Abstract
Oral squamous cell carcinoma (OSCC) represents 95% of all forms of head and neck cancer, and over the last decade its incidence has increased by 50%. Oral carcinogenesis is a multistage process, which simultaneously involves precancerous lesions, invasion and metastasis. Degradation of the cell cycle and the proliferation of malignant cells results in the loss of control mechanisms that ensure the normal function of tissues. The aim of the current review is to present the histopathological features of OSCC, including potentially malignant changes, the international classification of tumors, the tumor invasion front and tumor biomarkers (Ki-67, p53, homeobox genes and collagen type IV), as well as the tumor microenvironment and function of cancer-associated fibroblasts in the most common type of oral cancer that is encountered by dental surgeons. In OSCC, associations have been identified between the proliferation, basal lamina degradation and connective tissue modulation. Therefore, the comparison of these factors with the survival time of OSCC patients from the histopathological diagnosis is of interest.
Keywords: mouth neoplasms, oral squamous cell carcinoma, oral cancer, p53, Ki-67, collagen type IV
1. Introduction
Head and neck cancer is one of the 10 most common types of cancer worldwide, afflicting >500,000 individuals each year. Oral cancer is considered to be a preventable condition, due to the possibility of early detection and treatment (1). Oral squamous cell carcinoma (OSCC) represents 95% of all forms of head and neck cancer, and during the past decade its incidence has increased by 50% (2,3). Snuff and alcohol consumption are associated with 90% of patients that exhibit oral cancer (1) and the two factors appear to have a synergistic effect (4).
The majority of OSCC are diagnosed at a late phase (5), in stages III or IV (6,7), which markedly decreases the chances of survival and leads to a significant deterioration in patient quality of life.
Despite the currently available therapeutic strategies, which include the excision of malignant tissue and combination of radiotherapy and chemotherapy, the five-year survival rate is only 53% (3). In addition, a high percentage of patients have a poor response to therapy and high recurrence rates (8).
The purpose of the current review was to present the histological and molecular characteristics of the most common type of oral cancer encountered by dental surgeons.
2. Histology
In general, cancers, including OSCC, emerge from the accumulation of genetic changes and epigenetic anomalies in the signaling pathways that are associated with cancer, resulting in phenotypes that facilitate OSCC development. This process was summarized by Hanahan and Weinberg in ‘Hallmarks of Cancer’ (9).
OSCC is a malignant neoplasm derived from the stratified squamous epithelium of the oral mucosa (10). Its pathogenesis is multifactorial, associated with cigarette smoke, alcohol (11) and snuff, as well as the papilloma virus, among others (12). The malignant neoplasm occurs at various sites, the most frequent being the lip, lateral edges of the tongue (Fig. 1A) (13) and floor of the oral cavity. The incidence of OSCC increases with age, with the majority of OSCC occuring in patients >40 years (14).
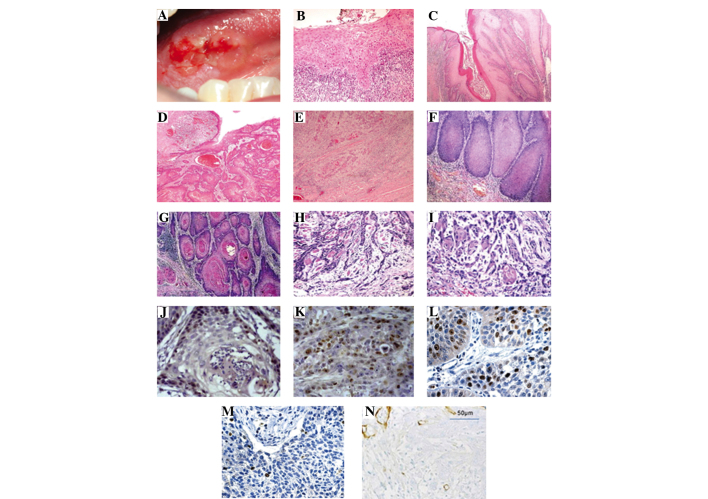
Figure 1.
