These data suggest that EspG preferentially binds and sequesters ARF proteins in the active GTP-bound conformation. An EspG ARF-binding mutant had no effect on GAP activity, indicating the specificity of EspG’s stearic hindrance.
ARF proteins are required for the recycling of vesicles to the plasma membrane and for organization of vesicle trafficking.68–70 EspG was shown to localize to the Golgi apparatus and disrupt Golgi stacks, suggesting that EspG interferes with vesicle trafficking. An EspG ARF-binding mutant did not induce Golgi disruption. Interestingly, Golgi dispersal is a well-established indicator of microtubule disruption, however in this study microinjection of 10 nM recombinant EspG into rat kidney cells did not disrupt microtubule networks. 71–75 The absence of this phenotype may be due to differences in protocols, including the use of different cell types (rat kidney versus IEC), microinjection versus infection or transfection and/or the time points examined. Previous ectopic expression studies were performed using either stable or transient transfection of espG and microtubules were examined at later time points. Microtubules were not examined in cells transfected with espG in this publication, only EspG microinjected cells.
Most interesting was the demonstration that, in contrast to the sequestration of active ARF proteins by EspG, this effector protein also binds p21-activated kinase 2 (PAK2) and promotes its activity. In the auto-inhibited homodimer conformation, the region of PAK that interacts with EspG has three functions—it blocks substrate binding, it blocks the catalytic site, and it stabilizes the homodimer structure. In the presence of EspG, PAK2 activity is increased 7.6-fold, suggesting the reversal of these inhibitions. PAK and ARF proteins bind EspG on adjacent nonoverlapping surfaces. In addition, PAK binding is entirely dependent on the formation of the EspG-ARF complex as in the absence of ARF interaction, PAK2 fails to associate with EspG (Fig. 3). The PAK family of proteins transduce signals from the Rac1 and Cdc42 GTPases, regulating cytoskeletal dynamics, and cell motility.76 Of note, both Rac1 and CdC42 have also been determined to play a role in TJ disruption.77,78 By linking GTPase inhibition and Rac1/Cdc42 signal trans-duction, EspG may serve as a “catalytic scaffold” that permits EPEC to interfere with host cell processes in a specific location within the cell, namely at the Golgi membranes. A separate study also reported that EspG binds to the Rac/Cdc42-binding site of PAK1 leading to the conclusion that by imitating a small GTPase, EspG permits EPEC to bypass host cell GTPases and enable PAK-dependent actin remodeling regardless of the status of native Rac/Cdc42.51 In summary, while the impact of EspG-PAK1 binding on TJs is currently not known, these studies identify cytoskeletal remodeling as a major function of EspG and suggest that this effector plays multiple roles in EPEC pathogenesis.
Figure 3.
EspG regulates the GTPase cycle. (A) Active, GTP-bound ARF is membrane bound. GTPase activating proteins (GAPs) stimulate GTP hydrolysis and release from the membrane. Guanine nucleotide exchange factors (GEFs) catalyze the exchange of GDP for GTP and the reactivation of ARF. EspG is unable to bind to inactive GDP-bound ARF and preferentially binds GTP-bound ARF. PAK2 will not bind to EspG independently of ARF. (B) The formation of the ARFGTP-EspG complex renders GAPs unable to stimulate GTP hydrolysis. (C) Once the ARFGTP-EspG complex has formed, PAK is able to bind EspG.
Another role for EspG may be linked to its localization to the Golgi apparatus.52 Ectopic expression of EspG from EPEC, and EHEC, EspG2 from EPEC, and VirA from S. flexneri–induced Golgi disruption. Expression of these proteins significantly inhibited Golgi-based secretion as determined by the release of a reporter protein. Another EPEC effector, NleA, also inhibits the secretory pathway by binding to SEC24 and inhibiting COPII anterograde trafficking.79 Comparison of the activity levels of NleA to EspG from EPEC and EHEC, EspG2 from EPEC, and VirA from S. flexneri revealed that all EspG and VirA inhibit protein secretion to a significantly greater extent than NleA. A yeast two-hybrid screen identified Golgi matrix protein GM130 as a binding partner for EPEC EspG. GM130 is a Golgi-associated protein that interacts with vesicle docking protein p115 as well as Rab1 and syntaxin 5.80 The link between the EspG-GM130 interaction and EspG-mediated interruption of protein transport is currently unclear. Transient expression of EHEC EspG in this study was not observed to induce dramatic fragmentation of microtubules. Instead, “thickening” of tubular structures was observed, as well as membrane ruffling, indicating that EspG may have hitherto undefined effects on both microtubule and actin cytoskeletal components.
