Abstract
BACKGROUND
The discovery of kisspeptin as key central regulator of GnRH secretion has led to a new level of understanding of the neuroendocrine regulation of human reproduction. The related discovery of the kisspeptin-neurokinin B-dynorphin (KNDy) pathway in the last decade has further strengthened our understanding of the modulation of GnRH secretion by endocrine, metabolic and environmental inputs. In this review, we summarize current understanding of the physiological roles of these novel neuropeptides, and discuss the clinical relevance of these discoveries and their potential translational applications.
METHODS
A systematic literature search was performed using PUBMED for all English language articles up to January 2014. In addition, the reference lists of all relevant original research articles and reviews were examined. This review focuses mainly on published human studies but also draws on relevant animal data.
RESULTS
Kisspeptin is a principal regulator of the secretion of gonadotrophins, and through this key role it is critical for the onset of puberty, the regulation of sex steroid-mediated feedback and the control of adult fertility. Although there is some sexual dimorphism, both neuroanatomically and functionally, these functions are apparent in both men and women. Kisspeptin acts upstream of GnRH and, following paracrine stimulatory and inhibitory inputs from neurokinin B and dynorphin (KNDy neuropeptides), signals directly to GnRH neurones to control pulsatile GnRH release. When administered to humans in different isoforms, routes and doses, kisspeptin robustly stimulates LH secretion and LH pulse frequency. Manipulation of the KNDy system is currently the focus of translational research with the possibility of future clinical application to regulate LH pulsatility, increasing gonadal sex steroid secretion in reproductive disorders characterized by decreased LH pulsatility, including hypothalamic amenorrhoea and hypogonadotropic hypogonadism. Conversely there may be scope to reduce the activity of the KNDy system to reduce LH secretion where hypersecretion of LH adds to the phenotype, such as in polycystic ovary syndrome.
CONCLUSIONS
Kisspeptin is a recently discovered neuromodulator that controls GnRH secretion mediating endocrine and metabolic inputs to the regulation of human reproduction. Manipulation of kisspeptin signalling has the potential for novel therapies in patients with pathologically low or high LH pulsatility.
Keywords: kisspeptin, kisspeptin-neurokinin B-dynorphin, GnRH, LH pulsatility
Introduction
Since its discovery, hypothalamic secretion of GnRH has been robustly established as the key pathway that initiates and controls reproductive function. Whilst the pivotal central role played by GnRH remains undisputed, a number of functional limitations of the GnRH neuronal network have been identified. For example, in rats, GnRH neurones lack estrogen receptor (ER)-alpha (Herbison and Theodosis, 1992), the principal ER, thus suggesting the need for an intermediary signalling pathway mediating gonadal feedback.
It was only a decade ago that the discovery of the obligate role of kisspeptin in human puberty revolutionized current understanding of the neuroendocrine regulation of human reproduction (de Roux et al., 2003; Seminara et al., 2003). Kisspeptin, a hypothalamic peptide coded by the KiSS1 gene, is a novel neuromodulator that acts upstream of GnRH, and is sensitive to sex steroid feedback and metabolic cues. Kisspeptin is now recognized as a crucial regulator of the onset of puberty, the regulation of sex hormone-mediated secretion of gonadotrophins, and the control of fertility (Pinilla et al., 2012). The related discovery of a reproductive role for neurokinin B has stimulated further interest in the field. The same functional neuronal network secretes kisspeptin and neurokinin B—now called kisspeptin-neurokinin B-dynorphin (KNDy) neurones as they also co-secrete dynorphin, a well-established opioid inhibitor (Goodman et al., 2007). Exogenous kisspeptin has been administered to healthy volunteers and a limited number of patients, with a view to restoring reproductive function in certain conditions.
In this review, we summarize current understanding of the physiological regulation of GnRH pulse frequency by kisspeptin, and appraise the clinical relevance of the discoveries of kisspeptin and neurokinin B. The focus will predominantly be on human findings, using animal data where human studies are lacking but where there is direct translational potential.
Methods
A systematic literature search was performed using PUBMED for all English language articles published up to January 2014 using the terms ‘kisspeptin’ and ‘reproduction’. The search was performed without limitations by species although subsequent priority was given to human studies, where available. The initial search identified 390 manuscripts, which were used as background material for the review. In addition, the reference lists of all relevant original research articles and reviews were examined and selected if judged to be relevant. Relevant abstracts from recent scientific meetings were included in the review.
Discovery of the kisspeptin and KNDy neuronal network
KISS1 gene, peptide and its receptor
KISS1, the gene encoding kisspeptins, was originally identified in 1996 as a suppressor of metastasis in human malignant melanoma (Lee et al., 1996). As it was discovered in Hershey (PA, USA), the gene was named after the famous chocolate ‘Kisses’ produced in the town. The SS in KiSS1 acknowledges that it is a ‘suppressor sequence’.
The KISS1 gene is localized to chromosome 1q32 and has four exons, the first two of which are not translated (West et al., 1998). The gene encodes the precursor 145 amino acid peptide, which is cleaved to a 54 amino acid protein (West et al., 1998). To acknowledge its metastasis inhibitory properties, the 54 amino acid transcript was named ‘metastin’ (Ohtaki et al., 2001). This can be further cleaved to 14, 13 and 10 amino acid peptides. The 54 amino acid and the shorter peptides belong to the RF amide group of peptides, sharing the C-terminal sequence of Arg-Phe-NH2, and are now collectively referred to as kisspeptins (Kotani et al., 2001).
In 2001, kisspeptin was identified as a ligand for the orphan G-protein coupled receptor 54 (GPR54), which was first described in the rat brain and subsequently in human (then named AXOR12 and hOT7T175) (Lee et al., 1999; Muir et al., 2001; Ohtaki et al., 2001), now termed KISS1R (Gottsch et al., 2009). KISS1R maps to chromosome 19p13.3 and includes five exons, encoding a 398 amino acid protein with seven hydrophobic trans-membrane domains (Muir et al., 2001). It has an amino acid sequence close to that of the galanin receptor family (40% identity), although it does not bind either galanin or galanin-like peptide (Lee et al., 1999). Upon binding by kisspeptin, KISS1R activates phospholipase C and recruits secondary intracellular messengers, inositol triphosphate and diacylglycerol, which in turn mediate calcium release and protein kinase C activation to mediate kisspeptin's function (Muir et al., 2001; Liu et al., 2008; Constantin et al., 2009). Activation of Kiss1r results in a biphasic increase in intracellular calcium, with a rapid increase followed by a more sustained second phase (Min et al., 2014). To maintain this second phase and therefore sustain signalling, kisspeptin receptor trafficking involving internalization, recycling and recruitment from an intracellular pool, is required (Min et al., 2014). Without receptor trafficking, the kisspeptin receptor undergoes desensitization following an initial acute phase (Min et al., 2014). Since the discovery of kisspeptin-KISS1R signalling, many different terms have been used to describe its components. The nomenclature used in this review for kisspeptin and its receptor will be that recently recommended by Gottsch et al. (2009).
Functional neuroanatomy of kisspeptin signalling
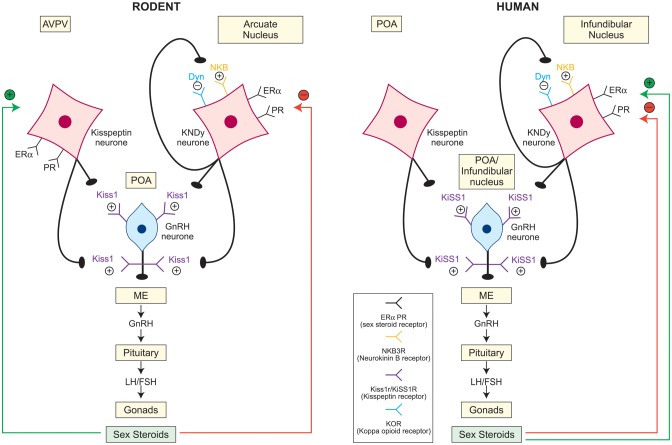
GnRH neurones extend from the preoptic area through to the infundibular nucleus (homologue to the arcuate nucleus in other species) of the hypothalamus in humans, whereas in rodents GnRH neurones reside predominantly in the preoptic area (Lehman et al., 1986; Schwanzel-Fukuda and Pfaff, 1989; Clifton and Steiner, 2009) (Fig. 1). GnRH axons project from these nuclei to the median eminence, where GnRH is secreted into the portal circulation in a coordinated and pulsatile manner. Similarly, kisspeptin neurones are located in the rostral preoptic area and the infundibular nucleus in the human hypothalamus (Rometo et al., 2007; Hrabovszky et al., 2010). The anatomical distribution of kisspeptin neurones and their appositions with other hypothalamic endocrine networks are described below. Areas of incongruity between data from human studies and those carried out in other species are also highlighted.
Figure 1.
Schematic diagram showing the neuroanatomy of the kisspeptin-GnRH pathway and the relationship between KNDy neurones and GnRH neurones in humans and rodents. Kisspeptin signals directly to the GnRH neurones, which express kisspeptin receptor. The location of kisspeptin neurone populations within the hypothalamus is species specific, residing within the anteroventral periventricular nucleus (AVPV) and the arcuate nucleus in rodents, and within the preoptic area (POA) and the infundibular nucleus in humans. Kisspeptin neurones in the infundibular (humans)/arcuate (rodents) nucleus co-express neurokinin B and dynorphin (KNDy neurones), which via neurokinin B receptor and kappa opioid peptide receptor autosynaptically regulate pulsatile kisspeptin secretion, with neurokinin B being stimulatory and dynorphin inhibitory. Negative (red) and positive (green) sex steroid feedback is mediated via distinct kisspeptin populations in rodents, via the AVPV and the arcuate nucleus, respectively. In humans KNDy neurones in the infundibular nucleus relay both negative (red) and positive (green) feedback. The role of the POA kisspeptin population in mediating sex steroid feedback in humans is incompletely explored. ME, median eminence; +, stimulatory; −, inhibitory; ERα, estrogen receptor alpha; PR, progesterone receptor; Kiss1/KiSS1, kisspeptin; NKB, neurokinin B; Dyn, dynorphin.
Kisspeptin neurone localization in humans
Initial studies of the neuroanatomical distribution of kisspeptin neurones in the human brain carried out in autopsy samples from premenopausal and post-menopausal women localized KISS1 expression to the infundibular nucleus only (Rometo et al., 2007). A more recent study, using both male and female autopsy samples, has confirmed the localization of the majority of kisspeptin cell bodies in the infundibular nucleus, but identified a second dense population of kisspeptin cells in the rostral preoptic area (Hrabovszky et al., 2010).
Whilst kisspeptin neurones are located in the infundibular/arcuate nucleus across all species, including humans, the rostral population is species specific (Clarkson and Herbison, 2006; Pompolo et al., 2006; Ramaswamy et al., 2008; Clarkson et al., 2009; Hrabovszky et al., 2010). In rodents, the rostral population is located in the anteroventral periventricular nucleus (AVPV) and the periventricular nucleus (PeN), the continuum of this region known as the rostral periventricular region of the third ventricle (RP3V) (Clarkson and Herbison, 2006; Clarkson et al., 2009) (Fig. 1). In contrast, humans and ruminants lack this well-defined RP3V population and have more scattered kisspeptin cell bodies within the preoptic region (Pompolo et al., 2006; Oakley et al., 2009; Hrabovszky et al., 2010). Unlike in humans and ruminants, Kiss1 mRNA was not detectable in the preoptic area in the adult rhesus monkey (Ramaswamy et al., 2008).