(A) Oral squamous cell carcinoma (OSCC) of the lateral edge of the tongue (13). (B) Severe dysplasia of the surface epithelium associated with chronic inflammatory infiltration at the stromal-epithelial interface of the dysplastic epithelium (stain, H&E; magnification, ×50) (13). Histological grades of tumor differentiation of OSCC: (C) Well-differentiated, hyperkeratosis and inflammation associated with the stromal-epithelial interface; (D) moderately differentiated; and (E) undifferentiated infiltrating and dispersed cells with no clear demarcation between the front and surrounding tissue invasion (stain, H&E; magnification, ×25) (13). Different patterns of invasion at the tumor invasion front according to the cell morphology: (F) Wide fronts of invasion (score 1); (G) islet cell widths (score 1); (H) thin infiltrating cords (score 2); and (I) individual cells invading the interface (score 3) (1). OSCC patients (J) with recurrence and (K) without recurrence. Antibody staining for Ki-67 with a high degree of nuclear staining (magnification, ×400) (16). Representative samples of homeobox protein, HOXB7 immunohistochemical expression in OSCC with (L) high and (M) low expression (32). (N) Immunohistochemical expression of type IV collagen α2 chain in undifferentiated OSCC (38).
OSCC is characterized by histopathological and clinical manifestations. All carcinogenesis evolves from initial cell injury to the formation of a malignant neoplasm (9). Histologically, the lesion passes through various phases (preneoplastic damage) until the ultimate formation of a cancer. This carcinogenesis may be associated with precancerous lesions (such as leukoplakia, erythroplakia and mixed). However, it is necessary to consider that not all reactional lestions or potentially malignant lesions result in the subsequent development of malignant neoplasms (15).
Potentially malignant changes
According to their histological appearance, lesions that present in the epithelium during the process of carcinogenesis may be classified according to their reactive epithelial changes (such as hyperkeratosis, hyperplasia and acanthosis) or preneoplastic changes (including mild, moderate and severe dysplasia; Fig. 1B) (16) prior to the establishment of an invasive carcinoma (12,14,17). Oral cancer originates as an epithelial dysplasia and is characterized by the altered proliferation of dysplastic squamous cells on the surface of the epithelial layer, which subsequently degrades the subepithelial basement membrane (BM). Degradation of the BM results in local destruction and distant invasion via metastasis. Local invasion to the underlying tissue occurs via the islets and cords of epithelial cells (18).
The ability to metastasize is directly associated with the differential grade of tumor cells, similar to that of the neoplastic tissue architecture and normal epithelium (14).
International Classification of Tumors (World Health Organization) and the tumor invasion front (TIF)
Currently, two systems are used to histologically classify tumor lesions; the International Histological Classification of Tumors (Fig. 1C–E) and the pattern of the TIF (19). The initial classification of lesions is based on the degree of tumor differentiation (well-, moderately- and undifferentiated) (20), which is essential to evaluate the tumor’s growth rate and ability to metastasize (14).
The TIF constitutes the area of the lesion with the greatest depth of invasion and progression into the surrounding tissues (21). In addition, the cells of the TIF have differing molecular characteristics when compared with the cells at the superficial areas of the tumor (10,22). The TIF is considered to be the most representative area of the tumor (23) and is identified by four characteristics; the degree of keratinization, nuclear polymorphism, lymphocytic infiltration and pattern of invasion (PI) (23,24). Of these, the PI is considered to be a good prognostic factor in OSCC (1). To evaluate the severity of the invasion, several morphological criteria exist, associated with certain PIs, according to the following three categories (Fig. 1F–I): i) Islet-infiltrating cells with wide fronts of invasion; ii) thin infiltrating cords; and iii) individual infiltrating cells (1).
