Abstract
Background
Protein kinases are proven targets for drug development with an increasing number of eukaryotic Protein Kinase (ePK) inhibitors now approved as drugs. Mitogen-activated protein kinase (MAPK) family members connect cell-surface receptors to regulatory targets within cells and influence a number of tissue-specific biological activities such as cell proliferation, differentiation and survival. However, the contributions of members of the MAPK pathway to schistosome development and survival are unclear.
Methodology/Principal Findings
We employed RNA interference (RNAi) to elucidate the functional roles of five S. mansoni genes (SmCaMK2, SmJNK, SmERK1, SmERK2 and SmRas) involved in MAPK signaling pathway. Mice were injected with post-infective larvae (schistosomula) subsequent to RNAi and the development of adult worms observed. The data demonstrate that SmJNK participates in parasite maturation and survival of the parasites, whereas SmERK are involved in egg production as infected mice had significantly lower egg burdens with female worms presenting underdeveloped ovaries. Furthermore, it was shown that the c-fos transcription factor was overexpressed in parasites submitted to RNAi of SmERK1, SmJNK and SmCaMK2 indicating its putative involvement in gene regulation in this parasite's MAPK signaling cascade.
Conclusions
We conclude that MAPKs proteins play important roles in the parasite in vivo survival, being essential for normal development and successful survival and reproduction of the schistosome parasite. Moreover SmERK and SmJNK are potential targets for drug development.
Author Summary
Enzymes known as mitogen-activated protein kinases (MAP kinases/MAPKs) influence a number of essential biological activities, such as cell proliferation, differentiation and survival. However, for the Schistosoma mansoni flatworm parasite, very little is known about these enzymes. We used RNA interference (RNAi), a technique designed to decrease or stop the production of specific proteins of interest, to examine the contributions of five Schistosoma mansoni MAPKs to parasite growth and survival. After inducing the RNAi effect in young parasites, we then transferred the worms into mice and after 37 days, counted the number of surviving adult worms in the bloodstream, eggs in the liver, and examined those surviving worms for morphological defects. We found that RNAi of SmJNK decreases parasite survival by 56%, whereas RNAi of SmERK slows the maturation of the ovary and, thus, egg-laying. We also noted that c-fos, that is responsible for activating genes in the genome, was upregulated after RNAi of MAPKs. Our results help define the importance of MAPKs in the normal development and survival of the schistosome parasite and suggest one or more of these enzymes may be useful as drug targets to treat schistosomiasis.
Introduction
Schistosomes are parasitic flatworms (Phylum Platyhelminths) that can survive for years or decades in the mammalian host [1], [2]. Besides strategies to inhibit or modulate host immune responses, the maintenance of homeostasis and complex cellular adaptations, Schistosoma integrates specific extracellular signals to generate an appropriate cellular response [3]. In this context, signal transduction has essential functions in the cell control involving non-linear integrated networks that interact mostly by switching the activity status of proteins.
The mitogen-activated protein kinase (MAP kinase/MAPK) signaling pathway is activated by a variety of extracellular growth factor-receptor interactions in response to environmental stimuli and leads to the downstream transcriptional activation of specific genes [4]. For example, in mammals, activated ERK MAPKs can translocate into the nucleus and induce phosphorylation of specific transcription factors such as ELK-1 [5]. ELK-1 forms a complex with another transcription factor, SRF (serum response factor), and the ELK-1/SRF complex is then able to bind to the promoter of the c-fos gene and trigger transcription [6]. MAPKs influence a number of tissue-specific biological activities like cell proliferation, survival and differentiation through the activation of other protein kinases, metabolic enzymes or by the phosphorylation of transcription factors and components of the cytoskeleton [7].
Recently we showed by in silico analyses that the MAPK signaling components are well conserved in the three main Schistosoma species that infect humans, namely S. mansoni, S. japonicum and S. haematobium [8]. These include representatives of the MAPK subfamilies ERK (extracellular signal-regulated kinase), p38, JNK (c-Jun N-terminal kinase) and nmo (nemo MAPK). However, a detailed understanding of MAPK pathway in schistosome development and survival remains to be elucidated.
In planarians, ERK plays a pivotal role in stem cell dynamics during regeneration. Activation of ERK signaling induces stem cells to exit proliferative state and enter the differentiating state [9]. In the C. elegans model nematode, ERK MAPKs are required for multiple developmental events, including the induction of vulval, uterine and spicule cell fates, and the promotion of germ line meiosis [10]. In S. mansoni Vicogne and colleagues (2004) [11] showed that the human epidermal growth factor (EGF) can activate the Ras/ERK pathway, which induces meiosis in oocytes. This is a relevant observation because oviposition is responsible for the pathogenesis of schistosomiasis. Females can release, on average, 300 highly immunoreactive eggs a day. Although, many eggs escape via body wastes, others become trapped in various tissues to elicit eosinophilic and granulomatous inflammatory reactions that give way to progressive fibrosis that can lead to organ dysfunction and, sometimes, death. These observations have led to our hypothesis that ERK MAPK pathway is involved in Schistosoma reproduction.
Apart from MAPKs, c-Jun N-terminal kinase (JNK) proteins also have evolutionary conserved functions, including the control of cellular responses to stress stimuli induced by a range of intrinsic and environmental aggression, e.g., UV irradiation, DNA damage, heat, bacterial antigens and inflammatory cytokines [12]. In addition, JNK signaling plays a crucial role during planarian regeneration by regulating the G2/M transition in the cell cycle of pluripotent stem cells [13]. In our in silico analyses, we showed that only one member of the MAPK JNK sub-family is encoded in the S. mansoni genome, in contrast to five genes expressed in Caenorhabditis elegans and three genes in humans [8]. This evolutionary constriction of the JNK subfamily in S. mansoni to just one enzyme suggests that SmJNK may be particularly worthy of investigation to understand its potential target for drug development as drug effectiveness can be marked when a single-copy gene is targeted [14].
The divalent cátion calcium (Ca2+) is one of the most widely ion used as a second messenger in cell signaling, and much of this process is controlled by calmodulin-binding kinase (CaMK) [15]. As SmJNK, only one SmCaMK2 gene is encoded in the S. mansoni genome. In C. elegans, a JNK cell-specific pathway that is responsible for worm development, is activated by CaMK2 [10], [16]. Against this background, our study aimed at elucidating the function of ERK, JNK, CAMK2 and RAS, proteins involved in the MAPKs signaling pathways in the parasite S. mansoni, using RNA interference (RNAi). We show that RNAi of SmERK decreases egg production by female worms recovered from mice, which was consistent with the observations of an under-developed ovary and immature oocytes, and suggesting a direct involvement of SmERK in parasite reproduction. Furthermore, suppression of SmJNK gene expression killed the parasite and was associated with damage to the worm's tegument.
Methods
Ethics statement
Brazilian national guidelines set out in the Law 11794/08 were followed, stipulating the conditions for the use of animals in scientific research and setting up the National Council for the Control of Animal Experimentation (CONCEA) requiring the establishment of ethics committees on the use of animals (CEUA) by institutions under operational standards set out in Decree 6899/2009, including the principles of the Brazilian Society of Science in Laboratory Animals (SBCAL). Accordingly, animal experiments carried out in this work were approved by the Ethics Commission for Animal Use (CEUA) of Fundação Oswaldo Cruz under the number P49/12-5.
Parasites
The LE strain of Schistosoma mansoni was maintained at Centro de Pesquisas René Rachou – FIOCRUZ using Biomphalaria glabrata as the intermediate snail host. Schistosomula were obtained by mechanical transformation of cercariae according to Howells et al (1974) [17] and cultured in MEM medium (Minimum Essential Medium Eagle) supplemented with 20 mM Hepes, 2 mM glutamate, 1×10−6 M serotonin, 5×10−7 M hypoxanthine, 2×10−7 M hydrocortisone, 0.5% MEM vitamin solution 100X, antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin), and 2% fetal bovine serum (FBS).