Kisspeptin axons form dense pericapillary plexuses in the human infundibular stalk, the site of neurosecretion of GnRH (Hrabovszky et al., 2010). Axo-somatic, axo-dendritic and axo-axonal contacts between kisspeptin and GnRH axons were also demonstrated in the infundibular stalk, in keeping with data from rodents, sheep and monkeys, where kisspeptin and GnRH neuronal networks are in close proximity (Clarkson and Herbison, 2006; Ramaswamy et al., 2008; Smith et al., 2008a; Hrabovszky et al., 2010; Uenoyama et al., 2011). GnRH neurones express Kiss1r mRNA (Irwig et al., 2004; Han et al., 2005; Messager et al., 2005). These findings indicate direct involvement of kisspeptin in the neurosecretion of GnRH. However, in humans as well as other species studied to date, not all GnRH neurones receive kisspeptin neurone contacts and the incidence of these contacts seems low (Clarkson and Herbison, 2006; Ramaswamy et al., 2008; Smith et al., 2008a; Hrabovszky et al., 2010), suggesting a subtle regulation of GnRH secretion by kisspeptin and other neuropeptides.
Kisspeptin neurone populations co-express other neuropeptides
There is considerable overlap in the distribution of kisspeptin, neurokinin B and dynorphin in the hypothalamus, with frequent colocalization. Mapping of kisspeptin and neurokinin B neurones was similar in the infundibular nucleus of post-menopausal women, prompting the identification of a subpopulation of kisspeptin neurones which express neurokinin B and dynorphin in the human infundibular nucleus (Rometo et al., 2007; Hrabovszky et al., 2010). This unique region expressing kisspeptin, neurokinin B and dynorphin is conserved across species and resides in the hypothalamic arcuate nucleus in sheep and rodents (Burke et al., 2006; Goodman et al., 2007; Navarro et al., 2009). Neurokinin B and dynorphin are, however, absent from the kisspeptin population in the preoptic area/RP3V. These infundibular (human)E/arcuate (rodent and ruminant) nucleus neurones which co-express all three neuropeptides are referred to as KNDy neurones (Cheng et al., 2010) (Fig. 1).
KNDy neurones in rats and sheep also co-localize with the glutamate transporter-2, and glutamate has been implicated in mediating estrogen positive feedback resulting in the pre-ovulatory GnRH surge. Whether KNDy cells express glutamate receptors remains to be determined (Pompolo et al., 2003; Ciofi et al., 2006). Kisspeptin neurones in the preoptic area/RP3V are not KNDy neurones, but in the mouse AVPV they co-express tyrosine hydroxylase (the key regulatory step in dopamine synthesis) (Oakley et al., 2009). This differential expression of neuropeptides reflects complex signalling within the hypothalamus and distinct functions of the two kisspeptin populations (Ojeda et al., 2010; Tello et al., 2010).
Kisspeptin neurone populations differ in physiological function
KNDy neurones of the infundibular/arcuate nucleus influence the activity of GnRH by acting on both GnRH cell bodies and neurosecretory terminals (Krajewski et al., 2005; Ciofi et al., 2006; Ramaswamy et al., 2008) (Fig. 1). KNDy neurones make direct contact with GnRH cell bodies and dendrites in humans and project to the median eminence in rodents, sheep and monkeys (Krajewski et al., 2005; Ciofi et al., 2006; Clarkson and Herbison, 2006; Ramaswamy et al., 2008; Dahl et al., 2009). KNDy cells act synergistically to produce coordinated and pulsatile GnRH secretion by controlling the neuroactivity of other KNDy cells, as inferred from a reciprocally interconnected KNDy cell network within the arcuate nucleus in the sheep and rat (Foradori et al., 2002; Burke et al., 2006; Lehman et al., 2010). This is supported by the expression of neurokinin B receptors and the kappa opioid peptide receptor (the receptor for dynorphin) within the KNDy cells, but not the kisspeptin receptor, which predominantly co-localizes with GnRH neurones (Krajewski et al., 2005; Navarro et al., 2009; Herbison et al., 2010) (Fig. 1). This implies that the stimulatory role of neurokinin B and the inhibitory action of dynorphin autosynaptically coordinate the pulsatile release of kisspeptin, which in turn drives the pulsatile secretion of GnRH and LH (Navarro et al., 2009).
Kisspeptin-mediated GnRH stimulation is sex steroid dependent. Estrogen and progesterone modulate kisspeptin activity at both the AVPV nucleus and the arcuate/infundibular nucleus through sex steroid receptors (Ciofi et al., 1994; Goubillon et al., 2000; Foradori et al., 2002; Smith et al., 2005; Franceschini et al., 2006) (Fig. 1). It is becoming clear that not only do kisspeptin neurones mediate both negative and positive sex steroid feedback, but also that distinct subgroups, which are species specific, are involved in these two critical regulatory functions, described more fully below (sections: Kisspeptin mediates negative sex steroid feedback and Kisspeptin may also mediate estrogenic positive feedback). In rodents, the AVPV and the arcuate nucleus respond to positive and negative sex steroid feedback, respectively (Smith et al., 2005, 2006b, Herbison, 2008), whereas in humans, the infundibular nucleus alone relays sex steroid signalling (Rometo et al., 2007; Oakley et al., 2009) (Fig. 1). Thus while there is less marked anatomical differentiation of the two feedback pathways in humans, it remains possible (and perhaps likely) that the two functions are mediated by different neurones.
Kisspeptin neurones show sexual dimorphism
There is evidence for sexual dimorphism in kisspeptin pathways in the human, probably reflecting these functional differences discussed above. Female hypothalami have significantly more kisspeptin fibres in the infundibular nucleus and ventral periventricular zone than are seen in men (Hrabovszky et al., 2010). There is also a striking sex difference in the number and expression of kisspeptin cell bodies, which are observed in the rostral periventricular zone of the female only (Hrabovszky et al., 2010). Likewise only a few kisspeptin cell bodies are present in the infundibular nucleus in males in contrast to the abundant kisspeptin cell bodies in females (Hrabovszky et al., 2010). Similarly, sex differences have been reported in the arcuate nucleus of the sheep (Cheng et al., 2010). Pre-ovulatory positive sex steroid feedback is unique to the female, and the adult female mouse and rat hypothalamus contain 10-fold more kisspeptin neurones than males in the RP3V region, whereas the arcuate nucleus responsible for negative sex steroid feedback does not display such dimorphism (Clarkson and Herbison, 2006; Kauffman et al., 2007).
Kisspeptin and the regulation of GnRH secretion
Kisspeptin is a potent stimulator of the hypothalamic-pituitary-gonadal (HPG) axis in both animal models and humans. Kisspeptin signals directly to the GnRH neurones through the action on the kisspeptin receptor to release GnRH into the portal circulation, which in turn stimulates the secretion of LH and FSH from the gonadotrophs of the anterior pituitary. Evidence for this comes from multiple sources. Since GnRH cannot be measured in the peripheral circulation, LH pulse frequency remains a widely used and a well validated surrogate of hypophyseal GnRH pulsatility as each GnRH pulse is associated with an LH pulse (Clarke and Cummins, 1985).
Kisspeptin stimulates gonadotrophin release in humans
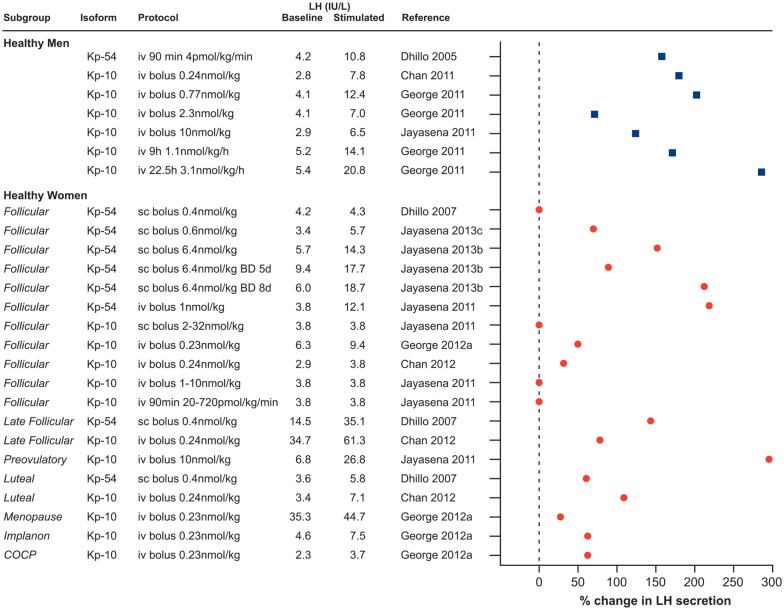
Kisspeptin stimulates the secretion of both LH and FSH in the human, although the effect on the former is much more marked (George and Seminara, 2012). Kisspeptin-54 was first administered in healthy men by intravenous infusion (4 pmol/kg/min (0.023 µg/kg/min) for 90 min) and resulted in a robust and dose-dependent increase (from 0.25 pmol/kg/min (0.001 µg/kg/min) to 12 pmol/kg/min (0.07 µg/kg/min)) in LH, and less marked rises in FSH and testosterone (Dhillo et al., 2005). Kisspeptin-54 clearance showed first-order kinetics with a half-life of 27.6 ± 1.1 min (Dhillo et al., 2005), which compares with about 4 min for kisspeptin-10 (Jayasena et al., 2011). The potency of kisspeptin to stimulate the secretion of gonadotrophins and its preferential effect on the release of LH has been consistently observed when kisspeptin is administered by different routes (intravenous or subcutaneous) and types of exposure (single boluses or continuous infusion), in different isoforms (kisspeptin-54 and kisspeptin-10), to men or women or in different disease models (Dhillo et al., 2005, 2007; Jayasena et al., 2009, 2010, 2011, 2013a, b; Chan et al., 2011, 2012; George et al., 2011, 2012, 2013; George and Seminara 2012; Abbara et al., 2013; Young et al., 2013). Figures 2 and 3 summarize knowledge of the stimulatory effect of exogenous kisspeptin on the secretion of LH in humans to date.
Figure 2.
Kisspeptin stimulates LH secretion in healthy men (filled squares) and women (filled circles). The stimulatory effect of kisspeptin on LH secretion is shown in both healthy men and women, when kisspeptin is administered in different isoforms (kisspeptin-54 and kisspeptin-10) and by different protocols (intravenous or subcutaneous, single boluses or continuous infusion). Note that stimulated LH values are either mean LH or peak LH concentrations depending on how the data are originally presented. Where authors do not state exact LH concentration following kisspeptin administration, this was obtained from the relevant figures. 0% change in LH secretion is reported if no statistically significant change in LH secretion was reported and authors do not show actual LH concentrations. iv, intravenous; sc, subcutaneous; BD, twice daily; Implanon, etonogestrel contraceptive implant; COCP, combined oral contraceptive pill.
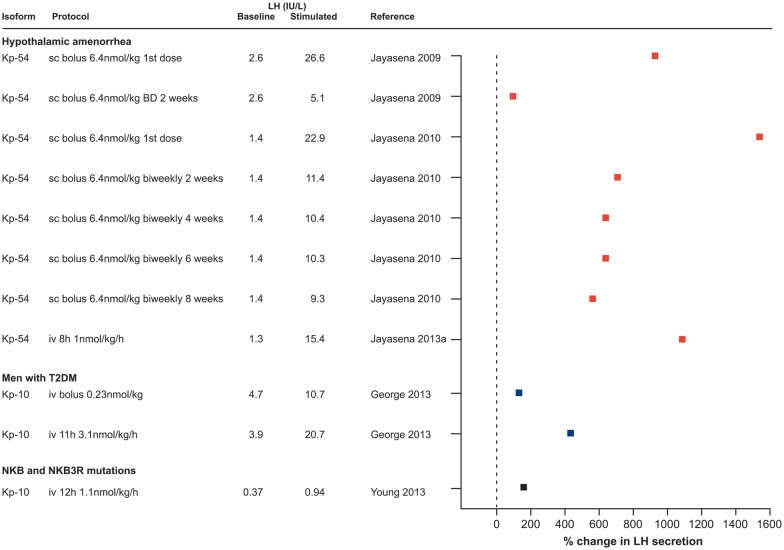
Figure 3.