In the clinical field, the majority of medical centers base their decisions upon the clinical and pathological information. The TNM stage (T, tumor size; N, regional lymph node compromise; and M, metastasis) (25) and the degree of tumor differentiation (20), combined with the patient’s health status, are the predominant factors that determine the therapeutic strategy. To advance the knowledge of OSCC, numerous pathological and molecular clinical markers have been identified for the prediction of prognosis (1).
3. Tumor biomarkers
Transformed neoplastic cells determine the biological behavior of the tumor. Aberrant cells, which posess common features, present a wide range of morphological and functional disorders.
Genetic and epigenetic alterations in OSCC lead to changes that include reduced expression or overexpression of proteins. The accumulation of these changes in oncogenes and tumor suppressor genes may lead to the formation of OSCC. The genes that are critically altered in OSCC include cyclin D1, p53, retinoblastoma, epidermal growth factor receptor, signal transducer and activator of transcription 3, and vascular endothelial growth factor receiver, as well as other molecules (26,27).
Ki-67 and p53
Ki-67 and p53 are the most commonly used tumor markers for studying cell proliferation. The p53 protein is one of the transcription factors that is implicated in cell cycle control, apoptosis and preservation of genetic stability (28). In addition, the p53 gene is one of the most commonly mutated genes in OSCC with mutations detected in >50% of OSCC cases (29). The activation of p53 has been reported in a number of processes, such as DNA damage, hypoxia and oncogene activation. In addition, p53 protects against tumor formation by preventing the accumulation of cells with DNA damage, which subsequently induces a loss of function in the majority of malignant neoplasms (30). Although not completely understood, Ki-67 is considered to be an important protein in cell division, as it has been observed that the antigen is expressed primarily during the cell cycle stages of G1, S, G2 and M, with a marked emphasis on the M phase. However, Ki-67 expression is not observed during the G0 phase and has a low expression in the G1 and S phases (31). Furthermore, Ki-67 is considered to be one of the best predictors of survival (Fig. 1J and K) (16) and recurrence (5).
Homeobox (HOX) genes
Recently, novel markers have been used to assess morphogenesis and cell differentiation. Previous studies have demonstrated that the aberrant expression of genes is associated with cancer embryogenesis, particularly the HOX genes that may induce embryological development, as well as contribute to the onset and progression of tumors (32,33). Furthermore, HOX gene overexpression has been associated with carcinogenesis, including head and neck neoplasms (34) and HOXB7, a member of the family of homeodomain transcription factors, is a critical regulator of development, controlling the proliferation and survival of progenitor cells. In OSCC, HOXB7 is overexpressed (Fig. 1L and M) (32), which has been confirmed to be associated with a poor prognosis in OSCC and other types of cancer (32,35).
Collagen type IV (ColIV)
Infiltration is a key prerequisite for cancer metastasis, making it a significant factor in the prognosis of patients with OSCC (36). For the activation of the process, degradation of the BM must occur between the epithelium and lamina propria, which is located around the nest of cancer cells and blood vessels. The BM has been identified as a crucial structure in the regulation of tumor invasion. Its molecular assembly is a barrier for the invasion of the connective tissue, in particular of the epithelial cells, unless a molecular rupture occurs (37).
ColIV is the most important protein component of the BM and its integrity is altered by the degradation of the BM via matrix metalloproteinases (MMP) 2 and 9 that are present in OSCC (Fig. 1N) (38) and the surrounding tissues (36). Furthermore, MMP 2 and 9 facilitate the development of lymph node metastases (38,39). Therefore, monitoring the changes in the expression of ColIV may have prognostic value in OSCC patients (36,40).
4. Tumor microenvironment (TME)
For a number of years, cancer has been considered a cell-autonomous process in which consecutive mutations in the oncogenes and tumor suppressor genes lead to the infinite proliferation of neoplastic cells (41). Thus, cancer therapeutic strategies have been focused and limited on such mutations within the tumor cells (4). However, increasing evidence indicates that the genesis and progression of the tumor is determined by tumor cells as well as by a low TME (42).