Disruption of Golgi stacks, via binding to GM130 or sequestration of ARF proteins, may be another mechanism by which EspG contributes to barrier dysfunction. As TJ proteins move through the Golgi, they are glycosylated, and directed to specific cell membrane domains.81 Disruption of the Golgi via a GM130-dependent mechanism or by sequestration of active ARF may prevent newly synthesized TJ proteins from reaching the plasma membrane and contributing to TJ reconstruction. The EPEC effector NleA has already been shown to contribute to disruption of TJ function mediated by inhibition of Golgi trafficking.17
These data open new avenues of investigation into the many potential roles of EspG1/G2 in EPEC pathogenesis and provide new insight into how they may contribute to EPEC-induced TJ disruption, potentially by disrupting microtubules, by as-yet undetermined mechanisms related to vesicle trafficking or cytoskeletal remodeling or by blocking secretion of TJ proteins.
Conclusion
Loss of TJ structure and function during EPEC infection is due to multiple effectors, each of which may have myriad effects on the host cell. For example, EspF has been termed the bacterial “Swiss army knife” and has been shown to associate with mitochondria, promote apoptosis, regulate host membrane interactions, promote invasion of host IEC and inhibit macrophage uptake in addition to disrupting TJs.82–87 While microtubule disruption was initially thought to be the sole function of EspG1/G2, new work uncovering inhibition of vesicle trafficking, promotion of cytoskeletal remodeling and inhibition of Golgi secretion as additional functions of these proteins paints a more complex picture. In the ongoing hunt for therapeutic targets, EPEC effectors with broad scopes of capabilities invite further investigation; disabling one potent effector with a large range of functions could lead to greatly reduced physiological disturbance. The expanding repertoire of activities of EspG1/G2 identified demonstrates that further investigation is needed to fully understand their role in EPEC-induced loss of epithelial barrier function.
Acknowledgments
This work was supported by Grants DK50694, DK58964, DK067887, and a VA Merit to GH and DK091151 to LG.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Fagundes-Neto U, Kallas MR, Patricio FR. Mor-phometric study of the small bowel mucosa in infants with diarrhea due to enteropathogenic Escherichia coli strains. Hepato-gastroenterology. 1997;44:1051–1056. [PubMed] [Google Scholar]
- 2.Hill SM, Phillips AD, Walker-Smith JA. En-teropathogenic Escherichia coli and life threatening chronic diarrhoea. Gut. 1991;32:154–158. doi: 10.1136/gut.32.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fagundes-Neto U, et al. Nutritional impact and ul-trastructural intestinal alterations in severe infections due to enteropathogenic Escherichia coli strains in infants. J. Am. Col. Nutri. 1996;15:180–185. doi: 10.1080/07315724.1996.10718586. [DOI] [PubMed] [Google Scholar]
- 4.Jarvis KG, et al. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc. Natl. Acad. Sci. USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvis KG, Kaper JB. Secretion of extracellular proteins by enterohemorrhagic Escherichia coli via a putative type III secretion system. Infect. Immun. 1996;64:4826–4829. doi: 10.1128/iai.64.11.4826-4829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolff C, et al. Protein translocation into host epithelial cells by infecting enteropathogenic Escherichia coli. Mol. Microbiol. 1998;28:143–155. doi: 10.1046/j.1365-2958.1998.00782.x. [DOI] [PubMed] [Google Scholar]
- 7.Karaolis DK, et al. Cloning of the RDEC-1 locus of enterocyte effacement (LEE) and functional analysis of the phenotypeonHEp-2cells. Ad. Exp. Medi.Biol. 1997;412:241–245. doi: 10.1007/978-1-4899-1828-4_36. [DOI] [PubMed] [Google Scholar]