ERK/JNK phylogenetic analysis
In order to establish the evolutionary relationships among ERK and JNK proteins, homologs from S. mansoni (NCBI TaxID: 6183), S. haematobium (NCBI TaxID: 6185), S. japonicum (NCBI TaxID: 6182), Caenorhabditis elegans (NCBI taxID: 6239), Drosophila melanogaster (NCBI TaxID: 7227), and Homo sapiens (NCBI taxID: 9606) were selected for phylogenetic analysis. Amino acid sequences corresponding to the conserved catalytic domain (PF00069), present in JNK and ERK proteins, were aligned using MAFFT 7 with iterative refinement by the G-INS-i strategy [18] (Figure S1). The multiple sequence alignment comprising 34 sequences with 300 sites was manually refined using Jalview [19] and further used in phylogenetic analysis. To reconstruct the phylogenetic tree we used MrBayes (version 3.2.1), which performs Bayesian inference using a variant of the Markov chain Monte Carlo (MCMC) [20]. MCMC analyses were run as four chains for 10,000,000 generations and sampled every 100 generations. Of the initial samples, 25% were discarded as “burn-in.” Mixed models were applied as a parameter to estimate the best-fit evolutionary model. Support values were estimated as Bayesian posterior probabilities.
Selected genes and primer design
The S. mansoni sequences were downloaded from SchistoDB, version 3.0 [21]. We selected five genes from the MAPK signaling pathway to perform the RNAi experiments: SmCaMK2 (Smp_011660.2), SmJNK (Smp_172240), SmERK1 (Smp_142050), SmERK2 (Smp_047900) and SmRas (Smp_179910, previously characterized by [22]). In addition to the evaluation of transcript levels of those five genes, the transcription factors SmSRF (Smp_097730), SmC-Fos1 (Smp_124600) and SmC-Fos2 (Smp_170130) were also included in the analysis, in order to evaluate downstream interactions.
Procedures for dsRNA preparation, qPCR primer design and analysis, isolation of parasite RNA and reverse transcription to cDNA have been detailed previously [23] Primers were designed using the Primer 3 software (http://frodo.wi.mit.edu/) [24], [25] following strictly the MIQE guidelines [26] employing a 150–200 bp target product size for qPCR and 500–600 bp for templates of double stranded-RNA (dsRNA) (Figure S2). A T7 promoter tag was added to the 5′ end of all PCR primers designed for dsRNA template amplification (Table 1). A fragment of ∼500 bp of open reading frame for GFP (from the plasmid vector pCRII-GFP) was used as non- schistosome RNAi control [23]. Each qPCR primer was designed to anneal outside the targeted region of dsRNAs and was tested for primer annealing efficiency and optimal concentration. SmCaMK2 (Smp_011660.1) has two predicted alternative splicing products (Smp_011660.2 and Smp_011660.3). The regions selected to design the dsRNA and to measure the transcription level by qPCR were identical in the three isoforms.
Table 1. Primer sequences.
| Gene ID | Primer name | Primer sequence | |
| DsRNA primers | Smp_142050 | SmERK1 | Fow:5′-taatacgactcactatagggTTGGTCAATTGGTTGTATTATGG-3′ |
| Rev:5′-taatacgactcactatagggGGAACAATGGCACCAGGAAT-3′ | |||
| Smp_047900 | SmERK2 | Fow:5′-taatacgactcactatagggTCTGCCAGCGAACATATCG-3′ | |
| Rev:5′-taatacgactcactatagggGGATCACCAAGTCGTGAAGA-3′ | |||
| Smp_011660.2 | SmCaMK2 | Fow:5′-taatacgactcactatagggGATGACATTCAGGACGAAGG-3′ | |
| Rev:5′-taatacgactcactatagggTCGCAGGACTGACTGTTAG-3′ | |||
| Smp_172240 | SmJNK | Fow:5′-taatacgactcactatagggACATGCAGCCGGTATAATCC-3′ | |
| Rev:5′-taatacgactcactatagggTTACTTCAGAGTCTTCATACCATACG-3′ | |||
| Smp_179910 | SmRas | Fow:5′-taatacgactcactatagggTGGCACCAGAACTTATCAGG-3′ | |
| Rev:5′-taatacgactcactatagggGATATAGAGCAGTCATTGCATTCC-3′ | |||
| pCRII-GFP | GFP | Fow:5′-taatacgactcactatagggTCTTCAAGTCCGCCATG-3′ | |
| Rev:5′-taatacgactcactatagggTGCTCAGGTAGTGGTTGTC-3′ | |||
| qPCR primers | Smp_142050 | qSmERK1 | Fow:5′-TGCAACATCTTGTTGAATGC-3′ |
| Rev:5′-GCACGATACCAACGTGTACG-3′ | |||
| Smp_047900 | qSmERK2 | Fow:5′-TTATCCTTCGGCGGATGC-3′ | |
| Rev:5′-AGCAACAGGCTCATCACTAGG-3′ | |||
| Smp_011660.2 | qSmCaMK2 | Fow:5′-ACGACTATGCTAGCCACACG-3′ | |
| Rev:5′-CAGACGATTCCTTAATACCATCG-3′ | |||
| Smp_172240 | qSmJNK | Fow:5′-TCCTCCTGGGTATCATGTCG-3′ | |
| Rev:5′-GCTACAACAAAGCCCTGAGC-3′ | |||
| Smp_179910 | qSmRas | Fow:5′-GACTGAGTACAAGTTAGTTGTTGTTGG-3′ | |
| Rev:5′-TTCTATAAGAGTCCTCTATCGTTGG-3′ | |||
| Smp_124600 | qSmc-Fos1 | Fow:5′-GAGGCTGCAAGAGAATGTCG-3′ | |
| Rev:5′-CAAAGTGCTTTAACTTTCTGAAGC-3′ | |||
| Smp_170130 | qSmc-Fos2 | Fow:5′-TTGTTTCTCGTCCATCCACA-3′ | |
| Rev:5′-GAAACAGCTTGACGTTGTGC-3′ | |||
| Smp_097730 | qSmSRF | Fow:5′-GATACCTATTGAATTTATTTCTGATCG-3′ | |
| Rev:5′-CGGTTAATTCAGCCAATTCC-3′ | |||
| AF216698.1 | COX | Fow:5′-TACGGTTGGTGGTGTCACAG-3′ | |
| Rev:5′-ACGGCCATCACCATACTAGC-3′ |
dsRNA synthesis and parasite exposure
Following amplification, PCR products were separated on 1% agarose gels and purified using QIAquick Gel Extraction Kit (QIAGEN). DsRNAs targeting specific S. mansoni genes were generated from PCR products of approximately 500 bp that had been amplified from schistosomula cDNA using the T7 RiboMAX Express RNAi Kit (Promega) as described elsewhere [23], [27]. Final dsRNA synthesis reactions were allowed to incubate for 16 h at 37°C prior to DNAse treatment. DsRNA was analyzed by electrophoresis in 1% agarose gels to ensure that the correct length of product was generated: sequence identity was confirmed by DNA Sanger sequencing.
Schistosomula (2,000 worms) were cultivated in 24-well polystyrene plates containing 2 mL MEM supplemented with 1% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. For each treatment, 100 nM of dsRNA were added in the first day. Incubations were carried for 2, 4 or 7 days at 37°C under 5% CO2. The experiments were performed in duplicate and in three biological replicates.
Gene expression analyses (qPCR)
For qPCR, total RNA was extracted using the RNeasy Mini Kit (Qiagen). Residual DNA was removed by DNase digestion using the Turbo DNA-free kit (Ambion, Life Technologies). RNA (100 ng) was used to synthesize cDNA with the Superscript III cDNA Synthesis kit (Life Technologies). Each cDNA sample was tested in three technical replicates per plate using a minimum of 3 biological replicates. Experiments were carried out in a 7500 Real Time PCR System (Life Technologies) using the Power SYBR Green Master mix (Life Technologies). Reactions were carried out in a final volume of 25 µl in 96 well plates. S. mansoni cytochrome C oxidase I (GenBank AF216698) was used as the sample normalizing transcript [28], [29], as it has been shown to be highly and constitutively expressed in various S. mansoni life-cycle stages [30], [27] and GFP cDNA was used as endogenous control [31]. Two internal controls assessing both possible genomic DNA contaminations (no reverse transcriptase) and purity of the reagents (no cDNA) were included. The 2−ΔΔCt method was used to measure transcript levels post-RNAi [32]. Transcript levels were expressed as percentage of difference compared to those following exposure to the schistosome- unspecific GFP dsRNA. Statistical analysis employed the Mann-Whitney U-test (p<0.05).