Recent findings have indicated that for the effective control of cancer, the genesis and progression of the tumor must not only be considered to be cell-autonomous, but predominantly as a disease that involves complex heterotypic multicellular interactions within the newly formed tissue and the original cancerous tissue. Furthermore, the disease must be considered to be a a systemic, solid-tumor tissue disease rather than a single disease entity. Therefore, the concept of the TME has been proposed as an integral aspect and essential area of cancerous tissues. Recent evidence from a study concerning the TME has emerged, forcing the scientific community to review the basics of cancer biology (43).
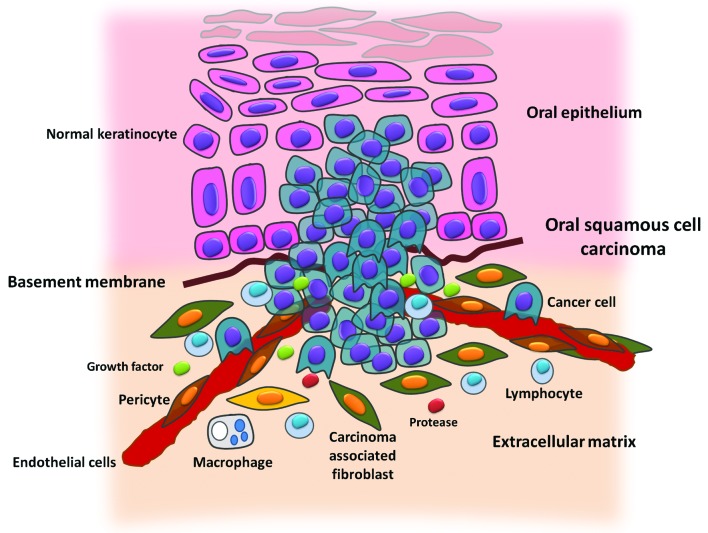
The TME contains numerous types of cells, including fibroblasts, cancer-associated fibroblasts (CAFs), myofibroblasts, smooth muscle cells, endothelial cells and their precursors, pericytes, neutrophils, eosinophils, basophils, mast cells, T and B cells, natural killer cells, and antigen presenting cells, such as macrophages and dendritic cells (Fig. 2).
Figure 2.
In the tumoral microenvironment (TME), different stromal cells, as well as tumor cells were observed, including vascular and lymphatic endothelial cells, and pericyte support fibroblast innate and adaptive immune cells. Furthermore, the TME contained no cellular components, including the extracellular matrix, growth factors, proteases, protease inhibitors or other signaling molecules that are significant in the reactions of the stroma in the TME (4).
CAFs
Despite a marked recruitment of immune cells in the TME, immune cells do not represent the main population of tumor stromal cells; CAFs are the most abundant cells of the TME. CAFs are generally identified by the expression of α-smooth muscle actin, which is similar to the expression of myofibroblasts that occurrs at the site of wound healing and chronic inflammation, however, is absent in normal skin fibroblasts (44,45).
CAFs may be locally differentiated from normal fibroblasts or surrounding stromal stem cells that are derived from the mesenchymal cells of bone marrow, which is recruited by the tumor (46). The tumor stroma is rich in CAFs, which may be scattered or found in the tumor periphery. Certain evidence indicates that CAFs mechanically reshape the extracellular matrix, via the use of proteases, to facilitate the invasion of cancer cells (4). Previous studies have also demonstrated the existence of a molecular dialogue between CAFs and tumor cells, the latter of which secrete interleukin 1α, which stimulates the secretion of chemokine (CC motif) ligand 7 from the CAFs, resulting in tumor progression (6). The increased presence of CAFs observed in OSCC has been associated with a diffuse invasion pattern, preparing the environment for tumor invasion and metastasis (47), and is associated with a poor prognosis (48).