- 8.McDaniel TK, et al. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean P, Kenny B. The effector repertoire of enteropathogenic E. coli: ganging up on the host cell. Curr. Opin. Microbiol. 2009;12:101–109. doi: 10.1016/j.mib.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean P, Maresca M, Kenny B. EPEC’s weapons of mass subversion. Curr. Opin. Microbiol. 2005;8:28–34. doi: 10.1016/j.mib.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Dean P, et al. Potent diarrheagenic mechanism mediated by the cooperative action of three enteropathogenic Escherichia coli-injected effector proteins. Proc. Natl. Acad. Sci. USA. 2006;103:1876–1881. doi: 10.1073/pnas.0509451103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guttman JA, et al. Aquaporins contribute to diarrhoea caused by attaching and effacing bacterial pathogens. Cell Microbiol. 2007;9:131–141. doi: 10.1111/j.1462-5822.2006.00773.x. [DOI] [PubMed] [Google Scholar]
- 13.Hecht G, et al. Differential regulation of Na+/H+ exchange isoform activities by enteropathogenic E. coli in human intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G370–G378. doi: 10.1152/ajpgi.00432.2003. [DOI] [PubMed] [Google Scholar]
- 14.Hodges K, et al. The enteropathogenic Escherichia coli effector protein EspF decreases sodium hydrogen exchanger 3 activity. Cell Microbiol. 2008;10:1735–1745. doi: 10.1111/j.1462-5822.2008.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill RK, et al. Mechanism underlying inhibition of intestinal apical Cl–/OH– exchange following infection with enteropathogenic E. coli. J. Clin. Invest. 2007;117:428–437. doi: 10.1172/JCI29625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dean P, Kenny B. Intestinal barrier dysfunction by enteropathogenic Escherichia coli is mediated by two effector molecules and a bacterial surface protein. Mol. Microbiol. 2004;54:665–675. doi: 10.1111/j.1365-2958.2004.04308.x. [DOI] [PubMed] [Google Scholar]
- 17.Thanabalasuriar A, et al. The bacterial virulence factor NleA is required for the disruption of intestinal tight junctions by enteropathogenic Escherichia coli. Cell Microbiol. 2010;12:31–41. doi: 10.1111/j.1462-5822.2009.01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomson FL, et al. Enteropathogenic Escherichia coli EspG disrupts microtubules and in conjunction with Orf3 enhances perturbation of the tight junction barrier. Mol. Microbiol. 2005;56:447–464. doi: 10.1111/j.1365-2958.2005.04571.x. [DOI] [PubMed] [Google Scholar]
- 19.Pearson AD, et al. Intestinal permeability in children with Crohn's disease and coeliac disease. Br. Medi. J. Clin.Res. 1982;285:20–21. doi: 10.1136/bmj.285.6334.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ukabam SO, Clamp JR, Cooper BT. Abnormal small intestinal permeability to sugars in patients with Crohn’s disease of the terminal ileum and colon. Digestion. 1983;27:70–74. doi: 10.1159/000198932. [DOI] [PubMed] [Google Scholar]
- 21.Blair SA, et al. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab. Invest. 2006;86:191–201. doi: 10.1038/labinvest.3700373. [DOI] [PubMed] [Google Scholar]
- 22.Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G496–G504. doi: 10.1152/ajpgi.00318.2005. [DOI] [PubMed] [Google Scholar]
- 23.Marchiando AM, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J. Cell Biol. 2010;189:111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuss IJ, et al. Nonclassical CD1d–restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J. Clin. Invest. 2004;113:1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heller F, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junc-stions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Rosen MJ, et al. STAT6 activation in ulcerative colitis: a new target for prevention of IL-13-induced colon epithelial cell dysfunction. Inflammat. Bowel Dis. 2011;17:2224–2234. doi: 10.1002/ibd.21628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng CY, et al. Environmental toxicants and male reproductive function. Spermatogenesis. 2011;1:2–13. doi: 10.4161/spmg.1.1.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang XW, et al. Mechanisms involved in the blood-testis barrier increased permeability induced by EMP. Toxicology. 2010;276:58–63. doi: 10.1016/j.tox.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Wong EW, et al. Regulation of blood-testis barrier dynamics by TGF-beta3 is a Cdc42-dependent protein trafficking event. Proc. Natl. Acad. Sci. USA. 2010;107:11399–11404. doi: 10.1073/pnas.1001077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong EWP, et al. Cell Junctions in the Testis as Targets for Toxicants. In: Charlene AM, editor. Comprehensive Toxicology. Second Edition. Oxford: Elsevier; 2010. Nov 09, pp. 167–188. [Google Scholar]
