Abstract
Background
Predictive models for febrile neutropenia (FN) would be informative for physicians in clinical decision making. This study aims to validate a predictive model (Jenkin’s model) that comprises pretreatment hematological parameters in early-stage breast cancer patients.
Patients and Methods
A total of 428 breast cancer patients who received neoadjuvant/adjuvant chemotherapy without any prophylactic use of colony-stimulating factor were included. Pretreatment absolute neutrophil counts (ANC) and absolute lymphocyte counts (ALC) were used by the Jenkin’s model to assess the risk of FN. In addition, we modified the threshold of Jenkin’s model and generated Model-A and B. We also developed Model-C by incorporating the absolute monocyte count (AMC) as a predictor into Model-A. The rates of FN in the 1st chemotherapy cycle were calculated. A valid model should be able to significantly identify high-risk subgroup of patients with FN rate >20%.
Results
Jenkin’s model (Predicted as high-risk when ANC≦3.1*10∧9/L;ALC≦1.5*10∧9/L) did not identify any subgroups with significantly high risk (>20%) of FN in our population, even if we used different thresholds in Model-A(ANC≦4.4*10∧9/L;ALC≦2.1*10∧9/L) or B(ANC≦3.8*10∧9/L;ALC≦1.8*10∧9/L). However, with AMC added as an additional predictor, Model-C(ANC≦4.4*10∧9/L;ALC≦2.1*10∧9/L; AMC≦0.28*10∧9/L) identified a subgroup of patients with a significantly high risk of FN (23.1%).
Conclusions
In our population, Jenkin’s model, cannot accurately identify patients with a significant risk of FN. The threshold should be changed and the AMC should be incorporated as a predictor, to have excellent predictive ability.
Introduction
Febrile neutropenia (FN) is one of the most common complications in breast cancer patients treated with chemotherapy. Approximately 25–40% of treatment-naïve patients develop FN [1]. FN may predispose patients to life-threatening infection and/or broad-spectrum antibiotic use, prolonged hospitalization, treatment delay or dose reductions[2]. Therefore, prophylactic use of colony stimulating-factor (CSF) in selected patients is critical. Many guidelines recommend that the decision to use CSF prophylactically should depend on the risk of FN with the chemotherapy regimens[3]–[8], which have been categorized into high-risk (>20%), intermediate-risk (10–20%) and low-risk (<20%) regimens of FN.
Although the chemotherapy regimen is the most critical external reason for FN in breast cancer patients, it should not be ignored that even for those patients receiving dose-dense chemotherapy regimens, 30–50% of them will not experience FN [9]–[11]. Therefore, internal reasons exist that may account for FN. Advanced or metastatic disease, age, comorbidity status, history of some chronic diseases, liver function and renal function have all been reported to be associated with FN[12]–[17]. These factors, however, are not a direct reflection of the granulocyte reservoir or the stem cell pool of the bone marrow. Therefore, pretreatment hematological parameters, such as white blood cell count[18], platelet count[19], absolute neutrophil count (ANC) [20], [21], absolute lymphocyte count (ALC) [22], [23] or absolute monocyte count (AMC) [16], [19], [24], are hypothesized to reflect, to some extent, the patient’s predisposition to FN. Jenkins et al. developed a model using pretreatment ANC and ALC in breast cancer patients receiving CEF (5-fluorouracil, epirubicin and cyclophosphamide) chemotherapy[20]. They categorized patients into five subgroups based on different combinations of quintiles of ANC and ALC values [20], [21]. Group V (ANC≤3.1×109/L & ALC≤1.5×109/L) was defined as a high-risk subgroup in their studies with an FN risk higher than 20%. Their model has been externally validated in breast cancer patients receiving the TAC (docetaxel, adriamycin and cyclophosphamide) regimen, which showed a high risk of FN (>20%) [21].
The aims of this study are 1) to evaluate whether the pretreatment hematological parameters are predictive of FN and 2) to validate Jenkin’s predictive model in our population.
Methods
Patients and Data Collection
We searched our database for early-stage breast cancer patients who received neoadjuvant/adjuvant chemotherapy between 2005 and 2013 at our Sun Yat-sen Memorial Hospital. Exclusion criteria include 1) stage IV breast cancer, 2) history of other cancers, 3) essential data unavailable, 4) history of anemia or other hematological disorders, 5) the first chemotherapy cycle was not administered at our hospital, and 6) prophylactic use of CSF. A total of 428 patients were finally identified and included. All of the included patients received breast-conserving surgery or mastectomy when appropriate. FN was defined as a temperature >38.5°C and an ANC<0.5×109/L or<1.0×109/L and expected to fall below 0.5×109/L. In the current study, we only focused on FN occurring in the 1st cycle of chemotherapy. Based on the policy of our institution, we did not administer prophylactic CSF for chemotherapy in early-stage breast cancer patients, except for those who received dose-dense regimens. Clinicopathological features of the patients and the results of the hematological tests were extracted from the medical records. For patients with no FN events recorded in our database, we performed telephone interviews for confirmation. This study was approved by the Institutional Review Broad of Sun Yat-sen Memorial Hospital. Written informed consents were obtained from the included patients.