5. Conclusion
In conclusion, an association between cell proliferation markers in the basal lamina and connective tissue has been identified in OSCC. In addition, hyperproliferative neoplastic cells may induce ColIV degradation and facilitate tumor invasion. Once installed in the connective tissue, the invading tumor cells may stimulate fibroblasts, which results in an increase in the presence of CAFs. This scenario may be associated with clinical and histopathological characteristics, in terms of a more aggressive stage of disease and a poor differentiation grade of tumor invasion, as well as the decreased survival time of patients with increased rates of cell proliferation, loss of BM integrity and CAF expression within the connective tissue.
Therefore, the comparison of these factors with the survival time of OSCC patients, from the time of histopathological diagnosis, is of interest. The results of the present review may be useful to clarify the tumor-stromal interaction, and its significance regarding the clinical and histological characteristics of OSCC, in order to expand the quantity of specific prognostic factors available as alternatives to the classic TNM.
Acknowledgements
The authors would like to thank the Investigations Directorate (DI) and the Master Program of Biomedical Sciences, University of Talca (Talca, Chile) for its cooperation.
References
- 1.Dissanayaka WL, Pitiyage G, Kumarasiri PV, Liyanage RL, Dias KD, Tilakaratne WM. Clinical and histopathologic parameters in survival of oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:518–525. doi: 10.1016/j.oooo.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Sankila R, Ferlay J, Parkin DM. Estimates of cancer incidence and mortality in Europe in 1995. Eur J Cancer. 2002;38:99–166. doi: 10.1016/s0959-8049(01)00350-1. [DOI] [PubMed] [Google Scholar]
- 3.Parkin D, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Koontongkaew S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and necksquamous cell carcinomas. J Cancer. 2013;4:66–83. doi: 10.7150/jca.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wangsa D, Ryott M, Avall-Lundqvist E, et al. Ki-67 expression predicts locoregional recurrence in stage I oral tongue carcinoma. Br J Cancer. 2008;99:1121–1128. doi: 10.1038/sj.bjc.6604633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung DW, Che ZM, Kim J, Kim K, Kim KY, Williams D. Tumor-stromal crosstalk in invasion of oral squamous cell carcinoma: a pivotal role of CCL7. Int J Cancer. 2010;127:332–344. doi: 10.1002/ijc.25060. [DOI] [PubMed] [Google Scholar]
- 7.Centelles PV, Seoane-Romero JM, Gómez I, Diz-Dios P, de Melo NS, Seoane J. Timing of oral cancer diagnosis: Implications for prognosis and survival. In: Ogbureke KUE, editor. Oral Cancer. InTech; 2012. pp. 173–188. [Google Scholar]
- 8.Bettendorf O, Piffkò J, Bànkfalvi A. Prognostic and predictive factors in oral squamous cell cancer: important tools for planning individual therapy? Oral Oncol. 2004;40:110–119. doi: 10.1016/j.oraloncology.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 10.Tumuluri V, Thomas GA, Fraser IS. Analysis of the Ki-67 antigen at the invasive tumour front of human oral squamous cell carcinoma. J Oral Pathol Med. 2002;31:598–604. doi: 10.1034/j.1600-0714.2002.00042.x. [DOI] [PubMed] [Google Scholar]
- 11.Wilkey JF, Buchberger G, Saucier K, et al. Cyclin D1 overexpression increases susceptibility to 4-nitroquinoline-1 -oxide-induced dysplasia and neoplasia in murine squamous oral epithelium. Mol Carcinog. 2009;48:853–861. doi: 10.1002/mc.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neville B, Damm D, Allen C, Bouquot J. Oral and Maxillofacial Pathology. 3rd edition. Saunders Elsevier; Philadelphia, PA: 2009. pp. 356–367. [Google Scholar]