In vivo experiments - adult worm and egg recovery
Swiss Webster mice were subcutaneously injected with 300 schistosomula 2 days after dsRNA treatment (3 independent experiments, 6 animals per group). After 37 days when the parasite has matured, mice were perfused according to Pellegrino and Siqueira (1956) [33] and the adult worms counted. Livers from infected animals were weighed and the eggs counted after digestion with 10% KOH. Statistical significance of the data was analyzed using the Mann-Whitney test (Wilcoxon-Sum of Ranks, p<0.05, N = 3).
Adult worm samples recovered after perfusion were analyzed by confocal microscopy. The parasites were fixed in AFA (2% acetic acid, 10% formaldehyde and 48% ethanol) and stored at room temperature. Whole worms were stained with 2.5% hydrochloric carmine, dehydrated by passage through 70, 90 and 100% ethanol, clarified with methyl salicylate and Canada balsam (1∶2), and individually mounted on glass slides.
Morphometric analyzes were performed on male and female worms using computer images (Image Pro Plus - Media Cybernetics, USA) captured by a Sony camera (640×480 pixels, RGB) coupled to a light microscope (Olympus BX50). The following parameters were determined: number and area of testicular lobes, area of the ovary, the presence of eggs and vitelline glands, the integrity of tegument and presence of surface tubercles. Statistical significance of the data was analyzed using the Mann-Whitney test (Wilcoxon-Sum of Ranks, p<0.05). It was analyzed 5 females that were treated with SmERK dsRNA, 6 for SmJNK, 8 for SMCaMK2 and 6 GFP control; 13 males that were treated with SmERK dsRNA, 5 for SmJNK, 6 for SmCaMK2 and 9 for GFP control.
Confocal microscopy images of the reproductive system and tegument were taken using a LSM-410, (Zeiss) equipped with a 488 nm HeNe laser and a LP 585 filter in reflected mode.
Results
ERK and JNK conservation
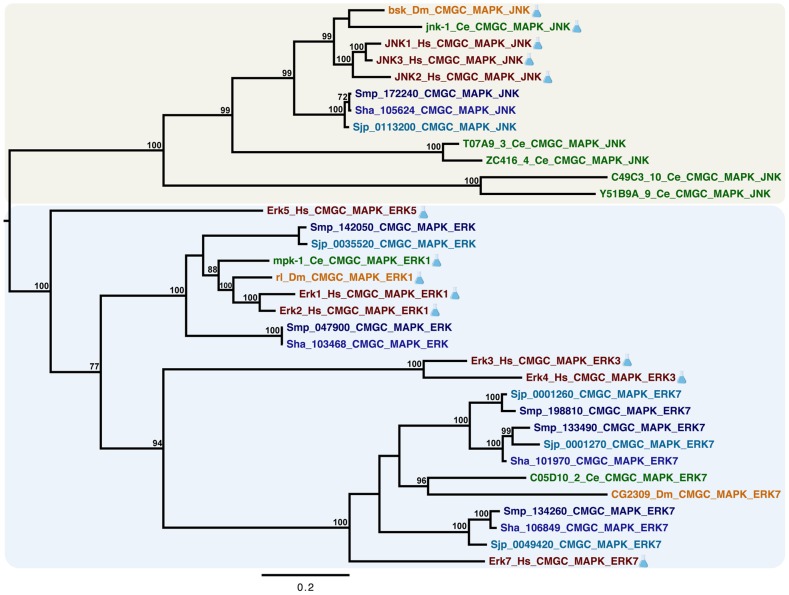
It has previously been shown that S. mansoni expresses only one JNK sub-family member, which contrasts to the presence of five and three homologs in C. elegans and humans, respectively [8]. This evolutionary constriction of the JNK subfamily in S. mansoni suggests that the SmJNK protein may be a potential target for drug development. Aiming at characterizing the evolutionary relationships between ERK and JNK proteins encoded by parasites and free-living organisms, we performed phylogenetic analyses on selected homologs from three Platyhelminths (S. mansoni, S. haematobium and S. japonicum), one nematode (Caenorhabditis elegans), one arthropod (Drosophila melanogaster) and one chordate (Homo sapiens). This taxon sampling covers important evolutionary innovations in processes in which kinases are directly involved in responses to environmental stimuli such as reproduction and development. As shown by Figure 1, gene duplication followed by divergence was probably the main evolutionary mechanism driving the evolution of the ERK and JNK subfamily members. The tree topology shows two well-supported clades grouping ERK and JNK proteins, thus revealing that the catalytic domain (PF00069) is sufficiently divergent to discriminate these two protein subfamilies. The number of orthologs in schistosomes and other metazoans varies and the presence of sequence variants may implicate structural and/or functional specializations. In most cases, when orthologs were identified in the three Schistosoma species, the relationships among them reflected the current knowledge regarding the origin and evolution of the Schistosoma lineage [34]. Together, these findings demonstrate that ERK and JNK proteins are evolutionarily conserved in metazoan species transducing signals from the cell surface to the nucleus.
Figure 1. Evolutionary relationships of ERK and JNK proteins.
Evolutionary relationships of 34 ERK and JNK proteins encoded by schistosome parasites (S. haematobium, S. japonicum and S. mansoni [different shades of blue]), Caenorhabditis elegans (green), Drosophila melanogaster (yellow), and Homo sapiens (red) as inferred by Bayesian analysis. Experimentally characterized proteins are indicated by an Erlenmeyer symbol. Different background colors highlight two clades: one containing ERK proteins and another containing JNK proteins. Support values were computed by posterior probability. The analysis was performed with conserved amino acid sequences corresponding to the catalytic domain (PF00069). Mixed models were selected as implemented in MrBayes with 10 million generations sampled every 100 generations.
MAPK members are susceptible to RNAi but knockdown efficiency varies
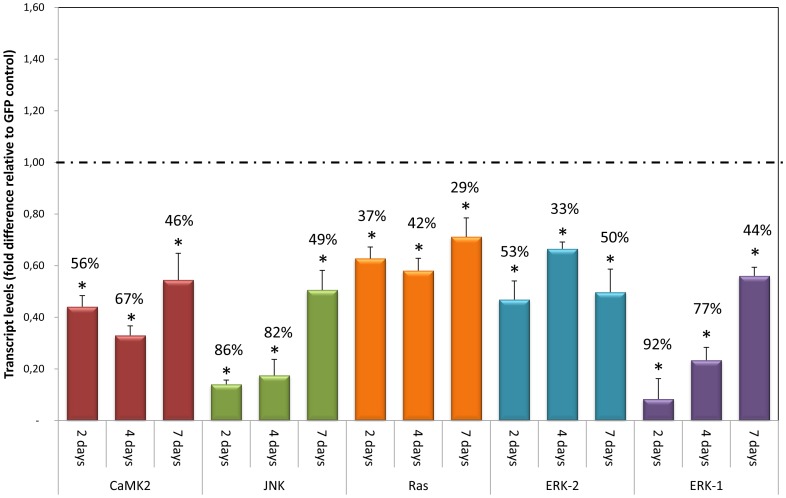
To functionally characterize MAPK pathway members (SmERK1, SmERK2, SmJNK, SmCaMK2 and SmRas) by RNAi, we co-incubated schistosomula with synthetic double-strand RNAs (dsRNA) in vitro. Relative to schistosome-unspecific controls using dsRNA to GFP, all genes targeted were sensitive to RNAi and were substantially suppressed after two, four or seven days (Figure 2). Transcript levels were reduced by up to 92%, for SmERK1after two days (transcription levels relative to controls = 0,08+/−0,0079) to 42% for SmRas after four days (transcription levels relative to controls = 0,58+/−0,04) (Figure 2). Additionally, decreased transcript levels of SmERK2 were observed in parasites two days after SmERK1 dsRNA exposure (56% of inhibition: transcription levels relative to controls = 0,44+/−0,006) (data not shown), this could be due to the similarity between SmERK1 and SmERK2. After this observation we decided to call the ERK1 treatment ERK1/2.