Increase in LH secretion following the administration of kisspeptin in human disease models. The stimulatory effect of kisspeptin on LH secretion is shown in reproductive endocrine disorders, when kisspeptin is administered in different isoforms (kisspeptin-54 and kisspeptin-10) and by different protocols (i.v. or s.c., single boluses or continuous infusion) as indicated. Note that stimulated LH values are either mean LH or peak LH concentrations depending on how the data are originally presented. Where authors do not state an exact LH concentration following kisspeptin administration, this is obtained from the relevant figures. BD, twice daily; T2DM, type 2 diabetes; NKB (TAC3), neurokinin B; NKB3R (TAC3R), neurokinin B receptor.
While kisspeptin stimulates LH release 2- to 3-fold in most circumstances, the stimulatory effect on FSH is much smaller and is less consistent (Dhillo et al., 2005, 2007; George et al., 2011, 2012; Jayasena et al., 2011; Chan et al., 2012). A more potent effect of kisspeptin on LH secretion than FSH in humans is concordant with studies in rodents (Thompson et al., 2004; Navarro et al., 2005a).
Kisspeptin operates upstream of GnRH neurones and coordinates GnRH and LH pulsatility
Kisspeptin acts directly on GnRH neurones
Evidence that kisspeptin exerts its stimulatory function on gonadotrophin secretion through a direct action on the hypothalamic GnRH system is provided by findings from animal studies, consistent with the anatomical studies described above. Kisspeptin causes depolarization of and increases in firing rate of GnRH neurones in vitro (Han et al., 2005; Zhang et al., 2008); kisspeptin stimulates the secretion of GnRH in hypothalamic explants (Thompson et al., 2004; Tovar et al., 2006); c-Fos immunoreactivity (a marker of neuronal activity) (Matsui et al., 2004; Han et al., 2005) and the expression of GnRH mRNA is up-regulated within the cell bodies of GnRH neurones following the kisspeptin exposure (Novaira et al., 2009; Oakley et al., 2009). In sheep, intracerebroventricular infusion of kisspeptin caused a dramatic increase in the cerebrospinal fluid GnRH content and simultaneously in serum LH and FSH (Messager et al., 2005).
Some studies suggest that kisspeptin directly stimulates pituitary gonadotrophs to release LH and FSH, based on the expression of Kiss1 and Kiss1r genes in gonadotrophs, the secretion of gonadotrophins from pituitary explants treated with kisspeptin (Kotani et al., 2001; Navarro et al., 2005b, Gutierrez-Pascual et al., 2007; Richard et al., 2008) and the detection of kisspeptin (although in low levels) in the hypophyseal portal circulation in the sheep (Smith et al., 2008b). The ability of kisspeptin to induce gonadotrophin release from the pituitary fragments might be explained by the pharmacological concentrations of kisspeptin used (Navarro et al., 2005a, Gutierrez-Pascual et al., 2007). Whilst kisspeptin may have a direct stimulatory action on gonadotrophs, indirect stimulation through enhancing GnRH secretion appears to be the principal physiologic pathway for the stimulation of gonadotrophin secretion (Gottsch et al., 2004; Irwig et al., 2004; Smith et al., 2008b).
The physiological role of kisspeptin in the regulation of GnRH secretion was further demonstrated by studies using a kisspeptin antagonist (Millar et al., 2010). Kisspeptin-induced GnRH neurone firing was abolished by the kisspeptin antagonist (Irwig et al., 2004; Liu et al., 2008; Roseweir et al., 2009). Kisspeptin is needed for the pulsatile release of GnRH, as when injected into the median eminence of pubertal rhesus monkeys, kisspeptin antagonist suppressed both mean GnRH and GnRH pulses (Roseweir et al., 2009). Kisspeptin modulates the secretion of GnRH at the arcuate nucleus, the site of the GnRH pulse generator: kisspeptin antagonist reduced LH pulse frequency when administered to the arcuate nucleus but not when administered to the preoptic area in the rat (Li et al., 2009).
That the effect of kisspeptin on LH release was prevented by pretreatment with GnRH antagonist further points to the action of kisspeptin upstream of GnRH (Gottsch et al., 2004; Shahab et al., 2005). Although there are no studies in humans administering GnRH antagonist followed by kisspeptin, the direct action of kisspeptin on GnRH neurones is inferred from consistent findings in rodents and nonhuman primates. Humans with ‘inactivating’ mutations in kisspeptin and/or its receptor show hypogonadotropic hypogonadism and delayed puberty (de Roux et al., 2003; Seminara et al., 2003), whist those with ‘activating’ mutations undergo precocious puberty (Teles et al., 2008; Silveira et al., 2010), suggesting that kisspeptin modulates GnRH pulsatility.
Kisspeptin increases LH pulsatility in humans
As GnRH secretion is pulsatile, the effect of kisspeptin on the characteristics of that pulsatility (as reflected in LH pulses) has been investigated. Intravenous infusion of kisspeptin-10 (1.5 µg/kg/h (1.1 nmol/kg/h) for 9 h) in healthy men (George et al., 2011) and kisspeptin-54 (subcutaneous bolus 0.3 nmol/kg (1.76 µg/kg) and 0.6 nmol/kg (3.5 µg/kg)) in healthy women (Jayasena et al., 2013c) increased LH pulse frequency and amplitude. The ability of kisspeptin to enhance LH pulsatility has also been demonstrated in human reproductive disorders, including in hypothalamic amenorrhoea (Jayasena et al., 2013a), in hypogonadal men with type 2 diabetes (George et al., 2013) and in neurokinin B signalling defects (Young et al., 2013), described more fully below. Kisspeptin not only drives the pulsatile secretion of GnRH, but also appears to reset the hypothalamic clock of GnRH pulsatility in men. Acute injection of kisspeptin-10 delayed the next endogenous LH pulse by the interval that would be observed between the two consecutive endogenous LH pulses (Chan et al., 2011). However, the same kisspeptin dosing protocol did not support the ability of kisspeptin to reset the GnRH pulse generator in women across the different phases of the menstrual cycle (Chan et al., 2012). The authors suggest that the GnRH pulse generator in men operates differently to women and that it is the changes in the sex steroid milieu across the menstrual cycle in women that might be responsible for this discrepancy (Chan et al., 2012). The marked sexual dimorphism in the anatomy of the kisspeptin system described above may underlie this intriguing observation and even determine the frequency of GnRH secretion in women across the normal menstrual cycle. This variability in the frequency of GnRH pulsatility is central to the differential regulation of LH and FSH (McNeilly et al., 2003) and thus ovarian follicle development, the correct selection of a single dominant follicle for ovulation, and the luteal phase with limited follicle development.
Men and women show sexual dimorphism in their response to kisspeptin
Men and women display sexual dimorphism in their response to exogenous kisspeptin (Fig. 2). Whilst kisspeptin potently stimulates the release of LH in men, the effect of kisspeptin is more variable in women and depends on the phase of the menstrual cycle. It has been proposed that in the early follicular phase, the impact of exogenous kisspeptin is limited due to high endogenous kisspeptin activity (Chan et al., 2012), although this is speculative. Sex steroid-deficient post-menopausal women were more responsive to kisspeptin-10 than women in the early follicular phase (George et al., 2012) (Fig. 2). Women taking combined estrogen and progestogen contraceptives showed a minimal response to kisspeptin-10 (George et al., 2012), contrasting to the larger response in the physiological luteal phase (Dhillo et al., 2007) (Fig. 2). These complex relationships suggest that other mechanisms, in addition to the absolute or relative levels of estrogen and progesterone, appear to regulate kisspeptin sensitivity across the menstrual cycle and clearly there remains much to be learnt regarding these inter-relationships. The expression of kisspeptin receptor in different sex steroid environments has not been described in primates and data in lower species remain contradictory (Navarro et al., 2004a, Yamada et al., 2007; Li et al., 2012). Changes in pituitary sensitivity to GnRH and sex steroid feedback at that level (Hall et al., 1994; Shaw et al., 2010) add to the complexity of analysis of in vivo studies.
The sexual dimorphism in the responsiveness of men and women has been elegantly illustrated using the different isoforms of kisspeptin (Jayasena et al., 2011). Men respond to modest doses of both kisspeptin-54 and kisspeptin-10. In a study of healthy women in the early follicular phase, kisspeptin-10 administered as an intravenous bolus, subcutaneous bolus or as an intravenous infusion did not result in a detectable LH response (Jayasena et al., 2011) (Fig. 2). In another study however, a low-dose intravenous bolus of kisspeptin-10 induced an LH response in normal women in the early follicular phase (George et al., 2012). These differences may have been methodological—as the Jayasena et al. (2011) protocol did not involve baseline sampling. In the study by George et al. (2012), a 10-min baseline LH sampling for 180 min was employed prior to kisspeptin administration, enabling comparison of LH secretion before and after kisspeptin-10 infusion in the same individual. Given the small sample number, small effect size and inter-individual variability in baseline LH, this lack of baseline data to enable intra-individual comparisons diminishes statistical sensitivity to identify small changes in LH. A small but significant increase in LH in response to kisspeptin-54 administered intravenously or subcutaneously in the early follicular phase was also observed (Dhillo et al., 2007; Jayasena et al., 2011), indicating that the response to the longer isoform is substantially more robust, perhaps reflecting its longer half-life. Sexual dimorphism in the kisspeptin system is also seen in rodent models: females have significantly more kisspeptin neurones than males in the AVPV nucleus (Oakley et al., 2009). This sexual variation in anatomical distribution of the kisspeptin pathway and the response to kisspeptin administration may reflect sexually dimorphic roles of kisspeptin, notably in the generation of the pre-ovulatory LH surge, which is unique to the female.
Kisspeptin mediates negative sex steroid feedback
Patterns of GnRH and subsequently LH secretion across the menstrual cycle are modulated by gonadal steroid feedback. During the follicular phase of the menstrual cycle GnRH activity and thus LH secretion is limited by estradiol (E2)-mediated negative feedback (with additional action on pituitary gonadotrophs) (Karsch, 1987; Shaw et al., 2010). The basis for the change to positive feedback with elaboration of the mid-cycle LH surge has long been unclear. GnRH neurones do not express ERs, suggesting that a separate population of neurones acts as a mediator to relay the ovulation-inducing message from gonads to the hypothalamic GnRH neurones. Recent evidence suggests that KNDy neurones appear to constitute this ‘missing link’, mediating both negative and positive sex steroid feedback.
Kisspeptin in the infundibular nucleus mediates negative feedback of estrogen in humans (Fig. 1). In post-menopausal women kisspeptin neurones in the infundibular nucleus were hypertrophied and expressed more KISS1 mRNA than in premenopausal women (Rometo et al., 2007). These hypertrophied neurones expressed both ESR1 (encoding ER alpha) and neurokinin B mRNA, had increased expression of neurokinin B and showed a similar distribution to that of kisspeptin neurones (Rance et al., 1990; Rance and Young, 1991). The suggestion that kisspeptin and neurokinin B in the infundibular nucleus act synergistically to mediate estrogen negative feedback is supported by animal data, showing an up-regulation of Kiss1 mRNA expression in ovariectomised rodents, sheep and monkeys in the arcuate nucleus (equivalent to the infundibular nucleus in humans) but not in more rostral areas, and that this was prevented by E2 replacement (Oakley et al., 2009; Lehman et al., 2010). Consistent with this, the intracerebroventricular administration of kisspeptin antagonist prevented the LH rise in castrated rodents (Roseweir et al., 2009). Similarly, ovariectomy increased and estrogen replacement reduced neurokinin B gene expression in the infundibular nucleus of monkeys (Abel et al., 1999; Sandoval-Guzman et al., 2004).