Chemotherapy
The chemotherapy regimens were employed as follows: CMF, cyclophosphamide + methotrexate+5-fluouracil; EC, epirubicin + cyclophosphamide; TC, paclitaxel + cyclophosphamide; DC, docetaxel + cyclophosphamide; CEF, cyclophosphamide + epirubicin+5-fluouracil; ET, epirubicin + paclitaxel; TEC, epirubicin + paclitaxel+ cyclophosphamide; ED, epirubicin + docetaxel; and DEC, epirubicin + docetaxel + cyclophosphamide. Patients were required to have whole blood counts measured at baseline, as well as on the 7th, 9th and 14th days of each chemotherapy cycle, and the results and/or any febrile events were reported to their physician. CSF (filgrastim 5 mcg/kg until post-nadir ANC recovery) was employed for ANC <1.0×109/L at the 7th or 9th day of each cycle. Antibiotics were employed at any time when FN occurred. No prophylactic antibiotics were used before treatment.
Statistical Consideration
For the comparison of FN rates in patients with different pathological features, Fisher’s exact test/chi-squared test and the Mann-Whitney U test were used for categorical and continuous variables, respectively. The Mann-Whitney U test was also used in univariate analysis to screen the pretreatment blood count variables for independent risk factors of FN.
In the Jenkin’s model, patients were classified into five subgroups (Group I–V) based on their ANC and ALC values [21], [25]. To validate the Jenkin’s model, we calculated the FN rate of each subgroup (Table 1). The model was considered valid if the actual rate of FN in the predicted high-risk group (Group V) was higher than 20% and the FN rates among subgroups were of statistical significance. In addition, we modified Jenkin’s model by combining the five subgroups into two subgroups (low-risk and high-risk). The modified Jenkin’s models A and B (referred to as Model-A and Model-B hereafter) were generated as follows:
Table 1. Patients features of the included patients.
| Items | n | FN | no-FN | P | ||||
| n | % | n | % | |||||
| Age (yrs, Mean±std) | 47.3±9.8 | 45.9±9.3 | 47.5±9.9 | NS | ||||
| BMI (Mean±std) | 23.2±4.0 | 22.7±3.8 | 23.3±4.0 | NS | ||||
| BSA (m∧2 Mean±std) | 1.6±0.2 | 1.52±0.2 | 1.56±0.2 | NS | ||||
| Hypertension history | ||||||||
| No | 386 | 54 | 14 | 332 | <0.05 | |||
| Yes | 41 | 1 | 2 | 40 | 98 | |||
| Diabetes history | ||||||||
| No | 411 | 53 | 13 | 358 | NS | |||
| Yes | 17 | 2 | 12 | 15 | 88 | |||
| Menopausal status | ||||||||
| Pre/peri-menopausal status | 276 | 38 | 14 | 238 | NS | |||
| Post menopausal status | 146 | 15 | 10 | 131 | 90 | |||
| T-stage | ||||||||
| T1 | 196 | 22 | 11 | 174 | NS | |||
| T2 | 214 | 31 | 14 | 183 | 86 | |||
| T3 | 12 | 2 | 17 | 10 | 83 | |||
| N-stage | ||||||||
| N0 | 277 | 28 | 10 | 249 | <0.05 | |||
| N1 | 95 | 17 | 18 | 78 | 82 | |||
| N2 | 32 | 4 | 13 | 28 | 88 | |||
| N3 | 22 | 6 | 27 | 16 | 73 | |||
| Pathology subtype | ||||||||
| IDC | 376 | 15 | 4 | 361 | NS | |||
| ILC | 7 | 1 | 14 | 6 | 86 | |||
| IDC+ILC | 12 | 1 | 8 | 11 | 92 | |||
| Others | 33 | 2 | 6 | 31 | 94 | |||
| ER status | ||||||||
| Negative | 81 | 12 | 15 | 69 | NS | |||
| Positive | 340 | 43 | 13 | 297 | 87 | |||
| PR status | ||||||||
| Negative | 121 | 12 | 10 | 109 | NS | |||