- 13.Jerjes W, Upile T, Petrie A, et al. Clinicopathological parameters, recurrence, locoregional and distant metastasis in 115 T1–T2 oral squamous cell carcinoma patients. Head Neck Oncol. 2010;2:9. doi: 10.1186/1758-3284-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sapp JP, Eversole LR, Wysocki GP. Contemporary Oral and Maxillofacial Pathology Chapter 6: Epithelial Disorders. 2nd edition. Mosby Year Book Inc; Maryland Heights, MO: 2004. pp. 184–193. [Google Scholar]
- 15.Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52:195–215. doi: 10.3322/canjclin.52.4.195. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Zhang B, Jiang L, et al. RACK1, an excellent predictor for poor clinical outcome in oral squamous carcinoma, similar to Ki67. Eur J Cancer. 2009;45:490–496. doi: 10.1016/j.ejca.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Rivera MCA. 4NQO carcinogenesis: A model of oral squamous cell carcinoma. Int J Morphol. 2012;30:309–314. [Google Scholar]
- 18.Fuentes B, Duaso J, Droguett D, et al. Progressive extracellular matrix disorganization in chemically induced murine oral squamous cell carcinoma. ISRN Pathology. 2012 [Google Scholar]
- 19.Rivera CA, Droguett DA, Kemmerling U, Venegas BA. Chronic restraint stress in oral squamous cell carcinoma. J Dent Res. 2011;90:799–803. doi: 10.1177/0022034511399911. [DOI] [PubMed] [Google Scholar]
- 20.Pindborg JJ, Reichart PA, Smith CJ, Van der Waal I. WHO International Histological Classification of Tumours Histological typing of cancer and precancer of the oral mucosa. Springer-Verlag; New York: 1997. [Google Scholar]
- 21.Wang X, Zhang J, Fan M, et al. The expression of E-cadherin at the invasive tumor front of oral squamous cell carcinoma: immunohistochemical and RT-PCR analysis with clinicopathological correlation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:547–554. doi: 10.1016/j.tripleo.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Bànkfalvi A, Piffkò J. Prognostic and predictive factors in oral cancer: the role of the invasive tumour front. J Oral Pathol Med. 2000;29:291–298. doi: 10.1034/j.1600-0714.2000.290701.x. [DOI] [PubMed] [Google Scholar]
- 23.Kurokawa H, Zhang M, Matsumoto S, et al. The high prognostic value of the histologic grade at the deep invasive front of tongue squamous cell carcinoma. J Oral Pathol Med. 2005;34:329–333. doi: 10.1111/j.1600-0714.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- 24.Bryne M, Koppang HS, Lilleng R, Kjaerheim A. Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J Pathol. 2005;166:375–381. doi: 10.1002/path.1711660409. [DOI] [PubMed] [Google Scholar]
- 25.Oliveira L, Ribeiro-Silva A, Costa J, Simões A, Matteo M, Zucoloto S. Prognostic factors and survival analysis in a sample of oral squamous cell carcinoma patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:685–695. doi: 10.1016/j.tripleo.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2010;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 27.Choi S, Myers J. Molecular pathogenesis of oral squamous cell carcinoma: implications for therapy. J Dent Res. 2008;87:14–32. doi: 10.1177/154405910808700104. [DOI] [PubMed] [Google Scholar]
- 28.Massano J, Regateiro F, Januário G, Ferreira A. Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:67–76. doi: 10.1016/j.tripleo.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 29.van Houten VM, Tabor MP, van den Brekel MW, et al. Mutated p53 as a molecular marker for the diagnosis of head and neck cancer. J Pathol. 2002;198:476–486. doi: 10.1002/path.1242. [DOI] [PubMed] [Google Scholar]