These findings together imply that estrogen mediates its negative feedback by suppressing kisspeptin and neurokinin B release from KNDy neurones, which reduces their stimulatory input to GnRH neurones (Fig. 1). The converse, i.e. inhibitory, involvement of the opioid component of this signalling system has long been recognized. The colocalization of dynorphin in at least some kisspeptin and neurokinin B-containing neurones in the human is discussed above (section: Kisspeptin neurone populations co-express other neuropeptides). Naloxone, an opioid receptor antagonist, increased serum LH levels in late follicular and luteal phases of the menstrual cycle (Quigley and Yen, 1980; Shoupe et al., 1985). This effect was not apparent in post-menopausal and oophorectomized young women, whereas replacement of estrogen or progesterone restored the ability of naloxone to release LH (Melis et al., 1984; Casper and Alapin-Rubillovitz, 1985; Shoupe et al., 1985). The endogenous opioid peptide dynorphin mediates this role physiologically, and inhibited GnRH and LH pulse frequency following progesterone administration (Ferin et al., 1984; Karsch, 1987; Goodman et al., 2004). In contrast, the opioid receptor antagonist naltrexone increased serum LH concentrations and LH pulse amplitude in women with hypothalamic amenorrhoea (Genazzani et al., 1995). The relative deficiency of dynorphin signalling as part of negative estrogen feedback in the post-menopausal and oophorectomized states would appear a likely explanation for the lack of response to naloxone in hypergonadotrophic states, in contrast to potentially increased dynorphin signalling contributing to the hypogonadotrophic state in hypothalamic amenorrhoea. It is however possible that already near maximal LH secretion in sex steroid deficient post-menopausal and oophorectomized women would account for the inability of naloxone to further stimulate gonadotrophin release although kisspeptin-10 induced LH secretion in post-menopausal women (George et al., 2012). In post-menopausal women, the expression of prodynorphin mRNA in the infundibular nucleus is reduced (Rometo and Rance, 2008). The role of dynorphin as a mediator of sex steroid negative feedback is consistent with data from the ewe, where dynorphin is coexpressed with kisspeptin and neurokinin B, both of which show a high degree of colocalization with ER alpha and progesterone receptors in the arcuate nucleus, and the expression of prodynorphin mRNA is suppressed by ovariectomy (Goubillon et al., 2000; Foradori et al., 2002, 2005; Franceschini et al., 2006; Goodman et al., 2007). This is distinct from the apparent situation in rodents where, despite colocalization of KNDy neurones with both estrogen and progesterone receptors, dynorphin does not seem to mediate estrogen negative feedback (Navarro et al., 2009).
In summary, it appears that in humans KNDy neurones mediate negative sex steroid feedback in the infundibular nucleus by suppressing the secretion of kisspeptin and neurokinin B and stimulating the secretion of dynorphin, which act synergistically to reduce the activity of the GnRH neuronal system, and thus gonadotrophin secretion.
Kisspeptin may also mediate estrogenic positive feedback
Estrogen feedback switches from negative to positive in the late follicular phase to induce the GnRH/LH surge at the time of ovulation. However, the neuroendocrine mechanisms involved in this critical physiological event have been unclear. Emerging data suggest that although the negative feedback of sex steroids is mediated by KNDy neurones in the infundibular/arcuate nucleus, the positive feedback of sex steroids is more site and species specific (Fig. 1).
Recent data support a potential role for kisspeptin in generating the ovulatory LH surge in humans. Kisspeptin-54 (subcutaneous in doses of 1.6–12.8 nmol/kg (9.4–75 µg/kg), used instead of hCG during an FSH/GnRH antagonist assisted conception ovulation induction protocol, induced an LH surge and triggered oocyte maturation, with subsequently a live term birth reported (Abbara et al., 2013). Repeated twice-daily administration of kisspeptin-54 shortened the menstrual cycle and advanced the onset of the LH peak in healthy women (Jayasena et al., 2013b). This is in keeping with data from animal models. Kisspeptin administration results in an early LH surge in sheep, and conversely administration of kisspeptin antiserum or antagonists to rats and sheep prevented or blunted the LH surge (Kinoshita et al., 2005; Caraty et al., 2007; Clarkson et al., 2008; Pineda et al., 2010).
The anatomical site of kisspeptin that relays positive sex steroid feedback is different in rodents compared with humans and other species. In rodents the AVPV nucleus is the location of estrogen positive feedback, which is not matched in humans, other primates and sheep, where kisspeptin neurones in the infundibular/arcuate exert this function (Fig. 1). The expression of Kiss1 mRNA in the AVPV nucleus is dramatically increased after estrogen replacement and at the time of the GnRH/LH surge (Smith et al., 2005, 2006b). There are no studies looking at the anatomical region of kisspeptin expression that mediates estrogen positive feedback in humans and evidence comes from other species, which, like humans, have no homologous area to the AVPV nucleus. In sheep, the expression of Kiss1 mRNA in the arcuate nucleus is markedly enhanced during the pre-ovulatory LH surge (Smith et al., 2008b). In rodents the AVPV nucleus receives afferent fibres from the suprachiasmatic nucleus, the location of circadian clock, which coordinates and provides precise timing for the LH surge and the kisspeptin system in now being integrated into our understanding of the neurobiology of this system across species, including the human (Christian and Moenter, 2010; Khan and Kauffman, 2012).
KNDy neurones may have a role in positive estrogen feedback. In sheep, the neurokinin B receptor agonist senktide increased LH secretion close to levels seen during the pre-ovulatory LH surge (Billings et al., 2010). KNDy neurones do not mediate positive estrogen feedback in rodents based on their location in the arcuate nucleus only (Burke et al., 2006; Goodman et al., 2007; Navarro et al., 2009). Other neurotransmitters may also contribute to the kisspeptin-mediated LH surge, as kisspeptin populations in the preoptic area/RP3V and the infundibular/arcuate nucleus co-localize with different peptides (see above section: Kisspeptin neurone populations co-express different neuropeptides).
Kisspeptin stimulates gonadotrophin release in disease models
In addition to being a potent stimulator of LH secretion in healthy men and women, the ability of kisspeptin to induce LH release in human disease models characterized by low gonadotrophin secretion has been investigated (Fig. 3).
Hypothalamic amenorrhoea
Hypothalamic amenorrhoea is characterized by slow GnRH pulsatile secretion, resulting in a preferential decline in LH compared with FSH secretion and low ovarian follicular activity. As, by definition, this is a functional rather than pathological condition, it might be readily corrected by administration of kisspeptin to increase GnRH secretion. Hypothalamic amenorrhoea was the first disease model used to explore the therapeutic potential of kisspeptin-54, which when administrated as subcutaneous bolus at 6.4 nmol/kg (37 µg/kg) resulted in a 10-fold increase in LH and 2.5-fold increase in FSH secretion, both to normal physiological levels (Jayasena et al., 2009) (Fig. 3). However, this increase in gonadotrophins did not translate into a significant elevation in E2 secretion, suggesting that folliculogenesis was not restored, confirmed by ovarian quiescence on ultrasound scan (Jayasena et al., 2009). The lack of ovarian activity may relate to the limited effect on FSH secretion and the short timescale of study. Despite the initial stimulation of LH and FSH secretion, when kisspeptin-54 was injected twice daily for 2 weeks, these increases were not sustained with LH falling to pretreatment levels, suggesting tachyphylaxis (Jayasena et al., 2009, 2010) (see section: Continuous exposure to kisspeptin can cause desensitization). However, sustained secretion of gonadotrophins at physiological levels was achieved with intermittent subcutaneous injection of kisspeptin-54 twice weekly (6.4 nmol/kg (37 µg/kg)) for 8 weeks, although it did not result in increased E2 secretion or follicular development (Jayasena et al., 2010). It has subsequently been shown that an infusion of kisspeptin-54 (0.01 nmol/kg/h (0.059 µg/kg/h) to 1 nmol/kg/h (5.9 µg/kg/h)) for 8 h in women with hypothalamic amenorrhoea can induce LH pulsatility with a 3-fold increase in LH pulse frequency and mass per pulse (Jayasena et al., 2013a). The ability of the increased gonadotrophin secretion, and perhaps the relative effects of LH and FSH, to bring about ovarian activity and menstrual cycles will determine the therapeutic application of kisspeptin in this condition.
In all the studies above, regardless of the dose and route of administration, the LH response to kisspeptin is ∼5-fold greater in hypothalamic amenorrhoea than in healthy women in the early follicular phase. This is consistent with data from a rodent model of undernutrition showing up-regulated hypothalamic Kiss1r mRNA expression (Castellano et al., 2005). Altered GnRH sensitivity is unlikely as the effect of GnRH on LH secretion is similar in hypothalamic amenorrhoea and healthy women in the early follicular phase (Jayasena et al., 2009; George et al., 2012).
Hypogonadism in men with type 2 diabetes
Men with type 2 diabetes often have low testosterone concentrations, and inappropriately low LH indicating a hypothalamic/pituitary basis (George et al., 2010). As with hypothalamic amenorrhoea, increasing LH secretion by administration of kisspeptin might therefore have therapeutic potential. This has been explored in a small number of such men, investigating the response to both bolus administration and infusion of kisspeptin-10 (George et al., 2013) (Fig. 3). Kisspeptin-10 intravenous bolus administration (0.3 µg/kg (0.23 nmol/kg)) increased LH secretion 2-fold in diabetic hypogonadal men, i.e. of the same magnitude as in healthy men with peak mean LH 10.7 IU/l and 14.5 IU/l, respectively (George et al., 2013). An infusion of kisspeptin-10 for 11 h at a higher dose (4 µg/kg/h (3.1 nmol/kg/h)) produced a more profound 5-fold increase in LH release (George et al., 2013), also comparable to the response in healthy men (George et al., 2011). Kisspeptin-10 also stimulated LH pulse frequency in diabetic men with hypogonadotropic hypogonadism, which was sustained for the duration of the infusion (11 h) with no evidence of a decline in LH (i.e. no desensitization) over that timescale (George et al., 2013). Importantly, serum testosterone was also elevated into the normal physiological range (George et al., 2013). The ability of kisspeptin to robustly increase LH pulsatility with an associated increase in testosterone is very encouraging, but the potential of kisspeptin to maintain gonadotrophin and sex steroid release for longer periods of time relevant to therapeutic use has yet to be determined.
Neurokinin B signalling deficiencies
Patients with loss-of-function mutation in neurokinin B (TAC3) and its receptor (TAC3R) show hypogonadotropic hypogonadism characterized by failure to progress through puberty (Topaloglu et al., 2009). It is postulated that inability of neurokinin B in an autocrine and/or paracrine manner to stimulate kisspeptin secretion results in low GnRH pulse frequency with correspondingly low LH and gonadal steroid levels but normal or near-normal levels of FSH typically seen in these patients. Neurokinin B, being potentially upstream of kisspeptin in neuroendocrine signalling, makes kisspeptin an attractive therapeutic agent to restore GnRH secretion in patients with defects in the neurokinin B system. Indeed, continuous infusion of kisspeptin-10 (1.5 µg/kg/h (1.1 nmol/kg/h) for 12 h) in two patients with TAC3 and two patients with TAC3R mutation stimulated the LH response 2.5-fold (Young et al., 2013) (Fig. 3). Overall, the LH response to kisspeptin was more limited than that achieved in healthy men using the same protocol (George et al., 2011) with lower LH mass per pulse, although pulse frequency was normalized, consistent with complex neuropeptide interactions associated with KNDy neurone function rather than a linear hierarchy, as described above. Nevertheless, a significant increase in testosterone levels in male patients and in E2 levels in the single female patient was achieved (Young et al., 2013).