| Positive | 301 | 43 | 14 | 258 | 86 | |||
| HER2 | ||||||||
| Negative | 292 | 34 | 12 | 258 | <0.05 | |||
| Intermediate | 31 | 1 | 3 | 30 | 97 | |||
| Positive | 104 | 20 | 19 | 84 | 81 | |||
| Ki67 | ||||||||
| Negative | 101 | 11 | 11 | 90 | NS | |||
| Positive | 303 | 39 | 13 | 264 | 87 | |||
| Neoadjuvant chemotherapy | ||||||||
| No | 98 | 20 | 20 | 78 | <0.05 | |||
| Yes | 328 | 35 | 11 | 293 | 89 | |||
| Blood type | ||||||||
| A | 120 | 12 | 10 | 108 | NS | |||
| B | 108 | 20 | 19 | 88 | 81 | |||
| O | 172 | 20 | 12 | 152 | 88 | |||
| AB | 22 | 2 | 9 | 20 | 91 | |||
| Chemotherapy regimens? | ||||||||
| CMF | 18 | 0 | 0 | 18 | <0.01 | |||
| CEF | 21 | 1 | 5 | 20 | 95 | |||
| EC | 22 | 5 | 23 | 17 | 77 | |||
| TC | 53 | 1 | 2 | 52 | 98 | |||
| DC | 29 | 2 | 7 | 27 | 93 | |||
| TEC | 16 | 3 | 19 | 13 | 81 | |||
| DEC | 43 | 14 | 33 | 29 | 67 | |||
| ET | 78 | 11 | 14 | 67 | 86 | |||
| ED | 62 | 17 | 27 | 45 | 73 | |||
| Others | 7 | 1 | 14 | 6 | 86 | |||
| Chemotherapy regimens with different risk of FN?. | ||||||||
| Low | 222 | 9 | 4 | 213 | <0.01 | |||
| Intermediate | 101 | 15 | 15 | 86 | 85 | |||
| High | 105 | 31 | 30 | 74 | 70 | |||
BMI, body mass index. BSA, body surface area ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; FN, febrile neutropenia.?High risk regimen(DEC, DE); Intermediate risk regimen (TEC,ET OTHERS); ?C, Cyclophosphomide;M, Methotrexate; E, epirubicin; F, 5-Fluorouracil; T, Paclitaxle; D, Docetaxel;Others included regimens contained herceptins cisplatin, or nolvelbine; NS, non-significant;
Model-A
Group I and II as a low-risk subgroup; Group III, IV and V as a high-risk subgroup.
Model-B
Group I, II and III as a low-risk subgroup; Group IV and V as a high-risk subgroup.
To improve the performance of Model-A, we incorporated the AMC value as one of the predictors and generated Model-C. Patients in the high-risk subgroup of Model-A were classified as high-risk in Model-C when their AMC values were lower than a specific threshold. To determine the optimal threshold of AMC for Model-C, we used ROC curves and calculated the corresponding AUC and P values when a different threshold of AMC was used. The AMC value that enabled the AUC and P value of Model-C to reach a significant level was used as the threshold of AMC. Multivariate analysis was performed using logistic regression. All tests of significance were two tailed. Statistical analyses were carried out using SPSS v18.0 (Chicago, IL, USA).
Results
Clinicopathological Features
The clinicopathological features and hematological test results of the 428 patients are summarized in Table 1 and Figure 1. The mean and median pretreatment WBC, ANC and ALC values of our population are comparable to those in Jenkin’s studies (Table 2). Fifty-five patients (12.8%) developed FN during the 1st cycle of chemotherapy. The median and mean ANC nadir in FN patients was 0.06×109/L and 0.20×109/L, respectively.
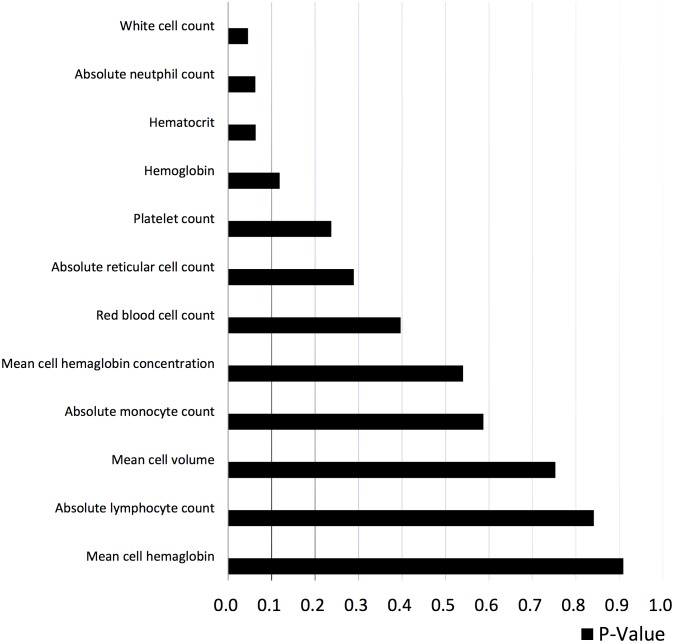
Figure 1. Univariate analysis of predictive hematological factors for FN.
Mann-Whitney U test was used as a univariate analysis and the P-value was shown. White cell count, absolute neutrophil count and hematocrit with P-value less than 0.1 was incorporated into multivariate analysis.
