Abstract
BACKGROUND
Endometriosis is defined as the colonization and growth of endometrial tissue at anatomic sites outside the uterine cavity. Up to 15% of reproductive-aged women in the USA suffer from painful symptoms of endometriosis, such as infertility, pelvic pain, menstrual cycle abnormalities and increased risk of certain cancers. However, many of the current clinical treatments for endometriosis are not sufficiently effective and yield unacceptable side effects. There is clearly an urgent need to identify new molecular mechanisms that critically underpin the initiation and progression of endometriosis in order to develop more specific and effective therapeutics which lack the side effects of current therapies. The aim of this review is to discuss how nuclear receptors (NRs) and their coregulators promote the progression of endometriosis. Understanding the pathogenic molecular mechanisms for the genesis and maintenance of endometriosis as modulated by NRs and coregulators can reveal new therapeutic targets for alternative endometriosis treatments.
METHODS
This review was prepared using published gene expression microarray data sets obtained from patients with endometriosis and published literature on NRs and their coregulators that deal with endometriosis progression. Using the above observations, our current understanding of how NRs and NR coregulators are involved in the progression of endometriosis is summarized.
RESULTS
Aberrant levels of NRs and NR coregulators in ectopic endometriosis lesions are associated with the progression of endometriosis. As an example, endometriotic cell-specific alterations in gene expression are correlated with a differential methylation status of the genome compared with the normal endometrium. These differential epigenetic regulations can generate favorable cell-specific NR and coregulator milieus for endometriosis progression. Genetic alterations, such as single nucleotide polymorphisms and insertion/deletion polymorphisms of NR and coregulator genes, are frequently detected in ectopic lesions compared with the normal endometrium. These genetic variations impart new molecular properties to NRs and coregulators to increase their capacity to stimulate progression of endometriosis. Finally, post-translational modifications of NR coregulators, such as proteolytic processing, generate endometriosis-specific isoforms. Compared with the unmodified coregulators, these coregulator isoforms have unique functions that enhance the pathogenesis of endometriosis.
CONCLUSIONS
Epigenetic/genetic variations and posttranslational modifications of NRs and coregulators alter their original function so that they become potent ‘drivers’ of endometriosis progression.
Keywords: nuclear receptor, nuclear receptor coregulator, endometriosis
Introduction
Endometriosis is an estrogen-dependent pro-inflammatory disease defined as the colonization and growth of endometrial tissue at anatomic sites outside the uterine cavity, primarily the pelvic peritoneum and ovaries (Bulun, 2009). Up to 15% of reproductive-aged women in the USA chronically suffer from the symptoms of endometriosis (Bulun, 2009; Karaman et al., 2012), which include infertility, pelvic pain, menstrual cycle abnormalities and increased risk of certain cancers, such as ovarian, lymphatic, breast and skin cancers (Swiersz, 2002; Giudice and Kao, 2004; Bertelsen et al., 2007). Due to the severe and chronic morbidity associated with this gynecological disorder, past studies have attempted to identify distinguishing molecular features of the endometriotic lesions with the aim of developing more effective prognostic, diagnostic and/or treatment strategies for the clinical management of this debilitating disease. Despite such efforts, however, many current clinical treatments are inadequate for symptom relief and have unacceptable side effects. Thus, more detailed molecular studies are needed in order to identify novel essential molecular pathways for endometriosis progression. Nevertheless, it is clear from currently available data that estrogen-mediated cell signaling and inflammatory signaling both are essential for the progression of endometriosis. A summary of data implicating nuclear receptors (NRs) and NR coregulators in these processes and in the progression of ectopic lesion growth is given below. We have attempted to catalog all published contributory causes of this complex disease with a short discussion of how each might influence survival/progression of endometriotic lesions. At the end, however, we will provide our best opinion as to the key regulators that deserve major attention for therapeutic development in the future.
The role of NRs and coregulators in estrogen signaling in endometriosis
Compared with the normal endometrium, endometriotic tissues have higher concentrations of estradiol-17β (E2) and locally elevated levels of steroidogenic factor 1 (SF-1) and aromatase, along with reduced activity of 17β-hydroxysteroid dehydrogenase-2 (Zeitoun and Bulun, 1999; Bulun, 2009; Bulun et al., 2009, 2010a, b). In response to these aberrant E2 levels in endometriotic tissues, a unique NR pattern is maintained in endometriotic tissues compared with the normal endometrium. For example, estrogen receptor (ER)β levels in endometriotic tissues are much higher than in the normal endometrium, whereas ERα levels are not substantially different in endometriotic tissue versus normal endometrium; often they are reduced in endometriotic tissues compared with the normal endometrium (Fujimoto et al., 1999). ERα and ERβ knockout mice with surgically induced endometriosis revealed that the ERα gene is required for normal uterine growth and for ectopic lesion growth; in addition, the ERβ gene is involved in ectopic lesion growth (Burns et al., 2012). However, in mice with surgically induced endometriosis the numbers and size of ERα knockout ectopic lesions are smaller than those of ERβ knockout ectopic lesions. Furthermore, endometriotic tissues have decreased progesterone signaling, and this ‘progesterone resistance’ is commonly observed in women with endometriosis (Aghajanova et al., 2010; Fazleabas, 2010). Interestingly, elevated ERβ enhances progesterone resistance in endometriotic tissues (Bulun et al., 2012). Therefore, these two different ER isoforms have unique functions in endometriosis progression.
To precisely regulate NR-mediated gene expression caused by external stimuli, NRs recruit specific coregulators in tissue-, temporal- and spatially specific manners. Accordingly, alteration of the NR coregulator milieu is very frequently associated with the progression of human disease (Lonard et al., 2007). In the case of endometriosis, alternations in the molecular properties of NR coregulators, such as RA-binding protein 2 (Pavone et al., 2010), Breast Cancer 1 (Goumenou et al., 2003), tumor protein 53 (TP53) (Paskulin et al., 2012), hydrogen-peroxide inducible clone 5 (Hic-5) (Aghajanova et al., 2009), DNA (cytosine-5-)-methyltransferase (DNMT) (Wu et al., 2007a, b), steroid receptor coactivator-1 (SRC-1) (Han et al., 2012), heat shock protein 70 (HSP70) (Chehna-Patel et al., 2011) and Cyclin D1 (Pellegrini et al., 2012), are detected more frequently in endometriotic tissues compared with the normal endometrium. The altered molecular properties of these NR coregulators change estrogen-mediated signaling pathways to increase ectopic lesion growth.
The role of NRs and coregulators in inflammatory signaling in endometriosis
In addition to estrogen dependency, pro-inflammatory signaling plays an essential role in endometriosis progression because levels of tumor necrosis factor (TNF)α, prostaglandin E2, cyclo-oxygenase (COX)-2 and various cytokines are highly elevated in endometriotic tissues relative to normal endometrium and these factors synergistically stimulate ectopic lesion growth (Tseng et al., 1996; Noble et al., 1997; Berkkanoglu and Arici, 2003; Szyllo et al., 2003; Bulun, 2009). Therefore, this pro-inflammatory response is a major component of endometriosis progression.
How do NRs modulate inflammatory signaling in ectopic lesions leading to the development of endometriosis and vice versa? In addition to ligands of NRs, pro-inflammatory cytokines can enhance the activities of NRs by modulating functional interactions between NRs and coregulators in the absence of the normal cognate NR ligands (Barish et al., 2005). This observation suggests that the activation of cytokine signaling by endometriosis induces ligand independent NR activation in ectopic lesions to support NR-dependent ectopic lesion growth. Alternatively, ligand-bound NRs also can modulate inflammatory signaling in ectopic lesions. Previous studies have revealed that certain types of NR family members, such as Liver X Receptor (LXR), peroxisome proliferator-activated receptor (PPAR) and glucocorticoid receptor (GR), negatively modulate inflammatory signaling pathways by ligand-dependent binding of NRs to negative regulatory elements of inflammatory genes or by positively regulating the expression of inflammatory inhibitor genes in a ligand-dependent manner (Delerive et al., 2001; Galon et al., 2002; Joseph et al., 2003; Welch et al., 2003). Therefore, activation of PPAR-γ with thiazolidinedione (PPAR-γ agonists) inhibits the progression of endometriosis by preventing activation of inflammatory genes in ectopic lesions (Lebovic et al., 2013).
Alternative isoforms of NR coregulators also have been implicated in the regulation of inflammatory signaling in endometriotic tissues. For example, six DNA variants of the receptor-interacting protein 140 (RIP140) gene were identified in endometriosis patients (Caballero et al., 2005). Therefore, different molecular properties of RIP140 variants might be involved in the progression of endometriosis by differentially regulating inflammatory signaling in endometriotic tissues compared with wild-type RIP140. In recently published work dealing with the SRC-1 coactivator, we demonstrated that a 70-kDa SRC-1 isoform generated by matrix metalloprotein (MMP) 9 from full-length SRC-1 is highly elevated in mouse and human endometriotic tissues. This SRC-1 isoform, but not full-length SRC-1, prevents TNF alpha-mediated apoptosis and sustains the ectopic endometriotic lesions (Han et al., 2012).
Collectively, NRs and NR coregulators differentially contribute to effectively modulate the process of endometriosis. In this manuscript, we summarize available data dealing with NRs and their coregulators that have been associated with endometriosis (such as altered protein levels, genetic variations and post-translational modifications). These altered molecular properties often present opportunities to develop new molecular therapeutic approaches for the next generation of endometriosis therapies.
Methods
To identify NRs and NR coregulator genes that are differentially expressed between endometriotic tissues and normal endometrium, genes were selected from Gene Expression Omnibus (GEO) profiles using the NCBI database (http://www.ncbi.nlm.nih.gov/geoprofiles/), with the following key words: ‘endometriosis and nuclear receptor’, ‘endometriosis and coactivator’ and ‘endometriosis and corepressor’. The lists of NR and NR coregulator genes were taken from the Nuclear Receptor Signaling Atlas (NURSA; http://www.nursa.org/) database. Integrations of GEO profile data and the NR and coregulator gene lists generated a list of NRs and coregulators that have differential expression patterns between endometriotic tissues and normal endometrium (Tables I and II). Based on this list, a systematic literature search was conducted in PubMed (http://www.ncbi.nlm.nih.gov/sites/entrez/) of articles published on or before 1 September 2013 using the search terms ‘endometriosis and NR gene name listed Table I’ or ‘endometriosis and NR coregulator gene listed in Table II’. These data provide NR and NR coregulator genes that have aberrant gene expression in endometriotic tissues and could be involved in the progression of endometriosis.
Table I.
The list of NRs that have different expression levels in endometriotic tissue when compared with the normal endometrium.
| Gene | Average folda | GEO file nameb |
|---|---|---|
| PGR | 0.25 | GDS2835, GDS3092, GDS3975 |
| RORB | 0.36 | GDS2835, GDS3060, GDS3092, GDS3975 |
| ESR1 | 0.43 | GDS2385,GDS3060, GDS3092, GDS3975 |
| RORC | 0.47 | GDS2835, GDS3092, GDS3975 |
| NR2F6 | 0.50 | GDS3092, GDS3975 |
| NR5A1 | 0.55 | GDS3092 |
| DNMT2 | 0.59 | GDS2835 |
| NR4A3 | 0.61 | GDS3092 |
| NR2E1 | 0.62 | GDS3092 |
| PPARG | 0.64 | GDS2737 |
| NR2F1 | 0.65 | GDS3060 |
| ESRRG | 0.65 | GDS2835, GDS3092 |
| ESRRB | 0.66 | GDS3092 |
| DNMT1 | 0.67 | GDS3975 |
| RXRG | 0.68 | GDS3060 |
| NR2C1 | 0.69 | GDS3092 |
| NR2F1 | 1.50 | GDS2835 |
| RARB | 1.55 | GDS3060 |
| NR1D2 | 1.56 | GDS3975 |
| NR2E3 | 1.64 | GDS3060 |
| NR2F2 | 1.64 | GDS3060 |
| NR4A1 | 1.66 | GDS2737, GDS2835, GDS3060, GDS3975 |
| VDR | 1.67 | GDS2737, GDS2835, GDS3975 |
| ESR2 | 1.69 | GDS3060, GDS3975 |
| HNF4A | 1.81 | GDS3060 |
| THRA | 1.83 | GDS3975 |
| NR3C2 | 2.27 | GDS2835, GDS3060, GDS3092 |
| NR5A2 | 2.29 | GDS2835, GDS3060 |
| NR1H4 | 2.61 | GDS2835, GDS3092, GDS3975 |
| NR1I2 | 2.65 | GDS3060 |
| NR5A1 | 2.97 | GDS3975 |
| NR4A3 | 3.02 | GDS2835, GDS3975 |
| NR0B1 | 3.28 | GDS2835, GDS3092 |
| NR4A2 | 3.73 | GDS2835, GDS3975 |
| THRB | 4.02 | GDS3092 |
NR, nuclear receptor.
aAverage fold: average fold expression levels for endometriotic tissue/normal endometrium.
bGEO data file: these data are from Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/).
Table II.
The list of coregulators that have different expression levels in endometriotic tissue when compared with the normal endometrium.
| Gene ID | Average folda | GEO file nameb |
|---|---|---|
| BRCA1 | 0.33 | GDS3092, GDS2835, GDS3975 |
| CRABP2 | 0.35 | GDS2835, GDS3092, GDS3975 |
| CCNE1 | 0.37 | GDS3092 |
| MED24 | 0.41 | GDS2835, GDS3975 |
| MUC-1 | 0.42 | GDS2835, GDS3092, GDS3975 |
| NRIP1 | 0.49 | GDS3092, GDS2835 |
| COPS5 | 0.51 | GDS3092 |
| PPARGC1A | 0.54 | GDS3975 |
| TP-53 | 0.54 | GDS2835, GDS3092 |
| TADA3 | 0.57 | GDS3092 |
| CDK7 | 0.61 | GDS3092 |
| DDX17 | 0.61 | GDS3975 |
| SMARCA4 | 0.63 | GDS2835, GDS3975 |
| RARA | 0.66 | GDS2737 |
| RAN | 0.67 | GDS3092, GDS2835 |
| KDM1A | 0.69 | GDS3092 |
| PSMC3 | 0.69 | GDS2835 |
| GMEB2 | 0.70 | GDS3092 |
| SRC-1 | 1.3 | GDS2835 |
| PIAS1 | 1.5 | GDS2835 |
| SMARCA2 | 1.5 | GDS2835 |
| SRC-3 | 1.5 | GDS3060 |
| PPARGC1A | 1.5 | GDS3092 |
| RB1 | 1.6 | GDS3060 |
| PRMT2 | 1.6 | GDS2835, GDS3975 |
| TGFB1I1 | 1.7 | GDS3060, GDS3092 |
| CCND1 | 1.8 | GDS3060 |
| DCAF6 | 1.8 | GDS3060 |
| KAT2B | 1.9 | GDS2835 |
| CITED2 | 2.0 | GDS2835, GDS3092, GDS3975 |
| TRERF1 | 2.0 | GDS3060 |
| SRCAP | 3.0 | GDS3060 |
| FHL2 | 4.0 | GDS2835, GDS3092, GDS3975 |
| ISL1 | 4.4 | GDS3092 |
| ZFPM2 | 6.1 | GDS2835, GDS3060, GDS3092, GDS3975 |
aAverag fold: average fold expression levels in endometriotic tissue/normal endometrium.
bGEO data file: these data are from Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/).
To identify NR and NR coregulator genes that have genetic variations or post-translational modifications in endometriotic tissues, a systematic literature search was conducted in PubMed (http://www.ncbi.nlm.nih.gov/sites/entrez/) of articles published on or before 1 September 2013, using the search terms ‘endometriosis plus polymorphism’ and ‘ endometriosis plus post-translational modification’. In all of the above cases, only papers that included information on both case and controls were included.
Results
Alterations in levels of NRs and NR coregulators in endometriosis
Because NRs and their coregulators play essential roles in maintaining normal cellular physiology in various tissues, their levels are precisely regulated. Consequently, dysregulation of NR and coregulator levels is highly associated with the progression of human diseases (Lonard et al., 2007; Dasgupta et al., 2013). In this content, aberrant levels of NRs and their coregulators also could be associated with the progression of endometriosis due to up-regulated estrogen and inflammation-mediated signaling in endometriotic tissues. Based on the integrative analysis described in the methods section, NRs and NR coregulators that exhibit >1.5-fold changes in the expression between endometriosis groups and normal controls were selected and classified as ‘endometriosis-specific NRs (ESNRs)’ and ‘endometriosis specific coregulators (ESCRs)’ (Tables I and II). Using these tables and the PubMed database, only those NRs and coregulators that have aberrant expression patterns in endometriotic tissues and are involved in the progression of endometriosis are selected and described as below.