Figure 2. Transcript levels of target genes in schistosomula 2, 4, and 7 days after exposure to dsRNA.
Bar graph indicating the relative steady-state transcript levels of SmCaMK2 (red), SmJNK (green), SmRas (orange), SmERK-2 (blue), and SmERK1 (purple) genes after 2, 4, and 7 days after dsRNA exposure. For each dsRNA tested, data are represented as mean fold-differences (+/−SE) relative to GFP control (1.00 – dashed line). Transcript levels were determined by qPCR and data analyzed using the ΔΔCt method [24], followed by statistical analysis using the Mann-Whitney U-test. Data were generated from 3 independent experiments, each one in duplicate, and all the data shown is statistically different from GFP controls.
RNAi of SmJNK and SmERK limits parasite survival and/or fecundity in vivo
To investigate whether RNAi of SmCaMK2, SmJNK and SmERK1/2 impacts parasite viability in vivo, schistosomula were first incubated for two days with dsRNA and then transferred into mice (n = 6 per treatment). After 37 days, adult worms were perfused from the hepatic portal system and eggs recovered from livers. Due to the lack of effective RNAi knockdown, parasites treated with SmRas-dsRNA was not included in the in vivo test.
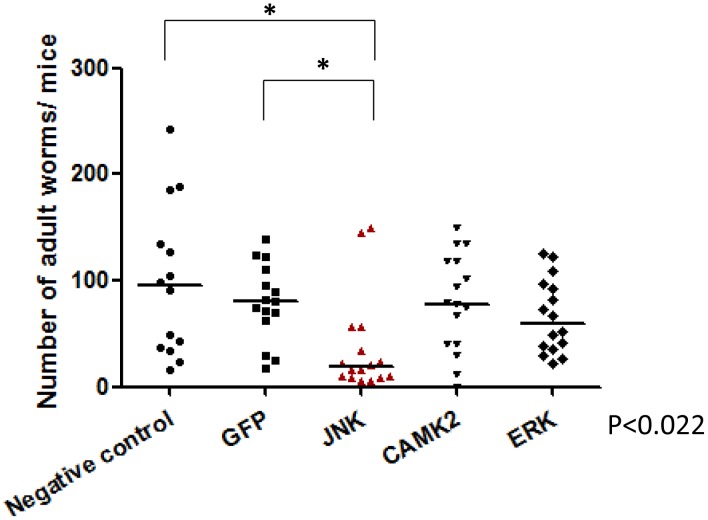
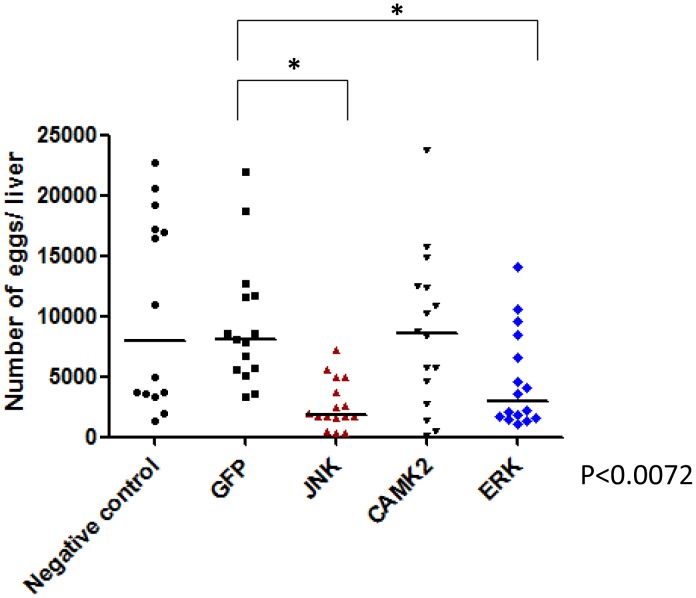
RNAi of SmJNK in schistosomula resulted in the death of 56% of parasites relative to the GFP control (Figure 3). Also, the number of hepatic eggs was decreased by 59% (Figure 4). For SmCaMK2 and SmERK1/2, no significant changes in the number of adult worms were observed post-RNAi (Figure 3); also, RNAi of CaMK2 did not alter egg output. On the other hand, although the knockdown of SmERK1/2 did not seem to affect parasite survival, egg production was decreased by 44% relative to parasites GFP-dsRNA treated (control) (Figure 4). Transcript levels of SmCaMK2, SmJNK and SmERK1/2 returned to its normal level of expression in 37 day-old worms (data not shown).
Figure 3. Survival of the parasite after RNAi of MAPKs in vitro and subsequent transfer into mice.
Schistosomula were treated with GFP, SmJNK, SmCaMK2, and SmERK1 dsRNAs for two days and then injected into mice. After 37 days adult worms were recovered and counted. Each symbol in the chart represents worm counts from one mouse and the horizontal lines are median values per treatment group. Data were generated from 3 independent experiments and all treatments were statistical analyzed using Mann-Whitney U-test within each experiment (P≤0.05). The asterisk indicates a significance value of P<0.022 for RNAi of SmJNK relative to the GFP control.
Figure 4. Hepatic egg counts after RNAi of MAPKs in vitro and subsequent transfer of parasites into mice.
Schistosomula were treated with GFP, SmJNK, SmCaMK2 and SmERK1 dsRNAs for two days in vitro and then injected into mice. After 37 days of parasite eggs per liver digest were counted. Each symbol in the chart represents worm counts from one mouse and the horizontal lines are median values per treatment group. Data were generated from 3 independent experiments and all treatments were statistical analyzed using Mann-Whitney U-test within each experiment (P≤0.05). The asterisk indicates a significance value of SmJNK and SmERK P<0.0072 relative to the GFP control.
RNAi of SmJNK and SmERK alters parasite morphology
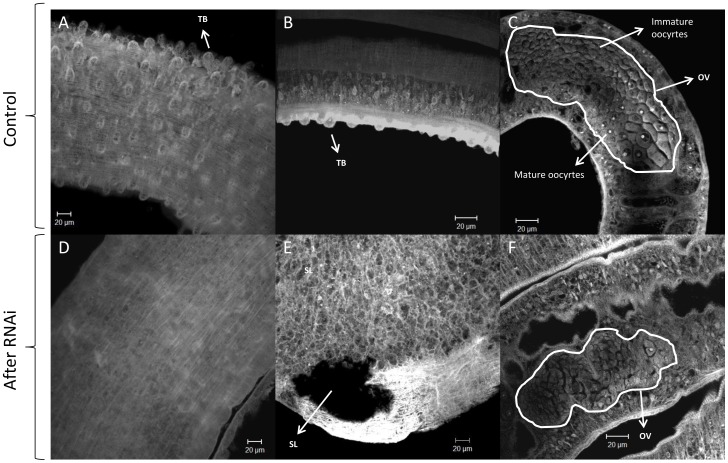
Confocal microscopy was employed to understand whether parasite morphology was also altered in association with the decreased viability and/or egg production after RNAi of SmJNK and SmERK1/2. It was possible to observe that RNAi of SmJNK damaged the adult male tegument (Figure 5) in which the tubercles were reduced (Figure 5D) and unusual dilations were observed (Figure 5E). In addition, in the females control (Figure 5C) the ovary presents oocytes ranging from immature cells to mature cells with large and clearly nuclei and evident nucleolus, but the females worms treated with JNK dsRNA presented undifferentiated oocytes (cells throughout the uterus present the same size) (Figure 5F).
Figure 5. Morphology of adult worms after RNAi of SmJNK in vitro and subsequent transfer of parasites into mice.