Table 2. Pretreatment WBC, ANC and ALC values in our pupulation are comparable to Jenkins study.
| Items | 2009 Dataset in Jenkin’s study | Our dataset | Our dataset | |||||
| Mean±SD(10∧9/L) | Normal range (10∧9/L) | Mean±SD(10∧9/L) | Normal range (10∧9/L) | Median (10∧9/L) | Range (10∧9/L) | Median (10∧9/L) | Range (10∧9/L) | |
| WBC | 6.96±1.82 | 3.60–11.00 | 6.70±2.00 | 4.00–10.00 | 6.90 | 3.80–19.5 | 6.46 | 2.39–13.47 |
| ANC | 4.32±1.48 | 1.80–7.50 | 4.30±1.80 | 2.00–7.50 | 4.30 | 1.60–17.9 | 3.93 | 0.35–12.47 |
| ALC | 2.02±0.64 | 1.50–4.00 | 1.90±0.60 | 0.80–4.00 | 1.90 | 0.30–4.4 | 1.82 | 0.07–4.63 |
Univariate Analysis of Clinicopathological Factors and Pretreatment Hematological Parameters
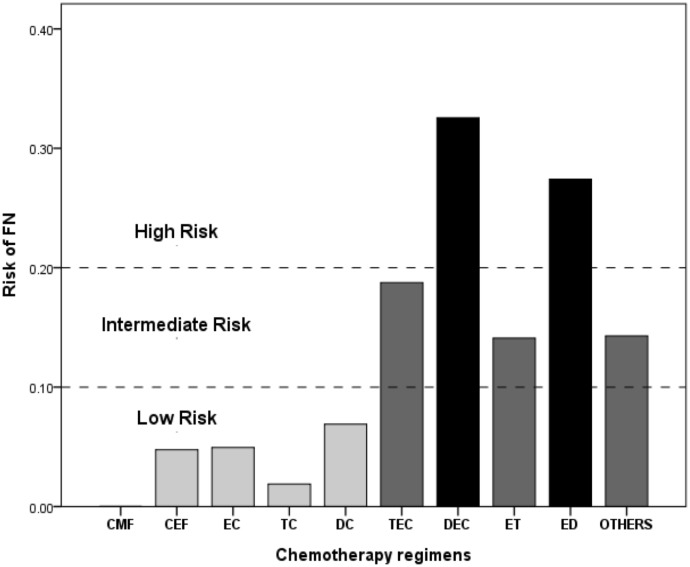
History of hypertension (P<0.05), N stage (P<0.05), Her2 status (P<0.05), neoadjuvant chemotherapy (P<0.05), white cell count (P<0.05) and chemotherapy regimens (P<0.01) were significantly associated with FN. Hematocrit (P = 0.06) and ANC (P = 0.06) were marginally significant in predicting FN. The FN rates of different regimens are shown in Figure 2. DEC and ED were classified as high-risk regimens (>20%), whereas TEC, ET and others (carboplatin- and/or trastuzumab-based regimens) were classified as intermediate-risk regimens (10–20%). CMF, CEF, EC, ET, TC and DC regimens were classified as low risk (<10%).
Figure 2. FN rate in patients receiving different chemotherapy regimens.
Chemotherapy regimens were catagorized into high-, intermediate- or low-risk regimens based on their probability of having FN events.
Validation of Jenkin’s Model
Jenkin’s model classified patients into five subgroups. The number of patients distributed in these subgroups in our dataset is similar to that reported by Jenkin et al. in 2009[20] and 2012[21] (see Figure S1 and Table S1). Based on Jenkin’s model, the FN rates were not significantly different among the five subgroups in our patients, and none of them had an FN rate higher than 20% (Table 3). Model-A, rather than Model-B, could identify patients with a significantly higher FN rate (17.2% vs. 9.7, P<0.05), but did not reach the 20% high-risk threshold.
Table 3. Validation of Jenkin’s model and Modified Jenkin’s model.
| Group* | ANC (×10∧9/L) | ALC (×10∧9/L) | AMC (×10∧9/L) | Total No. | FN in the 1st cycle | P† | |
| No. | % | ||||||
| Jenkin’s model | |||||||
| Group I | >5.2 | >2.4 | n/a | 155 | 15 | 9.7 | NS |
| Group II | ≦5.2 | ≦2.4 | n/a | 93 | 9 | 9.7 | |
| Group III | ≦4.4 | ≦2.1 | n/a | 72 | 14 | 19.4 | |
| Group IV | ≦3.8 | ≦1.8 | n/a | 69 | 13 | 18.8 | |
| Group V (High-risk subgroup) | ≦3.1 | ≦1.5 | n/a | 39 | 4 | 10.3 | |
| Model-A | |||||||
| Low-risk subgroup (Group I & II) | >4.4 | >2.1 | n/a | 248 | 24 | 9.7 | <0.05 |
| High-risk subgroup (Group III,IV & V) | ≦4.4 | ≦2.1 | n/a | 180 | 31 | 17.2 | |
| Model-B | |||||||
| Low-risk subgroup (Group I,II & III) | >3.8 | >1.8 | n/a | 320 | 38 | 11.9 | NS |
| High-risk subgroup(Group IV & V) | ≦3.8 | ≦1.8 | n/a | 108 | 17 | 15.7 | |
| Model-C | |||||||
| Low-risk subgroup | Not fulfill the criteria of high-risk group | 337 | 34 | 10.1 | <0.01 | ||
| High-risk subgroup | ≦4.4 | ≦2.1 | ≦0.28 | 91 | 21 | 23.1 | |
*In Jenkin’s model, patients were classified into different groups without overlaps. For example, group IV comprises patients with ANC ≦3.8 and ALC ≦1.8 who do not fulfil the criteria for group V.