NRs associated with endometriosis progression
Progesterone receptor
The progesterone receptor (PR) plays an essential role in normal female reproductive function, such as initiation and maintenance of pregnancy (Wetendorf and DeMayo, 2012). To dynamically modulate progesterone-mediated cellular processes, PR has two isoforms, PR-A and PR-B, and the ratio of PR-B to PR-A is tissue-specifically regulated (Vegeto et al., 1993; Condon et al., 2006; Bellance et al., 2013). Notably, PR-A and PR-B govern distinct endogenous gene networks in progesterone-responsive cells (Richer et al., 2002). More importantly, studies with PR isoform-specific gene ablation mice have revealed that only PR-A plays a crucial role in ovarian and uterine function, whereas PR-B, but not PR-A, is required for mammary gland development (Conneely et al., 2002; Mulac-Jericevic et al., 2003). Because of their crucial roles in female reproductive tissues, alterations of the progesterone levels and/or PR-mediated signaling pathways are closely associated with human endometrial disease progression. Endometriotic tissues do not respond well to progesterone stimuli; this progesterone resistance is associated with infertility in women with endometriosis (Bulun et al., 2006). How do endometriotic tissues become resistant to progesterone? One possible mechanism leading to progesterone resistance is loss of PR expression in endometriotic tissues compared with normal endometrial cells (Al-Sabbagh et al., 2012). The comparative analysis of PR RNA levels via quantitative RT–PCR and PR protein level assessment using immunohistochemistry revealed that endometriotic tissues have a lower ratio of PR-B/PR-A compared with the normal endometrium (Hayashi et al., 2012). This suggests that a reduction of PR-B levels in endometriotic tissue could be associated in part with the development of progesterone resistance. Interestingly, the proliferative activity of immortalized endometrial stromal cells has been reported to be significantly enhanced by knockdown of PR-B using small interfering RNA (Wu et al., 2008). In addition to the progression of progesterone resistance in endometriotic tissues, the down-regulation of PR-B might be responsible for some of the increased proliferative activity observed in endometriotic tissues in women with endometriosis. In general, the down-regulation of PR-B generates a tendency toward progesterone resistance in endometriosis and may promote proliferative activity in endometriotic tissues and survival of ectopic lesions in vivo.
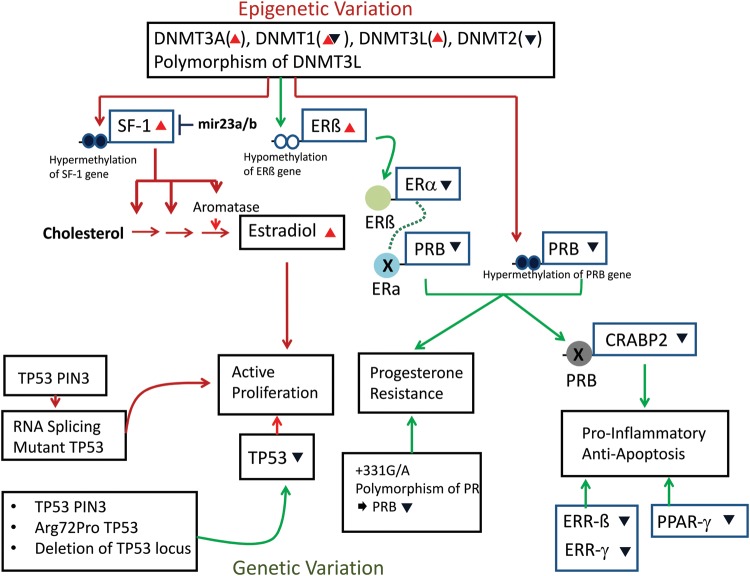
Another question is how PR-B gene expression is down-regulated in endometriotic tissues when compared with normal? This may be due to hypermethylation of the PR promoter region leading to transcriptional repression and silencing; the methylation status of the PR-A and PR-B promoter regions in endometriotic tissues has been investigated and it is reported that the promoter region of PR-B, but not PR-A, is hypermethylated in endometriosis compared with the normal endometrium (Wu et al., 2006). This hypermethylation of the PR-B-specific promoter can be a cause for the down-regulation of PR-B gene expression and the development of progesterone resistance in endometriotic tissues (Fig. 1).
Figure 1.
Epigenetic and genetic variation involved in the progression of endometriosis. (1) Epigenetic variation: the endometriotic tissues have aberrant levels of DNA methyltransferase (DNMT)s and polymorphism of DNMT3L compared with the normal endometrium. The alternation of DNMTs might be associated with hypermethylation of the SF-1 gene to enhance its expression in endometriotic tissues. The overexpression of SF-1 actively involves in estradiol production for the ectopic lesion proliferation. In contrast to SF-1, the alternation of DNMTs is correlated with hypomethylation of the estrogen receptor (ER)β gene to increase ERβ expression in endometriotic tissues. The increased ERβ binds to ERα promoter region to down-regulate ERα in endometriotic tissues. The reduced levels of ERα did not induce progesterone receptor (PR) gene expression in endometriotic tissue in response to estradiol. The alteration of DNMTs also may be directly involved in the hypomethylation of the PR gene promoter to down-regulate PR gene expression in endometriotic tissues. Therefore, both types of down-regulation of PR gene are associated with the development of progesterone resistance in endometriotic tissues. In addition to progesterone resistance, down-regulation of the PRB gene is associated with reduction of CRABP2 gene expression in endometriotic tissues to enhance pro-inflammatory signaling for survival of the ectopic lesion. (2) Genetic variation: the tumor protein 53 (TP53) PIN3 mutant, Arg72Pro TP53 mutant and deletion mutant of TP53 locus were frequently detected in endometriotic tissues. These TP53 mutations are correlated with the reduced levels of TP53 in endometriotic tissues. In addition, a PIN3 mutant can generate an RNA splicing mutant of TP53 lacking the N-terminal activation domain. Therefore, both types of alterations of TP53 induce proliferation activity of endometriotic tissues compared with wild-type endometrium. The +331G/A polymorphism of the PRB gene is correlated with the alteration of PRB gene expression in endometriotic tissues that might be associated with the development of progesterone resistance. (3) Unknown mechanism: estrogen receptor-related (ERR)-β, ERR-γ and PPAR-γ levels are reduced in endometriotic tissues compared with the normal endometrium, with an unknown mechanism preventing apoptosis signaling resulting in the survival of ectopic lesions. (Red triangles): increasing, (dark blue inverted triangles): decreasing, (red arrows): positive regulation and (green arrows): negative regulation.
In contrast with the above observations, however, some studies reported higher levels of PR-B in endometriotic tissues (Misao et al., 1999; Bukulmez et al., 2008). For examples, ovarian endometrioma inner lining samples had significantly higher levels of PR-B mRNA when compared with the eutopic endometrium (Misao et al., 1999), and endometrioma capsules manifested robust immunoreactivity for PR-B, whereas PR-A immunoreactivity was barely detectable (Bukulmez et al., 2008). However, how and why PR-B gene expression is highly elevated in these endometriotic tissues is not clear. In addition, Smuc et al. (2009) have reported that there is no difference in the expression of the PR-B isoform at both mRNA and protein levels in endometriotic tissue versus normal endometrium. Therefore, varied expression patterns have been reported for PR-B among endometriotic tissues.
Estrogen receptor α
ERα is the classical human ER that was cloned in 1986 (Green et al., 1986). Because ERα recognizes estrogen and modulates estrogen-mediated cellular processes in various estrogen-target tissues, dysregulation of ERα gene expression is associated with human disease progression, including cancers and endometrial disease (Burns et al., 2012). In the case of endometriosis, ERα expression has been identified in endometriotic tissues as well as in the normal endometrium (Lyndrup et al., 1987; Lessey et al., 1989; Prentice et al., 1992; Bergqvist and Fernö, 1993; Jones et al., 1995), and the relative expression pattern of ERα in endometriotic tissues versus the normal endometrium is dynamically regulated. In the case of endometriosis progression, ERα mRNA expression levels have been reported to be increased in ectopic tissues in comparison with both normal and eutopic endometrial tissue (Pellegrini et al., 2012). Recent studies using the ERα null mouse with surgically induced endometriosis have revealed that the ERα gene is required for the development of endometriosis because ectopic lesions in ERα knockout animals are smaller and fewer in number compared with wild-type ectopic lesions (Burns et al., 2012). Therefore, ERα expression in ectopic lesions is required for the full growth of ectopic lesions in mice with endometriosis. However, why endometriotic cells have a higher level of ERα is not yet clear.
Because of the essential role that ERα plays in endometriosis progression, selective ER modulators can be employed to suppress ERα activity in ectopic lesions and to obtain growth regression. For example, raloxifene and bazedoxifene, which inhibit endometrial ERα activity, have caused significant regression of experimental endometriosis in an animal model (Yao et al., 2005; Altintas et al., 2010; Kulak et al., 2011). These data suggest that targeting ERα activity could be an alternative endometriosis treatment that could reduce some of the side effects of current total systemic estrogen depletion therapy. In a clinical trial, however, raloxifene treatment did not impact ectopic lesion growth and shortened the time before pelvic pain returned (Stratton et al., 2008).
In addition to elevations of ERα, however, certain previous studies have revealed that endometriotic cells have a lower level of ERα than normal endometrium (Matsuzaki et al., 2001; Smuc et al., 2009; Trukhacheva et al., 2009) (Table I). This is confusing at first glance. For example, ERα levels are significantly down-regulated in endometriotic tissues that exhibit progesterone resistance. Reduced ERα is associated with lower PR levels in endometriotic tissues because PR gene expression is directly regulated by ERα in the endometrium (Boney-Montoya et al., 2010), and the down-regulation of PR is correlated with progesterone resistance and progression in endometriotic tissues. How do endometriotic cells down-regulate ERα levels during the progression of progesterone resistance? Decreased ERα levels are closely associated with increased ERβ levels in endometriosis (Trukhacheva et al., 2009; Bulun et al., 2010a, b). In endometriotic tissues, increased ERβ may be recruited to the ERα promoter to suppress ERα expression (Trukhacheva et al., 2009) (Fig. 1). It is possible that ERα expression patterns differ depending upon the endometriotic sites (Matsuzaki et al., 2001; Colette et al., 2013).
ER-related receptors
ER-related receptors (ERRs) are members of the orphan NR family. ERRs can bind to estrogen response elements in DNA to modulate target gene expression. However, endogenous ligands for the ERR have not yet been clearly identified. The ERR family consists of three members: ERR-α, ERR-β and ERR-γ (Heard et al., 2000). Expression of ERRs has been detected in the normal endometrium; however, their expression was not clearly detected in endometriotic lesions (Bombail et al., 2008; Fujimoto and Sato, 2009). Consistent with these observations, our ESNR analysis also revealed that endometriotic tissues have a lower level of ERR isoforms when compared with the normal endometrium (Table I). Overall, expression of ERRs is down-regulated in the ectopic endometrium compared with the normal endometrium. However, the molecular mechanism of down-regulation of ERRs in endometriotic tissue has not been clearly elucidated.
Why might the down-regulation of ERRs be associated with endometriosis? A previous study revealed that increased ERR-γ expression promotes mesenchymal-to-epithelial transition (MET), suppressing breast cancer progression (Tiraby et al., 2011). Endometriosis progression itself is associated with an epithelial-to-mesenchymal transition (EMT) in order to infiltrate target tissues and organs. Therefore, down-regulation of ERRs might inhibit MET processes in endometriotic tissues and encourage the induction of EMT. Generally, endometriotic lesions have apoptotic resistance and a modified protein pattern in their mitochondrial membranes because most apoptotic proteins are anchored to the mitochondrial membrane (Agic et al., 2009; Ding et al., 2010). Interestingly, ERRβ and ERRγ modulate the expression of the mitochondrial genes involved in apoptotic processes (Giguere, 2008). Therefore, decreased levels of ERR isoforms may provide a beneficial effect for ectopic lesions, allowing for their survival by preventing mitochondria-mediated apoptosis signaling (Fig. 1).
Peroxisome proliferator-activated receptors
Three subtypes of PPARs have been identified and characterized (α, β and γ), and the activity of PPARs is regulated by polyunsaturated fatty acids and eicosanoid metabolites (Willson and Wahli, 1997; Michalik and Wahli, 1999). Their specific endogenous ligands are yet to be identified.
ESNR pattern analysis has revealed that endometriotic tissues have a lower level of PPAR-γ compared with the normal endometrium (Table I). In the case of cancer, expression of the PPAR-γ gene is modulated by DNA methylation (Pancione et al., 2010). However, whether an alteration of the methylation status of the PPAR-γ gene is correlated with a down-regulation of PPAR-γ in endometriotic tissues has not been elucidated.
Does down-regulation of PPAR-γ in endometriotic cells play a role in endometriosis progression? During endometriosis progression, the chemokine Regulated Upon Activation Normal T Cell Expressed and Secreted (RANTES) plays an essential role in endometriosis because it has been shown to mediate inflammatory cell chemotaxis (Wang et al., 2010; Yang et al., 2013). Therefore, high levels of RANTES excreted from endometriotic tissues recruit macrophages and can contribute to progression of endometriosis. Interestingly, PPAR-γ inhibits RANTES production from human endometriotic stromal cells (Pritts et al., 2002, 2003). Moreover, ciglitazone, a PPAR-γ agonist, significantly decreased the attachment of endometrial cells to peritoneal mesothelial cells in an in vitro model of the early endometriotic lesion (Kavoussi et al., 2009). In addition, rosiglitazone and pioglitazone (PPAR-γ agonists) significantly decreased the size of ectopic uterine tissues and the mean wet weight of explants (Lebovic et al., 2004, 2010; Demirturk et al., 2006). Activation of PPAR-γ with agonists suppressed ectopic lesion growth in rat and baboon models of endometriosis (Aytan et al., 2007; Lebovic et al., 2010). For the survival of ectopic cells with activated inflammatory signaling, the ectopic lesions could suppress PPAR-γ gene expression to encourage further endometriosis progression (Fig. 1).
NR subfamily 5, group A, member 1 (NR5A1)/SF-1
SF-1 has been known to be a key modulator of steroidogenesis since the early 1990s (Lala et al., 1992; Morohashi et al., 1992; Giguere, 2008). SF-1 is involved in controlling many physiological pathways to precisely regulate adrenal and reproductive functions; many essential factors involved in the development of adrenal and reproductive tissue are controlled by SF-1 (Morohashi et al., 1992; Lin and Achermann, 2008; Schimmer and White, 2010). Therefore, dysregulation of SF-1 gene expression might be associated with estrogen-dependent endometriosis progression. ESNR expression profiling analysis revealed that levels of SF-1 RNAs were highly elevated in endometriotic tissues compared with the normal endometrium (Table I). Consistent with RNA levels of SF-1, protein levels of SF-1 were also significantly elevated in the ectopic endometrium compared with normal endometria, and the elevation of SF-1 levels was significantly correlated with the severity of endometriosis (Tian et al., 2009).
The above observation raises the question of how endometriotic tissues maintain a higher level of SF-1 compared with the normal endometrium. A previous study revealed that aberrant alteration of DNA methylation and alteration of the gene expression of DNA methyltransferases, which regulate DNA methylation, are correlated with endometriosis progression (Nasu et al., 2011). Based on this observation, the methylation status of the SF-1 gene in endometriotic tissue and the normal endometrium was investigated. Bisulfite sequencing revealed more intense methylation in CpG islands that span from exon II to intron III of the SF-1 gene in endometriotic cells compared with normal endometrial cells (Xue et al., 2011). There was a strong correlation between mRNA levels of SF-1 and the percentage of methylation of exon II/intron III of the SF-1 gene. Therefore, the methylation of a coding exon/intron sequence in the SF-1 gene may enhance gene expression in endometriotic tissues, enhancing lesion survival; whereas hypomethylation of exon II/intron III of the SF-1 gene in the normal endometrium was associated with drastically lower levels of SF-1. In addition, a recent study revealed that the methylation status of the intron I region of the SF-1 gene is strongly correlated with SF-1 mRNA in endometriotic cells (Xue et al., 2013). Collectively, published data suggest that the methylation of CpG islands in intron I and exon II/Intron III of the SF-1 gene increases SF-1 gene expression in endometriotic tissues (Fig. 1).
How are these endometriotic-specific DNA methylation patterns in the SF-1 gene generated in endometriotic tissue? Interestingly, expression levels of DNMT3A and DNMT3B are highly elevated in the ectopic endometrium compared with the normal endometrium or the eutopic endometrium of women with endometriosis (Wu et al., 2007a, b; van Kaam et al., 2011). Therefore, increased DNMT3A and DNMT3B gene expression might be actively involved in methylation of intron I and exon II/Intron III of the SF-1 gene, leading to enhanced SF-1 gene expression in endometriotic tissues. In addition to DNA hypermethylation, levels of SF-1 RNA are post-transcriptionally regulated by microRNAs (miRNAs) in endometriotic tissues. For example, both miR23a and miR23b are down-regulated in endometriotic tissues compared with the normal endometrium, and their expression levels are inversely correlated with SF-1 mRNA levels (Shen et al., 2013) (Fig. 1). Therefore, mRNA levels of SF-1 are significantly reduced when eutopic endometrial stromal cells overexpress miR23a/b, whereas SF-1 levels are highly up-regulated when miR23a/b levels are down-regulated in normal endometrial stromal cells (Shen et al., 2013). However, the detailed molecular mechanism of endometriotic cell-specific down-regulation of miR23a/b has not been clearly elucidated.
Since the induction of SF-1 gene expression could drive the initiation, development and establishment of endometriosis, reducing SF-1 levels or inhibiting SF-1 activity could be possible molecular therapeutic approaches for endometriosis treatment. For example, the Scripps Research Institute has synthesized small molecule inhibitors against SF-1 based on isoquinolinore scaffolds to improve potency and selectivity and to lower cellular toxicity (Roth et al., 2008). Therefore, such a small molecule inhibitor against SF-1 could be employed in endometriosis treatment.
Estrogen receptor β
E2 is a key component of ectopic lesion growth and in the persistence of endometriotic tissue, as well as for the inflammation and pain associated with the disease. In addition to E2, its cognate ERs play crucial roles in the development of endometriosis (Burns and Korach, 2012; Burns et al., 2012). In contrast to the modest changes in ERα expression described above, markedly elevated levels of ERβ are detected in endometriotic tissues in mice with endometriosis and in human endometriotic tissues compared with normal endometrial tissues (Brandenberger et al., 1999; Han et al., 2012).
