Abstract
Endoscopic ultrasound-guided fine-needle aspiration cytology (EUS-FNAC) has proven to be of significant value as a diagnostic method for the evaluation of esophageal mesenchymal tumors, such as true leiomyomas. Utilizing the cell block procedure, the present study reports the diagnostic approach of EUS-FNAC in two patients affected by this lesion, describing the cytological and immunocytochemical findings. Spindle-shaped elements with elongated nuclei were appreciable; moreover, the cytoplasmatic immunohistochemical positivity for smooth muscle actin and desmin strongly supported the diagnosis of leiomyoma when also taking into account the constant negativity for CD34, CD117 and S100. The differential diagnosis between spindle cell mesenchymal tumors and leiomyomas, and the clinico-therapeutic management of the latter are also discussed in the study.
Keywords: esophageal leiomyomas, immunocytochemistry, cytopathology, cell block, differential diagnosis
Introduction
Mesenchymal tumors of the gastrointestinal tract are uncommon, representing only a small percentage of gastrointestinal neoplasms (1–7). The tumors are generally localized within the submucosa as intramural nodules that can to lead to obstruction, ulceration and bleeding (3,8,9). The majority of these tumors, including leiomyomas, schwannomas and neurofibromas, show benign behavior, even if their malignant counterparts have been reported (2,7,8). Nevertheless, among spindle cell tumors, gastrointestinal stromal tumors (GISTs) represent the most commonly occurring event, but they are characterized by a different prognosis and clinical management, and therefore require a diagnostic distinction from the other entities, mainly from leiomyomas (3,7,10–14). The differential diagnosis between GISTs and gastrointestinal leiomyomas offers certain difficulties, not only due to their overlapping clinical and ultrasound presentations, but also due to their cytological appearance, largely represented by spindle cells.
Endoscopic ultrasound-guided fine-needle aspiration cytology (EUS-FNAC) has proven itself to be a reliable method for the diagnosis of GISTs (15) and other gastrointestinal mesenchymal tumors, including true leiomyomas (15). The present study reports two cases of true intramural leiomyomas of the esophagus, in which EUS-FNAC allowed the sampling of the submucosal lesions, which are otherwise difficult to biopsy by traditional methods; moreover, the immunophenotypic profile readily obtained from cell blocks aided in the definition of these lesions, distinguishing them from other gastrointestinal stromal or mesenchymal tumors. Patients provided written informed consent.
Case reports
Case 1
A 43-year-old male presented with dysphagia that had been apparent for 2 months. A physical examination revealed no abnormalities, and the standard serum laboratory tests were in the normal range. Computed tomography (CT) scans of the chest revealed a hypodense mass developing in the distal esophagus and causing substenosis of the lumen, which was extended to 65 mm in length, with non-homogeneous contrast enhancement (Fig. 1A). EUS examination revealed a 45-mm hypoechoic, round lesion with well demarcated margins, originating from the muscle layer of the distal esophagus in contact with the inferior caval vein and right atrium (Fig. 1B). EUS-FNAC was performed by using a convex array echoendoscope (EG-3870 UTK; Pentax, Co., Ltd., Tokyo, Japan) and by making two passes with a 22G needle. The specimens were processed by an in-room cytopathologist and immediately examined for adequate cellularity following staining by hematoxylin and eosin. A second slide was immediately fixed in 98% ethanol and stained with Papanicolaou. Any excess materials, including the needle and syringe utilized in the procedure, were rinsed in 10 ml 50% ethanol in a specimen container. All content was centrifuged in a 10-ml disposable centrifuge tube at 5,017 × g for 6 min to create 1 or 2 pellets; the supernatant fluid was decanted and the pelleted material was immediately fixed in a freshly prepared solution of 4% neutral buffered formalin for 45 min. The cell pellets were then placed in a cassette and stored at 80% ethanol until ready for processing in an automatic tissue processor (Leica TP1020; Leica, Buckinghamshire, UK). The cell blocks obtained were embedded in paraffin at 56°C, and 3-μm thick successive sections were cut and routinely stained by hematoxylin and eosin; parallel serial sections of the same thickness were mounted on silane-coated glasses and submitted to immunohistochemical procedures, as described previously (16,17).