Chi-square test was used.
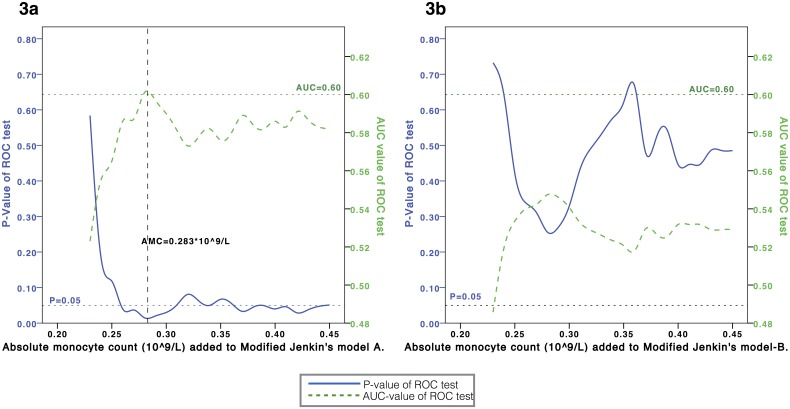
Therefore, we investigated whether incorporating the AMC value could improve the performance. As shown in Figure 3a, the performance of Model-A can reach a plateau with AUC≈0.58–0.60 and P≈0.05 for an AMC threshold value>0.28×109/L. By contrast, the performance of Model-B could not be improved regardless of the AMC value used (Figure 3b). Therefore, the optimal threshold of AMC to be used should be 0.28×109/L, and a new model (Model-C) was generated based on Model-A:
Figure 3. Optimal threshold of AMC.
To incorporate AMC into Model-A (3a) or Model-B (3b), we calculated the AUC and P-value of the new model when different threshold of AMC was used. A new model (Model-C) could be developd from Model-A (3a) with the highest AUC value and lowest P value, when the threshold of AMC = 0.283*10∧9/L. No valid model could be established when AMC was incorporated into Model-B (3b).
Model-C
High-risk subgroup: ANC≤4.4×109/L, ALC≤2.1×109/L and AMC≤0.28×109/L.
Low-risk subgroup: Patients do not fulfill the criteria for inclusion in the high-risk subgroup.
The high-risk subgroup in Model-C demonstrated a significantly higher FN rate compared with the low-risk subgroup (23.1% vs. 10.1%; P<0.01). The sensitivity, specificity, false-negative rate, false-positive rate, positive predictive value and negative predictive value were 38.2%, 81.2%, 61.8%, 18.8%, 23.1% and 89.1%, respectively.
Multivariate Analysis
Clinicopathological factors and pretreatment hematological factors that were shown to be associated with FN in the univariate analysis, together with the chemotherapy regimen (classified as low-, intermediate- and high-risk) and Model-C (low-risk vs. high-risk subgroup), were included in logistic regression as the multivariate analysis. The chemotherapy regimen (intermediate- vs. low-risk regimen (HR = 3.51; P<0.01; 95% CI: 1.45–8.53); high- vs. low-risk regimen (HR = 9.48; P<0.01; 95% CI: 4.26–21.1)) and the Model-C subgroup (high- vs. low-risk group; HR = 2.77; P<0.01; 95% CI: 1.42–5.37) are the only two independent predictors for FN.
Discussion
Chemotherapy Regimens and FN
Assessing the risk of FN would be informative for physicians in clinical decision making before chemotherapy. The regimens and dosage are the major considerations when evaluating the risk of FN. The estimated risk of FN from each regimen suggested by the current guidelines was limited by the specific populations, study methods and different clinical scenarios. For example, the CMF (cyclophosphamide, methotrexate, fluorouracil) regimen is classified as a low-risk (<10%) or intermediate-risk (10–20%) regimen in the EORTC [3] or NCCN [5] guidelines, respectively. Hence, we assessed the FN rate in different chemotherapy regimens in our population. Consistent with the NCCN and EORTC guidelines, the FN rate of our DAC regimen was higher than 20%. When paclitaxel, instead of docetaxel, was used in combination with anthracycline +/− cyclophosphamide, the FN rate fell into the 10–20% range. In the NCCN guidelines, docetaxel every 21 days and CMF regimens are considered intermediate-risk regimens. However, in our population, these two regimens had a low risk of FN (<10%)[5], consistent with the EORTC guidelines [3]. Therefore, physicians should summarize the FN rate of each regimen in their own population to gain reliable reference information for clinical decision making. Applying any of the guidelines without prior validation is not appropriate.