ERβ has several alternatively spliced forms, and each ERβ isoform has a tissue- and disease-specific expression pattern (Gaskell et al., 2003; Leung et al., 2006). ERβ1, ERβ2 and ERβ5 isoforms are expressed in human endometrium; a decreased ratio of ERα/ERβ1 has been detected in the proliferative endometrium of endometriosis patients compared with the normal endometrium (Juhasz-Boss et al., 2011). This observation suggests that elevated levels of ERβ1, but not ERβ2 or ERβ5, might be associated with the pathogenesis of endometriosis.
The above observation raised the question of how endometriotic tissues maintain higher levels of ERβ compared with the normal endometrium. The methylation status of the ERβ gene promoter is differentially maintained between endometriotic cells and normal endometrial cells, similar to SF-1 (Xue et al., 2007a, b). For example, a CpG island in the promoter region (−197/+359) of the ERβ gene is hypomethylated in endometriotic tissues compared with the normal endometrium, and the activity of the ERβ promoter containing this CpG island is strongly inactivated by in vitro methylation (Xue et al., 2007a, b). In general, it appears that hypomethylation of the ERβ gene promoter increases ERβ gene expression in endometriotic tissues compared with the normal endometrium. How do endometriotic cells induce hypomethylation of the ERβ gene? Interestingly, down-regulation of DNAMT1 and DNMT2 has been detected in ectopic lesions compared with the normal endometrium (Eyster et al., 2007; Borghese et al., 2008). Therefore, reductions of DNAMT1 and DNMT2 might be causal for hypomethylation of the ERβ gene promoter region, leading to increased ERβ gene expression in endometriotic tissues (Fig. 1).
What is the role of overexpressed ERβ in the progression of endometriosis? Chromatin immunoprecipitation assays have demonstrated that ERβ binds to the promoter locus of the ERα gene, which has non-classical activator protein 1 and specificity protein 1 binding motifs and a classic estrogen response element; together they function to suppress ERα expression in endometriotic stromal cells (Trukhacheva et al., 2009). This repressive role of ERβ on ERα gene expression in endometriotic cells also has been confirmed by experiments knocking down and overexpressing ERβ (Trukhacheva et al., 2009). Because ERα directly regulates endometrial PR gene expression (Boney-Montoya et al., 2010), ERα deficiency in endometriotic tissues might be responsible for the failure of E2 activity to induce PR gene expression. This secondary PR deficiency in turn causes progesterone resistance in women with endometriosis (Bulun et al., 2012). Therefore, the ERα deficiency mediated by overexpressing ERβ could promote the progesterone resistance in endometriotic tissues (Fig. 1).
A study on ERβ knockout mice with surgically induced endometriosis revealed that the ERβ gene is required for ectopic lesion growth, even though the impact of an ERβ gene knockout is less than ERα gene deletion in the suppression of ectopic lesion growth (Burns et al., 2012). This observation suggests that ERβ could have other functions that support the growth and/or maintenance of ectopic lesions in addition to the development of progesterone resistance in endometriotic tissues. Thus, questions regarding the roles of ERβ in endometriosis have not been fully addressed to date.
Vitamin D receptor
The vitamin D receptor (VDR) is expressed in cycling human endometrial tissues (van Kaam et al., 2008), and vitamin D3 plays a functional role in fertility and uterine growth (Kwiecinksi et al., 1989; Yoshizawa et al., 1997). Therefore, the vitamin D3/VDR signaling axis could play some role in normal female reproductive function. In addition, VDR-mediated cellular pathways might be involved in endometriosis progression, as immunohistochemistry has shown stronger staining for VDR in endometriotic tissues and endometrial cancer compared with the normal endometrium (Agic et al., 2007; Zelenko et al., 2012). Although the role of VDR in the progression of endometriosis has not been clearly elucidated, VDR-agonist treatment inhibits leukocyte infiltration into inflammatory sites and this VDR-agonist-mediated anti-inflammatory effect is associated with inhibition of nuclear factor (NF)-kB-mediated cell survival signaling (Griffin et al., 2003). Since endometriosis is known as an inflammatory disease, and anti-inflammatory drugs suppress ectopic lesions in mouse models of endometriosis (Efstathiou et al., 2005), an inhibition of NF-kB activity by ligand-bound VDR should suppress ectopic lesion growth because ectopic lesions maintain higher levels of VDR expression. As expected, elocalcitol, a VDR agonist, was claimed to inhibit ectopic lesion growth in a validated mouse model of endometriosis (Mariani et al., 2012). Although the role of increased levels of VDR in ectopic lesion growth is not clear, endometriotic VDR expression might be targeted by alternative therapies to lessen the side effects of current endometriosis treatments.
NR coregulators associated with the development of endometriosis
Cellular retinoic acid-binding protein 2
Retinoic acid (RA) is a dual effector that determines cell fate in a tissue type-dependent manner (Schug et al., 2007, 2008). In normal cells, for example, RA classically enhances differentiation and apoptosis and inhibits proliferation. RA binds to cellular RA-binding protein 2 (CRABP2) to deliver RA to the RA receptor (RAR)α, which eventually enhances differentiation and apoptosis and inhibits proliferation (Schug et al., 2007). In pathological cell types, however, RA paradoxically induces cell survival signaling pathways because RA binds to fatty acid-binding protein 5 (FABP5), which delivers RA to a different NR, PPAR β/δ and induces the expression of pro-survival genes (Schug et al., 2008).
Endometriosis-specific coregulator (ESCR) expression pattern analysis revealed that levels of CRABP2 are lower in endometriotic tissues compared with the normal endometrium (Table II). How can endometriotic cells maintain lower levels of CRABP2? PR is actively involved in the up-regulation of CRABP2 expression in endometrial stromal cells to control retinol uptake and to support the growth-suppressor actions of RA (Pavone et al., 2010). In contrast to the normal endometrium, however, endometriotic cells with progesterone resistance have low levels of PR. Therefore, the reduction of PR could lead to down-regulation of CRABP2 levels, and down-regulation of CRABP2 could lead to enhancement of FABP5-mediated pro-survival activity in response to RA (Pavone et al., 2010).
The above observation suggests that if CRABP2 is up-regulated in ectopic lesions, it could suppress the growth of the lesion by causing CRABP2/RARα-mediated apoptosis in response to RA. Therefore, regulation of the pathway leading to CRABP2 gene expression could be a potential molecular therapeutic target for endometriosis treatment. For example, simvastatin enhances the inhibitory effects of RA on cell growth by increasing apoptotic effects because simvastatin stimulates the expression of CRABP2 but not FABP5 (Sokalska et al., 2013). Therefore, the combination of simvastatin with RA could be employed for endometriosis treatment to increase the CRABP2/RARα-mediated apoptosis pathway in ectopic lesions and to suppress ectopic lesion growth.
Breast cancer 1, early onset gene
The Breast Cancer 1, Early Onset (BRCA1) gene was identified as a tumor suppressor because its main function is to repair damaged DNA and ensure genomic stability (Chen and Parmigiani, 2007). When the BRCA1 gene is mutated or its expression levels are altered, DNA damage is not repaired properly. As a result, cells develop additional genetic alterations that can lead to diseases such as cancer (Satagopan et al., 2002). The ESCR gene expression pattern analysis revealed that BRCA1 RNA is significantly reduced in endometriotic tissues compared with the normal endometrium (Table II). Therefore, endometriotic tissues have a higher potential to acquire genetic variations in their genomes due to the down-regulation of BRCA1. Why do endometriotic tissues have a lower level of BRCA1 compared with the normal endometrium? In the case of breast cancer, hypermethylation of the BRCA1 gene promoter is detected in breast cancer cells compared with normal breast cells, and hypermethylation of the BRCA1 gene is strongly correlated with a reduction of BRCA1 mRNA levels in some clinical breast cancer specimens (Rice et al., 2000; Xu et al., 2013). As with cancer, endometriosis-specific aberrant genomic DNA methylation patterns also are found in endometriotic tissues (Nasu et al., 2011). As described previously, some genes, such as PRB (Wu et al., 2006), E-Cadherin (Wu et al., 2007a, b) and HOXA10 (Wu et al., 2005), show hypermethylation in their promoter and other regions in endometriosis compared with the normal endometrium. However, ERβ (Xue et al., 2007a, b), SF-1 (Xue et al., 2007a, b) and aromatase (Izawa et al., 2008) genes are hypomethylated in endometriotic tissues. Although the methylation status of the BRCA-1 gene in endometriotic tissues has not been investigated, altered methylation of the BRCA-1 gene potentially could be associated with the development of endometriosis.
Tumor protein 53
TP53 has been described as a tumor suppressor gene because it has anticancer functions and plays a role in apoptosis, genomic stability and inhibition of angiogenesis (Matlashewski et al., 1984; Isobe et al., 1986). The level and/or activity of TP53 is significantly down-regulated in most types of cancer cells compared with normal tissues (Semczuk et al., 2010). As in cancer, TP53 levels also are down-regulated in endometriotic tissues compared with the normal endometrium (Arimoto et al., 2003) (Table II). Why do endometriotic tissues have a reduced TP53 level compared with normal the endometrium? Notably, alterations of chromosome 17 in general and the p53 locus in particular are frequently detected in severe and late-stage endometriosis (Bischoff et al., 2002). Therefore, somatic genome perturbation of the TP53 locus might be associated with down-regulated TP53 levels in endometriotic tissues compared with the normal endometrium (Fig. 1). How does a TP53 deficiency lead to the development of endometriosis? Unfortunately, detailed studies to address this question have not yet been conducted. However, down-regulated TP53 might lead to uncontrolled proliferative activity and the development of ectopic lesions in women with endometriosis, as it does in cancer.
Transforming growth factor beta 1 induced transcript 1Hic-5
Hic-5 is also known as 55 kDa androgen receptor-associated protein or transforming growth factor β1-induced transcript 1 (TGFB1I1). Functionally, Hic-5 is involved in cellular senescence (Fujita et al., 1998). In the case of NR signaling, Hic-5 potentiates transactivation of the GR, androgen receptor, mineralocorticoid receptor and PR, but it does not alter ER activity (Yang et al., 2000). Interestingly, expression levels of Hic-5 are significantly reduced in human endometrial stromal fibroblasts from women with endometriosis compared with the normal endometrium (Aghajanova et al., 2009). Therefore, a deficiency in Hic-5 gene expression appears to be closely associated with the development of endometriosis. However, the molecular mechanisms leading to down-regulation of Hic-5 levels in endometriotic tissue have not yet been elucidated. What is the physiological meaning of the down-regulation of Hic-5 levels in endometriotic tissues? Endometriotic tissues display progesterone resistance due to low levels of PR. However, some endometriotic tissues have higher levels of PR-B expression compared with the normal endometrium (Misao et al., 1999; Bukulmez et al., 2008). Since Hic-5 works as a PR coactivator in certain PR-mediated cellular processes (Aghajanova et al., 2009), down-regulation of Hic-5 might impair PR activity in ectopic lesions and promote progesterone resistance in PR-positive ectopic lesions. Hic-5 also has a differential expression pattern between ectopic lesions related to their location (Table II) (Colette et al., 2013).
DNA (cytosine-5-)-methyltransferase
The genome-wide profiling of methylation patterns in gene promoter regions has revealed an unbalanced and unique distribution of DNA methylation patterns in endometriotic tissues when compared with the normal endometrium (Borghese et al., 2010). For example, subtelomeric hypermethylated regions are associated with endometriotic tissues, whereas hypermethylated regions are uniformly distributed on the chromosomes of normal endometrium (Borghese et al., 2010). This endometriotic cell-specific DNA methylation pattern could be associated with variations in DNMT gene expression. For example, levels of DNMT1 and DNMT2 are down-regulated in endometriotic tissues compared with the normal endometrium (Borghese et al., 2008) (Table II). In addition to aberrant levels, altered activity of these DNMTs could induce defective methylation of gene promoter regions during the genesis and/or progression of endometriosis. Therefore, deletion of DNMT1 and DNMT2 might be associated with the hypomethylation of the ERβ gene in endometriotic tissues, which could induce ERβ gene expression for the survival of ectopic lesions.
In contrast to DNMT1 and DNMT2, DNMT3A and DNMT3B are over-expressed in the ectopic endometrium compared with the normal endometrium as well as in the eutopic endometrium in women with endometriosis (Wu et al., 2007a, b; Borghese et al., 2008) (Table II). The hypermethylation of CpG islands in intron I and exon II/Intron III of the SF-1 gene is associated with elevated SF-1 gene expression in endometriotic tissues. In contrast to SF-1, however, the hypermethylation of the PR-B-specific promoter is associated with low levels of PR-B in endometriotic tissues, causing progesterone resistance. Therefore, overexpression of DNMT3A and DNMT3B genes could influence hypermethylation of SF-1 and PR-B for the development of endometriosis (Fig. 1).
Cyclin D1
The typical function of cyclin D1 (CCND1) is to regulate the cell cycle by modulating cyclin-dependent kinases (CDKs). In addition to cell cycle control, CCND1 synergistically regulates ERα-mediated cellular processes in a ligand-independent fashion with SRCs; CCND1 recruits SRCs to ERα in the absence of ligand so as to enhance ERα activity (Zwijsen et al., 1998). Therefore, CCND1 works as an ERα coregulator to modulate ERα-mediated cellular processes. Interestingly, CCND1 expression levels are highly elevated in endometriotic tissues compared with the normal endometrium (Pellegrini et al., 2012). This observation suggests an essential role for CCND1 in the progression of endometriosis. However, how endometriotic tissues achieve a higher level of CCDN1 has not yet been elucidated. What could be the functional link between overexpression of the CCND1 gene and the progression of endometriosis? Because CCDN1 works as an ERα coactivator, the elevated activity of the CCDN1/ERα functional axis could up-regulate ERα-mediated signaling in endometriotic tissues in a feed-forward manner. In this way, elevated levels of CCDN1 might increase the activity of ERα in ERα-mediated cellular processes required for ectopic lesion progression.
Because CCDN1 plays an essential role in estrogen signaling in ectopic lesions, targeting CCDN1 also could be employed as an alternative endometriosis treatment. For example, Dienogest, a specific PR agonist, has been used for endometriosis treatment because it directly inhibits the proliferation of human endometrial epithelial cells by suppressing CCND1 gene expression (Shimizu et al., 2009). In addition to Dienogest, Puerarin causes regression of the growth of endometriotic implants in animal models of endometriosis by preventing CCDN1-mediated estrogen signaling (Chen et al., 2011). Puerarin also suppresses proliferative activity of human endometriotic stromal cells via the down-regulation of CCDN1 (Cheng et al., 2012). Therefore, the regulation of CCDN1 expression levels in ectopic lesions could be another novel molecular therapeutic approach for endometriosis treatment by preventing ERα-mediated cellular processes.
NR coactivator 1 (NCOA1)/SRC-1
The estrogen/ER signal axis plays a crucial role in endometriosis progression (Bulun, 2009; Burns et al., 2012). For the precise regulation of ER-mediated gene regulation, ERs recruit various coregulators onto ER at target gene promoter regions (Foulds et al., 2013). Therefore, the discovery of an endometriosis-specific partnership between ERα and coregulators could shed new light onto the molecular mechanisms of estrogen-dependent endometriosis progression. SRC-1 is an ERα coregulator, and alterations in SRC-1 levels in specific tissues is correlated with the progression of human disease, such as prostate, breast and other cancers (Lonard et al., 2007). In the case of endometriosis, expression levels of SRC-1 and the number of SRC-1-positive cells in ovarian endometriosis lesions have been reported to be greater than those of SRC-2 and SRC-3. In addition, immunohistochemistry has revealed that SRC-1 is co-localized with ERα in almost all glandular and many stromal cells in ovarian endometriotic tissues (Kumagami et al., 2011).
The proliferative activity is significantly lower in endometriotic epithelia than eutopic endometrial epithelia in the proliferative phase of the menstrual cycle in the absence of alterations in ER and PR (Suzuki et al., 2010). Notably, the expression of SRC-1 in endometriotic epithelia in the proliferative phase is significantly lower than in eutopic endometrioma (Suzuki et al., 2010). This observation of reduced proliferative activity in endometriotic epithelial cells can be correlated with reduced expression of SRC-1 levels. At first glance, the meaning of this correlation may be unclear. However, in a later section below, we will explain the important interactions of the SRC-1 gene in endometriosis. SRC-1 has a predominant role in endometriosis progression compared with other SRC family members.
Genetic variation of NR and coregulator genes in endometriosis
In addition to identifying alterations in expression levels of NRs and their coregulators, a number of different studies revealed that genetic variations in genes encoding NRs and their coregulators also are associated with endometriosis susceptibility (Stefansson et al., 2002; Rotman et al., 2013). For example, the rate of endometriosis among the relatives of endometriosis patients is higher than controls in both hospital-based (Kennedy et al., 1995; Simpson and Bischoff, 2002) and population-based (Stefansson et al., 2002) control samples. In addition to studies of relatives, twin studies of endometriosis have revealed an increased concordance of endometriosis in monozygotic twins compared with dizygotic twins (Treloar et al., 1999, 2002). Collectively, the above observations strongly suggest that a genetic component(s) is associated with the progression of endometriosis. To this end, various factors have been identified across the genome and investigated to determine how genetics may play a role in endometriosis progression (Montgomery et al., 2008; Rahmioglu et al., 2012). For example, rs10965235, located in an intron of CDKN2BAS on chromosome 9p21, is closely associated with a Japanese endometriosis cohort (Uno et al., 2010), and the intergenic single nucleotide polymorphism (SNP) rs12700667 on chromosome 7p15.2 is associated with a European endometriosis cohort (Painter et al., 2011). Using the PubMed database, we investigated whether genetic variation in NRs and their coregulators is associated with the progression of endometriosis.