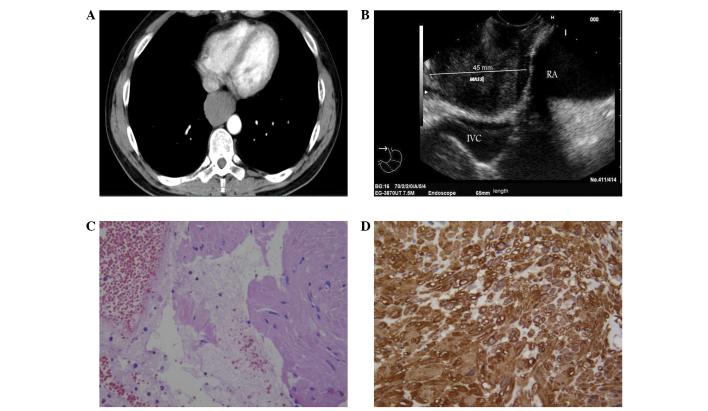
Figure 1.
Case 1: (A) CT scan showing a hypodense mass with non-homogeneous contrast enhancement developing from the distal esophagus causing sub-stenosis of the lumen. (B) EUS scanning results revealing a 45-mm, hypoechoic round mass, originating from the muscle layer in contact with the inferior vena cava (IVC) and the right atrium (RA). (C) Cytological smear results exhibiting aggregates of spindle cell elements with elongated nuclei (hematoxylin and eosin staining; magnification, ×160). (D) The same elements were intensely immunoreactive for SMA (immunoperoxidase and Mayer’s hemalum counterstain; magnification, ×200). CT, computed tomography; EUS, endoscopic ultrasound; SMA, smooth muscle actin.
Case 2
A 39-year-old female presented with dyspepsia and esophageal reflux that had been apparent for 4 weeks. There was no weight loss, but nausea and mild vomiting were occasionally present. Upon physical examination, local peri-gastric discomfort and pain were noted. EUS scanning showed a 27.8×16.4-mm ovoid, homogeneous and hypoechoic well-delimited mass originating from the esophageal sub-mucosa (Fig. 2A). No lesions were evident elsewhere in the abdominal organs or lymph nodes. EUS-FNAC was performed with the same procedure as utilized in case 1; again, adequate cellularity and one cell block were obtained.
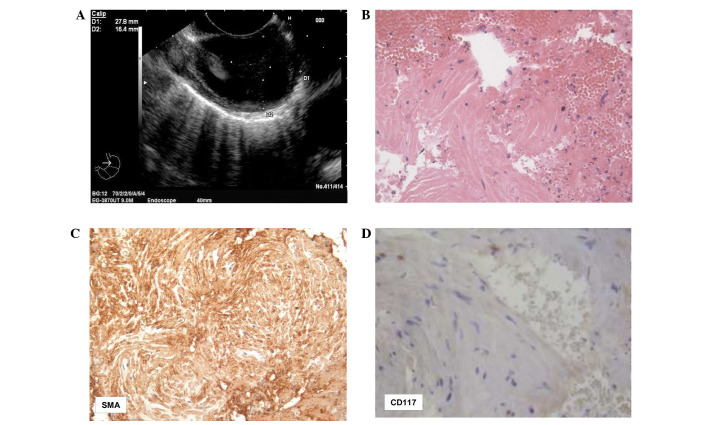
Figure 2.
Case 2: (A) EUS scanning results revealing a 27.8×16.4-mm, hypoechoic, ovoid, well-delimited lesion originating from the muscle layer of the distal esophagus. (B) Clusters of spindle cells intermingled with red blood cells are indicative of leiomyoma (hematoxylin and eosin staining; magnification, ×160). (C) These elements were reactive for SMA (immunoperoxidase and Mayer’s hemalum counterstain; magnification, ×120), (D) while no immunoreactivity was found with CD117 (immunoperoxidase and Mayer’s hemalum counterstain; magnification, ×160). EUS, endoscopic ultrasound; SMA, smooth muscle actin.