- 31.Tai HY, et al. Pen ch 13 allergen induces secretion of mediators and degradation of occludin protein of human lung epithelial cells. Allergy. 2006;61:382–388. doi: 10.1111/j.1398-9995.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 32.Vermeer PD, et al. MMP9 modulates tight junction integrity and cell viability in human airway epithelia. Am. J. Physiol. Lung Cel. Mol. Physiol. 2009;296:L751–762. doi: 10.1152/ajplung.90578.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinhas R, et al. Pollen proteases compromise the airway epithelial barrier through degradation of transmem-brane adhesion proteins and lung bioactive peptides. Allergy. 2011;66:1088–1098. doi: 10.1111/j.1398-9995.2011.02598.x. [DOI] [PubMed] [Google Scholar]
- 34.Morimoto S, et al. Rab13 mediates the continuous endocytic recycling of occludin to the cell surface. J. Biol. Chem. 2005;280:2220–2228. doi: 10.1074/jbc.M406906200. [DOI] [PubMed] [Google Scholar]
- 35.Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J. Cell Biol. 2008;181:683–695. doi: 10.1083/jcb.200711165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol. Biol. Cell. 2005;16:3919–3936. doi: 10.1091/mbc.E04-12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subramanian VS, et al. Tight junction targeting and intracellular trafficking of occludin in polarized epithelial cells. Am. J. Physiol. Cell Physiol. 2007;293:C1717–C1726. doi: 10.1152/ajpcell.00309.2007. [DOI] [PubMed] [Google Scholar]
- 38.Banan A, et al. Oxidant-induced intestinal barrier disruption and its prevention by growth factors in a human colonic cell line: role of the microtubule cytoskeleton. Free Radi. Biol. Medi. 2000;28:727–738. doi: 10.1016/s0891-5849(00)00160-x. [DOI] [PubMed] [Google Scholar]
- 39.Simonovic I, et al. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell Microbiol. 2000;2:305–315. doi: 10.1046/j.1462-5822.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- 40.Muza-Moons MM, Schneeberger EE, Hecht GA. Enteropathogenic Escherichia coli infection leads to appearance of aberrant tight junctions strands in the lateral membrane of intestinal epithelial cells. Cell Microbiol. 2004;6:783–793. doi: 10.1111/j.1462-5822.2004.00404.x. [DOI] [PubMed] [Google Scholar]
- 41.Shifflett DE, et al. Enteropathogenic E. coli disrupts tight junction barrier function and structure in vivo. Lab. Invest. 2005;85:1308–1324. doi: 10.1038/labinvest.3700330. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Q, et al. Redistribution of Tight Junction Proteins During EPEC Infection In Vivo. Inflammation. 2010 doi: 10.1007/s10753-010-9285-1. [DOI] [PubMed] [Google Scholar]
- 43.Bonazzi M, et al. Clathrin phosphorylation is required for actin recruitment at sites of bacterial adhesion and internalization. J. Cell Biol. 2011;195:525–536. doi: 10.1083/jcb.201105152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Grado M, et al. Enteropathogenic Escherichia coli infection induces expression of the early growth response factor by activating mitogen-activated protein kinase cascades in epithelial cells. Infect. Immun. 2001;69:6217–6224. doi: 10.1128/IAI.69.10.6217-6224.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hardwidge PR, et al. Proteomic analysis of the intestinal epithelial cell response to enteropathogenic Escherichia coli. J. Biol. Chem. 2004;279:20127–20136. doi: 10.1074/jbc.M401228200. [DOI] [PubMed] [Google Scholar]
- 46.Bhatt S, Romeo T, Kalman D. Honing the message: post-transcriptional and post-translational control in attaching and effacing pathogens. Tre. Microbiol. 2011;19:217–224. doi: 10.1016/j.tim.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elliott SJ, et al. EspG, a novel type III system-secreted protein from enteropathogenic Escherichia coli with similarities to VirA of Shigella flexneri. Infect. Immun. 2001;69:4027–4033. doi: 10.1128/IAI.69.6.4027-4033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaw RK, et al. Enteropathogenic Escherichia coli type III effectors EspG and EspG2 disrupt the microtubule network of intestinal epithelial cells. Infect. Immun. 2005;73:4385–4390. doi: 10.1128/IAI.73.7.4385-4390.