Adult 37-day-old worms were fixed and stained, and visualized by confocal microscopy as described in the text. A, B and C show normal worms treated with GFP dsRNA, whereas D, E and F show morphological changes in worms treated with SmJNK dsRNA. A and B - the tubercules (TB) are highlighted on the tegument; C – female worm ovary (OV) showing immature and mature oocytes; D – muscular structure of a worm without tubercules; E- subtegumentar lesion (SL); F- immature ovary.
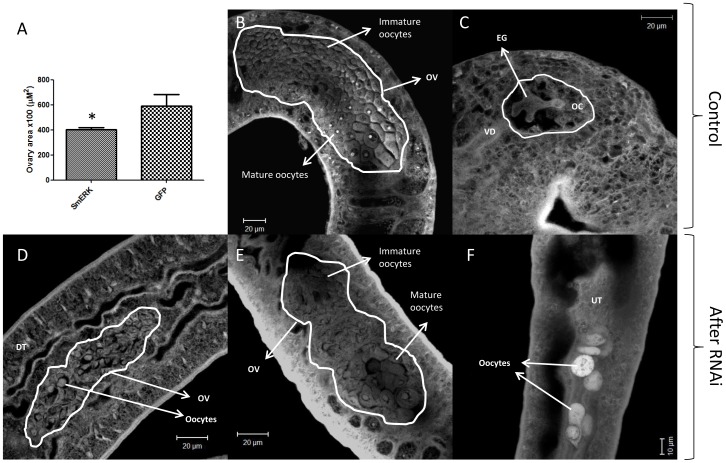
The knockdown of SmERK1/2 did not cause changes in male worms (data not shown). The tegument and testicular lobes appeared to be normal and the seminal vesicle was full of spermatozoids. However, the females showed alterations in the reproductive system such as small ovaries (∼44% smaller than GFP controls) (Figure 6A) containing immature oocytes (Figure 6D) or, even when mature oocytes were observed (Figure 6E), a higher number of oocytes were present in the uterus (Figure 6F) whereas eggs were expected in this location, like the ones observed in the control group (Figure 6C).
Figure 6. Morphology of adult female worms after RNAi of SmERK1/2 in vitro and subsequent transfer of parasites into mice.
Adult 37-day-old worms were fixed and stained, and visualized by confocal microscopy as described in the text. A - mean of females' ovary area (µM2) of SmERK-knockdown and control showing a significant size reduction; B and C show normal worms treated with GFP dsRNA where the ovary (OV) with immature and mature oocytes, an egg (EG) and the vitelloduct (VD) are visible; D, E and F show morphological changes in worms treated with SmERK dsRNA where the ovary (D) present no mature oocytes (D) or even when mature oocytes are visible (E) an unexpected phenotype (a lot of oocytes) is observed in the uterus (UT) (F). The eggs shoud be fully formed in the uterus as showing in (C). Statistical analyses were performed using Mann-Whitney U-test, P≤0.05; n = 5).
In addition, RNAi of SmCaMK2 induced no apparent morphological alterations (Figure S3).
RNAi of MAPK genes alters the transcript levels of downstream target genes
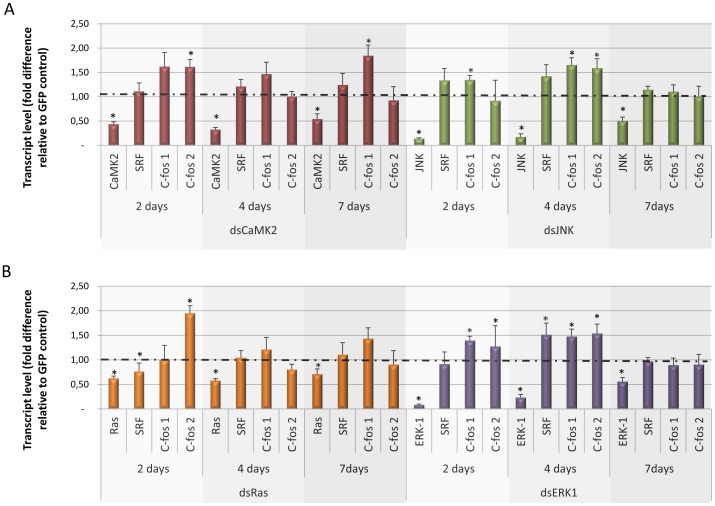
In other organisms MAPK pathway, the downstream genes are transcribed when the ELK1/SRF complex binds to the promoter region of c-fos gene. To study the conservation of the MAPK pathway in S. mansoni compared to other metazoans, we measured the transcript levels of the SRF transcription factor and c-fos genes after RNAi of SmCaMK2, SmJNK, SmRas and SmERK1. RNAi of the first three targets caused the over expression of Smc-fos1 (by 1.62+/−0.28; 1.65+/−0.14; 1.21+/−019 and 1.47+/−0.06, respectively) and Smc-fos2 (by 1.65+/−0.15; 1.59+/−0.19; 1.95+/−0.24 and 1.53+/−0.02, respectively) (relative to the GFP control) (Figure 7). In addition, as the MAPKs (SmJNK and SmERK-1) transcript levels increased up to seven days, the Smc-fos1 and Smc-fos2 RNA levels decreased (1.10+/−0.2 and 0.89+/−0.18) and (0.60+/−0.20 and 0.9+/−0.14), respectively (Figure 7). SmSRF gene expression, in most cases, did not exhibit variation. A minor alteration in Smc-fos1 transcript level was observed after RNAi of SmRas (Figure 7B).
Figure 7. Transcript levels of SmSRF and Smc-fos1 and Smc-fos2 genes 2, 4, and 7 days after schistosomula were exposed to various MAPK dsRNAs.
Bar graph indicating the relative steady-state transcript levels of (A) SmCaMK2 (red), SmJNK (green) and (B) SmRas (orange), SmERK1(purple) genes after 2, 4, and 7 days of dsRNA exposure. For each dsRNA tested, data are represented as mean fold-differences (+/−SE) relative to GFP control (1.00). Transcript levels were determined by qPCR and data analyzed using the ΔΔCt method [24] followed by statistical analysis using the Mann-Whitney U-test. Data were generated from 3 independent experiments, each one in duplicate. Significance levels (*) were set at P≤0.05.
Discussion
MAPKs connect cell-surface receptors to regulatory targets within cells to coordinate gene expression. Members of this family regulate essential cellular processes and are conserved in eukaryotes [4]. It would be expected that MAPKs also have important functions in the schistosome parasite, however, little was known. Here, we demonstrate RNAi for genes related to MAPK signaling pathway, namely: SmJNK, SmERK-1, SmERK-2, SmCaMK2, SmRas. Knockdown efficiency reached levels of up to 92% for SmERK-1, whereas SmERK-2 was less susceptible with a 33% knockdown. Other authors [31], [27] also reported variable efficiencies of RNAi across different targets and for specific dsRNA sequences. It is possible that some genes are expressed in cells and tissues that are inaccessible to dsRNA and/or that the employed delivery method (soaking) did provide for maximal penetration of the RNAi effect. Also, the secondary structure of some mRNA targets might prevent or affect activation of the RISC complex [35].
Having demonstrated RNAi for the MAPKs studied, we next asked whether RNAi would limit survival and development of the parasites upon their transfer to mice. Thus, RNAi of SmJNK seems to be partially lethal and 56% of the parasites did not survive to 37 days, at which time worms were harvested from mice and counted. In addition, the recovered worms had morphological changes in the tegument. It's important to emphasize that survived worms may be affected to a lesser extent or maybe even not affected by RNAi treatment. Mourão and colleagues [31], after labeling the RNAi molecule with a fluorescent label, demonstrated that RNAi uptake was not equal among all parasites. So, the results suggest that SmJNK is an essential protein. This fact is reinforced by previous knowledge of JNK signaling pathway influencing metabolism, growth, regeneration, and stress tolerance in Drosophila lifespan regulation [36]. Moreover, in flies, the JNK signaling pathway is also involved in midgut epithelial homeostasis and may be important in other contexts, such as oxidative stress for protection against gut infections [37]. A strong inhibition of JNK signaling activity in Drosophila shortens lifespan due to complete inhibition of intestinal stem cells proliferation [36]. JNK signaling misregulation has also been implicated in regeneration, neurodegenerative diseases, diabetes, and cancer [38], [39], [40], [41], [13]. Moreover, JNK is only encoded by one gene in Schistosoma and Drosophila which is in contrast to the five subfamily members in C. elegans and three in humans. Accordingly, it's conceivable pivotal importance in the MAPK pathway and for downstream signaling may prove a valuable target point for small molecule interventions.