NRs that show genetic alterations in endometriotic tissue
Estrogen receptor α
The human ERα gene has 8 exons spanning >140 kb on chromosome 6q25 (Ponglikitmongkol et al., 1988). Notably, polymorphisms of ERα are associated with estrogen-dependent progression of human diseases, such as breast cancer, osteoporosis and Parkinson's disease (Hill et al., 1989; Kobayashi et al., 1996; Isoe-Wada et al., 1999). In addition, ERα polymorphisms have been hypothesized to be associated with the risk of endometriosis in many epidemiological studies. For example, the PvuII and XbaI polymorphisms of the ERα gene that are located in intron 1 are reported to be associated with an increased risk of endometriosis (Govindan et al., 2009). However, another meta-analysis revealed that the PvuII and XbaI polymorphisms in the ERα gene may not be associated with endometriosis risk (Hu et al., 2012; Li et al., 2012). Therefore, it is uncertain whether PvuII and XbaI polymorphisms of the ERα gene are associated with endometriosis progression. In addition to SNPs, a polymorphic (TA)n repeat insertion 1174 bp upstream of the human ERα gene was reported in a patient with endometriosis (Georgiou et al., 1999; Hsieh et al., 2005), but the functional correlation between the polymorphic (TA)n repeat and endometriosis is far from clear.
Estrogen receptor β
Several genetic polymorphisms in the ERβ gene have been identified: (i) a 21 bp deletion encompassing codon 238 and 244 in exon4; (ii) a 846G>A transition in exon 4 that substitutes the amino acid G (250) to S(250); (iii) a 1421T>C transition in exon 7 that is a silent mutation; (iv) a 1082G>A transition within the LDB in exon 5 and finally, (v) a 1730 (A>G) mutation in the 3′ untranslated region of exon 8 (Rosenkranz et al., 1998; Wang et al., 2004). However, there are no significant differences in the frequency of either AluI polymorphism in exon 8 or the RsaI polymorphism in exon 5 of the ERβ gene between endometriosis patients and control group (Wang et al., 2004; Trabert et al., 2011; Hu et al., 2012). In a Japanese population, however, a positive association was reported between the AluI polymorphism in the ERβ gene and stage IV endometriosis (Wang et al., 2004).
Progesterone
Progesterone is a potent antagonist of estrogen-induced endometrial proliferation. Therefore, alterations in progesterone-mediated signaling may be associated with the pathogenesis of endometriosis. For example, a lower level of PR-B gene expression is associated with the progression of progesterone resistance in endometriotic tissues. Interestingly, a genomic polymorphism analysis detected a 306-base pair insertion from the PV/HS-1 Alu subfamily into intron G of the PR gene (PROGINS) (Rowe et al., 1995). This polymorphism is closely associated with the progression of breast and ovarian cancer as well as the progression of endometriosis (Wieser et al., 2002; Lattuada et al., 2004; Pearce et al., 2005; Hu et al., 2012). However, other studies reported that PROGINS is not associated with endometriosis when compared with healthy women (Treloar et al., 2005; Trabert et al., 2011).
In addition to PROGINS, a +331G/A polymorphism in the PR gene was detected in endometriosis patients (van Kaam et al., 2007). The +331G/A polymorphism is located in the promoter region of the PR gene (De Vivo et al., 2002). Interestingly, the +331G/A polymorphism enhances the synthesis of PR-B by generating an additional TATA box, thereby altering the ratio of PR-A to PR-B (De Vivo et al., 2002), leading to higher expression levels of PR-B in the endometrial epithelium of +331A carriers (Berchuck et al., 2004). Therefore, the presence of the +331A allele is considered to reduce the risk of developing deep infiltrating endometriosis and clear cell ovarian cancers (Berchuck et al., 2004; van Kaam et al., 2007) (Fig. 1).
Coregulators that have genetic modifications in endometriotic tissue
Tumor protein 53
As described above, endometriotic cells have lower levels of TP53 than normal endometrial cells. In addition to alterations of TP53 levels, genetic alteration of the TP53 gene is associated with the pathogenesis of endometriosis. Gene-specific PCRs have revealed that codon 72 in TP53 has a polymorphism (Arg/Arg, Arg/Pro, Pro/Pro) in women with severe endometriosis but not in women without endometriosis and this polymorphism is closely associated with the progression of endometriosis in Taiwanese and Mexican populations (Chang et al., 2002; Gallegos-Arreola et al., 2012). For example, p53 Arg/Arg homozygotes have a lower risk for endometriosis, whereas Arg/Pro heterozygotes and Pro/Pro homozygotes have a higher risk for endometriosis progression. How the Arg72Pro TP53 polymorphism changes the molecular properties of TP53 leading to endometriosis progression has not been elucidated. In contrast to the Taiwanese and Mexican populations, however, the Arg72Pro TP53 polymorphism was not associated with a higher risk of endometriosis in Italian and Brazilian populations (Vietri et al., 2007; Bianco et al., 2011).
In addition to the Arg72Pro TP53 polymorphism, a polymorphism in Intron 3 of the TP53 gene (PIN3, rs17878362, 16 bp duplication) was claimed to be associated with the progression of endometriosis (Paskulin et al., 2012). How does the TP53-PIN3 mutation impact the progression of endometriosis? A previous study revealed that lymphoblastoid cell lines with the TP53-PIN3 allele have reduced levels of TP53 mRNA (Gemignani et al., 2004). In a previous section, we reported that endometriotic cells have lower levels of TP53 compared with the normal endometrium. Therefore, the TP53-PIN3 mutation might be associated with reduced TP53 levels in endometriotic tissues, contributing to the progression of endometriosis (Fig. 1).
In addition to down-regulation of TP53 levels, PIN3 can change TP53 splicing patterns in endometriotic tissues because the PIN3 region is involved in splicing intron 2 of the TP53 gene. In silico models predict that PIN3 may alter the topology of G-quadruplex structures, generating alternatively spliced p53 mutants retaining intron 2 (Gemignani et al., 2004; Marcel et al., 2011). This TP53 splice variant lacks the N-terminal domain containing the transactivation motif of p53 (Gemignani et al., 2004; Marcel et al., 2011). Therefore, the TP53-PIN3 mutant gene might produce an N-terminally truncated TP53, which binds DNA but does not activate transcription through p53-response elements. This TP53-PIN3 mutant could work as a dominant negative mutant of TP53 to enhance the progression of endometriosis. Together, the down-regulation of TP53 gene expression and loss of transactivation ability of TP53 in endometriotic tissues might be associated with the TP53-PIN3 mutation (Fig. 1).
DNA (cytosine-5-)-methyltransferase 3
Epigenetic mechanisms have been proposed to play a role in endometriosis progression since expression levels of DNMTs are regulated differentially in endometriotic tissues versus normal endometrium. In addition to aberrant expression, genetic polymorphisms of DNMT genes have been identified (Borghese et al., 2012; Szczepanska et al., 2013). For example, a rare DNMT3L variant (rs113593938), located in exon 10, is associated with significant hypomethylation of subtelomeric regions (El-Maarri et al., 2009). Among polymorphisms of DNMT genes, the rs8129776 polymorphism in intron 9 of the DNMT 3L gene is significantly associated with an increased risk of ovarian endometrioma progression (Borghese et al., 2012). However, the rs8129776 polymorphism of DNMT3L was not reported to be associated with alterations in DNMT3L levels in endometriotic tissues (Huang et al., 2012). Therefore, even though the functional consequence of the rs8129776 polymorphism of the DNMT 3L gene in endometriosis progression is not clear at present, the role of polymorphism of DNMT3L should remain a consideration due to the reported methylation status of NR genes in endometriosis (above and in Fig. 1).
Receptor-interacting protein 140
RIP140 is a bi-functional coregulator for multiple members of the NR super-family, such as ER, PR, RAR and GR. RIP140 null male mice are viable, but RIP140 null females are infertile because of the complete failure of mature follicles to release oocytes at the ovulation stage (White et al., 2000). Therefore, RIP140 clearly plays an essential role in female reproductive biology. A small case–control study revealed that some SNPs, such as Gly75Gly, Arg448Gly and Ser803Leu of RIP140, are present in the RIP140 gene locus (Caballero et al., 2005). Among them, the Arg448Gly polymorphism of RIP140 appears to be weakly associated with endometriosis (Caballero et al., 2005). It is possible that an Arg448Gly polymorphism could act as a low penetrance allele contributing to human endometriosis progression. However, the molecular mechanism of how this mutation impacts the development of endometriosis is not well understood. This mutation might affect a protein–protein interaction of RIP140 because the carboxyl terminal-binding protein interacting motif of RIP140 is located close to the Arg448 region.
Post-translational modifications of NR coregulators in the pathogenesis of endometriosis
To modulate diverse cellular processes with limited gene numbers, the molecular properties of NR and NR coregulators, such as stability, structure, function, activity, intracellular localization and interaction with other proteins, are dynamically regulated by post-translational modifications via reversible chemical reactions (e.g. phosphorylation, acetylation, methylation, hydroxylation, glycosylation and nitrosylation) and structural changes (e.g. disulfide-bridge formation and proteolytic cleavage) or the addition of small protein tags (e.g. ubiquitination and neddylation) (Han et al., 2009). These alterations in the molecular properties of a specific target protein by post-translational modifications are associated frequently with human disease progression. For example, estrogen-induced phosphorylation of SRC-3 by atypical protein kinase Cζ protects SRC-3 from proteosomal degradation, promoting increased estrogenic gene activity and proliferation of breast cancer cells through increased SRC-3 stability and levels (Yi et al., 2008). In the case of endometriosis, a proteomic approach identified proteins with aberrant levels in the eutopic endometrium of endometriosis patients in the mid-secretory phase of the menstrual cycle (Stephens et al., 2010). Interestingly, alterations in protein abundance do not always correlate with microarray RNA data, and there is no reason to think that endometriosis is an exception. This suggests that further proteomic analyses are required. It also suggests that, considering the extensive interplay between signaling pathways and kinases in endometriosis, extensive post-translational modification changes likely play a significant role in endometriotic tissues to change the stability and function of regulatory proteins so as to promote endometriosis progression. Although a comprehensive PubMed analysis revealed that some NR coregulators have endometriotic cell-specific post-translational modifications, the number of such NR coregulators published in PubMed to date was quite small. These are summarized below.
The 20 kDa fragment of HSP70
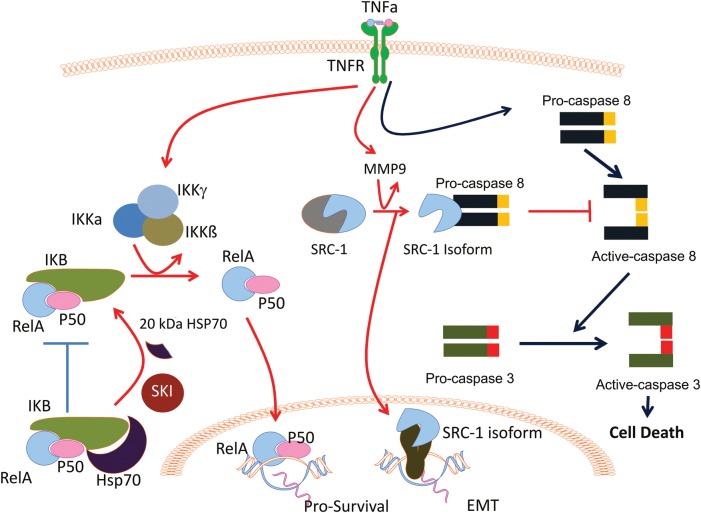
HSP70 proteins function as molecular chaperones that protect and assist the folding process of newly synthesized proteins, helping in translocation, function and general quality control (Morishima, 2005). A 20-kDa fragment of HSP70 has been detected in ectopic tissue lysates isolated from endometriosis patients when compared with the normal endometrium (Chehna-Patel et al., 2011). The 20 kDa fragment of HSP70 is generated from full-length HSP70 by subtilisin/kexin isozyme (SKI)-1 in ectopic tissue. Interestingly, SKI-1 and HSP70 are spatially proximal in endometriotic tissues, but not in the normal endometrium (Chehna-Patel et al., 2011). These findings suggest that the 20 kDa fragment of HSP70 is generated by SKI-1, and may contribute to survival of endometriotic cells. What could be a role for the 20 kDa fragment of HSP70 in endometriosis progression compared with full-length HSP70? HSP70 is designed to interact with transcription factors, such as NF-γB and non-ligand-bound steroid receptors, and to maintain them in an inactive form by physically restricting them within the cytoplasmic compartment (Guzhova et al., 1997; Malhotra and Wong, 2002; Ran et al., 2004). Thus, full-length HSP70 can minimize the NF-κB mediated signaling. In contrast to full-length HSP70, however, the 20 kDa fragment of HSP70 does not interact with transcription factors such as NF-κB. Unrestricted NF-κB then can freely translocate into the nucleus to enhance NF-κB-mediated inflammation. In response to inflammatory signaling, endometriotic tissues could further activate SKI to generate more of the 20 kDa fragment of HSP70 (Fig. 2). In addition, the 20 kDa fragment of HSP70 might be actively involved in the development of endometriosis in ways that have not yet been investigated.
Figure 2.
Post-translational modification of NR coregulators in endometriotic tissues. (1) 20 kDa HSP70: in endometriotic tissue, SKI-1 protease actively processes HSP70 to the 20 kDa HSP70 isoform that does not bind I-kappa-B (IKB) protein. Therefore, V-Rel reticuloendotheliosis viral oncogene homolog A (RelA) and P50 easily are generated by the IκB kinase (IKK) complex activated by TNFα in endometriotic tissues. Therefore, RelA/P50 translocate into the nucleus to activate pro-survival gene expression in endometriotic cells. (2) 70 kDa steroid receptor coactivator (SRC)-1 isoform: in normal endometrial cells, TNFα activates caspase8/caspase3 cell death signaling to induce apoptosis (blue line). In endometriotic cells, however, TNFα-activated matrix metallopeptidase (MMP) 9 to proteolytically process the SRC-1 full-length protein to the 70 kDa SRC-1 C-terminal isoform (red line). The resulting 70 kDa SRC-1 isoform interacts with pro-caspase 8 to prevent its activation in response to TNFα. Therefore, inactivation of caspase 8 by the SRC-1 isoform effectively prevents apoptosis progression in ectopic lesions. In addition to anti-apoptosis, the SRC-1 isoform enhances gene expression involved in epithelial-to-mesenchymal transition (EMT) progression in ectopic lesions, supporting their survival.
The 70 kDa isoform of SRC-1 coactivator
As described in the previous section, SRC-1 is a major coregulator involved in endometriosis progression (Kumagami et al., 2011). We have recently shown that a specific 70 kDa isoform of SRC-1 is selectively generated at high levels in both human and mouse ectopic endometriotic lesions, whereas the parent full-length SRC-1 is ‘reduced’ in endometriotic tissues (Han et al., 2012). Molecular and biochemical analyses revealed that this SRC-1 isoform is a 70-kDa SRC-1 C-terminal fragment that is proteolytically generated from full-length SRC-1 by MMP9. Typically, MMP9 is secreted from cells and is involved in tissue remodeling during cell migration and invasion. Because of its tissue remodeling activity, MMP9 is actively associated with cancer initiation and metastasis. In addition to its role in the development of cancer, MMP9 is up-regulated in endometriotic cells during their migration and implantation into distant anatomic sites. Interestingly, we found that MMP9 also has an intracellular function to generate the 70 kDa fragment of SRC-1 from full-length SRC-1 in endometriosis conditions.
How is MMP9 specifically activated in endometriotic tissues but not in the normal endometrium? MMP9 is activated by increased levels of TNF-α (TNFα), a cytokine that is secreted from activated peritoneal leukocytes as part of the pro-inflammatory response to ectopic menstrual effluents during endometriosis progression. Therefore, this newly identified TNFα-MMP9-SRC-1 isoform signaling axis appears to be a key module for the pathogenic progression of endometriosis. What is the specific role of the SRC-1 isoform rather than full-length SRC-1 in the pathogenesis of endometriosis? During endometriosis progression, retrograde menstrual tissue usually activates an inflammatory response and the host immune surveillance system attacks and flushes the unwelcome cells from the pelvic area. In patients with endometriosis, however, these cells escape the immune surveillance system by generating the SRC-1 isoform in ectopic lesions because the SRC-1 isoform prevents TNFα-mediated apoptosis in the endometriotic cells. This non-genomic mechanism of action of the cytoplasmic SRC-1 isoform involves an interaction between the isoform and cytosolic caspase 8 that prevents caspase 8 activation and acts to block TNFα-induced apoptosis (Han et al., 2012) (Fig. 2). In contrast to the SRC-1 isoform, however, full-length SRC-1 does not prevent, but rather allows TNFα-mediated apoptosis in human endometrial cells because nuclear full-length SRC-1 does not interact with caspase 8 (Han et al., 2012). Evading TNFα-induced apoptosis, endometriotic cells are then free to undergo the EMT process that drives cell motility and invasion, phenotypic hallmarks of aggressive endometriosis in women at high risk for this debilitating disease. In fact, overexpression of the SRC-1 isoform enhances EMT processing in endometriotic tissues to support the growth of ectopic lesions (Han et al., 2012) (Fig. 2). Full-length SRC-1 does not induce the EMT process. Consequently, for survival, ectopic tissues change the molecular properties of SRC-1 by proteolytic processing, leading to anti-apoptosis activity and enhanced EMT activity by the SRC-1 isoform. This pathological dysregulation of a nuclear coactivator appears to be a critical predisposing event for establishment and survival and growth of endometriotic lesions.