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mellies JL, et al. espC pathogenicity island of enteropathogenic Escherichia coli encodes an enterotoxin. Infect. Immun. 2001;69:315–324. doi: 10.1128/IAI.69.1.315-324.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smollett K, et al. Function and distribution of EspG2, a type III secretion system effector of enteropathogenic Escherichia coli. Microbes Infect. 2006;8:2220–2227. doi: 10.1016/j.micinf.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Germane KL, Spiller BW. Structural and functional studies indicate that the EPEC effector, EspG, directly binds p21-activated kinase. Biochemistry. 2011;50:917–919. doi: 10.1021/bi1020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Selyunin AS, et al. The assembly of a GTPase-kinase signalling complex by a bacterial catalytic scaffold. Nature. 2011;469:107–111. doi: 10.1038/nature09593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hardwidge PR, et al. Modulation of host cytoskeleton function by the enteropathogenic Escherichia coli and Citrobacter rodentium effector protein EspG. Infect. Immun. 2005;73:2586–2594. doi: 10.1128/IAI.73.5.2586-2594.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsuzawa T, Kuwae A, Abe A. Enteropathogenic Escherichia coli type III effectors EspG and EspG2 alter epithelial paracellular permeability. Infect. Immun. 2005;73:6283–6289. doi: 10.1128/IAI.73.10.6283-6289.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Savkovic SD, et al. EPEC-activated ERK1/2 participate in inflammatory response but not tight junction barrier disruption. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G890–G898. doi: 10.1152/ajpgi.2001.281.4.G890. [DOI] [PubMed] [Google Scholar]
- 56.Yoshida S;, et al. Microtubule-severing activity of Shigella is pivotal for intercellular spreading. Science. 2006;314:985–989. doi: 10.1126/science.1133174. [DOI] [PubMed] [Google Scholar]
- 57.Matsuzawa T, et al. Enteropathogenic Escherichia coli activates the RhoA signaling pathway via the stimulation of GEF-H1. EMBO J. 2004;23:3570–3582. doi: 10.1038/sj.emboj.7600359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ivanov AI, et al. Microtubules regulate disassembly of epithelial apical junctions. BMC Cell Biol. 2006;7:12. doi: 10.1186/1471-2121-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amano M, et al. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 60.Chang YC, et al. GEF-H1 couples nocodazole-induced microtubule disassembly to cell contractility via RhoA. Mol. Biol. Cell. 2008;19:2147–2153. doi: 10.1091/mbc.E07-12-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Enomoto T. Microtubule disruption induces the formation of actin stress fibers and focal adhesions in cultured cells: possible involvement of the rho signal cascade. Cell Stru. Fun. 1996;21:317–326. doi: 10.1247/csf.21.317. [DOI] [PubMed] [Google Scholar]
- 62.Kimura K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 63.Manjarrez-Hernandez HA, et al. Phosphorylation of myosin light chain at distinct sites and its association with the cytoskeleton during enteropathogenic Escherichia coli-infection. Infect. Immun. 1996;64:2368–2370. doi: 10.1128/iai.64.6.2368-2370.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuhan R, et al. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;113:1873–1882. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]
- 65.Zolotarevsky Y, et al. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology. 2002;123:163–172. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- 66.Shen L, et al. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J. Cell Sci. 2006;119:2095–2106. doi: 10.1242/jcs.02915. [DOI] [PubMed] [Google Scholar]