Validation of Jenkin’s Model in the Population
Developing a predictive model for FN is important. In patients who receive high-risk regimens with the support of prophylactic CSF, an accurate model may enable the identification of those who may still have FN and the subsequent dose deduction. Patients could also be well informed about the possible complications. A predictive model could also be helpful for patients with an intermediate risk of FN (10–20%) when the use of prophylactic CSF is determined by the physician. Several models have been developed and widely validated in cancer patients[9], [14], [16], [19], [22], [24]. However, few models have been developed specifically for breast cancer patients. The INC-EU (Impact of Neutropenia in Chemotherapy European study group) reported a multivariate model in breast cancer patients[15], but they did not present it as an applicable formula or nomogram for external validation. In the present study, we tested whether Jenkin’s model is valid in our patients. Prior to that, we screened our pretreatment hematological parameters and found that only the ANC was marginally associated with FN status. ANC, ALC or AMC alone was not associated with FN. Similar findings were also observed in one of Jenkin’s studies, in which the ANC and ALC were not by themselves correlated with the frequency of FN. However, their patients, when combined into five groups based on the Jenkin’s model, had significant differences in the risk of FN in any cycle or in the 1st cycle[21]. When testing the Jenkin’s model in our population, we noticed that group V patients (ANC≤3.1×109/L & ALC≤1.5×109/L), who are defined as a high-risk subgroup in the Jenkin’s model, did not have an FN rate higher than 20%. The following explanations for the failure of the Jenkin’s model were considered:
The distribution of baseline hematological parameters differed among our population and Jenkin’s. This explanation could be ruled out because we compared the mean and median values of the WBC, ANC and ALC in our populations with those in the Jenkin’s studies and did not observe any significant differences (Table 2). In addition, the number of the patients distributed in the different subgroups was also similar among the populations (see Figure S1 and Table S1).
The FN rate among our populations (12.8%) and those in Jenkin’s studies are different (8% and 6% in the 2009 and 2012 studies, respectively). In addition, Jenkin et al. used the same regimen (CEF in the 2009 study and TEC in the 2012 study) in their population, whereas different chemotherapy regimens were used in our patients. These might be the most likely reasons that cannot be ruled out. Our study did not have a sufficiently large sample size to validate Jenkin’s model in patients receiving the same chemotherapy regimens.
In Jenkin’s model, they considered patients in Group V to be the high-risk subgroup. Because Group V patients did not have a significant higher risk of FN in our populations, we tried to use different thresholds of Jenkin’s model by combining the five subgroups into two and generated Model-A and -B (described in the Methods and Results sections). As shown in Table 3, Model-A and -B did not perform well either.
Taken together, our data suggested that the Jenkin’s model may not be valid in our population.
Incorporation of AMC into the Jenkin’s Model
To improve the Jenkin’s model, we incorporated the AMC as a predictor based on our hypothesis that the combination of ANC, ALC and AMC could comprehensively reflect the bone marrow granulocyte reservoir and, therefore, predict the chemotherapy-induced FN. Kondo et al. and Oguz et al. reported that an AMC<0.15×109/L was an independent factor for FN in solid tumors[13], [24]. With the same threshold, Moreau’s study also suggested that the baseline AMC could independently predict FN in hematological malignancies[26]. In our study, the quintile values of AMC were 0.23×109/L, 0.30×109/L, 0.36×109/L and 0.45×109/L. There were only 16 (3.7%) patients with a pretreatment AMC<0.15×109/L, and none of them had FN events. Therefore, an AMC<0.15×109/L may not be an optimal threshold. Our study revealed that the AMC threshold should be higher than 0.28×109/L to enable the AUC to reach a significant level (Figure 3). We applied 0.28×109/L as the AMC threshold in Model-C, which identified patients with a significantly high risk of FN (23%). This result is very surprising because AMC only constitutes a small percentage of the WBC but plays such a critical role in risk assessment. Model-C might be able to reflect the patients’ internal reasons that determine their predisposition to FN. In addition, the predictive ability of Model-C was independent of the chemotherapy regimens, as shown by our multivariate analysis. Therefore, to comprehensively evaluate the risk of FN, we propose that the pretreatment ANC, ALC and AMC values should all be considered, in addition to the chemotherapy regimens.