Following the FNAC procedures, the two patients were observed for a period of 48 h for any procedure-related complications.
Cytological and immunocytochemical findings
The smears from the two cases exhibited a hemorrhagic background, with loose clusters or small aggregates of spindle-shaped cells (Figs. 1C and 2B) that had elongated nuclei, occasionally showing finely granular chromatin. No mitotic figures were found. The corresponding cell blocks documented an equivalent morphology characterized by small tissue fragments, with relatively low to moderate cellularity composed of monomorphic-uniform spindle cells, eosinophilic cytoplasm and vesicular nuclei (Figs. 1C and 2B). The nuclear chromatin was finely granular and evenly dispersed, while micronucleoli were inconspicuous. No atypia or mitoses were noted.
Immunohistochemical procedures were carried out on the 3-μm serial sections, utilizing the following commercially obtained antisera (all DakoCytomation, Copenhagen, Denmark): Vimentin [working dilution (w.d.), 1:250], smooth muscle actin (SMA; w.d. 1:200), desmin (w.d., 1:250), CD117 (w.d., 1:150), CD34 (w.d. 1:200), S-100 (w.d., 1:400) and Ki67 (MIB-1; w.d., 1:50). In each of the two cases, strong and diffuse cytoplasmic immunostaining was encountered for vimentin, desmin and SMA (Figs. 1D and 2C). No immunostaining was recorded for S100, CD34 and CD117 (Fig. 2D). The growth fraction, determined using Ki67 as the MIB-1 labeling index, was extremely low and quite inconspicuous, showing <1% positively-labeled nuclei.
In light of the microscopic examination and immunohistochemical findings, the two esophageal lesions were diagnosed as intraparietal true leyomiomas, without atypia. The patients refused surgical procedures, and were lost to follow-up subsequent to a period of 12 months.
Discussion
It is well known that the diagnostic yield of EUS-FNAC greatly depends on the site, size and characteristics of the target tissues, as well as certain procedural aspects (9,15,18). By contrast, although conventional endoscopy and CT scans may identify esophageal lesions, these procedures cannot reveal the nature, size or origin of sub-mucosal neoplasms (7,9). However, the efficacy of EUS-FNAC as a main diagnostic procedure is also largely dependent on the expertise, training and interaction between the endosonographer and cytopathologist (15). In the present study, adequate cellular smears and corresponding cell blocks were obtained using the EUS-FNAC approach that is used on esophageal mesenchymal tumors, particularly true leiomyomas. Even if the observed spindle-shaped cells with elongated nuclei could also be confused with other gastrointestinal non-epithelial tumors, the serial immunohistochemical procedures performed on the cell blocks allowed acquisition of the final diagnosis. In fact, the coexistence of desmin and SMA strongly supported the smooth muscle nature of the observed esophageal neoplastic lesions, while the constant negativity for CD34, CD117 and S-100 excluded other diagnostic hypotheses, including inflammatory fibroid polyps, GISTs and schwannomas. Consequently, the availability of an adequate number of serial sections obtained from tissue blocks appears to be an additional diagnostic aid in order to perform the indicated immunohistochemical algorithm, as described previously (15,19,20). Finally, the low growth fraction, revealed by the Ki67 labeling index in the present study, further indicates the benign nature of leiomyomas, thus discounting the diagnostic hypotheses of highly malignant neoplasms, including leiomyosarcomas, spindle-cell amelanotic melanomas and undifferentiated sarcomatoid carcinomas (14,15).
Esophageal leiomyomas are rare benign tumors, with a frequent asymptomatic occurrence, that do not metastasize (21). In fact, patients with these tumors more commonly seek care due to difficulty in swallowing or as a result of the tumors being detected during the endoscopic workup for other diseases, as documented in case 2 of the present study. Moreover, the progression of these neoplasms shows a slow growing phase and the size of the lesions remains stable during the first year of follow-up. Therefore for those patients who refuse to receive surgical excision, as in the present cases, a periodic follow-up with EUS has been considered preferable and more accepted (22,23). On the other hand, the surgical treatment for esophageal leiomyomas depends on multiple factors, including tumor size, location, gross morphology and the patient’s symptoms and overall condition (21,24,25). Furthermore, indications for surgical treatment include unremitting symptoms, a progressive increase in tumor size, mucosal ulceration or the requirement to achieve the histopathological diagnosis due to an inconclusive EUS-FNAC procedure (25–27).