Continuous exposure to kisspeptin can cause desensitization
Continuing administration of GnRH desensitizes the HPG axis after an initial stimulation, by down-regulation of GnRH receptors and desensitization of gonadotrophes (Belchetz et al., 1978; McArdle et al., 1987; Mason et al., 1994). There is evidence for pulsatile (i.e. non-continuous) secretion of kisspeptin within the hypothalamic median eminence of the monkey (Keen et al., 2008). Continuous administration of kisspeptin-10 (intravenous 200 µg/h (154 nmol/kg) or 400 µg/h (307 nmol/kg) for 98 h) to rhesus monkeys resulted in suppressed LH secretion, indicative of kisspeptin receptor desensitization (Ramaswamy et al., 2007). The kisspeptin receptor has also been shown to desensitize in vitro (Pampillo et al., 2009). Consistent with this, repeated subcutaneous administration of kisspeptin-54 (6.4 nmol/kg (37 µg/kg) twice daily) for 2 weeks in women with hypothalamic amenorrhoea resulted in an initial stimulation of LH and FSH which was not maintained (Jayasena et al., 2009) (Fig. 3). However other studies in humans using infusions or repeated administration of kisspeptin have not provided consistent evidence for desensitization (Figs 2 and 3). More recently, infusion of a lower dose of kisspeptin-54 for 8 h (from 0.01 nmol/kg/h (0.059 µg/kg/h) to 1 nmol/kg/h (5.9 µg/kg/h)) in women with hypothalamic amenorrhoea not only caused sustained LH secretion but also restored LH pulsatility (Jayasena et al., 2013a) (Fig. 3). Continuous exposure to kisspeptin-54 administered twice daily for 1 week advanced the menstrual cycle in healthy women (Jayasena et al., 2013b). Similarly, continuous kisspeptin-10 infusion at 4 µg/kg/h (3.1 nmol/kg/h) for 22.5 h in healthy men showed continuing stimulation of LH secretion, with no evidence of desensitization (George et al., 2011) (Fig. 2). In contrast, LH secretion was not sustained in three healthy men during infusion of kisspeptin 10 for 24 h at 12 µg/kg/h (9.2 nmol/kg/h), the highest dose used in humans to date (Lippincott et al., 2013). However, LH secretion remained well above baseline at the end of infusion, in contrast to the marked desensitization observed with a high dose of kisspeptin-54 in women with hypothalamic amenorrhoea (Jayasena et al., 2009) (Fig. 2). It would be interesting to determine if LH secretion remains above baseline or if LH decreases to castrate levels with kisspeptin infusion for longer than 24 h. These data therefore suggest that while high doses of both kisspeptin-54 and kisspeptin-10 may induce desensitization, this does not occur at lower doses. In a dose-finding study involving bolus injection of kisspeptin-10, the highest dose (3 µg/kg (2.3 nmol/kg)) resulted in a sub-maximal LH response, consistent with desensitization even with bolus administration of kisspeptin-10 (George et al., 2011) (Fig. 2). An alternative explanation for this observation is that at that high dose kisspeptin might have stimulated another RF-amine receptor, such as gonadotrophin inhibitory hormone receptor, known to have an inhibitory effect on GnRH and LH (George et al., 2011).
Consistent with these findings, intermittent administration of kisspeptin results in sustained GnRH and LH pulsatility. Intermittent administration of kisspeptin-10 in juvenile male monkeys (intravenous hourly for 2 days) and juvenile female rats (intracerebroventricular twice daily for 5 days) caused precocious puberty (Navarro et al., 2004b, Plant et al., 2006). Kisspeptin-54 (6.4 nmol/kg (37 µg/kg/h)) injected twice weekly sustained the secretion of gonadotrophins for an 8-week period after a brief initial suppression (Jayasena et al., 2010) (Fig. 3).
The ability of natural forms of kisspeptin to induce desensitization in humans thus remains unclear, with the discrepancies between studies possibly reflecting the duration of kisspeptin injection (8–22.5 h versus 2 weeks), lower doses of kisspeptin infused in the human studies compared with primate studies, variation in the isoforms of kisspeptin used, the mode of kisspeptin administration, differences between the human and animal models and sex and even health status (healthy men versus women with hypothalamic amenorrhoea). Kisspeptin receptor agonist analogues have also been developed, and two of these, TAK-448 and TAK-683, are potent inducers of desensitization with potential use to suppress gonadotrophin secretion and thus gonadal function, similar to the GnRH analogues widely used today. Phase I clinical studies in healthy men show that subcutaneous infusion of TAK-448 (0.01–1 mg/day) and TAK-683 (0.01–2 mg/day) for 2 weeks rapidly suppress testosterone below castration levels (MacLean et al., 2013; Scott et al., 2013).
Kisspeptin and puberty
The demonstration of the obligate role of kisspeptin-GPR54 signalling in human puberty was the finding that firmly established kisspeptin as a crucial regulator of reproductive function. In 2003, two independent groups identified ‘inactivating’ point mutations and deletions in KISS1R that were associated with impaired pubertal development in some patients with hypogonadotropic hypogonadism (de Roux et al., 2003; Seminara et al., 2003). Mutations in KISS1R were demonstrated in both consanguineous families and in unrelated patients. In addition, Kiss1r- and Kiss1-deficient mice displayed a virtually identical phenotype (Funes et al., 2003; Seminara et al., 2003; d'Anglemont de Tassigny et al., 2007).
Conversely an ‘activating’ mutation (Arg386Pro) in the kisspeptin receptor gene was identified in a girl with precocious puberty, although inheritance could not be determined as the biological family was not available for genetic testing (Teles et al., 2008). Cells transfected with the mutant kisspeptin receptor showed prolonged accumulation of inositol phosphate and phosphorylation of extracellular signal-regulated kinase, indicating extended intracellular signalling (Teles et al., 2008). Missense mutations have also been described in the KISS1 gene in three unrelated children with central precocious puberty (Silveira et al., 2010). This mutant kisspeptin is more resistant to in vitro degradation, suggesting greater bioavailability as the cause of precocious puberty (Silveira et al., 2010).
Hypothalamic expression of Kiss1 and Kiss1r mRNA is dramatically up-regulated at puberty in rodents and primates (Navarro et al., 2004a, Han et al., 2005; Shahab et al., 2005) and the percentage of GnRH neurones depolarizing in response to kisspeptin increases from juvenile (25%) to prepubertal (50%) to adult mice (>90%), suggesting that GnRH neurones acquire sensitivity to kisspeptin across puberty (Han et al., 2005). Kisspeptin-54 secretion and specifically kisspeptin-54 pulse frequency increase at the onset of puberty in monkeys (Keen et al., 2008). In addition to these physiological changes linking kisspeptin signalling to the timing of puberty, the administration of exogenous kisspeptin resulted in earlier puberty in rats and monkeys (Navarro et al., 2004b, Plant et al., 2006). Conversely, administration of a kisspeptin antagonist inhibited pulsatile GnRH release in pubertal monkeys and delayed puberty in rats (Roseweir et al., 2009; Pineda et al., 2010). The findings strongly support a requirement for KISS1/GPR54 signalling to initiate and progress through puberty in a range of species.
Kisspeptin and metabolism
Human reproductive function is influenced by both extremes of nutrition—undernutrition and obesity. Kisspeptin may provide a link between nutritional/metabolic status and reproduction by sensing energy stores and translating this information into the pulsatile secretion of GnRH. The expression of Kiss1 mRNA and gonadotrophin secretion is reduced in mice, pubertal rats and monkeys subject to fasting (Castellano et al., 2005; Cota et al., 2006; Roa et al., 2009; Wahab et al., 2011). Kisspeptin is able to restore delayed vaginal opening and increases low gonadotrophin and estrogen levels associated with chronic undernutrition in pre-pubertal rats (Navarro et al., 2004b, Castellano et al., 2005).
Humans with mutations in leptin or leptin receptor show hypogonadism (Farooqi and O'Rahilly, 2009). The leptin receptor (Ob-Rb) is not present on GnRH neurones, but 40% of kisspeptin neurones in the mouse arcuate nucleus express the leptin receptor (Smith et al., 2006a), suggesting a role for kisspeptin in mediating the metabolic signals of leptin on the HPG axis. Leptin-deficient mice show decreased expression of Kiss1 mRNA, which is partially up-regulated by leptin (Smith et al., 2006a). Incomplete restoration of Kiss1 mRNA expression suggests that other mediators are involved in inhibiting kisspeptin signalling in leptin deficiency. Furthermore, mice with selective deletion of leptin receptor from kisspeptin neurones display normal pubertal development, sexual maturation and fertility, demonstrating that leptin action on kisspeptin neurones is not obligatory for these processes (Donato et al., 2011).
Low levels of testosterone have also been observed in men with obesity and type 2 diabetes, where decreased secretion of GnRH is thought to be the causative factor (Dandona et al., 2008). A rat model of diabetes (streptozocin treated) has reduced levels of hypothalamic Kiss1 mRNA with subsequently low levels of circulating gonadotrophins and sex steroids, which are corrected by kisspeptin (Castellano et al., 2006, 2009). This raises the possibility that diminished kisspeptin secretion is a potential mechanism for hypogonadotropic hypogonadism in patients with obesity and diabetes (George et al., 2010). Indeed, as described above, kisspeptin-10 increased LH pulse frequency and LH secretion in hypogonadal men with type 2 diabetes (George et al., 2013). The likely pathways for down-regulation of kisspeptin signalling include negative feedback by estrogen, which is markedly elevated in obesity (Schneider et al., 1979), resistance to leptin, also seen in human obesity (Finn et al., 1998), insulin resistance and hyperglycaemia (Castellano et al., 2006, 2009), and inflammation, which is up-regulated in hypogonadal men with diabetes (Dandona et al., 2008) and is associated with decreased kisspeptin expression in rats (Iwasa et al., 2008).
Current data indicate that kisspeptin acts downstream to metabolic signals and conveys information about energy stores to GnRH neurones, thereby regulating reproduction. This gives promise for a potential novel therapeutic role of kisspeptin to restore the reproductive axis in conditions of negative energy balance, such as anorexia nervosa, and in diabetes.
Clinical applications of KNDy manipulation
GnRH analogues are extensively used in clinical practice in the treatment of hormone-dependent diseases and infertility. Current therapies manipulate the HPG axis at the level of GnRH receptors on pituitary gonadotrophs, largely to suppress gonadal function, e.g. in the treatment of prostate and breast cancer, endometriosis and uterine fibroids. As reproductive endocrine conditions can be broadly categorized into those with pathologically diminished (delayed puberty, hypothalamic amenorrhoea, hypogonadism in diabetes) and pathologically enhanced (polycystic ovary syndrome (PCOS), menopause, precocious puberty) GnRH and associated gonadotrophin pulsatility, the newly discovered hypothalamic peptides kisspeptin and neurokinin B offer a novel therapeutic approach with potential advantages over the existing therapies in several clinical contexts (Fig. 4).
Figure 4.
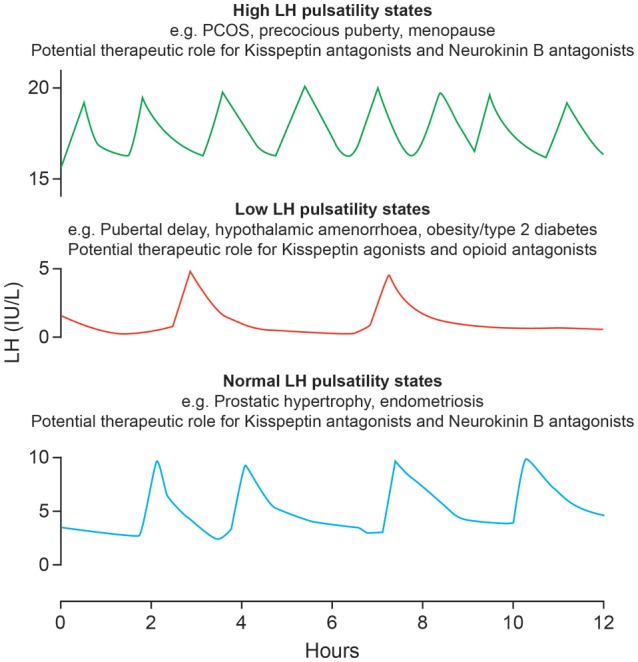
Potential clinical applications of novel kisspeptin-based modulation of LH secretion. Schematic presentation of LH pulses in health and in reproductive endocrine disorders. In health, an LH pulse occurs about every 90 min. The frequency of LH pulses is diminished in patients with hypothalamic amenorrhoea, male hypogonadism and pubertal delay, whereas LH pulse frequency is enhanced in women with polycystic ovary syndrome, menopause and precocious puberty. Therapeutic opportunities to correct abnormal LH pulse frequency by manipulating KNDy neurones with relevant agonists and antagonists are emerging. PCOS, polycystic ovary syndrome.