All of the patients received surgical treatment in our study. However, it is unknown whether the sequence of chemotherapy and surgery would have any influences on the model predicting accuracy. As a confounding factor, neoadjuvant chemotherapy was associated with FN in univariate analysis, but not in multivariate analysis, suggesting that the sequence of chemotherapy and surgery was not independently associated with FN. In multivariate analysis, we also assessed but did not observe any interaction between neoadjuvant chemotherapy and Model-C, which indicated that the surgical treatment or not would have no impact on the model prediction accuracy in this study.
Limitations of our Study
Several limitations of our study should be addressed.
We only focused on the FN that occurred during the 1st cycle of chemotherapy. We are uncertain whether our model can be predictive for FN occurring for the duration of chemotherapy. However, because approximately 80% of FN occurred during the 1st cycle of thermotherapy, our study may still be valid for testing the predictive models. To predict the risk of FN occurred in the 2nd cycle of chemotherapy or later, more “post-chemo” hematological parameters could be incorporated to improve the model performance.
The sample size of our population was not sufficiently large to assess the performance of the models in each chemotherapy regimen. The dosage of chemotherapy regimen might also have influences on model prediction, which could not be assessed in this study. In addition, we had no patients who received dose-dense chemotherapy, which is presently widely used. However, the multivariate analysis in our study suggested that Model-C is an independent predictor of FN when adjusted for the chemotherapy regimens. Thus, we believe that our Model-C, with AMC as one of the predictors, could predict the patient’s predisposition for FN regardless of the chemotherapy regimens.
Chia et al.[17] had studied the association between chronic comorbid condition and the risk of FN and showed that congestive heart failure, osteoarthritis, previous cancer and thyroid disorder were associated with increased risk of FN. In addition, the pretreatment renal function, liver function and chemotherapy dosage, which were shown to be associated with FN, were not included in this study. Therefore, it remains unknown whether these factors may affect the performance of the models in our study.
We do not have an external dataset to validate our Model-C.
Conclusions
In summary, our study suggested that 1) the FN rate of each chemotherapy regimen should be evaluated prior to following any guidelines on the prophylactic use of CSF. 2) The chemotherapy regimen is critical as an external factor when assessing the risk of FN in breast cancer patients. Hematological parameters alone cannot predict FN in our population. 3) Jenkin’s model did not pass the validation test in our populations. 4) Modification of Jenkin’s model with AMC incorporated as a predictor to create a new model (Model-C) was developed with excellent predictive capability.
Further investigations, including external validation of our new model, are needed. Can our model be used to predict the FN rate for the entire duration of chemotherapy or in metastatic breast cancer patients? Can additional parameters, such as indexes of the liver or renal function, be incorporated to improve our model? Can our model be used in patients receiving dose-dense chemotherapy with prophylactic CSF support? Are there any differences of the performance of our model when used in patients with different regimens of chemotherapy? Future studies are needed to help clarify these issues.
Supporting Information
Comparison of the population distribution patterns between our dataset and those of Jenkin’s. Figure S1 suggested that the population distribution patterns were similar between our datasets and those of Jenkin’s.
(TIFF)
Distribution of total patients in different risk groups.
(DOCX)
Acknowledgments
We appreciate all the support from the staff at our breast tumor center, Sun Yat-Memorial Hospital.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grant No. 81172524/H1622 and 81372817/ H1622). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dale DC (2002) Colony-stimulating factors for the management of neutropenia in cancer patients. Drugs 62 Suppl 1 1–15. [DOI] [PubMed] [Google Scholar]
- 2. Renner P, Milazzo S, Liu JP, Zwahlen M, Birkmann J, et al. (2012) Primary prophylactic colony-stimulating factors for the prevention of chemotherapy-induced febrile neutropenia in breast cancer patients. Cochrane Database Syst Rev 10: CD007913. [DOI] [PubMed] [Google Scholar]
- 3. Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, et al. (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47: 8–32. [DOI] [PubMed] [Google Scholar]
- 4. Crawford J, Caserta C, Roila F (2010) Hematopoietic growth factors: ESMO Clinical Practice Guidelines for the applications. Ann Oncol 21 Suppl 5 v248–251. [DOI] [PubMed] [Google Scholar]
- 5.NCCN (2013) Myeloid Growth Factors. NCCNorg Version 2.