- 67.Guillemot L, et al. Paracingulin regulates the activity of Rac1 and RhoA GTPases by recruiting Tiam1 and GEF-H1 to epithelial junctions. Mol. Biol. Cell. 2008;19:4442–4453. doi: 10.1091/mbc.E08-06-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.D’Souza-Schorey C, et al. ARF6 targets recycling vesicles to the plasma membrane: insights from an ultrastructural investigation. J. Cell Biol. 1998;140:603–616. doi: 10.1083/jcb.140.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Franco M, et al. EFA6, a sec7 domain-containing exchange factor for ARF6, coordinates membrane recycling and actin cytoskeleton organization. EMBO J. 1999;18:1480–1491. doi: 10.1093/emboj/18.6.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kahn RA. Toward a model for Arf GTPases as regulators of traffic at the Golgi. FEBS letters. 2009;583:3872–3879. doi: 10.1016/j.febslet.2009.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cole NB, et al. Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endo-plasmic reticulum exit sites. Mol. Biol. Cell. 1996;7:631–650. doi: 10.1091/mbc.7.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ho WC, et al. Reclustering of scattered Golgi elements occurs along microtubules. Eur. J. Cell Biol. 1989;48:250–263. [PubMed] [Google Scholar]
- 73.Rogalski AA, Singer SJ. Associations of elements of the Golgi apparatus with microtubules. J. Cell Biol. 1984;99:1092–1100. doi: 10.1083/jcb.99.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thyberg J, Moskalewski S. Role of microtubules in the organization of the Golgi complex. Exp. Cell Res. 1999;246:263–279. doi: 10.1006/excr.1998.4326. [DOI] [PubMed] [Google Scholar]
- 75.Wehland J, et al. Role of microtubules in the distribution of the Golgi apparatus: effect of taxol and microinjected anti-alpha-tubulin antibodies. Proc. Natl. Acad. Sci. USA. 1983;80:4286–4290. doi: 10.1073/pnas.80.14.4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bokoch GM. Biology of the p21-activated kinases. Ann. Rev. Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 77.Bruewer M, et al. RhoA, Rac1, and Cdc42 exert distinct effects on epithelial barrier via selective structural and biochemical modulation of junctional proteins and F-actin. Am. J. Physiol. Cell Physiol. 2004;287:C327–C335. doi: 10.1152/ajpcell.00087.2004. [DOI] [PubMed] [Google Scholar]
- 78.Jou TS, Schneeberger EE, Nelson WJ. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J. Cell Biol. 1998;142:101–115. doi: 10.1083/jcb.142.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim J, et al. The bacterial virulence factor NleA inhibits cellular protein secretion by disrupting mammalian COPII function. Cell Host. Microbe. 2007;2:160–171. doi: 10.1016/j.chom.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 80.Clements A, et al. EspG of enteropathogenic and enterohemorrhagic E. coli binds the Golgi matrix protein GM130 and disrupts the Golgi structure and function. Cell Microbiol. 2011;13:1429–1439. doi: 10.1111/j.1462-5822.2011.01631.x. [DOI] [PubMed] [Google Scholar]
- 81.Gut A, et al. Carbohydrate-mediated Golgi to cell surface transport and apical targeting of membrane proteins. EMBO J. 1998;17:1919–1929. doi: 10.1093/emboj/17.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Holmes A, et al. The EspF effector, a bacterial pathogen’s Swiss army knife. Infect. Immun. 78:4445–4453. doi: 10.1128/IAI.00635-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alto NM, et al. The type III effector EspF coordinates membrane trafficking by the spatiotemporal activation of two eukaryotic signaling pathways. J. Cell Biol. 2007;178:1265–1278. doi: 10.1083/jcb.200705021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McNamara BP, et al. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J. Clin. Invest. 2001;107:621–629. doi: 10.1172/JCI11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagai T, Abe A, Sasakawa C. Targeting of enteropathogenic Escherichia coli EspF to host mitochondria is essential for bacterial pathogenesis: critical role of the 16th leucine residue in EspF. J. Biol. Chem. 2005;280:2998–3011. doi: 10.1074/jbc.M411550200. [DOI] [PubMed] [Google Scholar]
- 86.Nougayrede JP, Donnenberg MS. En-teropathogenic Escherichia coli EspF is targeted to mitochondria and is required to initiate the mitochondrial death pathway. Cell Microbiol. 2004;6:1097–1111. doi: 10.1111/j.1462-5822.2004.00421.x. [DOI] [PubMed] [Google Scholar]
- 87.Tahoun A, et al. Comparative analysis of EspF variants in inhibition of Escherichia coli phagocytosis by macrophages and inhibition of E. coli translocation through human- and bovine-derived M cells. Infect. Immun. 2011;79:4716–4729. doi: 10.1128/IAI.00023-11. [DOI] [PMC free article] [PubMed] [Google Scholar]