Manipulation of KNDy neurones to stimulate HPG axis
Enhancing the stimulatory tone of kisspeptin and neurokinin B by appropriate agonists and suppressing the inhibitory tone of dynorphin by its antagonists, may have therapeutic potential for diseases with decreased gonadotrophin secretion. Exogenous kisspeptin enhances diminished LH pulsatility in hypogonadal men with diabetes and stimulates LH secretion in women with hypothalamic amenorrhoea (Jayasena et al., 2010; George et al., 2013). Kisspeptin initiates puberty in monkeys and rodents, but this has not been tested in children with delayed puberty (Navarro et al., 2004b, Plant et al., 2006). Pulsatile gonadotrophin secretion is restored by kisspeptin administered to patients with hypogonadotropic hypogonadism secondary to mutations in neurokinin B and/or its receptor (Young et al., 2013). The role of dynorphin antagonists, such as naloxone, in patients with abnormally low LH secretion remains to be elucidated.
Kisspeptin therapy has the potential to ‘fine tune’ IVF techniques. Kisspeptin triggered the LH surge during following ovulation induction for assisted reproduction (Abbara et al., 2013) with successful achievement of a live birth. Kisspeptin might stimulate a more physiological pattern of gonadotrophin secretion, avoiding the risk of ovarian hyperstimulation syndrome associated with currently used hCG injections although clearly much remains to be discovered regarding potential advantages and disadvantages over current approaches.
Manipulation of KNDy neurones to inhibit HPG axis
Suppressing the stimulatory role of kisspeptin and neurokinin B by specific receptor antagonists and enhancing the inhibitory action of dynorphin by its receptor agonist is desirable in scenarios of increased GnRH pulsatility where a reduction rather than complete suppression of GnRH is required. Increased frequency of GnRH and therefore LH pulsatile secretion (with little effect on FSH secretion) is central to the pathophysiology of PCOS, the most common endocrinopathy in women. As GnRH pulse frequency primarily determines LH but not FSH secretion (McNeilly et al., 2003), slowing GnRH might normalize the relative LH hypersecretion often seen in PCOS. Normalization of LH secretion (and perhaps the consequent hyperandrogenism) in PCOS may promote folliculogenesis and ovulation. Studies using a neurokinin B antagonist are currently underway to reduce high LH secretion in PCOS.
The ability of kisspeptin antagonists to limit follicular development and inhibit ovulation offers potential for a novel female contraceptive, perhaps being specifically advantageous in the scenarios where exogenous estrogen is contraindicated. However it might be limited by the resulting lack of progesterone exposure and adverse effects on the endometrium. Given the preferential stimulation of LH secretion in response to kisspeptin in humans (Dhillo et al., 2005, 2007; George et al., 2011, 2012), kisspeptin antagonists might potentially result in relative sparing of FSH compared with LH, which might reduce or prevent the unwanted side effects of estrogen deficiency, such as vasomotor symptoms and risk of osteoporosis, associated with GnRH analogue administration.
A similar therapeutic approach might support the use of kisspeptin and neurokinin B suppressive therapies in the treatment of precocious puberty. Similarly, it may alleviate menopausal hot flushes since KNDy neurones project to preoptic thermoregulatory areas that express neurokinin B receptor in rats and KNDy neurone ablation reduces cutaneous vasodilation (Burke et al., 2006; Rance, 2009; Hrabovszky et al., 2010; Krajewski et al., 2010; Mittelman-Smith et al., 2012; Rance et al., 2013). Although the inhibitory role of opioids on GnRH and LH pulsatility is well known, manipulation of this system does not have the apparent specificity of the kisspeptin or neurokinin B pathways.
The potential more subtle effects of kisspeptin antagonists reducing LH pulsatility contrast with the profound suppression resulting from GnRH analogue administration, decreasing gonadotrophin and sex steroid secretion to castration levels with consequent side effects, including hot flushes, loss of libido and decreased bone mineral density (Roseweir et al., 2009). Complete suppression of gonadotrophins and sex steroids is necessary is some conditions, such as prostate cancer, but partial suppression is more appropriate in benign prostatic hyperplasia, endometriosis and uterine fibroids. Clinical effectiveness in the management of endometriosis and uterine fibroids with GnRH suppression with add back, and with selective progesterone receptor modulators (Chabbert-Buffet et al., 2005), suggests that approaches not based on complete suppression of the HPG axis have clear clinical value. Targeted partial gonadotrophin suppression, such as that afforded via kisspeptin and/or neurokinin B inhibition, has the potential to overcome the existing drawbacks of GnRH analogues although the emerging data on kisspeptin analogues (MacLean et al., 2013; Scott et al., 2013) demonstrate the potential for profound suppression as well.
Conclusions
The discovery of kisspeptin has transformed our understanding of the neuroendocrine signals controlling the reproductive axis. Kisspeptin coordinates GnRH secretion, mediates gonadal steroid negative and positive feedback, controls the onset of puberty, and relays information regarding the body's energy stores. The last decade has thus seen a huge resurgence in interest in neuroendocrinology, and the potential for translational application is already being explored in human studies. However, much remains to be learnt before kisspeptin can replace or be used in conjunction with GnRH and gonadotrophin analogues, the current mainstay of infertility and reproductive endocrine disorder treatments.
The mode of kisspeptin administration, as with most peptides, remains a challenge and there is thus the need for novel approaches and the development of non-peptide analogues, which is already well underway. These will also allow refinement of experimental approaches to explore physiological pathways (such as elaboration of the importance of the sex steroid environment) as well as novel treatment strategies across a wide range of conditions requiring manipulation of gonadal function. Co-administration of kisspeptin, opioid and neurokinin B modifying agents will allow fine modulation of the HPG axis that may open new therapeutic avenues.
Authors' roles
K.S., J.T.G. and R.A.A. contributed equally to determining the scope of the review. K.S. and J.T.G. undertook the literature review. K.S. drafted the manuscript, which was edited by J.T.G. and R.A.A. All authors have approved the final manuscript for submission.
Funding
The authors' studies in this field are supported by the Medical Research Council (G0701682), the Novo Nordisk UK Research Foundation and Sanofi Excellence for Diabetes Research Awards. K.S.'s current position as a clinical research fellow is funded by the Wellcome Trust through the Scottish Translational Medicine and Therapeutics Initiative (STMTI). J.T.G.'s current position is part-funded by the University of Oxford Diabetes Trials Unit and the NIHR through the Oxford Biomedical Research Centre. Funding to pay the Open Access publication charges for this article was provided by the Wellcome Trust through the Scottish Translational Medicine and Therapeutics Initiative.
Conflict of interest
J.T.G. serves as the International Co-ordinating Investigator for an AstraZeneca sponsored clinical trial in PCOS, as a consultant for AstraZeneca and Takeda Pharmaceuticals; and has received educational grants, speaker fees or advisory board fees from most leading pharmaceutical companies active in the field of diabetes. R.A.A. has undertaken consultancy work for AstraZeneca and Takeda Pharmaceuticals.
Acknowledgements
The authors thank men and women who volunteered to take part in the studies and the staff at the Royal Infirmary of Edinburgh Clinical Research Facility and the Wellcome Trust Clinical Research Facility at the Western General Hospital, Edinburgh. We are pleased to recognise the contribution of Prof RP Millar in developing some of the concepts discussed, and are grateful to Ronnie Grant for his expert assistance with the graphics.
References
- Abbara A, Jayasena CN, Nijher GK, Comninos AN, Christopoulos G, Ashby D, Ghatei MA, Bloom SR, Carby A, Trew G, et al. Kisspeptin—a novel physiological trigger for oocyte maturation in IVF treatment. Hum Reprod. 2013 Supplement 1; European Society of Human Reproduction and Embryology 29th Annual Meeting, London, Abstract O-107. [Google Scholar]
- Abel TW, Voytko ML, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J Clin Endocrinol Metab. 1999;6:2111–2118. doi: 10.1210/jcem.84.6.5689. [DOI] [PubMed] [Google Scholar]
- Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;4368:631–633. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, McManus CJ, Nestor CC, Jacobs BH, Goodman RL. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology. 2010;8:3836–3846. doi: 10.1210/en.2010-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;5:712–726. doi: 10.1002/cne.21086. [DOI] [PubMed] [Google Scholar]
- Caraty A, Smith JT, Lomet D, Ben Said S, Morrissey A, Cognie J, Doughton B, Baril G, Briant C, Clarke IJ. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology. 2007;11:5258–5267. doi: 10.1210/en.2007-0554. [DOI] [PubMed] [Google Scholar]
- Casper RF, Alapin-Rubillovitz S. Progestins increase endogenous opioid peptide activity in postmenopausal women. J Clin Endocrinol Metab. 1985;1:34–36. doi: 10.1210/jcem-60-1-34. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;9:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernandez-Fernandez R, Roa J, Vigo E, Pineda R, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes. 2006;9:2602–2610. doi: 10.2337/db05-1584. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Roa J, Pineda R, Sanchez-Garrido MA, Garcia-Galiano D, Vigo E, Dieguez C, Aguilar E, Pinilla L, et al. Alterations in hypothalamic KiSS-1 system in experimental diabetes: early changes and functional consequences. Endocrinology. 2009;2:784–794. doi: 10.1210/en.2008-0849. [DOI] [PubMed] [Google Scholar]
- Chabbert-Buffet N, Meduri G, Bouchard P, Spitz IM. Selective progesterone receptor modulators and progesterone antagonists: mechanisms of action and clinical applications. Hum Reprod Update. 2005;3:293–307. doi: 10.1093/humupd/dmi002. [DOI] [PubMed] [Google Scholar]
- Chan YM, Butler JP, Pinnell NE, Pralong FP, Crowley WF, Jr, Ren C, Chan KK, Seminara SB. Kisspeptin resets the hypothalamic GnRH clock in men. J Clin Endocrinol Metab. 2011;6:E908–E915. doi: 10.1210/jc.2010-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YM, Butler JP, Sidhoum VF, Pinnell NE, Seminara SB. Kisspeptin administration to women: a window into endogenous kisspeptin secretion and GnRH responsiveness across the menstrual cycle. J Clin Endocrinol Metab. 2012;8:E1458–E1467. doi: 10.1210/jc.2012-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;1:301–311. doi: 10.1210/en.2009-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev. 2010;4:544–577. doi: 10.1210/er.2009-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofi P, Krause JE, Prins GS, Mazzuca M. Presence of nuclear androgen receptor-like immunoreactivity in neurokinin B-containing neurons of the hypothalamic arcuate nucleus of the adult male rat. Neurosci Lett. 1994;2:193–196. doi: 10.1016/0304-3940(94)90795-1. [DOI] [PubMed] [Google Scholar]
- Ciofi P, Leroy D, Tramu G. Sexual dimorphism in the organization of the rat hypothalamic infundibular area. Neuroscience. 2006;4:1731–1745. doi: 10.1016/j.neuroscience.2006.05.041. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT. GnRH pulse frequency determines LH pulse amplitude by altering the amount of releasable LH in the pituitary glands of ewes. J Reprod Fertil. 1985;2:425–431. doi: 10.1530/jrf.0.0730425. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;12:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;35:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, d'Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol. 2009;8:673–682. doi: 10.1111/j.1365-2826.2009.01892.x. [DOI] [PubMed] [Google Scholar]