In C. elegans, the JNK pathway is also activated by CaMK (unc-43, a calcium/calmodulin-dependent protein kinase) in a cell-specific signaling pathway [10], [16]. As JNK, only one CaMK2 protein was found in the predicted proteomes of S. mansoni [42], [8] and S. haematobium. Although SmCaMK2 has been predicted to be an essential gene and potential drug target [42], our present findings showed that RNAi of SmCaMK2 does not alter worm morphology or survival in mice. A simple explanation is that RNAi of CAMK2 was not efficient enough (ranged between 46 and 67%) to generate a phenotypic outcome. We also do not exclude the possibility that CaMK2 may regulate the JNK pathway only in particular cell type(s) or that the CaMK2 protein turnover is faster than that of SmJNK. Although, the same phenotype for SmJNK and SmCaMK2 was expected, since CaMK2 (UNC-53) can activate JNK signaling pathway in C. elegans, no alteration was observed after CaMK2 dsRNA treatment in S. mansoni. There are at least two possible explanations to this outcome: i) SmCaMK2 is not related to JNK signaling pathway or ii) SmCaMK2 is not the only activator of JNK signaling pathway in Schistosoma.
RNAi of SmERK1/2 decreased the number of parasite eggs recovered from the liver and apparently elicited morphological alterations only in the reproductive system of female worms. Our data are consistent with the known contributions of ERK to oocyte maturation and egg activation in other animals [43], [44]. Thus, in Xenopus laevis, the ERK protein is involved in the coordination of oocyte maturation [45]. In C. elegans, the knockout of ERK, affects the development of the vulva (necessary for egg-laying) and oocytes, resulting in a loss of egg production [46]. In a closely related organism, Echinococcus multilocularis it has been demonstrated that the Erk-like MAPK is activated by soluble host growth factors that are released by host hepatocytes and triggers metacestode development in vitro [47]. Moreover, the use of Erk-like MAPK pathway inhibitors affected E. multilucularis development and growth, but did not induce mortality [48]. In mice, the inactivation of the ERK signaling pathway is associated with embryonic death caused by abnormal placental development [49]. Sandler and colleagues also demonstrated that ERK is involved in starfish egg apoptosis [50]. These data are consistent with the current results and suggest a functional conservation of the ERK pathway across metazoans.
The effects observed after the knockdown of SmERK1/2 and SmJNK MAPKs were probably the consequence of gene transcription modulation that occur downstream of the MAPK signaling pathway. In other systems, SRF is a transcription factor known to regulate the transcription of the c-fos gene after MAPK activation [6]. For examples, in mammals, the c-fos gene has a variable level of transcription that is dependent on elk-1/SRF binding. The latter complex, in turn, has a less variable transcription rate as it presents a stable conformation in either active (On) or inactive (Off) modes [51]. In order to determine whether the MAPK pathway induces c-fos expression in S. mansoni, c-fos and SRF transcripts levels were evaluated after transcript knockdown of SmRas, SmERK1/2, SmJNK and SmCaMK2.
In general, we noted that the transcript levels of SmSRF remained constant. However, it was noted a different regulation (up or down) of SmSRF after SmRAS and SmERK dsRNA treatment (Figure 7) that may be an influence of some negative feedback regulation, a wide-spread mechanism among signaling molecules, especially in the MAPK pathway [52], [53]. On the other hand, the transcription of Smc-fos1 and Smc-fos2 is upregulated after RNAi of MAPK pathway genes. Thus, SmERK1/2, SmCaMK2 and SmJNK negatively regulated c-fos, whereby low levels of those proteins induce c-fos transcription. This contrasts with mammalian systems in which the inactivation of ERK and JNK prevents SRF activation, which, in turn, does not bind to the c-fos promoter region [5].
In contrast to the mammalian c-fos activation mechanism, C. elegans has a pathway that is consistent with our results observed for S. mansoni. Specifically, elk-1 (LIN-31 in C. elegans) and SRF (LIN-1 in C. elegans) form a complex when MAPKs are not phosphorylated that activates c-fos, which then inhibits vulval development. When MAPK is phosphorylated, the elk-1/SRF complex dissociates and elk-1 promotes vulval development in a signaling pathway that is activated by epidermal growth factor (EGF) [54]. Moreover, it was recently reported that pathways involved in activating c-fos gene expression might be themselves activated by calcium influx through the CaMK signaling pathway [50]. In this case, c-fos expression is induced by phosphorylation of CaMK2 which, in turn, phosphorylates SRF without a direct relationship to ERK or JNK proteins [55].
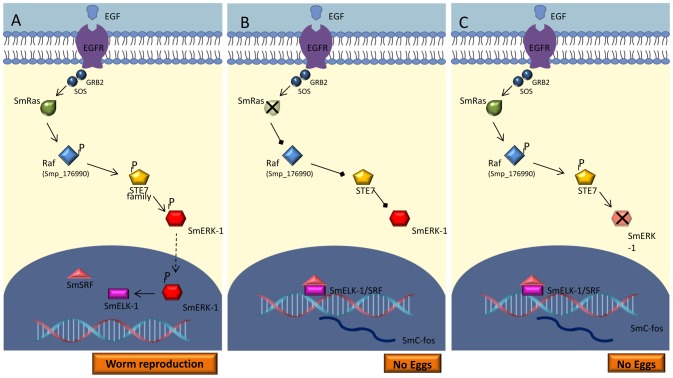
Together, it is possible that the high levels of Smc-fos1 and Smc-fos2 transcripts, as a result of RNAi of SmERK, SmJNK and SmCaMK2, is related to the inactivation of the MAPK signaling pathway which then induces the formation of the elk-1/SRF complex (Figure 8B–C). The elk-1/SRF complex is targeted by different signaling cascades and is involved in the regulation of c-fos. In S. mansoni, even though the outcomes of RNAi of SmJNK and SmCaMK2 were quite different, it does seem that both gene products contribute to the regulation of the c-fos gene. SmJNK and SmCaMK2 may be involved in independent pathways or they may simultaneously co-regulate the same gene in a particular cell type. On the other hand, SmRas and SmERK would act in the same pathway, as is the case for C. elegans, mammals and Drosophila, being directly involved in the development of S. mansoni eggs (Figure 8A).
Figure 8. Hypothetical S. mansoni MAPK signaling pathway.
(A) EGF activates the Ras/ERK signaling pathway. EGFR transmits the signal to the intracellular environment through the activation of Ras and sequential phosphorylation of SmRAF (ePK of TKL group and raf family), SmSTE7 (ePK of STE group and STE7family) and SmERK (ePK of CMGC group, MAPK family and ERK1/2 subfamily). Activated ERK translocates to the nucleus and inhibits the formation of the elk1/SRF complex and, in this case, oviposition remains constant. In B and C, SmRas or SmERK activity is interfered with (via RNAi) and the signal is not transmitted. Elk1 forms a complex with SRF which binds to the c-Fos promoter and this initiates c-Fos transcription that subsequently prevents the egg laying.
Our findings using RNAi demonstrate that S. mansoni MAPKs are essential to worm survival and/or reproduction suggesting that one or more of these kinases may be of interest in the development of new compounds to treat schistosomiasis. The complete mechanism by which MAPKs regulate those systems in Schistosoma still have to be elucidated to better focus on the most promising drug target.