Conclusion
Many of the current clinical endometriosis treatments are not sufficiently effective and have unacceptable side effects. For example, levels of prostaglandin E2, COX-2 and various cytokines are highly elevated in endometriotic tissue relative to normal endometrium (Tseng et al., 1996; Noble et al., 1997; Bulun, 2009), suggesting that a heightened pro-inflammatory response is a major component of this disease. Selective COX-2 inhibitors are often used as the first line of conventional treatment for this disorder (Ebert et al., 2005; Ozawa et al., 2006). However, the COX-2 selective inhibitors have gastrointestinal side effects (such as bleeding or perforation of an ulcer), even though their side effects are much less than older non-steroidal anti-inflammatory drugs (Jarupongprapa et al., 2013). Cox-2 inhibitor treatment also increases the risk of dyspepsia, and even may increase the risk for cardiovascular conditions (Mendes et al., 2012; Jarupongprapa et al., 2013). Since it is well established that increased concentrations of E2 arise in endometriotic tissues from locally elevated levels of aromatase along with reduced activity of 17β-hydroxysteroid dehydrogenase-2 (Brosens et al.; Bulun et al., 2010a, b), current endometriosis treatments also involve suppressing E2 levels through the use of GnRH agonists (Descamps and Lansac, 1998), oral contraceptives, synthetic progestins and/or aromatase inhibitors (Attar and Bulun, 2006). These therapies cause infertility and may have harmful side effects in other estrogen-target tissues such as bone and brain (Shepherd, 2001). In severe cases of endometriosis, however, a total hysterectomy may be the only current option when inflammatory and estrogen suppression therapies are ineffective.
To improve efficiency and overcome the side effects of current endometriosis treatments, there is a great need to identify the ‘key molecular driver mechanisms’ for this disease. Herein, we summarized how changes in the molecular properties of NRs and their coregulators might specifically contribute to endometriosis progression, and we suggest that these currently represent the highest probability for effective ‘driver targets’ for new alternative endometriosis treatments. For example, endometriotic cells have higher levels of SF-1 and ERβ because steroidogenesis plays an essential role in ectopic lesion growth. Therefore, regulation of these NR genes seems to be a good therapeutic target for endometriosis. In the case of DNMTs, aberrant levels and genetic variations can impact the expression levels of PR, ERβ, SF-1 and PPAR-γ in endometriotic tissues. Development of DNMT-specific inhibitors or activators might change the epigenetic status of endometrial cell-specific NR genes and suppress ectopic lesions in women. For example, 5-aza-2′-deoxycytidin (5-aza-dC), Zebularine, Procainamide, Procaine and Epigallocate chin-3-3-gallate have been used in cancer treatment to modulate DNMT activity (Miyamoto and Ushijima, 2005; Manoharan et al., 2007). The 5-aza-dC forms irreversible covalent bonds with DNMT1 after its incorporation into DNA, thereby inducing degradation of DNMT1 (Christman, 2002). This DNMT activity modulator could be employed to prevent endometriotic cell-specific NR gene expression for the suppression of ectopic lesion growth.
In addition to regulating endometriosis-specific levels of NRs, modulation of their activity could be employed for alternative endometriosis treatments. Small molecule inhibitors (SMIs) that impact SF-1 protein stability and/or inhibit SF-1 activity could be employed to enhance SF-1 activity in endometriotic tissues. A previous study revealed that two isoquinolinone analogs, SID7969543 and SID7970631, have submicromolar efficacy and selectivity for SF-1 via a panel of NR functional assays (Madoux et al., 2008). These SMIs could be applied for SF-1 targeted endometriosis treatment. Importantly, expression of ERβ is specifically and dramatically elevated in endometriotic tissues compared with the normal endometrium. This is especially notable because ERβ is generally expressed only at low levels in all tissues, and is very low in normal uterus. Therefore, ERβ-specific modulators could be employed to regulate endometriosis-specific ERβ activity for the suppression of endometriosis progression. ERB-041 is a selective ERβ agonist that has anti-inflammatory activity in preclinical models of arthritis and inflammatory bowel disease. ERB-041 treatment suppresses ectopic lesion growth by 40–75% compared with vehicle in a mouse endometriosis model (Harris et al., 2005). ERβ also may be involved in the progesterone resistance seen in endometriotic tissues. The combination of an ERβ antagonist plus progesterone might lead to greater suppression of ectopic lesions by reactivating the PR-mediated anti-proliferative activity in ectopic lesions. In any event, antagonists of ERβ remain a major target approach for future drug combinations for this disease.
In addition to NR-specific target drugs, NR coregulators can change their molecular properties in the face of active inflammation to enhance the progression of endometriosis. Compared with the direct regulation of NR function, modulation of coregulator molecular properties might have more dramatic effects on endometriosis due to the fact that NR coregulators systematically and globally regulate multiple endometriosis progression pathways, whereas an NR drug is likely to only modulate a restricted cellular pathway(s). Consequently, an endometriosis treatment based on down-regulation of a specific NR activity may not be as effective because the cell can activate alternative escape pathways to overcome the NR deficiency. In the case of NR coregulators, however, multiple important cellular pathways can be targeted simultaneously to prevent endometriosis progression. For example, the SRC-1 isoform prevents TNFα-mediated apoptosis and also modulates the EMT required for ectopic lesion maintenance and growth (Han et al., 2012). Since anti-apoptosis and EMT are two of the main essential cellular processes involved in the development of endometriosis, inhibition of the SRC-1 isoform could block these two essential cellular processes at the same time plus inhibit growth (by blocking full-length SRC-1) to completely shut down the essential endometriosis pathogenesis pathways. From this perspective, targeting endometriosis-specific coregulators may be the most effective method to prevent endometriosis progression. In that vein, our best guess is that the SRC-1 isoform may be the prime molecular therapeutic target for endometriosis treatment because normal endometrium (and other tissues) does not have the SRC-1 isoform. If ‘off-target’ effects of inhibitors are minimal, SRC-1 isoform-targeted treatment could significantly reduce side effects in normal tissues. Our laboratory has developed SMIs that selectively inhibit SRC-1 activity in various cancer cell lines (Wang et al., 2011). Based on these SRC-1-specific SMIs, SRC-1 isoform-specific SMIs can now be identified and are being used in laboratory trials for endometriosis treatment in animals.
Collectively, the NR/NR coregulator functional axis plays a critical role in the pathogenesis of endometriosis and the discovery of new molecular mechanisms for NR and NR coregulator functions that drive or maintain growth of endometriotic cells should generate new ideas for the next generation of therapies for endometriosis.
Authors' roles
Both of the authors (S.J.H. and B.W.O.) conceived the study, and participated in study design, collection, analysis of data, and in drafting and revising the manuscript.
Funding
Research supported by Eunice Kennedy Shriver NICHD through cooperative agreements U54HD007495; and as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research of NICHD (B.W.O.).
Conflict of interest
None declared.
References
- Aghajanova L, Velarde MC, Giudice LC. The progesterone receptor coactivator Hic-5 is involved in the pathophysiology of endometriosis. Endocrinology. 2009;150:3863–3870. doi: 10.1210/en.2009-0008. doi:10.1210/en.2009-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanova L, Velarde MC, Giudice LC. Altered gene expression profiling in endometrium: evidence for progesterone resistance. Semin Reprod Med. 2010;28:51–58. doi: 10.1055/s-0029-1242994. doi:10.1055/s-0029-1242994. [DOI] [PubMed] [Google Scholar]
- Agic A, Xu H, Altgassen C, Noack F, Wolfler MM, Diedrich K, Friedrich M, Taylor RN, Hornung D. Relative expression of 1,25-dihydroxyvitamin D3 receptor, vitamin D 1 alpha-hydroxylase, vitamin D 24-hydroxylase, and vitamin D 25-hydroxylase in endometriosis and gynecologic cancers. Reprod Sci. 2007;14:486–497. doi: 10.1177/1933719107304565. doi:10.1177/1933719107304565. [DOI] [PubMed] [Google Scholar]
- Agic A, Djalali S, Diedrich K, Hornung D. Apoptosis in endometriosis. Gynecol Obstet Invest. 2009;68:217–223. doi: 10.1159/000235871. doi:10.1159/000235871. [DOI] [PubMed] [Google Scholar]
- Al-Sabbagh M, Lam EWF, Brosens JJ. Mechanisms of endometrial progesterone resistance. Mol Cell Endocrinol. 2012;358:208–215. doi: 10.1016/j.mce.2011.10.035. doi:10.1016/j.mce.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Altintas D, Kokcu A, Kandemir B, Tosun M, Cetinkaya MB. Comparison of the effects of raloxifene and anastrozole on experimental endometriosis. Eur J Obstet Gynecol Reprod Biol. 2010;150:84–87. doi: 10.1016/j.ejogrb.2010.02.004. doi:10.1016/j.ejogrb.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Arimoto T, Katagiri T, Oda K, Tsunoda T, Yasugi T, Osuga Y, Yoshikawa H, Nishii O, Yano T, Taketani Y, et al. Genome-wide cDNA microarray analysis of gene-expression profiles involved in ovarian endometriosis. Int J Oncol. 2003;22:551–560. [PubMed] [Google Scholar]
- Attar E, Bulun SE. Aromatase inhibitors: the next generation of therapeutics for endometriosis? Fertil Steril. 2006;85:1307–1318. doi: 10.1016/j.fertnstert.2005.09.064. doi:10.1016/j.fertnstert.2005.09.064. [DOI] [PubMed] [Google Scholar]
- Aytan H, Caliskan AC, Demirturk F, Aytan P, Koseoglu DR. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone reduces the size of experimental endometriosis in the rat model. Aust N Z J Obstet Gynaecol. 2007;47:321–325. doi: 10.1111/j.1479-828X.2007.00744.x. doi:10.1111/j.1479-828X.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- Barish GD, Downes M, Alaynick WA, Yu RT, Ocampo CB, Bookout AL, Mangelsdorf DJ, Evans RM. A nuclear receptor atlas: macrophage activation. Mol Endocrinol. 2005;19:2466–2477. doi: 10.1210/me.2004-0529. doi:10.1210/me.2004-0529. [DOI] [PubMed] [Google Scholar]
- Bellance C, Khan JA, Meduri G, Guiochon-Mantel A, Lombes M, Loosfelt H. Progesterone receptor isoforms PRA and PRB differentially contribute to breast cancer cell migration through interaction with focal adhesion kinase complexes. Mol Biol Cell. 2013;24:1363–1374. doi: 10.1091/mbc.E12-11-0807. doi:10.1091/mbc.E12-11-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchuck A, Schildkraut JM, Wenham RM, Calingaert B, Ali S, Henriott A, Halabi S, Rodriguez GC, Gertig D, Purdie DM, et al. Progesterone receptor promoter +331A polymorphism is associated with a reduced risk of endometrioid and clear cell ovarian cancers. Cancer Epidemiol Biomarkers Prev. 2004;13:2141–2147. [PubMed] [Google Scholar]
- Bergqvist A, Fernö M. Endometriosis: oestrogen and progesterone receptors in endometriotic tissue and endometrium: comparison of different cycle phases and ages. Hum Reprod. 1993;8:2211–2217. doi: 10.1093/oxfordjournals.humrep.a138005. [DOI] [PubMed] [Google Scholar]
- Berkkanoglu M, Arici A. Immunology and endometriosis. Am J Reprod Immunol. 2003;50:48–59. doi: 10.1034/j.1600-0897.2003.00042.x. doi:10.1034/j.1600-0897.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- Bertelsen L, Mellemkjaer L, Frederiksen K, Kjaer SK, Brinton LA, Sakoda LC, van Valkengoed I, Olsen JH. Risk for breast cancer among women with endometriosis. Int J Cancer. 2007;120:1372–1375. doi: 10.1002/ijc.22490. doi:10.1002/ijc.22490. [DOI] [PubMed] [Google Scholar]
- Bianco B, Christofolini DM, Brandes A, Lerner TG, Goncalves-Filho RP, Souza AM, Barbosa CP. Analysis of codon 72 polymorphism of the TP53 gene in infertile women with and without endometriosis. Rev Bras Ginecol Obstet. 2011;33:37–42. doi: 10.1590/s0100-72032011000100006. doi:10.1590/S0100-72032011000100006. [DOI] [PubMed] [Google Scholar]
- Bischoff FZ, Heard M, Simpson JL. Somatic DNA alterations in endometriosis: high frequency of chromosome 17 and p53 loss in late-stage endometriosis. J Reprod Immunol. 2002;55:49–64. doi: 10.1016/s0165-0378(01)00131-0. doi:10.1016/S0165-0378(01)00131-0. [DOI] [PubMed] [Google Scholar]
- Bombail V, MacPherson S, Critchley HOD, Saunders PTK. Estrogen receptor related beta is expressed in human endometrium throughout the normal menstrual cycle. Hum Reprod. 2008;23:2782–2790. doi: 10.1093/humrep/den298. doi:10.1093/humrep/den298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boney-Montoya J, Ziegler YS, Curtis CD, Montoya JA, Nardulli AM. Long-range transcriptional control of progesterone receptor gene expression. Mol Endocrinol. 2010;24:346–358. doi: 10.1210/me.2009-0429. doi:10.1210/me.2009-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese B, Mondon F, Noël J-C, Fayt I, Mignot T-M, Vaiman D, Chapron C. Research resource: gene expression profile for ectopic versus eutopic endometrium provides new insights into endometriosis oncogenic potential. Mol Endocrinol. 2008;22:2557–2562. doi: 10.1210/me.2008-0322. doi:10.1210/me.2008-0322. [DOI] [PubMed] [Google Scholar]
- Borghese B, Barbaux S, Mondon F, Santulli P, Pierre G, Vinci G, Chapron C, Vaiman D. Research resource: genome-wide profiling of methylated promoters in endometriosis reveals a subtelomeric location of hypermethylation. Mol Endocrinol. 2010;24:1872–1885. doi: 10.1210/me.2010-0160. doi:10.1210/me.2010-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese B, Santulli P, Hequet D, Pierre G, de Ziegler D, Vaiman D, Chapron C. Genetic polymorphisms of DNMT3L involved in hypermethylation of chromosomal ends are associated with greater risk of developing ovarian endometriosis. Am J Pathol. 2012;180:1781–1786. doi: 10.1016/j.ajpath.2012.01.009. doi:10.1016/j.ajpath.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Brandenberger AW, Lebovic DI, Tee MK, Ryan IP, Tseng JF, Jaffe RB, Taylor RN. Oestrogen receptor (ER)-α and ER-β isoforms in normal endometrial and endometriosis-derived stromal cells. Mol Hum Reprod. 1999;5:651–655. doi: 10.1093/molehr/5.7.651. doi:10.1093/molehr/5.7.651. [DOI] [PubMed] [Google Scholar]
- Brosens I, Derwig I, Brosens J, Fusi L, Benagiano G, Pijnenborg R. The enigmatic uterine junctional zone: the missing link between reproductive disorders and major obstetrical disorders? Hum Reprod. 2010;25:569–574. doi: 10.1093/humrep/dep474. doi:10.1093/humrep/dep474. [DOI] [PubMed] [Google Scholar]