- Clifton D, Steiner RA. Neuroendocrinology of Reproduction. In: Strauss J, Barbieri R, editors. Yen & Jaffe's Reproductive Endocrinology. Philadelphia: Elsevier; 2009. pp. 3–33. [Google Scholar]
- Constantin S, Caligioni CS, Stojilkovic S, Wray S. Kisspeptin-10 facilitates a plasma membrane-driven calcium oscillator in gonadotropin-releasing hormone-1 neurons. Endocrinology. 2009;3:1400–1412. doi: 10.1210/en.2008-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;5775:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;25:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl SK, Amstalden M, Coolen L, Fitzgerald M, Lehman M. Dynorphin immunoreactive fibers contact GnRH neurons in the human hypothalamus. Reprod Sci. 2009;8:781–787. doi: 10.1177/1933719109336619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandona P, Dhindsa S, Chaudhuri A, Bhatia V, Topiwala S, Mohanty P. Hypogonadotrophic hypogonadism in type 2 diabetes, obesity and the metabolic syndrome. Curr Mol Med. 2008;8:816–828. doi: 10.2174/156652408786733658. [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;19:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;12:6609–6615. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- Dhillo WS, Chaudhri OB, Thompson EL, Murphy KG, Patterson M, Ramachandran R, Nijher GK, Amber V, Kokkinos A, Donaldson M, et al. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab. 2007;10:3958–3966. doi: 10.1210/jc.2007-1116. [DOI] [PubMed] [Google Scholar]
- Donato J, Jr, Cravo RM, Frazao R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, et al. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011;1:355–368. doi: 10.1172/JCI45106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi IS, O'Rahilly S. Leptin: a pivotal regulator of human energy homeostasis. Am J Clin Nutr. 2009;3:980S–984S. doi: 10.3945/ajcn.2008.26788C. [DOI] [PubMed] [Google Scholar]
- Ferin M, Van Vugt D, Wardlaw S. The hypothalamic control of the menstrual cycle and the role of endogenous opioid peptides. Recent Prog Horm Res. 1984;40:441–485. doi: 10.1016/b978-0-12-571140-1.50015-3. [DOI] [PubMed] [Google Scholar]
- Finn PD, Cunningham MJ, Pau KY, Spies HG, Clifton DK, Steiner RA. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology. 1998;11:4652–4662. doi: 10.1210/endo.139.11.6297. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology. 2002;11:4366–4374. doi: 10.1210/en.2002-220586. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic Acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;4:1835–1842. doi: 10.1210/en.2004-1326. [DOI] [PubMed] [Google Scholar]
- Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006;3:225–230. doi: 10.1016/j.neulet.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;4:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- Genazzani AD, Gastaldi M, Petraglia F, Battaglia C, Surico N, Volpe A, Genazzani AR. Naltrexone administration modulates the neuroendocrine control of luteinizing hormone secretion in hypothalamic amenorrhoea. Hum Reprod. 1995;11:2868–2871. doi: 10.1093/oxfordjournals.humrep.a135809. [DOI] [PubMed] [Google Scholar]
- George JT, Seminara SB. Kisspeptin and the hypothalamic control of reproduction: lessons from the human. Endocrinology. 2012;11:5130–5136. doi: 10.1210/en.2012-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JT, Millar RP, Anderson RA. Hypothesis: kisspeptin mediates male hypogonadism in obesity and type 2 diabetes. Neuroendocrinology. 2010;4:302–307. doi: 10.1159/000299767. [DOI] [PubMed] [Google Scholar]
- George JT, Veldhuis JD, Roseweir AK, Newton CL, Faccenda E, Millar RP, Anderson RA. Kisspeptin-10 is a potent stimulator of LH and increases pulse frequency in men. J Clin Endocrinol Metab. 2011;8:E1228–E1236. doi: 10.1210/jc.2011-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JT, Anderson RA, Millar RP. Kisspeptin-10 stimulation of gonadotrophin secretion in women is modulated by sex steroid feedback. Hum Reprod. 2012;12:3552–3559. doi: 10.1093/humrep/des326. [DOI] [PubMed] [Google Scholar]
- George JT, Veldhuis JD, Tena-Sempere M, Millar RP, Anderson RA. Exploring the pathophysiology of hypogonadism in men with type 2 diabetes: kisspeptin-10 stimulates serum testosterone and LH secretion in men with type 2 diabetes and mild biochemical hypogonadism. Clin Endocrinol (Oxf) 2013;1:100–104. doi: 10.1111/cen.12103. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;6:2959–2967. doi: 10.1210/en.2003-1305. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;12:5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;9:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Clifton DK, Steiner RA. From KISS1 to kisspeptins: an historical perspective and suggested nomenclature. Peptides. 2009;1:4–9. doi: 10.1016/j.peptides.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology. 2000;11:4218–4225. doi: 10.1210/endo.141.11.7743. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Pascual E, Martinez-Fuentes AJ, Pinilla L, Tena-Sempere M, Malagon MM, Castano JP. Direct pituitary effects of kisspeptin: activation of gonadotrophs and somatotrophs and stimulation of luteinising hormone and growth hormone secretion. J Neuroendocrinol. 2007;7:521–530. doi: 10.1111/j.1365-2826.2007.01558.x. [DOI] [PubMed] [Google Scholar]
- Hall JE, Taylor AE, Martin KA, Rivier J, Schoenfeld DA, Crowley WF., Jr Decreased release of gonadotropin-releasing hormone during the preovulatory midcycle luteinizing hormone surge in normal women. Proc Natl Acad Sci USA. 1994;15:6894–6898. doi: 10.1073/pnas.91.15.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;49:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V) Brain Res Rev. 2008;2:277–287. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, Theodosis DT. Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience. 1992;2:283–298. doi: 10.1016/0306-4522(92)90423-y. [DOI] [PubMed] [Google Scholar]
- Herbison AE, de Tassigny X, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology. 2010;1:312–321. doi: 10.1210/en.2009-0552. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, et al. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci. 2010;11:1984–1998. doi: 10.1111/j.1460-9568.2010.07239.x. [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;4:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Iwasa T, Matsuzaki T, Murakami M, Shimizu F, Kuwahara A, Yasui T, Irahara M. Decreased expression of kisspeptin mediates acute immune/inflammatory stress-induced suppression of gonadotropin secretion in female rat. J Endocrinol Invest. 2008;7:656–659. doi: 10.1007/BF03345620. [DOI] [PubMed] [Google Scholar]
- Jayasena CN, Nijher GM, Chaudhri OB, Murphy KG, Ranger A, Lim A, Patel D, Mehta A, Todd C, Ramachandran R, et al. Subcutaneous injection of kisspeptin-54 acutely stimulates gonadotropin secretion in women with hypothalamic amenorrhea, but chronic administration causes tachyphylaxis. J Clin Endocrinol Metab. 2009;11:4315–4323. doi: 10.1210/jc.2009-0406. [DOI] [PubMed] [Google Scholar]
- Jayasena CN, Nijher GM, Abbara A, Murphy KG, Lim A, Patel D, Mehta A, Todd C, Donaldson M, Trew GH, et al. Twice-weekly administration of kisspeptin-54 for 8 weeks stimulates release of reproductive hormones in women with hypothalamic amenorrhea. Clin Pharmacol Ther. 2010;6:840–847. doi: 10.1038/clpt.2010.204. [DOI] [PubMed] [Google Scholar]
- Jayasena CN, Nijher GM, Comninos AN, Abbara A, Januszewki A, Vaal ML, Sriskandarajah L, Murphy KG, Farzad Z, Ghatei MA, et al. The effects of kisspeptin-10 on reproductive hormone release show sexual dimorphism in humans. J Clin Endocrinol Metab. 2011;12:E1963–E1972. doi: 10.1210/jc.2011-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasena CN, Abbara A, Comninos AN, Ratnasabapathy R, Veldhuis JD, Nijher GK, Ganiyu-Dada Z, Mehta A, Todd C, Ghatei MA, et al. Kissppeptin-54 administration stimulates pulsatile luteinising hormone secretion in women with hypothalamic amenorrhea. Hum Reprod. 2013a Supplement 1: European Society of Human Reproduction and Embryology 29th Annual Meeting, London, Abstract O-268. [Google Scholar]
- Jayasena CN, Comninos A, Nijher G, Abbara A, De Silva A, Veldhuis J, Ratnasabapathy R, Izzi-Engbeaya C, Lim A, Patel D, et al. Twice-daily subcutaneous injection of kisspeptin-54 does not abolish menstrual cyclicity in healthy female volunteers. J Clin Endocrinol Metab. 2013b;11:4464–4474. doi: 10.1210/jc.2013-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasena CN, Comninos AN, Veldhuis JD, Misra S, Abbara A, Izzi-Engbeaya C, Donaldson M, Ghatei MA, Bloom SR, Dhillo WS. A single injection of kisspeptin-54 temporarily increases luteinizing hormone pulsatility in healthy women. Clin Endocrinol (Oxf) 2013c;4:558–563. doi: 10.1111/cen.12179. [DOI] [PubMed] [Google Scholar]
- Karsch FJ. Central actions of ovarian steroids in the feedback regulation of pulsatile secretion of luteinizing hormone. Annu Rev Physiol. 1987;49:365–382. doi: 10.1146/annurev.ph.49.030187.002053. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;4:1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;8:4151–4157. doi: 10.1210/en.2008-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AR, Kauffman AS. The role of kisspeptin and RFamide-related peptide-3 neurones in the circadian-timed preovulatory luteinising hormone surge. J Neuroendocrinol. 2012;1:131–143. doi: 10.1111/j.1365-2826.2011.02162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;10:4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;37:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;3:372–386. doi: 10.1002/cne.20626. [DOI] [PubMed] [Google Scholar]
- Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;2:680–697. doi: 10.1016/j.neuroscience.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;23:1731–1737. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- Lee DK, Nguyen T, O'Neill GP, Cheng R, Liu Y, Howard AD, Coulombe N, Tan CP, Tang-Nguyen AT, George SR, et al. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999;1:103–107. doi: 10.1016/s0014-5793(99)00009-5. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Robinson JE, Karsch FJ, Silverman AJ. Immunocytochemical localization of luteinizing hormone-releasing hormone (LHRH) pathways in the sheep brain during anestrus and the mid-luteal phase of the estrous cycle. J Comp Neurol. 1986;1:19–35. doi: 10.1002/cne.902440103. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;8:3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XF, Kinsey-Jones JS, Cheng Y, Knox AM, Lin Y, Petrou NA, Roseweir A, Lightman SL, Milligan SR, Millar RP, et al. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS One. 2009;12:e8334. doi: 10.1371/journal.pone.0008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Roa A, Clarke IJ, Smith JT. Seasonal variation in the gonadotropin-releasing hormone response to kisspeptin in sheep: possible kisspeptin regulation of the kisspeptin receptor. Neuroendocrinology. 2012;3:212–221. doi: 10.1159/000335998. [DOI] [PubMed] [Google Scholar]
- Lippincott MF, Y C, Sidhoum VF, Seminara S. Continuously administered kisspeptin as a pseudo-antagonist of the kisspeptin receptor. The Endocrine Society's 95th Annual Meeting, ENDO 2013; 2013. San Francisco, Abstract SAT 2134-2163. [Google Scholar]
- Liu X, Lee K, Herbison AE. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology. 2008;9:4605–4614. doi: 10.1210/en.2008-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean D, Matsui H, Suri A, Neuwirth R, Colombel M. The investigational Kisspeptin/GPR54 agonist peptide analog, TAK-448, given as single-dose then 13-day continuous subcutaneous infusion, stimulates then suppresses the LH-testosterone axis in healthy male volunteers. Endocr Rev. 2013;34 SAT-323, Abstracts. [Google Scholar]