Accession number for SchitoDB [21]: SmCaMK2 (Smp_011660.2), SmJNK (Smp_172240), SmERK1 (Smp_142050), SmERK2 (Smp_047900), SmRas (Smp_179910), SmSRF (Smp_097730), SmC-Fos (Smp_124600), SmC-Fos2 (Smp_170130).
Supporting Information
Multiple sequence alignment of ERK and JNK proteins encoded by parasites and free-living organisms. Amino acid sequences of the conserved catalytic domain (PF00069) were aligned using MAFFT 7 with iterative refinement by the G-INS-i strategy [18]. The multiple sequence alignment comprising 34 sequences with 300 sites was manually refined using Jalview [19]. The most conserved and important aminoacids for the catalitic activity are highlighted in the aligment (A–E).
(TIF)
S. mansoni dsRNA primers location. Protein ID is shown above each image. The total length of each gene and the DsRNA forward and reverse primer position are represented in the figure.
(TIF)
Morphology of adult male and female worms after RNAi of SmCaMK2 in vitro and subsequent transfer of parasites into mice. Adult 37-day-old worms were fixed and stained, and visualized by confocal microscopy as described in the text. A, C and E show male worms, whereas B, D and F shows female worms treated with SmCaMK2 dsRNA. No alterations are visible. It is possible to see that the testicular lobes (TL) are normal (A), seminal vesicle (SV), the duct for seminal pore (DP) and genital pore are visible (C), and tubercles (TB) are present in the tegument (E). The egg (EG) is fully formed (B), the ovary (OV) present mature and immature oocytes (D) and spermatozoides are visible in the spermathec (ES) (F).GP: genital pore; DP: duct for seminal pore; VD; vitelloduct; DT: digestive tract.
(TIF)
Acknowledgments
The authors acknowledge the use of the computing resources of the Center for Excellence in Bioinformatics, CEBio/FIOCRUZ, Brazil. The authors are grateful to Anderson Dominitini (CEBio), Adhemar Zerlotini (EMBRAPA), Eric Aguiar (CEBio) for bioinformatics technical support and to Mariana de Oliveira (CEBio) for help with illustrations for this work. We thank Brian M. Suzuki of the CDIPD-UCSF for expert technical support.
Funding Statement
This research was supported by the NIH/Fogarty International Center (TW007012 to GO), the National Council for Research and Development - CNPq (CNPq Research Fellowship 309312/2012-4 to GO, INCT-DT 573839/2008-5 to GO, fellowship to MdMM), and FAPEMIG (CBB - APQ-00520-13 to MdMM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Allen JE, Maizels RM (2011) Diversity and dialogue in immunity to helminths. Nature Reviews Immunology 11: 375–388. [DOI] [PubMed] [Google Scholar]
- 2. Pearce EJ, Sher A (1987) Mechanisms of immune evasion in schistosomiasis. Contrib Microbiol Immunol 8: 219–232. [PubMed] [Google Scholar]
- 3. Han ZG, Brindley PJ, Wang SY, Chen Z (2009) Schistosoma genomics: new perspectives on schistosome biology and host-parasite interaction. Annu.Rev.Genomics Hum.Genet 10: 211–240. [DOI] [PubMed] [Google Scholar]
- 4. Wilkinson MG, Millar JB (2000) Control of the eukaryotic cell cycle by MAP kinase signaling pathways. FASEB J 14: 2147–2157. [DOI] [PubMed] [Google Scholar]
- 5. Hazzalin CA, Mahadevan LC (2002) MAPK-regulated transcription: A continuously variable gene switch? Nature Reviews Molecular Cell Biology 3: 30–40. [DOI] [PubMed] [Google Scholar]
- 6. Cavigelli M, Dolfi F, Claret FX, Karin M (1995) Induction of C-Fos Expression Through Jnk-Mediated Tcf/Elk-1 Phosphorylation. Embo Journal 14: 5957–5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pearson G, Robinson F, Gibson TB, Xu BE, Karandikar M, et al. (2001) Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocrine Reviews 22: 153–183. [DOI] [PubMed] [Google Scholar]
- 8. Andrade LF, Nahum LA, Avelar LG, Silva LL, Zerlotini A, et al. (2011) Eukaryotic protein kinases (ePKs) of the helminth parasite Schistosoma mansoni. BMC.Genomics 12: 215-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tasaki J, Shibata N, Nishimura O, Itomi K, Tabata Y, et al. (2011) ERK signaling controls blastema cell differentiation during planarian regeneration. Development 138: 2417–2427. [DOI] [PubMed] [Google Scholar]
- 10. Sundaram MV. (2006) RTK/Ras/MAPK signaling. WormBook. 1–19. [Google Scholar]
- 11. Vicogne J, Cailliau K, Tulasne D, Browaeys E, Yan YT, et al. (2004) Conservation of epidermal growth factor receptor function in the human parasitic helminth Schistosoma mansoni. J.Biol.Chem 279: 37407–37414. [DOI] [PubMed] [Google Scholar]
- 12. Borsello T, Forloni G (2007) JNK signalling: A possible target to prevent neurodegeneration. Current Pharmaceutical Design 13: 1875–1886. [DOI] [PubMed] [Google Scholar]
- 13. Tasaki J, Shibata N, Sakurai T, Agata K, Umesono Y (2011) Role of c-Jun N-terminal kinase activation in blastema formation during planarian regeneration. Development Growth & Differentiation 53: 389–400. [DOI] [PubMed] [Google Scholar]
- 14. Emes RD, Yang ZH (2008) Duplicated Paralogous Genes Subject to Positive Selection in the Genome of Trypanosoma brucei. Plos One 3: 10.1371/journal.pone.0002295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Swulius MT, Waxham MN (2008) Ca(2+)/calmodulin-dependent protein kinases. Cell Mol.Life Sci 65: 2637–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sagasti A, Hisamoto N, Hyodo J, Tanaka-Hino M, Matsumoto K, et al. (2001) The CaMKII UNC-43 activates the MAPKKK NSY-1 to execute a lateral signaling decision required for asymmetric olfactory neuron fates. Cell 105: 221–232. [DOI] [PubMed] [Google Scholar]
- 17. Howells RE, Ramalhopinto FJ, Gazzinelli G, Deoliveira CC, Figueiredo EA, et al. (1974) Schistosoma-Mansoni - Mechanism of Cercarial Tail Loss and Its Significance to Host Penetration. Experimental Parasitology 36: 373–385. [DOI] [PubMed] [Google Scholar]
- 18. Katoh K, Asimenos G, Toh H (2009) Multiple alignment of DNA sequences with MAFFT. Methods Mol.Biol 537: 39–64. [DOI] [PubMed] [Google Scholar]
- 19. Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ (2009) Jalview Version 2-a multiple sequence alignment editor and analysis workbench. Bioinformatics 25: 1189–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, et al. (2012) MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zerlotini A, Aguiar ERGR, Yu FD, Xu HY, Li YX, et al. (2013) SchistoDB: an updated genome resource for the three key schistosomes of humans. Nucleic Acids Research 41: D728–D731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Osman A, Niles EG, LoVerde PT (1999) Characterization of the Ras homologue of Schistosoma mansoni. Mol.Biochem.Parasitol 100: 27–41. [DOI] [PubMed] [Google Scholar]
- 23. Marek M, Kannan S, Hauser AT, Moraes Mourão M, Caby S, et al. (2013) Structural basis for the inhibition of histone deacetylase 8 (HDAC8), a key epigenetic player in the blood fluke Schistosoma mansoni. Plos Pathogens 9: e1003645 10.1371/journal.ppat.1003645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koressaar T, Remm M (2007) Enhancements and modifications of primer design program Primer3. Bioinformatics 23: 1289–1291. [DOI] [PubMed] [Google Scholar]
- 25. Untergrasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, et al. (2012) Primer3 - new capabilities and interfaces. Nucleic Acids Res 40: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, et al. (2009) The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clinical Chemistry 55: 611–622. [DOI] [PubMed] [Google Scholar]