- 6. Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, et al. (2006) 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 24: 3187–3205. [DOI] [PubMed] [Google Scholar]
- 7. Kouroukis CT, Chia S, Verma S, Robson D, Desbiens C, et al. (2008) Canadian supportive care recommendations for the management of neutropenia in patients with cancer. Curr Oncol 15: 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Flowers CR, Seidenfeld J, Bow EJ, Karten C, Gleason C, et al. (2013) Antimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 31: 794–810. [DOI] [PubMed] [Google Scholar]
- 9. Ray-Coquard I, Borg C, Bachelot T, Sebban C, Philip I, et al. (2003) Baseline and early lymphopenia predict for the risk of febrile neutropenia after chemotherapy. Br J Cancer 88: 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dittrich C, Sevelda P, Salzer H, Obermair A, Speiser P, et al. (2003) Lack of impact of platinum dose intensity on the outcome of ovarian cancer patients. 10-year results of a prospective randomised phase III study comparing carboplatin-cisplatin with cyclophosphamide-cisplatin. Eur J Cancer 39: 1129–1140. [DOI] [PubMed] [Google Scholar]
- 11. Elias A, Ryan L, Sulkes A, Collins J, Aisner J, et al. (1989) Response to mesna, doxorubicin, ifosfamide, and dacarbazine in 108 patients with metastatic or unresectable sarcoma and no prior chemotherapy. J Clin Oncol 7: 1208–1216. [DOI] [PubMed] [Google Scholar]
- 12. Lyman GH, Lyman CH, Agboola O (2005) Risk models for predicting chemotherapy-induced neutropenia. Oncologist 10: 427–437. [DOI] [PubMed] [Google Scholar]
- 13. Oguz A, Karadeniz C, Ckitak EC, Cil V (2006) Which one is a risk factor for chemotherapy-induced febrile neutropenia in childhood solid tumors: early lymphopenia or monocytopenia? Pediatr Hematol Oncol 23: 143–151. [DOI] [PubMed] [Google Scholar]
- 14. Hosmer W, Malin J, Wong M (2010) Development and validation of a prediction model for the risk of developing febrile neutropenia in the first cycle of chemotherapy among elderly patients with breast, lung, colorectal, and prostate cancer. Supportive Care in Cancer 19: 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwenkglenks M, Pettengell R, Jackisch C, Paridaens R, Constenla M, et al. (2010) Risk factors for chemotherapy-induced neutropenia occurrence in breast cancer patients: data from the INC-EU Prospective Observational European Neutropenia Study. Supportive Care in Cancer 19: 483–490. [DOI] [PubMed] [Google Scholar]
- 16.Sato I (2012) Prediction of docetaxel monotherapy-induced neutropenia based on the monocyte percentage. Oncology Letters. [DOI] [PMC free article] [PubMed]
- 17. Chia VM, Page JH, Rodriguez R, Yang SJ, Huynh J, et al. (2013) Chronic comorbid conditions associated with risk of febrile neutropenia in breast cancer patients treated with chemotherapy. Breast Cancer Res Treat 138: 621–631. [DOI] [PubMed] [Google Scholar]
- 18. Lyman GH, Kuderer NM, Crawford J, Wolff DA, Culakova E, et al. (2011) Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer 117: 1917–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moreau M, Klastersky J, Schwarzbold A, Muanza F, Georgala A, et al. (2008) A general chemotherapy myelotoxicity score to predict febrile neutropenia in hematological malignancies. Annals of Oncology 20: 513–519. [DOI] [PubMed] [Google Scholar]
- 20. Jenkins P, Freeman S (2009) Pretreatment haematological laboratory values predict for excessive myelosuppression in patients receiving adjuvant FEC chemotherapy for breast cancer. Ann Oncol 20: 34–40. [DOI] [PubMed] [Google Scholar]
- 21. Jenkins P, Scaife J, Freeman S (2012) Validation of a predictive model that identifies patients at high risk of developing febrile neutropaenia following chemotherapy for breast cancer. Ann Oncol 23: 1766–1771. [DOI] [PubMed] [Google Scholar]
- 22. Choi CW, Sung HJ, Park KH, Yoon SY, Kim SJ, et al. (2003) Early lymphopenia as a risk factor for chemotherapy-induced febrile neutropenia. American Journal of Hematology 73: 263–266. [DOI] [PubMed] [Google Scholar]
- 23. Ray-Coquard I, Borg C, Bachelot T, Sebban C, Philip I, et al. (2003) Baseline and early lymphopenia predict for the risk of febrile neutropenia after chemotherapy. British Journal of Cancer 88: 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kondo M, Oshita F, Kato Y, Yamada K, Nomura I, et al. (1999) Early monocytopenia after chemotherapy as a risk factor for neutropenia. Am J Clin Oncol 22: 103–105. [DOI] [PubMed] [Google Scholar]
- 25. Jenkins P, Freeman S (2008) Pretreatment haematological laboratory values predict for excessive myelosuppression in patients receiving adjuvant FEC chemotherapy for breast cancer. Annals of Oncology 20: 34–40. [DOI] [PubMed] [Google Scholar]
- 26. Moreau M, Klastersky J, Schwarzbold A, Muanza F, Georgala A, et al. (2009) A general chemotherapy myelotoxicity score to predict febrile neutropenia in hematological malignancies. Ann Oncol 20: 513–519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of the population distribution patterns between our dataset and those of Jenkin’s. Figure S1 suggested that the population distribution patterns were similar between our datasets and those of Jenkin’s.
(TIFF)
Distribution of total patients in different risk groups.
(DOCX)