- Bukulmez O, Hardy DB, Carr BR, Word RA, Mendelson CR. Inflammatory status influences aromatase and steroid receptor expression in endometriosis. Endocrinology. 2008;149:1190–1204. doi: 10.1210/en.2007-0665. doi:10.1210/en.2007-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. doi:10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, Innes J, Julie Kim J. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248:94–103. doi: 10.1016/j.mce.2005.11.041. doi:10.1016/j.mce.2005.11.041. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Utsunomiya H, Lin Z, Yin P, Cheng YH, Pavone ME, Tokunaga H, Trukhacheva E, Attar E, Gurates B, et al. Steroidogenic factor-1 and endometriosis. Mol Cell Endocrinol. 2009;300:104–108. doi: 10.1016/j.mce.2008.12.012. doi:10.1016/j.mce.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Cheng YH, Pavone ME, Yin P, Imir G, Utsunomiya H, Thung S, Xue Q, Marsh EE, Tokunaga H, et al. 17Beta-hydroxysteroid dehydrogenase-2 deficiency and progesterone resistance in endometriosis. Semin Reprod Med. 2010a;28:44–50. doi: 10.1055/s-0029-1242992. doi:10.1055/s-0029-1242992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE, Cheng YH, Pavone ME, Xue Q, Attar E, Trukhacheva E, Tokunaga H, Utsunomiya H, Yin P, Luo X, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010b;28:36–43. doi: 10.1055/s-0029-1242991. doi:10.1055/s-0029-1242991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE, Monsavais D, Pavone ME, Dyson M, Xue Q, Attar E, Tokunaga H, Su EJ. Role of estrogen receptor-beta in endometriosis. Semin Reprod Med. 2012;30:39–45. doi: 10.1055/s-0031-1299596. doi:10.1055/s-0031-1299596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns KA, Korach KS. Estrogen receptors and human disease: an update. Arch Toxicol. 2012;86:1491–1504. doi: 10.1007/s00204-012-0868-5. doi:10.1007/s00204-012-0868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns KA, Rodriguez KF, Hewitt SC, Janardhan KS, Young SL, Korach KS. Role of estrogen receptor signaling required for endometriosis-like lesion establishment in a mouse model. Endocrinology. 2012;153:3960–3971. doi: 10.1210/en.2012-1294. doi:10.1210/en.2012-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero V, Ruiz R, Sainz JA, Cruz M, Lopez-Nevot MA, Galan JJ, Real LM, de Castro F, Lopez-Villaverde V, Ruiz A. Preliminary molecular genetic analysis of the receptor interacting protein 140 (RIP140) in women affected by endometriosis. J Exp Clin Assist Reprod. 2005;2:11. doi: 10.1186/1743-1050-2-11. doi:10.1186/1743-1050-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Hsieh YY, Tsai FJ, Tsai CH, Tsai HD, Lin CC. The proline form of p53 codon 72 polymorphism is associated with endometriosis. Fertil Steril. 2002;77:43–45. doi: 10.1016/s0015-0282(01)02938-7. doi:10.1016/S0015-0282(01)02938-7. [DOI] [PubMed] [Google Scholar]
- Chehna-Patel N, Warty N, Sachdeva G, Khole V. Proteolytic tailoring of the heat shock protein 70 and its implications in the pathogenesis of endometriosis. Fertil Steril. 2011;95:1560–1567, e1561–1563. doi: 10.1016/j.fertnstert.2011.01.122. doi:10.1016/j.fertnstert.2011.01.122. [DOI] [PubMed] [Google Scholar]
- Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–1333. doi: 10.1200/JCO.2006.09.1066. doi:10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen C, Shi S, Han J, Wang J, Hu J, Liu Y, Cai Z, Yu C. Endometriotic implants regress in rat models treated with puerarin by decreasing estradiol level. Reprod Sci. 2011;18:886–891. doi: 10.1177/1933719111398500. doi:10.1177/1933719111398500. [DOI] [PubMed] [Google Scholar]
- Cheng W, Chen L, Yang S, Han J, Zhai D, Ni J, Yu C, Cai Z. Puerarin suppresses proliferation of endometriotic stromal cells partly via the MAPK signaling pathway induced by 17ss-estradiol-BSA. PloS One. 2012;7:e45529. doi: 10.1371/journal.pone.0045529. doi:10.1371/journal.pone.0045529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman JK. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene. 2002;21:5483–5495. doi: 10.1038/sj.onc.1205699. doi:10.1038/sj.onc.1205699. [DOI] [PubMed] [Google Scholar]
- Colette S, Defrere S, Van Kerk O, Van Langendonckt A, Dolmans MM, Donnez J. Differential expression of steroidogenic enzymes according to endometriosis type. Fertil Steril. 2013;100:1642–1649. doi: 10.1016/j.fertnstert.2013.08.003. doi:10.1016/j.fertnstert.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20:764–775. doi: 10.1210/me.2005-0242. doi:10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O'Malley BW. Reproductive functions of progesterone receptors. Recent Progr Horm Res. 2002;57:339–355. doi: 10.1210/rp.57.1.339. doi:10.1210/rp.57.1.339. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Lonard DM, O'Malley BW. Nuclear receptor coactivators: master regulators of human health and disease. Ann Re Med. 2014;65:279–292. doi: 10.1146/annurev-med-051812-145316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delerive P, Monte D, Dubois G, Trottein F, Fruchart-Najib J, Mariani J, Fruchart JC, Staels B. The orphan nuclear receptor ROR alpha is a negative regulator of the inflammatory response. EMBO Rep. 2001;2:42–48. doi: 10.1093/embo-reports/kve007. doi:10.1093/embo-reports/kve007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirturk F, Aytan H, Caliskan AC, Aytan P, Koseoglu DR. Effect of peroxisome proliferator-activated receptor-gamma agonist rosiglitazone on the induction of endometriosis in an experimental rat model. J Soc Gynecol Invest. 2006;13:58–62. doi: 10.1016/j.jsgi.2005.10.002. doi:10.1016/j.jsgi.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Descamps P, Lansac J. Gn-RH agonists and ovarian endometriosis. Eur J Obstet Gynecol Reprod Biol. 1998;79:143–144. doi: 10.1016/s0301-2115(98)00068-2. doi:10.1016/S0301-2115(98)00068-2. [DOI] [PubMed] [Google Scholar]
- De Vivo I, Huggins GS, Hankinson SE, Lescault PJ, Boezen M, Colditz GA, Hunter DJ. A functional polymorphism in the promoter of the progesterone receptor gene associated with endometrial cancer risk. Proc Natl Acad Sci. 2002;99:12263–12268. doi: 10.1073/pnas.192172299. doi:10.1073/pnas.192172299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Wang L, Ren Y, Zheng Ws. Detection of mitochondrial biomarkers in eutopic endometria of endometriosis using surface-enhanced laser desorption/ionization time-of-flight mass spectrometry. Fertil Steril. 2010;94:2528–2530. doi: 10.1016/j.fertnstert.2010.04.054. doi:10.1016/j.fertnstert.2010.04.054. [DOI] [PubMed] [Google Scholar]
- Ebert AD, Bartley J, David M. Aromatase inhibitors and cyclooxygenase-2 (COX-2) inhibitors in endometriosis: new questions—old answers? Eur J Obstet Gynecol Reprod Biol. 2005;122:144–150. doi: 10.1016/j.ejogrb.2005.04.017. doi:10.1016/j.ejogrb.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Efstathiou JA, Sampson DA, Levine Z, Rohan RM, Zurakowski D, Folkman J, D'Amato RJ, Rupnick MA. Nonsteroidal antiinflammatory drugs differentially suppress endometriosis in a murine model. Fertil Steril. 2005;83:171–181. doi: 10.1016/j.fertnstert.2004.06.058. doi:10.1016/j.fertnstert.2004.06.058. [DOI] [PubMed] [Google Scholar]
- El-Maarri O, Kareta MS, Mikeska T, Becker T, Diaz-Lacava A, Junen J, Nusgen N, Behne F, Wienker T, Waha A, et al. A systematic search for DNA methyltransferase polymorphisms reveals a rare DNMT3L variant associated with subtelomeric hypomethylation. Hum Mol Genet. 2009;18:1755–1768. doi: 10.1093/hmg/ddp088. doi:10.1093/hmg/ddp088. [DOI] [PubMed] [Google Scholar]
- Eyster KM, Klinkova O, Kennedy V, Hansen KA. Whole genome deoxyribonucleic acid microarray analysis of gene expression in ectopic versus eutopic endometrium. Fertil Steril. 2007;88:1505–1533. doi: 10.1016/j.fertnstert.2007.01.056. doi:10.1016/j.fertnstert.2007.01.056. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT. Progesterone resistance in a baboon model of endometriosis. Semin Reprod Med. 2010;28:75–80. doi: 10.1055/s-0029-1242997. doi:10.1055/s-0029-1242997. [DOI] [PubMed] [Google Scholar]
- Foulds CE, Feng Q, Ding C, Bailey S, Hunsaker TL, Malovannaya A, Hamilton RA, Gates LA, Zhang Z, Li C, et al. Proteomic analysis of coregulators bound to ERalpha on DNA and nucleosomes reveals coregulator dynamics. Mol Cell. 2013;51:185–199. doi: 10.1016/j.molcel.2013.06.007. doi:10.1016/j.molcel.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto J, Hirose R, Sakaguchi H, Tamaya Ts. Expression of oestrogen receptor-alpha and -beta in ovarian endometriomata. Mol Hum Reprod. 1999;5:742–747. doi: 10.1093/molehr/5.8.742. doi:10.1093/molehr/5.8.742. [DOI] [PubMed] [Google Scholar]
- Fujimoto J, Sato E. Clinical implication of estrogen-related receptor (ERR) expression in uterine endometrial cancers. J Steroid Biochem Mol Biol. 2009;116:71–75. doi: 10.1016/j.jsbmb.2009.04.012. doi:10.1016/j.jsbmb.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Fujita H, Kamiguchi K, Cho D, Shibanuma M, Morimoto C, Tachibana K. Interaction of Hic-5, A senescence-related protein, with focal adhesion kinase. J Biol Chem. 1998;273:26516–26521. doi: 10.1074/jbc.273.41.26516. doi:10.1074/jbc.273.41.26516. [DOI] [PubMed] [Google Scholar]
- Gallegos-Arreola MP, Figuera-Villanueva LE, Puebla-Perez AM, Montoya-Fuentes H, Suarez-Rincon AE, Zuniga-Gonzalez GM. Association of TP53 gene codon 72 polymorphism with endometriosis in Mexican women. Genet Mol Res. 2012;11:1401–1408. doi: 10.4238/2012.May.15.10. doi:10.4238/2012.May.15.10. [DOI] [PubMed] [Google Scholar]
- Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, Ehrhart-Bornstein M, O'shea JJ, Chrousos GP, Bornstein SR. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 2002;16:61–71. doi: 10.1096/fj.01-0245com. doi:10.1096/fj.01-0245com. [DOI] [PubMed] [Google Scholar]
- Gaskell TL, Robinson LL, Groome NP, Anderson RA, Saunders PT. Differential expression of two estrogen receptor beta isoforms in the human fetal testis during the second trimester of pregnancy. J Clin Endocrinol Metab. 2003;88:424–432. doi: 10.1210/jc.2002-020811. doi:10.1210/jc.2002-020811. [DOI] [PubMed] [Google Scholar]
- Gemignani F, Moreno V, Landi S, Moullan N, Chabrier A, Gutierrez-Enriquez S, Hall J, Guino E, Peinado MA, Capella G, et al. A TP53 polymorphism is associated with increased risk of colorectal cancer and with reduced levels of TP53 mRNA. Oncogene. 2004;23:1954–1956. doi: 10.1038/sj.onc.1207305. doi:10.1038/sj.onc.1207305. [DOI] [PubMed] [Google Scholar]
- Georgiou I, Syrrou M, Bouba I, Dalkalitsis N, Paschopoulos M, Navrozoglou I, Lolis D. Association of estrogen receptor gene polymorphisms with endometriosis. Fertil Steril. 1999;72:164–166. doi: 10.1016/s0015-0282(99)00198-3. doi:10.1016/S0015-0282(99)00198-3. [DOI] [PubMed] [Google Scholar]
- Giguere V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev. 2008;29:677–696. doi: 10.1210/er.2008-0017. doi:10.1210/er.2008-0017. [DOI] [PubMed] [Google Scholar]
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. doi:10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- Goumenou AG, Vassiliadis S, Matalliotakis IM, Koumantakis EG, Lembessis P, Koutsilieris M. Mutation analysis of BrCA1, BrCA2, and p53 versus soluble HLA class I and class II in a case of familial endometriosis. Fertil Steril. 2003;79:445–448. doi: 10.1016/s0015-0282(02)04665-4. doi:10.1016/S0015-0282(02)04665-4. [DOI] [PubMed] [Google Scholar]
- Govindan S, Shaik NA, Vedicherla B, Kodati V, Rao KP, Hasan Q. Estrogen receptor-alpha gene (T/C) Pvu II polymorphism in endometriosis and uterine fibroids. Dis Markers. 2009;26:149–154. doi: 10.3233/DMA-2009-0625. doi:10.1155/2009/580260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S, Walter P, Kumar V, Krust A, Bornert J-M, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. doi:10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Griffin MD, Xing N, Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Ann Rev Nutr. 2003;23:117–145. doi: 10.1146/annurev.nutr.23.011702.073114. doi:10.1146/annurev.nutr.23.011702.073114. [DOI] [PubMed] [Google Scholar]
- Guzhova IV, Darieva ZA, Melo AR, Margulis BA. Major stress protein Hsp70 interacts with NF-kB regulatory complex in human T-lymphoma cells. Cell Stress Chaperones. 1997;2:132–139. doi: 10.1379/1466-1268(1997)002<0132:msphiw>2.3.co;2. doi:10.1379/1466-1268(1997)002<0132:MSPHIW>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Lonard DM, O'Malley BW. Multi-modulation of nuclear receptor coactivators through posttranslational modifications. Trends Endocrinol Metab. 2009;20:8–15. doi: 10.1016/j.tem.2008.10.001. doi:10.1016/j.tem.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Hawkins SM, Begum K, Jung SY, Kovanci E, Qin J, Lydon JP, DeMayo FJ, O'Malley BW. A new isoform of steroid receptor coactivator-1 is crucial for pathogenic progression of endometriosis. Nat Med. 2012;18:1102–1111. doi: 10.1038/nm.2826. doi:10.1038/nm.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HA, Bruner-Tran KL, Zhang X, Osteen KG, Lyttle CR. A selective estrogen receptor-beta agonist causes lesion regression in an experimentally induced model of endometriosis. Hum Reprod. 2005;20:936–941. doi: 10.1093/humrep/deh711. doi:10.1093/humrep/deh711. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Tanabe A, Kawabe S, Hayashi M, Yuguchi H, Yamashita Y, Okuda K, Ohmichi M. Dienogest increases the progesterone receptor isoform B/A ratio in patients with ovarian endometriosis. J Ovarian Res. 2012;5:31. doi: 10.1186/1757-2215-5-31. doi:10.1186/1757-2215-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard DJ, Norby PL, Holloway J, Vissing H. Human ERRgamma, a third member of the estrogen receptor-related receptor (ERR) subfamily of orphan nuclear receptors: tissue-specific isoforms are expressed during development and in the adult. Mol Endocrinol. 2000;14:382–392. doi: 10.1210/mend.14.3.0431. [DOI] [PubMed] [Google Scholar]
- Hill SM, Fuqua SAW, Chamness GC, Greene GL, McGuire WL. Estrogen receptor expression in human breast cancer associated with an estrogen receptor gene restriction fragment length polymorphism. Cancer Res. 1989;49:145–148. [PubMed] [Google Scholar]
- Hsieh Y-Y, Chang C-C, Tsai F-J, Lin C-C, Tsai C-H. Estrogen receptor α dinucleotide repeat and cytochrome P450c17α gene polymorphisms are associated with susceptibility to endometriosis. Fertil Steril. 2005;83:567–572. doi: 10.1016/j.fertnstert.2004.07.977. doi:10.1016/j.fertnstert.2004.07.977. [DOI] [PubMed] [Google Scholar]
- Hu X, Zhou Y, Feng Q, Wang R, Su L, Long J, Wei B. Association of endometriosis risk and genetic polymorphisms involving biosynthesis of sex steroids and their receptors: an updating meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2012;164:1–9. doi: 10.1016/j.ejogrb.2012.05.008. doi:10.1016/j.ejogrb.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Huang J-X, Scott MB, Pu X-Y, Zhou-Cun A. Association between single-nucleotide polymorphisms of DNMT3L and infertility with azoospermia in Chinese men. Reprod Biomed Online. 2012;24:66–71. doi: 10.1016/j.rbmo.2011.09.004. doi:10.1016/j.rbmo.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Isobe M, Emanuel BS, Givol D, Oren M, Croce CM. Localization of gene for human p53 tumour antigen to band 17p13. Nature. 1986;320:84–85. doi: 10.1038/320084a0. doi:10.1038/320084a0. [DOI] [PubMed] [Google Scholar]
- Isoe-Wada K, Maeda M, Yong J, Adachi Y, Harada H, Urakami K, Nakashima K. Positive association between an estrogen receptor gene polymorphism and Parkinson's disease with dementia. Eur J Neurol. 1999;6:431–435. doi: 10.1046/j.1468-1331.1999.640431.x. doi:10.1046/j.1468-1331.1999.640431.x. [DOI] [PubMed] [Google Scholar]
- Izawa M, Harada T, Taniguchi F, Ohama Y, Takenaka Y, Terakawa N. An epigenetic disorder may cause aberrant expression of aromatase gene in endometriotic stromal cells. Fertil Steril. 2008;89:1390–1396. doi: 10.1016/j.fertnstert.2007.03.078. doi:10.1016/j.fertnstert.2007.03.078. [DOI] [PubMed] [Google Scholar]
- Jarupongprapa S, Ussavasodhi P, Katchamart W. Comparison of gastrointestinal adverse effects between cyclooxygenase-2 inhibitors and non-selective, non-steroidal anti-inflammatory drugs plus proton pump inhibitors: a systematic review and meta-analysis. J Gastroenterol. 2013;48:830–838. doi: 10.1007/s00535-012-0717-6. doi:10.1007/s00535-012-0717-6. [DOI] [PubMed] [Google Scholar]
- Jones RK, Bulmer JN, Searle RF. Immunohistochemical characterization of proliferation, oestrogen receptor and progesterone receptor expression in endometriosis: comparison of eutopic and ectopic endometrium with normal cycling endometrium. Hum Reprod. 1995;10:3272–3279. doi: 10.1093/oxfordjournals.humrep.a135901. [DOI] [PubMed] [Google Scholar]
- Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. doi:10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- Juhasz-Boss I, Fischer C, Lattrich C, Skrzypczak M, Malik E, Ortmann O, Treeck O. Endometrial expression of estrogen receptor beta and its splice variants in patients with and without endometriosis. Arch Gynecol Obstet. 2011;284:885–891. doi: 10.1007/s00404-010-1768-7. doi:10.1007/s00404-010-1768-7. [DOI] [PubMed] [Google Scholar]