In summary, the present study provided further indications that EUS-FNAC has great clinico-diagnostic pre-surgical value, also allowing a correct differential diagnosis of other esophageal mesenchymal/stromal neoplasias with unpredictable biological behavior to be generated by immunohistochemistry.
References
- 1.Matsui M, Goto H, Niwa Y, Arisawa T, Hirooka Y, Hayakawa T. Preliminary results of fine needle aspiration biopsy histology in upper gastrointestinal submucosal tumors. Endoscopy. 1998;30:750–755. doi: 10.1055/s-2007-1001416. [DOI] [PubMed] [Google Scholar]
- 2.Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J. Esophageal stromal tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 17 cases and comparson with esophageal leiomyomas and leiomyosarcomas. Am J Surg Pathol. 2000;24:211–222. doi: 10.1097/00000478-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Miettinen M, Furlong M, Sarlomo-Rikala M, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: a clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. Am J Surg Pathol. 2001;25:1121–33. doi: 10.1097/00000478-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Wieczorek TJ, Faquin WC, Rubin BP, Cibas ES. Cytologic diagnosis of gastrointestinal stromal tumor with emphasis on the differential diagnosis with leiomyosarcoma. Cancer. 2001;93:276–287. doi: 10.1002/cncr.9042. [DOI] [PubMed] [Google Scholar]
- 5.Ando N, Goto H, Niwa Y, Hirooka Y, Ohmiya N, Nagasaka T, Hayakawa T. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002;55:37–43. doi: 10.1067/mge.2002.120323. [DOI] [PubMed] [Google Scholar]
- 6.Trupiano JK, Stewart RE, Misick C, Appelman HD, Goldblum JR. Gastric stromal tumors: a clinicopathologic study of 77 cases with correlation of features with nonaggressive and aggressive clinical behaviors. Am J Surg Pathol. 2002;26:705–714. doi: 10.1097/00000478-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Stelow EB, Stanley MW, Mallery S, Lai R, Linzie BM, Bardales RH. Endoscopic ultrasound-guided fine-needle aspiration findings of gastrointestinal leiomyomas and gastrointestinal stromal tumors. Am J Clin Pathol. 2003;119:703–708. doi: 10.1309/UWUV-Q001-0D9W-0HPN. [DOI] [PubMed] [Google Scholar]
- 8.Stelow EB, Jones DR, Shami VM. Esophageal leiomyosarcoma diagnosed by endoscopic ultrasound-guided fine-needle aspiration. Diagn Cytopathol. 2007;35:167–170. doi: 10.1002/dc.20606. [DOI] [PubMed] [Google Scholar]
- 9.Jenssen C, Dietrich CF. Endoscopic ultrasound-guided fine-needle aspiration biopsy and trucut biopsy in gastroenterology - An overview. Brest Pract Res Clin Gastroenterol. 2009;23:743–759. doi: 10.1016/j.bpg.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 11.Blay JY, Bonvalot S, Casali P, et al. GIST consensus meeting panelists. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20–21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16:566–578. doi: 10.1093/annonc/mdi127. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era - a population-based study in western Sweden. Cancer. 2005;103:821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 13.Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620–625. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 14.Maheshwari V, Alam K, Varshney M, Jain A, Asif Siddiqui F, Bhargava S. Fine-needle aspiration diagnosis of GIST: a diagnostic dilemma. Diagn Cytopathol. 2012;40:834–838. doi: 10.1002/dc.21734. [DOI] [PubMed] [Google Scholar]