- 27. Stefanic S, Dvorak J, Horn M, Braschi S, Sojka D, et al. (2010) RNA interference in Schistosoma mansoni schistosomula: selectivity, sensitivity and operation for larger-scale screening. PLoS.Negl.Trop.Dis 4: e850-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Le TH, Blair D, Agatsuma T, Humair PF, Campbell NJ, et al. (2000) Phylogenies inferred from mitochondrial gene orders-a cautionary tale from the parasitic flatworms. Mol.Biol.Evol 17: 1123–1125. [DOI] [PubMed] [Google Scholar]
- 29. Oliveira G, Busek S, Correa-Oliveira R (1998) Transcription levels of two actin genes (SmAct and SmAct2), cytochrome C oxidase subunit II (SmCOXII), triosephosphate isomerase (TPI), and a putative translation regulatory protein EIF-5 during the first seven days of in vitro development of Schistosoma mansoni schistosomula. Mem.Inst.Oswaldo Cruz 93 Suppl 1: 215–217. [DOI] [PubMed] [Google Scholar]
- 30. Jolly ER, Chin CS, Miller S, Baghat MM, Lim KC, et al. (2007) Gene expression patterns during adaptation of a helminth parasite to different environmental niches. Genome Biol 8: R65-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mourao MM, Dinguirard N, Franco GR, Yoshino TP (2009) Phenotypic screen of early-developing larvae of the blood fluke, schistosoma mansoni, using RNA interference. PLoS.Negl.Trop.Dis 3: e502-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 33. Pellegrino J, SIQUEIRA AF (1956) [A perfusion technic for recovery of Schistosoma mansoni from experimentally infected guinea pigs.]. Rev.Bras.Malariol.Doencas.Trop 8: 589–597. [PubMed] [Google Scholar]
- 34. Silva LL, Marcet-Houben M, Nahum LA, Zerlotini A, Gabaldón T, et al. (2012) The Schistosoma mansoni phylome: using evolutionary genomics to gain insight into a parasite's biology. BMC.Genomics 13: 617 10.1186/1471-2164-13-617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rao MK, Wilkinson MF (2006) Tissue-specific and cell type-specific RNA interference in vivo. Nature Protocols 1: 1494–1501. [DOI] [PubMed] [Google Scholar]
- 36. Biteau B, Karpac J, Supoyo S, DeGennaro M, Lehmann R, et al. (2010) Lifespan Extension by Preserving Proliferative Homeostasis in Drosophila. Plos Genetics 6: e1001159 10.1371/journal.pgen.1001159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ha EM, Oh CT, Ryu JH, Bae YS, Kang SW, et al. (2005) An antioxidant system required for host protection against gut infection in Drosophila. Developmental Cell 8: 125–132. [DOI] [PubMed] [Google Scholar]
- 38. Hotamisligil GS (2010) Endoplasmic reticulum stress and atherosclerosis. Nature Medicine 16: 396–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karin M, Gallagher E (2005) From JNK to pay dirt: Jun kinases, their biochemistry, physiology and clinical importance. Iubmb Life 57: 283–295. [DOI] [PubMed] [Google Scholar]
- 40. Sabio G, Davis RJ (2010) cJun NH2-terminal kinase 1 (JNK1): roles in metabolic regulation of insulin resistance. Trends in Biochemical Sciences 35: 490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weston CR, Davis RJ (2002) The JNK signal transduction pathway. Current Opinion in Genetics & Development 12: 14–21. [DOI] [PubMed] [Google Scholar]
- 42. Caffrey CR, Rohwer A, Oellien F, Marhofer RJ, Braschi S, et al. (2009) A comparative chemogenomics strategy to predict potential drug targets in the metazoan pathogen, Schistosoma mansoni. PLoS.One 4: e4413-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fan HY, Sun QY (2004) Involvement of mitogen-activated protein kinase cascade during oocyte maturation and fertilization in mammals. Biology of Reproduction 70: 535–547. [DOI] [PubMed] [Google Scholar]
- 44. Sackton KL, Buehner NA, Wolfner MF (2007) Modulation of MAPK activities during egg activation in Drosophila. Fly 1: 222–227. [DOI] [PubMed] [Google Scholar]
- 45. Nebreda AR, Ferby I (2000) Regulation of the meiotic cell cycle in oocytes. Curr.Opin.Cell Biol 12: 666–675. [DOI] [PubMed] [Google Scholar]
- 46. Hajnal A, Berset T (2002) The C.elegans MAPK phosphatase LIP-1 is required for the G(2)/M meiotic arrest of developing oocytes. EMBO J 21: 4317–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Spiliotis M, Konrad C, Gelmedin V, Tappe D, Brückner S, et al. (2006) Characterisation of EmMPK1, an ERK-like MAP kinase from Echinococcus multilocularis which is activated in response to human epidermal growth factor. Int.J.Parasitol 36: 1097–1112. [DOI] [PubMed] [Google Scholar]
- 48. Gelmedin V, Spiliotis M, Brehm K (2010) Molecular characterisation of MEK1/2- and MKK3/6-likemitogen-activatedproteinkinasekinases (MAPKK) from thefoxtape worm Echinococcus multilocularis. Int.J.Parasitol 40: 555–567. [DOI] [PubMed] [Google Scholar]
- 49. Giroux S, Tremblay M, Bernard D, Cadrin-Girard JF, Aubry S, et al. (1999) Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Current Biology 9: 369–372. [DOI] [PubMed] [Google Scholar]
- 50. Sadler KC, Yuce O, Hamaratoglu F, Verge V, Peaucellier G, et al. (2004) MAP kinases regulate unfertilized egg apoptosis and fertilization suppresses death via Ca2+ signaling. Molecular Reproduction and Development 67: 366–383. [DOI] [PubMed] [Google Scholar]
- 51. Johnson RS, Spiegelman BM, Papaioannou V (1992) Pleiotropic Effects of A Null Mutation in the C-Fos Protooncogene. Cell 71: 577–586. [DOI] [PubMed] [Google Scholar]
- 52. Owens DM, Keyse SM (2007) Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Nature Oncogene 26: 3203–3213. [DOI] [PubMed] [Google Scholar]
- 53. Kondoh K, Nishida E (2007) Regulation of MAP kinases by MAP kinase phosphatases. Biochim.Biophys.Acta 1773: 1227–1237. [DOI] [PubMed] [Google Scholar]
- 54. Tan PB, Lackner MR, Kim SK (1998) MAP kinase signaling specificity mediated by the LIN-1 Ets/LIN31 WH transcription factor complex during C-elegans vulval induction. Cell 93: 569–580. [DOI] [PubMed] [Google Scholar]
- 55. Ely HA, Mellon PL, Coss D (2011) GnRH Induces the c-Fos Gene via Phosphorylation of SRF by the Calcium/Calmodulin Kinase II Pathway. Molecular Endocrinology 25: 669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multiple sequence alignment of ERK and JNK proteins encoded by parasites and free-living organisms. Amino acid sequences of the conserved catalytic domain (PF00069) were aligned using MAFFT 7 with iterative refinement by the G-INS-i strategy [18]. The multiple sequence alignment comprising 34 sequences with 300 sites was manually refined using Jalview [19]. The most conserved and important aminoacids for the catalitic activity are highlighted in the aligment (A–E).
(TIF)
S. mansoni dsRNA primers location. Protein ID is shown above each image. The total length of each gene and the DsRNA forward and reverse primer position are represented in the figure.
(TIF)
Morphology of adult male and female worms after RNAi of SmCaMK2 in vitro and subsequent transfer of parasites into mice. Adult 37-day-old worms were fixed and stained, and visualized by confocal microscopy as described in the text. A, C and E show male worms, whereas B, D and F shows female worms treated with SmCaMK2 dsRNA. No alterations are visible. It is possible to see that the testicular lobes (TL) are normal (A), seminal vesicle (SV), the duct for seminal pore (DP) and genital pore are visible (C), and tubercles (TB) are present in the tegument (E). The egg (EG) is fully formed (B), the ovary (OV) present mature and immature oocytes (D) and spermatozoides are visible in the spermathec (ES) (F).GP: genital pore; DP: duct for seminal pore; VD; vitelloduct; DT: digestive tract.
(TIF)