- Karaman K, Pala EE, Bayol U, Akman O, Olmez M, Unluoglu S, Ozturk S. Endometriosis of the terminal ileum: a diagnostic dilemma. Case Rep Pathol. 2012;2012:742035. doi: 10.1155/2012/742035. doi:10.1155/2012/742035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavoussi SK, Witz CA, Binkley PA, Nair AS, Lebovic DI. Peroxisome-proliferator activator receptor-gamma activation decreases attachment of endometrial cells to peritoneal mesothelial cells in an in vitro model of the early endometriotic lesion. Mol Hum Reprod. 2009;15:687–692. doi: 10.1093/molehr/gap061. doi:10.1093/molehr/gap061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S, Mardon H, Barlow D. Familial endometriosis. J Assist Reprod Genet. 1995;12:32–34. doi: 10.1007/BF02214126. doi:10.1007/BF02214126. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Inoue S, Hosoi T, Ouchi Y, Shiraki M, Orimo H. Association of bone mineral density with polymorphism of the estrogen receptor gene. J Bone Miner Res. 1996;11:306–311. doi: 10.1002/jbmr.5650110304. doi:10.1002/jbmr.5650110304. [DOI] [PubMed] [Google Scholar]
- Kulak J, Jr, Fischer C, Komm B, Taylor HS. Treatment with bazedoxifene, a selective estrogen receptor modulator, causes regression of endometriosis in a mouse model. Endocrinology. 2011;152:3226–3232. doi: 10.1210/en.2010-1010. doi:10.1210/en.2010-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagami A, Ito A, Yoshida-Komiya H, Fujimori K, Sato A. Expression patterns of the steroid receptor coactivator family in human ovarian endometriosis. J Obstet Gynaecol Res. 2011;37:1269–1276. doi: 10.1111/j.1447-0756.2010.01509.x. doi:10.1111/j.1447-0756.2010.01509.x. [DOI] [PubMed] [Google Scholar]
- Kwiecinksi GG, Petrie GI, DeLuca HF. 1,25-Dihydroxyvitamin D3 restores fertility of vitamin D-deficient female rats. Am J Physiol. 1989;256:E483–E487. doi: 10.1152/ajpendo.1989.256.4.E483. [DOI] [PubMed] [Google Scholar]
- Lala DS, Rice DA, Parker KL. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol. 1992;6:1249–1258. doi: 10.1210/mend.6.8.1406703. [DOI] [PubMed] [Google Scholar]
- Lattuada D, Somigliana E, Vigano P, Candiani M, Pardi G, Di Blasio AM. Genetics of endometriosis: a role for the progesterone receptor gene polymorphism PROGINS? Clin Endocrinol. 2004;61:190–194. doi: 10.1111/j.1365-2265.2004.02076.x. doi:10.1111/j.1365-2265.2004.02076.x. [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Kir M, Casey CL. Peroxisome proliferator-activated receptor-gamma induces regression of endometrial explants in a rat model of endometriosis. Fertil Steril. 2004;82(Suppl. 3):1008–1013. doi: 10.1016/j.fertnstert.2004.02.148. doi:10.1016/j.fertnstert.2004.02.148. [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Mwenda JM, Chai DC, Santi A, Xu X, D'Hooghe T. Peroxisome proliferator-activated receptor-(gamma) receptor ligand partially prevents the development of endometrial explants in baboons: a prospective, randomized, placebo-controlled study. Endocrinology. 2010;151:1846–1852. doi: 10.1210/en.2009-1076. doi:10.1210/en.2009-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovic DI, Kavoussi SK, Lee J, Banu SK, Arosh JA. PPARgamma activation inhibits growth and survival of human endometriotic cells by suppressing estrogen biosynthesis and PGE2 signaling. Endocrinology. 2013;154:4803–4813. doi: 10.1210/en.2013-1168. doi:10.1210/en.2013-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessey BA, Metzger DA, Haney AF, McCarty KS. Immunohistochemical analysis of estrogen and progesterone receptors in endometriosis—comparison with normal endometrium during the menstrual-cycle and the effect of medical therapy. Fertil Steril. 1989;51:409–415. doi: 10.1016/s0015-0282(16)60545-9. [DOI] [PubMed] [Google Scholar]
- Leung YK, Mak P, Hassan S, Ho SM. Estrogen receptor (ER)-beta isoforms: a key to understanding ER-beta signaling. Proc Natl Acad Sci USA. 2006;103:13162–13167. doi: 10.1073/pnas.0605676103. doi:10.1073/pnas.0605676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu F, Tan S-Q, Wang Y, Li S-W. Estrogen receptor-alpha gene PvuII (T/C) and XbaI (A/G) polymorphisms and endometriosis risk: a meta-analysis. Gene. 2012;508:41–48. doi: 10.1016/j.gene.2012.07.049. doi:10.1016/j.gene.2012.07.049. [DOI] [PubMed] [Google Scholar]
- Lin L, Achermann JC. Steroidogenic factor-1 (SF-1, Ad4BP, NR5A1) and disorders of testis development. Sex Dev. 2008;2:200–209. doi: 10.1159/000152036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonard DM, Lanz RB, O'Malley BW. Nuclear receptor coregulators and human disease. Endocr Rev. 2007;28:575–587. doi: 10.1210/er.2007-0012. doi:10.1210/er.2007-0012. [DOI] [PubMed] [Google Scholar]
- Lyndrup J, Thorpe S, Glenthoj A, Obel E, Sele V. Altered progesterone estrogen-receptor ratios in endometriosis—a comparative-study of steroid-receptors and morphology in endometriosis and endometrium. Acta Obstet Gynecol Scand. 1987;66:625–629. doi: 10.3109/00016348709022068. doi:10.3109/00016348709022068. [DOI] [PubMed] [Google Scholar]
- Madoux F, Li X, Chase P, Zastrow G, Cameron MD, Conkright JJ, Griffin PR, Thacher S, Hodder P. Potent, selective and cell penetrant inhibitors of SF-1 by functional ultra-high-throughput screening. Mol Pharmacol. 2008;73:1776–1784. doi: 10.1124/mol.108.045963. doi:10.1124/mol.108.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra V, Wong HR. Interactions between the heat shock response and the nuclear factor-kappa B signaling pathway. Crit Care Med. 2002;30:S89–S95. doi:10.1097/00003246-200201001-00012. [PubMed] [Google Scholar]
- Manoharan M, Ramachandran K, Soloway MS, Singal R. Epigenetic targets in the diagnosis and treatment of prostate cancer. Int Braz J Urol. 2007;33:11–18. doi: 10.1590/s1677-55382007000100003. doi:10.1590/S1677-55382007000100003. [DOI] [PubMed] [Google Scholar]
- Marcel V, Tran PL, Sagne C, Martel-Planche G, Vaslin L, Teulade-Fichou MP, Hall J, Mergny JL, Hainaut P, Van Dyck E. G-quadruplex structures in TP53 intron 3: role in alternative splicing and in production of p53 mRNA isoforms. Carcinogenesis. 2011;32:271–278. doi: 10.1093/carcin/bgq253. doi:10.1093/carcin/bgq253. [DOI] [PubMed] [Google Scholar]
- Mariani M, Vigano P, Gentilini D, Camisa B, Caporizzo E, Di Lucia P, Monno A, Candiani M, Somigliana E, Panina-Bordignon P. The selective vitamin D receptor agonist, elocalcitol, reduces endometriosis development in a mouse model by inhibiting peritoneal inflammation. Hum Reprod. 2012;27:2010–2019. doi: 10.1093/humrep/des150. doi:10.1093/humrep/des150. [DOI] [PubMed] [Google Scholar]
- Matlashewski G, Lamb P, Pim D, Peacock J, Crawford L, Benchimol S. Isolation and characterization of a human p53 cDNA clone: expression of the human p53 gene. EMBO J. 1984;3:3257–3262. doi: 10.1002/j.1460-2075.1984.tb02287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki S, Murakami T, Uehara S, Canis M, Sasano H, Okamura K. Expression of estrogen receptor alpha and beta in peritoneal and ovarian endometriosis. Fertil Steril. 2001;75:1198–1205. doi: 10.1016/s0015-0282(01)01783-6. doi:10.1016/S0015-0282(01)01783-6. [DOI] [PubMed] [Google Scholar]
- Mendes RT, Stanczyk CP, Sordi R, Otuki MF, dos Santos FA, Fernandes D. Selective inhibition of cyclooxygenase-2: risks and benefits. Rev Bras Reumatol. 2012;52:767–782. doi:10.1590/S0482-50042012000500011. [PubMed] [Google Scholar]
- Michalik L, Wahli W. Peroxisome proliferator-activated receptors: three isotypes for a multitude of functions. Curr Opin Biotechnol. 1999;10:564–570. doi: 10.1016/s0958-1669(99)00030-0. doi:10.1016/S0958-1669(99)00030-0. [DOI] [PubMed] [Google Scholar]
- Misao R, Iwagaki S, Fujimoto J, Sun W, Tamaya T. Dominant expression of progesterone receptor form B mRNA in ovarian endometriosis. Horm Res. 1999;52:30–34. doi: 10.1159/000023429. doi:10.1159/000023429. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Ushijima T. Diagnostic and therapeutic applications of epigenetics. Japn J Clin Oncol. 2005;35:293–301. doi: 10.1093/jjco/hyi088. doi:10.1093/jjco/hyi088. [DOI] [PubMed] [Google Scholar]
- Montgomery GW, Nyholt DR, Zhao ZZ, Treloar SA, Painter JN, Missmer SA, Kennedy SH, Zondervan KT. The search for genes contributing to endometriosis risk. Hum Reprod Update. 2008;14:447–457. doi: 10.1093/humupd/dmn016. doi:10.1093/humupd/dmn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima N. Control of cell fate by Hsp70: more than an evanescent meeting. J Biochem. 2005;137:449–453. doi: 10.1093/jb/mvi057. doi:10.1093/jb/mvi057. [DOI] [PubMed] [Google Scholar]
- Morohashi K, Honda S, Inomata Y, Handa H, Omura T. A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J Biol Chem. 1992;267:17913–17919. [PubMed] [Google Scholar]
- Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA. 2003;100:9744–9749. doi: 10.1073/pnas.1732707100. doi:10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasu K, Kawano Y, Tsukamoto Y, Takano M, Takai N, Li H, Furukawa Y, Abe W, Moriyama M, Narahara H. Aberrant DNA methylation status of endometriosis: epigenetics as the pathogenesis, biomarker and therapeutic target. J Obstet Gynaecol Res. 2011;37:683–695. doi: 10.1111/j.1447-0756.2011.01663.x. doi:10.1111/j.1447-0756.2011.01663.x. [DOI] [PubMed] [Google Scholar]
- Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, Agarwal VR, Zhao Y, Carr BR, Bulun SE. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82:600–606. doi: 10.1210/jcem.82.2.3783. [DOI] [PubMed] [Google Scholar]
- Ozawa Y, Murakami T, Tamura M, Terada Y, Yaegashi N, Okamura K. A selective cyclooxygenase-2 inhibitor suppresses the growth of endometriosis xenografts via antiangiogenic activity in severe combined immunodeficiency mice. Fertil Steril. 2006;86:1146–1151. doi: 10.1016/j.fertnstert.2006.01.057. doi:10.1016/j.fertnstert.2006.01.057. [DOI] [PubMed] [Google Scholar]
- Painter JN, Anderson CA, Nyholt DR, Macgregor S, Lin J, Lee SH, Lambert A, Zhao ZZ, Roseman F, Guo Q, et al. Genome-wide association study identifies a locus at 7p15.2 associated with endometriosis. Nat Genet. 2011;43:51–54. doi: 10.1038/ng.731. doi:10.1038/ng.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancione M, Sabatino L, Fucci A, Carafa V, Nebbioso A, Forte N, Febbraro A, Parente D, Ambrosino C, Normanno N, et al. Epigenetic silencing of peroxisome proliferator-activated receptor gamma is a biomarker for colorectal cancer progression and adverse patients’ outcome. PloS One. 2010;5:e14229. doi: 10.1371/journal.pone.0014229. doi:10.1371/journal.pone.0014229. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Paskulin DD, Cunha-Filho JS, Souza CA, Bortolini MC, Hainaut P, Ashton-Prolla P. TP53 PIN3 and PEX4 polymorphisms and infertility associated with endometriosis or with post-in vitro fertilization implantation failure. Cell Death Dis. 2012;3:e392. doi: 10.1038/cddis.2012.116. doi:10.1038/cddis.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavone ME, Reierstad S, Sun H, Milad M, Bulun SE, Cheng Y-H. Altered retinoid uptake and action contributes to cell survival in endometriosis. J Clin Endocrinol Metab. 2010;95:E300–E309. doi: 10.1210/jc.2010-0459. doi:10.1210/jc.2010-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce CL, Hirschhorn JN, Wu AH, Burtt NP, Stram DO, Young S, Kolonel LN, Henderson BE, Altshuler D, Pike MC. Clarifying the PROGINS allele association in ovarian and breast cancer risk: a haplotype-based analysis. J Natl Cancer Instit. 2005;97:51–59. doi: 10.1093/jnci/dji007. doi:10.1093/jnci/dji007. [DOI] [PubMed] [Google Scholar]
- Pellegrini C, Gori I, Achtari C, Hornung D, Chardonnens E, Wunder D, Fiche M, Canny GO. The expression of estrogen receptors as well as GREB1, c-MYC, and cyclin D1, estrogen-regulated genes implicated in proliferation, is increased in peritoneal endometriosis. Fertil Steril. 2012;98:1200–1208. doi: 10.1016/j.fertnstert.2012.06.056. doi:10.1016/j.fertnstert.2012.06.056. [DOI] [PubMed] [Google Scholar]
- Ponglikitmongkol M, Green S, Chambon P. Genomic organization of the human oestrogen receptor gene. EMBO J. 1988;7:3385–3388. doi: 10.1002/j.1460-2075.1988.tb03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice A, Randall BJ, Weddell A, McGill A, Henry L, Horne CHW, Thomas EJ. Ovarian steroid receptor expression in endometriosis and in two potential parent epithelia: endometrium and peritoneal mesothelium. Hum Reprod. 1992;7:1318–1325. doi: 10.1093/oxfordjournals.humrep.a137848. [DOI] [PubMed] [Google Scholar]
- Pritts EA, Zhao D, Ricke E, Waite L, Taylor RN. PPAR-gamma decreases endometrial stromal cell transcription and translation of RANTES in vitro. J Clin Endocrinol Metab. 2002;87:1841–1844. doi: 10.1210/jcem.87.4.8409. [DOI] [PubMed] [Google Scholar]
- Pritts EA, Zhao D, Sohn SH, Chao VA, Waite LL, Taylor RN. Peroxisome proliferator-activated receptor-gamma ligand inhibition of RANTES production by human endometriotic stromal cells is mediated through an upstream promoter element. Fertil Steril. 2003;80:415–420. doi: 10.1016/s0015-0282(03)00600-9. doi:10.1016/S0015-0282(03)00600-9. [DOI] [PubMed] [Google Scholar]
- Rahmioglu N, Missmer SA, Montgomery GW, Zondervan KT. Insights into Assessing the Genetics of Endometriosis. Curr Obstet Gynecol Rep. 2012;1:124–137. doi: 10.1007/s13669-012-0016-5. doi:10.1007/s13669-012-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran R, Lu A, Zhang L, Tang Y, Zhu H, Xu H, Feng Y, Han C, Zhou G, Rigby AC, et al. Hsp70 promotes TNF-mediated apoptosis by binding IKK gamma and impairing NF-kappa B survival signaling. Genes Dev. 2004;18:1466–1481. doi: 10.1101/gad.1188204. doi:10.1101/gad.1188204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JC, Ozcelik H, Maxeiner P, Andrulis I, Futscher BW. Methylation of the BRCA1 promoter is associated with decreased BRCA1 mRNA levels in clinical breast cancer specimens. Carcinogenesis. 2000;21:1761–1765. doi: 10.1093/carcin/21.9.1761. doi:10.1093/carcin/21.9.1761. [DOI] [PubMed] [Google Scholar]
- Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277:5209–5218. doi: 10.1074/jbc.M110090200. doi:10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Hinney A, Ziegler A, Hermann H, Fichter M, Mayer H, Siegfried W, Young JK, Remschmidt H, Hebebrand J. Systematic mutation screening of the estrogen receptor beta gene in probands of different weight extremes: identification of several genetic variants. J Clin Endocrinol Metab. 1998;83:4524–4527. doi: 10.1210/jcem.83.12.5471. [DOI] [PubMed] [Google Scholar]
- Roth J, Madoux F, Hodder P, Roush WR. Synthesis of small molecule inhibitors of the orphan nuclear receptor steroidogenic factor-1 (NR5A1) based on isoquinolinone scaffolds. Bioorg Med Chem Lett. 2008;18:2628–2632. doi: 10.1016/j.bmcl.2008.03.027. doi:10.1016/j.bmcl.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman C, Fischel L, Cortez G, Greiss H, Rana N, Rinehart J, Coulam CB. A search to identify genetic risk factors for endometriosis. Am J Reprod Immunol. 2013;69:92–95. doi: 10.1111/aji.12034. doi:10.1111/aji.12034. [DOI] [PubMed] [Google Scholar]
- Rowe SM, Coughlan SJ, McKenna NJ, Garrett E, Kieback DG, Carney DN, Headon DR. Ovarian carcinoma-associated TaqI restriction fragment length polymorphism in intron G of the progesterone receptor gene is due to an Alu sequence insertion. Cancer Res. 1995;55:2743–2745. [PubMed] [Google Scholar]
- Satagopan JM, Boyd J, Kauff ND, Robson M, Scheuer L, Narod S, Offit K. Ovarian cancer risk in Ashkenazi Jewish carriers of BRCA1 and BRCA2 mutations. Clin Cancer Res. 2002;8:3776–3781. [PubMed] [Google Scholar]