- 15.Todaro P, Crinò SF, Pallio S, Fazzari C, Consolo P, Tuccari G. Gastrointestinal stromal tumors of the stomach: Cytological and immunocytochemical diagnostic features of two cases diagnosed by endoscopic ultrasound-guided fine needle aspiration. Oncol Lett. 2013;5:1862–1866. doi: 10.3892/ol.2013.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan NA, Narayan E, Smith MM, Horn MJ. Cell block cytology. Improved preparation and its efficacy in diagnostic cytology. Am J Clin Pathol. 2000;114:599–606. doi: 10.1309/G035-P2MM-D1TM-T5QE. [DOI] [PubMed] [Google Scholar]
- 17.Barresi V, Cerasoli S, Paioli G, Vitarelli E, Giuffrè G, Guiducci G, Tuccari G, Barresi G. Caveolin-1 in meningiomas: expression and clinico-pathological correlations. Acta Neuropathol. 2006;112:617–626. doi: 10.1007/s00401-006-0097-1. [DOI] [PubMed] [Google Scholar]
- 18.Yoshinaga S, Suzuki H, Oda I, Saito Y. Role of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for diagnosis of solid pancreatic masses. Dig Endosc. 2011;23(Suppl 1):29–33. doi: 10.1111/j.1443-1661.2011.01112.x. [DOI] [PubMed] [Google Scholar]
- 19.Wong DW, Lupton SC, Bhatt L, Gross L, Tanière P, Peake DR, Spooner D, Geh JI. Use of imatinib mesylate in gastrointestinal stromal tumors: Pan-Birmingham Cancer Network experience. Clin Oncol (R Coll Radiol) 2008;20:517–522. doi: 10.1016/j.clon.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Reid R, O’Dywer P, MacDuff E, et al. [Accessed November 13, 2012];Guidelines for the management of gastrointestinal stromal tumors (GIST) in Scotland. 2009 :53–55. http://www.pathologyscotland.org/download/sgpg/guidelines/gist.pdf.
- 21.Jiang W, Rice TW, Goldblum JR. Esophageal leiomyoma: experience from a single institution. Dis Esophagus. 2013;26:167–174. doi: 10.1111/j.1442-2050.2012.01345.x. [DOI] [PubMed] [Google Scholar]
- 22.Choi SH, Kim YT, Han KN, Ra YJ, Kang CH, Sung SW, Kim JH. Surgical management of the esophageal leiomyoma: lessons from a retrospective review. Dis Esophagus. 2011;24:325–329. doi: 10.1111/j.1442-2050.2010.01144.x. [DOI] [PubMed] [Google Scholar]
- 23.Xu GQ, Qian JJ, Chen MH, Ren GP, Che HT. Endoscopic ultrasonography for the diagnosis and selecting treatment of esophageal leiomyoma. J Gastroenterol Hepatol. 2012;27:521–525. doi: 10.1111/j.1440-1746.2011.06929.x. [DOI] [PubMed] [Google Scholar]
- 24.Hatch GF, 3rd, Wertheimer-Hatch L, Hatch KF, Davis GB, Blanchard DK, Foster RS, Jr, Skandalakis JE. Tumors of the esophagus. World J Surg. 2000;24:401–411. doi: 10.1007/s002689910065. [DOI] [PubMed] [Google Scholar]
- 25.Lee LS, Singhal S, Brinster CJ, Marshall B, Kochman ML, Kaiser LR, Kucharczuk JC. Current management of esophageal leiomyoma. J Am Coll Surg. 2004;198:136–146. doi: 10.1016/j.jamcollsurg.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Maish M. Esophagus. In: Townsend CM Jr, Beauchamp RD, Ever BM, Mattox KL, editors. Sabiston Textbook of Surgery. 18th edition. Saunders; Philadelphia, PA: 2007. pp. 1087–1088. [Google Scholar]
- 27.Jiang G, Zhao H, Yang F, Li J, Li Y, Liu Y, Liu J, Wang J. Thoracoscopic enucleation of esophageal leiomyoma: a retrospective study on 40 cases. Dis esophagus. 2009;22:279–283. doi: 10.1111/j.1442-2050.2008.00883.x. [DOI] [PubMed] [Google Scholar]