- Mason DR, Arora KK, Mertz LM, Catt KJ. Homologous down-regulation of gonadotropin-releasing hormone receptor sites and messenger ribonucleic acid transcripts in alpha T3-1 cells. Endocrinology. 1994;3:1165–1170. doi: 10.1210/endo.135.3.8070359. [DOI] [PubMed] [Google Scholar]
- Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;2:383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- McArdle CA, Gorospe WC, Huckle WR, Conn PM. Homologous down-regulation of gonadotropin-releasing hormone receptors and desensitization of gonadotropes: lack of dependence on protein kinase C. Mol Endocrinol. 1987;6:420–429. doi: 10.1210/mend-1-6-420. [DOI] [PubMed] [Google Scholar]
- McNeilly AS, Crawford JL, Taragnat C, Nicol L, McNeilly JR. The differential secretion of FSH and LH: regulation through genes, feedback and packaging. Reprod Suppl. 2003:463–476. [PubMed] [Google Scholar]
- Melis GB, Paoletti AM, Gambacciani M, Mais V, Fioretti P. Evidence that estrogens inhibit LH secretion through opioids in postmenopausal women using naloxone. Neuroendocrinology. 1984;1:60–63. doi: 10.1159/000123956. [DOI] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;5:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar RP, Roseweir AK, Tello JA, Anderson RA, George JT, Morgan K, Pawson AJ. Kisspeptin antagonists: unraveling the role of kisspeptin in reproductive physiology. Brain Res. 2010:81–89. doi: 10.1016/j.brainres.2010.09.044. [DOI] [PubMed] [Google Scholar]
- Min L, Soltis K, Reis AC, Xu S, Kuohung W, Jain M, Carroll RS, Kaiser UB. Dynamic kisspeptin receptor trafficking modulates kisspeptin-mediated calcium signaling. Mol Endocrinol. 2014;1:16–27. doi: 10.1210/me.2013-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci USA. 2012;48:19846–19851. doi: 10.1073/pnas.1211517109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;31:28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004a;10:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, et al. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004b;(Pt 2):379–386. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Barreiro ML, Casanueva FF, Aguilar E, Dieguez C, et al. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology. 2005a;4:1689–1697. doi: 10.1210/en.2004-1353. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P, et al. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005b;1:156–163. doi: 10.1210/en.2004-0836. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;38:11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novaira HJ, Ng Y, Wolfe A, Radovick S. Kisspeptin increases GnRH mRNA expression and secretion in GnRH secreting neuronal cell lines. Mol Cell Endocrinol. 2009;1–2:126–134. doi: 10.1016/j.mce.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;6:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;6837:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Dubay C, Lomniczi A, Kaidar G, Matagne V, Sandau US, Dissen GA. Gene networks and the neuroendocrine regulation of puberty. Mol Cell Endocrinol. 2010;1–2:3–11. doi: 10.1016/j.mce.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampillo M, Camuso N, Taylor JE, Szereszewski JM, Ahow MR, Zajac M, Millar RP, Bhattacharya M, Babwah AV. Regulation of GPR54 signaling by GRK2 and {beta}-arrestin. Mol Endocrinol. 2009;12:2060–2074. doi: 10.1210/me.2009-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda R, Garcia-Galiano D, Roseweir A, Romero M, Sanchez-Garrido MA, Ruiz-Pino F, Morgan K, Pinilla L, Millar RP, Tena-Sempere M. Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology. 2010;2:722–730. doi: 10.1210/en.2009-0803. [DOI] [PubMed] [Google Scholar]
- Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012;3:1235–1316. doi: 10.1152/physrev.00037.2010. [DOI] [PubMed] [Google Scholar]
- Plant TM, Ramaswamy S, Dipietro MJ. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;2:1007–1013. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- Pompolo S, Pereira A, Scott CJ, Fujiyma F, Clarke IJ. Evidence for estrogenic regulation of gonadotropin-releasing hormone neurons by glutamatergic neurons in the ewe brain: an immunohistochemical study using an antibody against vesicular glutamate transporter-2. J Comp Neurol. 2003;1:136–144. doi: 10.1002/cne.10805. [DOI] [PubMed] [Google Scholar]
- Pompolo S, Pereira A, Estrada KM, Clarke IJ. Colocalization of kisspeptin and gonadotropin-releasing hormone in the ovine brain. Endocrinology. 2006;2:804–810. doi: 10.1210/en.2005-1123. [DOI] [PubMed] [Google Scholar]
- Quigley ME, Yen SS. The role of endogenous opiates in LH secretion during the menstrual cycle. J Clin Endocrinol Metab. 1980;1:179–181. doi: 10.1210/jcem-51-1-179. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Seminara SB, Pohl CR, DiPietro MJ, Crowley WF, Jr, Plant TM. Effect of continuous intravenous administration of human metastin 45–54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (Macaca mulatta) Endocrinology. 2007;7:3364–3370. doi: 10.1210/en.2007-0207. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;9:4387–4395. doi: 10.1210/en.2008-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance NE. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;1:111–122. doi: 10.1016/j.peptides.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance NE, Young WS., III Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;5:2239–2247. doi: 10.1210/endo-128-5-2239. [DOI] [PubMed] [Google Scholar]
- Rance NE, McMullen NT, Smialek JE, Price DL, Young WS., III Postmenopausal hypertrophy of neurons expressing the estrogen receptor gene in the human hypothalamus. J Clin Endocrinol Metab. 1990;1:79–85. doi: 10.1210/jcem-71-1-79. [DOI] [PubMed] [Google Scholar]
- Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;3:211–227. doi: 10.1016/j.yfrne.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard N, Galmiche G, Corvaisier S, Caraty A, Kottler ML. KiSS-1 and GPR54 genes are co-expressed in rat gonadotrophs and differentially regulated in vivo by oestradiol and gonadotrophin-releasing hormone. J Neuroendocrinol. 2008;3:381–393. doi: 10.1111/j.1365-2826.2008.01653.x. [DOI] [PubMed] [Google Scholar]
- Roa J, Garcia-Galiano D, Varela L, Sanchez-Garrido MA, Pineda R, Castellano JM, Ruiz-Pino F, Romero M, Aguilar E, Lopez M, et al. The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Endocrinology. 2009;11:5016–5026. doi: 10.1210/en.2009-0096. [DOI] [PubMed] [Google Scholar]
- Rometo AM, Rance NE. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol. 2008;12:1376–1381. doi: 10.1111/j.1365-2826.2008.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;7:2744–2750. doi: 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, et al. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;12:3920–3929. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval-Guzman T, Stalcup ST, Krajewski SJ, Voytko ML, Rance NE. Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. J Neuroendocrinol. 2004;2:146–153. doi: 10.1111/j.0953-8194.2004.01143.x. [DOI] [PubMed] [Google Scholar]
- Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab. 1979;4:633–638. doi: 10.1210/jcem-48-4-633. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;6211:161–164. doi: 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- Scott G, Ahmad I, Howard K, MacLean D, Oliva C, Warrington S, Wilbraham D, Worthington P. Double-blind, randomized, placebo-controlled study of safety, tolerability, pharmacokinetics and pharmacodynamics of TAK-683, an investigational metastin analogue in healthy men. Br J Clin Pharmacol. 2013;2:381–391. doi: 10.1111/j.1365-2125.2012.04385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;17:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;6:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw ND, Histed SN, Srouji SS, Yang J, Lee H, Hall JE. Estrogen negative feedback on gonadotropin secretion: evidence for a direct pituitary effect in women. J Clin Endocrinol Metab. 2010;4:1955–1961. doi: 10.1210/jc.2009-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoupe D, Montz FJ, Lobo RA. The effects of estrogen and progestin on endogenous opioid activity in oophorectomized women. J Clin Endocrinol Metab. 1985;1:178–183. doi: 10.1210/jcem-60-1-178. [DOI] [PubMed] [Google Scholar]
- Silveira LG, Noel SD, Silveira-Neto AP, Abreu AP, Brito VN, Santos MG, Bianco SD, Kuohung W, Xu S, Gryngarten M, et al. Mutations of the KISS1 gene in disorders of puberty. J Clin Endocrinol Metab. 2010;5:2276–2280. doi: 10.1210/jc.2009-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;9:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006a;4:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006b;25:6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, et al. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008a;11:5770–5782. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Rao A, Pereira A, Caraty A, Millar RP, Clarke IJ. Kisspeptin is present in ovine hypophysial portal blood but does not increase during the preovulatory luteinizing hormone surge: evidence that gonadotropes are not direct targets of kisspeptin in vivo. Endocrinology. 2008b;4:1951–1959. doi: 10.1210/en.2007-1425. [DOI] [PubMed] [Google Scholar]
- Teles MG, Bianco SD, Brito VN, Trarbach EB, Kuohung W, Xu S, Seminara SB, Mendonca BB, Kaiser UB, Latronico AC. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med. 2008;7:709–715. doi: 10.1056/NEJMoa073443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tello JA, George JT, Millar M, Anderson RA, Millar RP. Laying the foundation for neuroendocrine control of human reproduction: an investigation into the development of kisspeptin and neurokinin B networks. Endocrine Abstracts. 2010 Society for Endocrinology, 2010, 2021, OC2012.2016. [Google Scholar]
- Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;10:850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;3:354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar S, Vazquez MJ, Navarro VM, Fernandez-Fernandez R, Castellano JM, Vigo E, Roa J, Casanueva FF, Aguilar E, Pinilla L, et al. Effects of single or repeated intravenous administration of kisspeptin upon dynamic LH secretion in conscious male rats. Endocrinology. 2006;6:2696–2704. doi: 10.1210/en.2005-1397. [DOI] [PubMed] [Google Scholar]
- Uenoyama Y, Inoue N, Pheng V, Homma T, Takase K, Yamada S, Ajiki K, Ichikawa M, Okamura H, Maeda KI, et al. Ultrastructural evidence of kisspeptin-gonadotrophin-releasing hormone (GnRH) interaction in the median eminence of female rats: implication of axo-axonal regulation of GnRH release. J Neuroendocrinol. 2011;10:863–870. doi: 10.1111/j.1365-2826.2011.02199.x. [DOI] [PubMed] [Google Scholar]
- Wahab F, Ullah F, Chan YM, Seminara SB, Shahab M. Decrease in hypothalamic Kiss1 and Kiss1r expression: a potential mechanism for fasting-induced suppression of the HPG axis in the adult male rhesus monkey (Macaca mulatta) Horm Metab Res. 2011;2:81–85. doi: 10.1055/s-0030-1269852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A, Vojta PJ, Welch DR, Weissman BE. Chromosome localization and genomic structure of the KiSS-1 metastasis suppressor gene (KISS1) Genomics. 1998;1:145–148. doi: 10.1006/geno.1998.5566. [DOI] [PubMed] [Google Scholar]
- Yamada S, Uenoyama Y, Kinoshita M, Iwata K, Takase K, Matsui H, Adachi S, Inoue K, Maeda KI, Tsukamura H. Inhibition of metastin (kisspeptin-54)-GPR54 signaling in the arcuate nucleus-median eminence region during lactation in rats. Endocrinology. 2007;5:2226–2232. doi: 10.1210/en.2006-1529. [DOI] [PubMed] [Google Scholar]
- Young J, George JT, Tello JA, Francou B, Bouligand J, Guiochon-Mantel A, Brailly-Tabard S, Anderson RA, Millar RP. Kisspeptin restores pulsatile LH secretion in patients with neurokinin B signaling deficiencies: physiological, pathophysiological and therapeutic implications. Neuroendocrinology. 2013;2:193–202. doi: 10.1159/000336376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Roepke TA, Kelly MJ, Ronnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci. 2008;17:4423–4434. doi: 10.1523/JNEUROSCI.5352-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]