- Schimmer BP, White PCs. Minireview: steroidogenic factor 1: its roles in differentiation, development, and disease. Mol Endocrinol. 2010;24:1322–1337. doi: 10.1210/me.2009-0519. doi:10.1210/me.2009-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–733. doi: 10.1016/j.cell.2007.02.050. doi:10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug TT, Berry DC, Toshkov IA, Cheng L, Nikitin AY, Noy N. Overcoming retinoic acid-resistance of mammary carcinomas by diverting retinoic acid from PPARβ/δ to RAR. Proc Natl Acad Sci. 2008;105:7546–7551. doi: 10.1073/pnas.0709981105. doi:10.1073/pnas.0709981105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semczuk A, Schneider-Stock R, Szewczuk W. Prevalence of allelic loss at TP53 in endometrial carcinomas. Oncology. 2010;78:220–228. doi: 10.1159/000314353. doi:10.1159/000314353. [DOI] [PubMed] [Google Scholar]
- Shen L, Yang S, Huang W, Xu W, Wang Q, Song Y, Liu Y. MicroRNA23a and microRNA23b deregulation derepresses SF-1 and upregulates estrogen signaling in ovarian endometriosis. J Clin Endocrinol Metab. 2013;98:1575–1582. doi: 10.1210/jc.2012-3010. doi:10.1210/jc.2012-3010. [DOI] [PubMed] [Google Scholar]
- Shepherd JE. Effects of estrogen on congnition mood, and degenerative brain diseases. J Am Pharm Assoc (Wash) 2001;41:221–228. doi: 10.1016/s1086-5802(16)31233-5. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Takeuchi T, Mita S, Mizuguchi K, Kiyono T, Inoue M, Kyo S. Dienogest, a synthetic progestin, inhibits the proliferation of immortalized human endometrial epithelial cells with suppression of cyclin D1 gene expression. Mol Hum Reprod. 2009;15:693–701. doi: 10.1093/molehr/gap042. doi:10.1093/molehr/gap042. [DOI] [PubMed] [Google Scholar]
- Simpson JL, Bischoff FZ. Heritability and molecular genetic studies of endometriosis. Ann N Y Acad Sci. 2002;955:239–251. doi: 10.1111/j.1749-6632.2002.tb02785.x. discussion 293-235, 396-406 doi:10.1111/j.1749-6632.2002.tb02785.x. [DOI] [PubMed] [Google Scholar]
- Smuc T, Hevir N, Ribic-Pucelj M, Husen B, Thole H, Rizner TL. Disturbed estrogen and progesterone action in ovarian endometriosis. Mol Cell Endocrinol. 2009;301:59–64. doi: 10.1016/j.mce.2008.07.020. doi:10.1016/j.mce.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Sokalska A, Anderson M, Villanueva J, Ortega I, Bruner-Tran KL, Osteen KG, Duleba AJ. Effects of simvastatin on retinoic acid system in primary human endometrial stromal cells and in a chimeric model of human endometriosis. J Clin Endocrinol Metab. 2013;98:E463–E471. doi: 10.1210/jc.2012-3402. doi:10.1210/jc.2012-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Geirsson RT, Steinthorsdottir V, Jonsson H, Manolescu A, Kong A, Ingadottir G, Gulcher J, Stefansson K. Genetic factors contribute to the risk of developing endometriosis. Hum Reprod. 2002;17:555–559. doi: 10.1093/humrep/17.3.555. doi:10.1093/humrep/17.3.555. [DOI] [PubMed] [Google Scholar]
- Stephens AN, Hannan NJ, Rainczuk A, Meehan KL, Chen J, Nicholls PK, Rombauts LJ, Stanton PG, Robertson DM, Salamonsen LA. Post-translational modifications and protein-specific isoforms in endometriosis revealed by 2D DIGE. J Proteome Res. 2010;9:2438–2449. doi: 10.1021/pr901131p. doi:10.1021/pr901131p. [DOI] [PubMed] [Google Scholar]
- Stratton P, Sinaii N, Segars J, Koziol D, Wesley R, Zimmer C, Winkel C, Nieman LK. Return of chronic pelvic pain from endometriosis after raloxifene treatment: a randomized controlled trial. Obstet Gynecol. 2008;111:88–96. doi: 10.1097/01.AOG.0000297307.35024.b5. doi:10.1097/01.AOG.0000297307.35024.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Horiuchi A, Oka K, Miyamoto T, Kashima H, Shiozawa T. Immunohistochemical detection of steroid receptor cofactors in ovarian endometriosis: involvement of down-regulated SRC-1 expression in the limited growth activity of the endometriotic epithelium. Virchows Arch. 2010;456:433–441. doi: 10.1007/s00428-010-0884-x. doi:10.1007/s00428-010-0884-x. [DOI] [PubMed] [Google Scholar]
- Swiersz LM. Role of endometriosis in cancer and tumor development. Ann N Y Acad Sci. 2002;955:281–292. doi: 10.1111/j.1749-6632.2002.tb02788.x. Discussion 293-285, 396-406 doi:10.1111/j.1749-6632.2002.tb02788.x. [DOI] [PubMed] [Google Scholar]
- Szczepanska M, Mostowska A, Wirstlein P, Malejczyk J, Ploski R, Skrzypczak J, Jagodzinski PP. Polymorphic variants of DNMT3A and the risk of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2013;166:81–85. doi: 10.1016/j.ejogrb.2012.09.003. doi:10.1016/j.ejogrb.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Szyllo K, Tchorzewski H, Banasik M, Glowacka E, Lewkowicz P, Kamer-Bartosinska As. The involvement of T lymphocytes in the pathogenesis of endometriotic tissues overgrowth in women with endometriosis. Mediators Inflamm. 2003;12:131–138. doi: 10.1080/0962935031000134842. doi:10.1080/0962935031000134842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Kong B, Zhu W, Su S, Kan Y. Expression of steroidogenic factor 1 (SF-1) and steroidogenic acute regulatory protein (StAR) in endometriosis is associated with endometriosis severity. J Int Med Res. 2009;37:1389–1395. doi: 10.1177/147323000903700513. doi:10.1177/147323000903700513. [DOI] [PubMed] [Google Scholar]
- Tiraby C, Hazen BC, Gantner ML, Kralli A. Estrogen-related receptor gamma promotes mesenchymal-to-epithelial transition and suppresses breast tumor growth. Cancer Res. 2011;71:2518–2528. doi: 10.1158/0008-5472.CAN-10-1315. doi:10.1158/0008-5472.CAN-10-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabert B, Schwartz SM, Peters U, De Roos AJ, Chen C, Scholes D, Holt VL. Genetic variation in the sex hormone metabolic pathway and endometriosis risk: an evaluation of candidate genes. Fertil Steril. 2011;96:1401–1406.e1403. doi: 10.1016/j.fertnstert.2011.09.004. doi:10.1016/j.fertnstert.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar SA, O'Connor DT, O'Connor VM, Martin NG. Genetic influences on endometriosis in an Australian twin sample. Fertil Steril. 1999;71:701–710. doi: 10.1016/s0015-0282(98)00540-8. doi:10.1016/S0015-0282(98)00540-8. [DOI] [PubMed] [Google Scholar]
- Treloar S, Hadfield R, Montgomery G, Lambert A, Wicks J, Barlow DH, O'Connor DT, Kennedy S. The International Endogene Study: a collection of families for genetic research in endometriosis. Fertil Steril. 2002;78:679–685. doi: 10.1016/s0015-0282(02)03341-1. doi:10.1016/S0015-0282(02)03341-1. [DOI] [PubMed] [Google Scholar]
- Treloar SA, Zhao ZZ, Armitage T, Duffy DL, Wicks J, O'Connor DT, Martin NG, Montgomery GW. Association between polymorphisms in the progesterone receptor gene and endometriosis. Mol Hum Reprod. 2005;11:641–647. doi: 10.1093/molehr/gah221. doi:10.1093/molehr/gah221. [DOI] [PubMed] [Google Scholar]
- Trukhacheva E, Lin Z, Reierstad S, Cheng YH, Milad M, Bulun SE. Estrogen receptor (ER) beta regulates ERalpha expression in stromal cells derived from ovarian endometriosis. J Clin Endocrinol Metab. 2009;94:615–622. doi: 10.1210/jc.2008-1466. doi:10.1210/jc.2008-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng JF, Ryan IP, Milam TD, Murai JT, Schriock ED, Landers DV, Taylor RN. Interleukin-6 secretion in vitro is up-regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis. J Clin Endocrinol Metab. 1996;81:1118–1122. doi: 10.1210/jcem.81.3.8772585. [DOI] [PubMed] [Google Scholar]
- Uno S, Zembutsu H, Hirasawa A, Takahashi A, Kubo M, Akahane T, Aoki D, Kamatani N, Hirata K, Nakamura Y. A genome-wide association study identifies genetic variants in the CDKN2BAS locus associated with endometriosis in Japanese. Nat Genet. 2010;42:707–710. doi: 10.1038/ng.612. doi:10.1038/ng.612. [DOI] [PubMed] [Google Scholar]
- van Kaam KJAF, Romano A, Schouten JP, Dunselman GAJ, Groothuis PG. Progesterone receptor polymorphism +331G/A is associated with a decreased risk of deep infiltrating endometriosis. Hum Reprod. 2007;22:129–135. doi: 10.1093/humrep/del325. doi:10.1093/humrep/del325. [DOI] [PubMed] [Google Scholar]
- van Kaam KJAF, Schouten JP, Nap AW, Dunselman GAJ, Groothuis PG. Fibromuscular differentiation in deeply infiltrating endometriosis is a reaction of resident fibroblasts to the presence of ectopic endometrium. Hum Reprod. 2008;23:2692–2700. doi: 10.1093/humrep/den153. doi:10.1093/humrep/den153. [DOI] [PubMed] [Google Scholar]
- van Kaam KJ, Delvoux B, Romano A, D'Hooghe T, Dunselman GA, Groothuis PG. Deoxyribonucleic acid methyltransferases and methyl-CpG-binding domain proteins in human endometrium and endometriosis. Fertil Steril. 2011;95:1421–1427. doi: 10.1016/j.fertnstert.2011.01.031. doi:10.1016/j.fertnstert.2011.01.031. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7:1244–1255. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- Vietri MT, Molinari AM, Iannella I, Cioffi M, Bontempo P, Ardovino M, Scaffa C, Colacurci N, Cobellis L. Arg72Pro p53 polymorphism in Italian women: no association with endometriosis. Fertil Steril. 2007;88:1468–1469. doi: 10.1016/j.fertnstert.2006.12.049. doi:10.1016/j.fertnstert.2006.12.049. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yoshida S, Negoro K, Kennedy S, Barlow D, Maruo T. Polymorphisms in the estrogen receptor beta gene but not estrogen receptor alpha gene affect the risk of developing endometriosis in a Japanese population. Fertil Steril. 2004;81:1650–1656. doi: 10.1016/j.fertnstert.2004.02.094. doi:10.1016/j.fertnstert.2004.02.094. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Yu J, Luo XZ, Shi YL, Wang Y, Wang L, Li DJs. The high level of RANTES in the ectopic milieu recruits macrophages and induces their tolerance in progression of endometriosis. J Mol Endocrinol. 2010;45:291–299. doi: 10.1677/JME-09-0177. doi:10.1677/JME-09-0177. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lonard DM, Yu Y, Chow DC, Palzkill TG, O'Malley BW. Small molecule inhibition of the steroid receptor coactivators, SRC-3 and SRC-1. Mol Endocrinol. 2011;25:2041–2053. doi: 10.1210/me.2011-1222. doi:10.1210/me.2011-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JS, Ricote M, Akiyama TE, Gonzalez FJ, Glass CK. PPARγ and PPARδ negatively regulate specific subsets of lipopolysaccharide and IFN-γ target genes in macrophages. Proc Natl Acad Sci. 2003;100:6712–6717. doi: 10.1073/pnas.1031789100. doi:10.1073/pnas.1031789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetendorf M, DeMayo FJ. The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Mol Cell Endocrinol. 2012;357:108–118. doi: 10.1016/j.mce.2011.10.028. doi:10.1016/j.mce.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Leonardsson G, Rosewell I, Ann Jacobs M, Milligan S, Parker M. The nuclear receptor co-repressor nrip1 (RIP140) is essential for female fertility. Nat Med. 2000;6:1368–1374. doi: 10.1038/82183. doi:10.1038/82183. [DOI] [PubMed] [Google Scholar]
- Wieser F, Schneeberger C, Tong D, Tempfer C, Huber JC, Wenzl R. PROGINS receptor gene polymorphism is associated with endometriosis. Fertil Steril. 2002;77:309–312. doi: 10.1016/s0015-0282(01)02984-3. doi:10.1016/S0015-0282(01)02984-3. [DOI] [PubMed] [Google Scholar]
- Willson TM, Wahli W. Peroxisome proliferator-activated receptor agonists. Curr Opin Chem Biol. 1997;1:235–241. doi: 10.1016/s1367-5931(97)80015-4. doi:10.1016/S1367-5931(97)80015-4. [DOI] [PubMed] [Google Scholar]
- Wu Y, Halverson G, Basir Z, Strawn E, Yan P, Guo SW. Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am J Obstet Gynecol. 2005;193:371–380. doi: 10.1016/j.ajog.2005.01.034. doi:10.1016/j.ajog.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics. 2006;1:106–111. doi: 10.4161/epi.1.2.2766. doi:10.4161/epi.1.2.2766. [DOI] [PubMed] [Google Scholar]
- Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Aberrant expression of deoxyribonucleic acid methyltransferases DNMT1, DNMT3A, and DNMT3B in women with endometriosis. Fertil Steril. 2007a;87:24–32. doi: 10.1016/j.fertnstert.2006.05.077. doi:10.1016/j.fertnstert.2006.05.077. [DOI] [PubMed] [Google Scholar]
- Wu Y, Starzinski-Powitz A, Guo SW. Trichostatin A, a histone deacetylase inhibitor, attenuates invasiveness and reactivates E-cadherin expression in immortalized endometriotic cells. Reprod Sci. 2007b;14:374–382. doi: 10.1177/1933719107302913. doi:10.1177/1933719107302913. [DOI] [PubMed] [Google Scholar]
- Wu Y, Shi X, Guo SW. The knockdown of progesterone receptor isoform B (PR-B) promotes proliferation in immortalized endometrial stromal cells. Fertil Steril. 2008;90:1320–1323. doi: 10.1016/j.fertnstert.2007.10.049. doi:10.1016/j.fertnstert.2007.10.049. [DOI] [PubMed] [Google Scholar]
- Xu Y, Diao L, Chen Y, Liu Y, Wang C, Ouyang T, Li J, Wang T, Fan Z, Fan T, et al. Promoter methylation of BRCA1 in triple-negative breast cancer predicts sensitivity to adjuvant chemotherapy. Ann Oncol. 2013;24:1498–1505. doi: 10.1093/annonc/mdt011. doi:10.1093/annonc/mdt011. [DOI] [PubMed] [Google Scholar]
- Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E, Yin P, Milad MP, Confino E, Reierstad S, Innes J, et al. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod. 2007a;77:681–687. doi: 10.1095/biolreprod.107.061804. doi:10.1095/biolreprod.107.061804. [DOI] [PubMed] [Google Scholar]
- Xue Q, Lin Z, Yin P, Milad MP, Cheng YH, Confino E, Reierstad S, Bulun SE. Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5′ CpG island in endometriosis. J Clin Endocrinol Metab. 2007b;92:3261–3267. doi: 10.1210/jc.2007-0494. doi:10.1210/jc.2007-0494. [DOI] [PubMed] [Google Scholar]
- Xue Q, Zhou YF, Zhu SN, Bulun SE. Hypermethylation of the CpG island spanning from exon II to intron III is associated with steroidogenic factor 1 expression in stromal cells of endometriosis. Reprod Sci. 2011;18:1080–1084. doi: 10.1177/1933719111404614. doi:10.1177/1933719111404614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Q, Xu Y, Yang H, Zhang L, Shang J, Zeng C, Yin P, Bulun SE. Methylation of a Novel CpG Island of Intron 1 Is Associated With Steroidogenic Factor 1 Expression in Endometriotic Stromal Cells. Reprod Sci. 2013 doi: 10.1177/1933719113497283. 1933719113497283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Guerrero J, Hong H, DeFranco DB, Stallcup MR. Interaction of the τ2 transcriptional activation domain of glucocorticoid receptor with a novel steroid receptor coactivator, Hic-5, which localizes to both focal adhesions and the nuclear matrix. Mol Biol Cell. 2000;11:2007–2018. doi: 10.1091/mbc.11.6.2007. doi:10.1091/mbc.11.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhang X, Zhou C, Huang X, Lin J, Xu H. Elevated immunoreactivity of RANTES and CCR1 correlate with the severity of stages and dysmenorrhea in women with deep infiltrating endometriosis. Acta Histochem. 2013;115:434–439. doi: 10.1016/j.acthis.2012.10.006. doi:10.1016/j.acthis.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Yao Z, Shen X, Capodanno I, Donnelly M, Fenyk-Melody J, Hausamann J, Nunes C, Strauss J, Vakerich K. Validation of rat endometriosis model by using raloxifene as a positive control for the evaluation of novel SERM compounds. J Invest Surg. 2005;18:177–183. doi: 10.1080/08941930591004412. doi:10.1080/08941930591004412. [DOI] [PubMed] [Google Scholar]
- Yi P, Feng Q, Amazit L, Lonard DM, Tsai SY, Tsai MJ, O'Malley BW. Atypical protein kinase C regulates dual pathways for degradation of the oncogenic coactivator SRC-3/AIB1. Mol Cell. 2008;29:465–476. doi: 10.1016/j.molcel.2007.12.030. doi:10.1016/j.molcel.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16:391–396. doi: 10.1038/ng0897-391. doi:10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- Zeitoun KM, Bulun SE. Aromatase: a key molecule in the pathophysiology of endometriosis and a therapeutic target. Fertil Steril. 1999;72:961–969. doi: 10.1016/s0015-0282(99)00393-3. doi:10.1016/S0015-0282(99)00393-3. [DOI] [PubMed] [Google Scholar]
- Zelenko Z, Aghajanova L, Irwin JC, Giudice LC. Nuclear receptor, coregulator signaling, and chromatin remodeling pathways suggest involvement of the epigenome in the steroid hormone response of endometrium and abnormalities in endometriosis. Reprod Sci. 2012;19:152–162. doi: 10.1177/1933719111415546. doi:10.1177/1933719111415546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwijsen RM, Buckle RS, Hijmans EM, Loomans CJ, Bernards R. Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev. 1998;12:3488–3498. doi: 10.1101/gad.12.22.3488. doi:10.1101/gad.12.22.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]