- 30.Maddocks OD, Vousden KH. Metabolic regulation by p53. J Mol Med (Berl) 2011;89:237–245. doi: 10.1007/s00109-011-0735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yerushalmi R, Woods R, Ravdin PM, Hayes M, Gelmon KA. Ki-67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11:174. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 32.Bitu CC, Carrera M, Lopes MA, Kowalski LP, Soares FA, Coletta RD. HOXB7 expression is a prognostic factor for oral squamous cell carcinoma. Histopathology. 2012;60:662–665. doi: 10.1111/j.1365-2559.2011.04102.x. [DOI] [PubMed] [Google Scholar]
- 33.Shah N, Sukumar S. The Hox genes and their roles in oncogenesis. Nat Rev Cancer. 2010;10:361–371. doi: 10.1038/nrc2826. [DOI] [PubMed] [Google Scholar]
- 34.Tucci R, Campos MS, Matizonkas-Antonio LF, Durazzo M, dos Pinto Junior DS, Nunes FD. HOXB5 expression in oral squamous cell carcinoma. J Appl Oral Sci. 2011;19:125–129. doi: 10.1590/S1678-77572011000200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao WT, Jiang D, Yuan J, et al. HOXB7 as a prognostic factor and mediator of colorectal cancer progression. Clin Cancer Res. 2011;17:3569–3578. doi: 10.1158/1078-0432.CCR-10-2533. [DOI] [PubMed] [Google Scholar]
- 36.Fan HX, Li HX, Chen D, Gao ZX, Zheng JH. Changes in the expression of MMP2, MMP9, and ColIV in stromal cells in oral squamous tongue cell carcinoma: relationships and prognostic implications. J Exp Clin Cancer Res. 2012;31:90. doi: 10.1186/1756-9966-31-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar V, Abbas AK, Aster JC. Robbins Basic Pathology Chapter 7: Neoplasia. 8th ed. Saunders; Philadelphia, PA: 2012. pp. 298–299. [Google Scholar]
- 38.Tamamura R, Nagatsuka H, Siar CH, et al. Comparative analysis of basal lamina type IV collagen alpha chains, matrix metalloproteinases-2 and −9 expressions in oral dysplasia and invasive carcinoma. Acta Histochem. 2013;115:113–119. doi: 10.1016/j.acthis.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 39.de Vicente JC, Fresno MF, Villalain L, Vega JA, Hernández Vallejo G. Expression and clinical significance of matrix metalloproteinase-2 and matrix metalloproteinase-9 in oral squamous cell carcinoma. Oral Oncol. 2005;41:283–293. doi: 10.1016/j.oraloncology.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 40.Baba Y, Iyama K, Ikeda K, et al. The Expression of type IV collagen α6 chain is related to the prognosis in patients with esophageal squamous cell carcinoma. Ann Surg Oncol. 2008;15:555–565. doi: 10.1245/s10434-007-9592-4. [DOI] [PubMed] [Google Scholar]
- 41.Kenny PA, Lee GY, Bissell MJ. Targeting the tumor microenvironment. Front Biosci. 2007;12:3468–3474. doi: 10.2741/2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Liu J. Tumor stroma as targets for cancer therapy. Pharmacol Ther. 2013;137:200–215. doi: 10.1016/j.pharmthera.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 44.Shimoda M, Mellody KT, Orimo A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol. 2010;21:19–25. doi: 10.1016/j.semcdb.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Räsänen K, Vaheri A. Activation of fibroblasts in cancer stroma. Exp Cell Res. 2010;316:2713–2722. doi: 10.1016/j.yexcr.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 46.Xouri G, Christian S. Origin and function of tumor stroma fibroblasts. Semin Cell Dev Biol. 2010;21:40–46. doi: 10.1016/j.semcdb.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 47.de-Assis EM, Pimenta LG, Costa-e-Silva E, Souza PE, Horta MC. Stromal myofibroblasts in oral leukoplakia and oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal. 2012;17:e733–e738. doi: 10.4317/medoral.17834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thode C, Jørgensen TG, Dabelsteen E, Mackenzie I, Dabelsteen S. Significance of myofibroblasts in oral squamous cell carcinoma. J Oral Pathol Med. 2011;40:201–207. doi: 10.1111/j.1600-0714.2010.00999.x. [DOI] [PubMed] [Google Scholar]