Abstract
Background
Biodegradable polymeric coatings have been proposed as a promising strategy to enhance biocompatibility and improve the delayed healing in the vessel. However, the efficacy and safety of biodegradable polymer drug-eluting stents (BP-DES) vs. bare metal stents (BMS) are unknown. The aim of this study was to perform a meta-analysis of randomized controlled trials (RCTs) comparing the outcomes of BP-DES vs. BMS.
Methods and Results
PubMed, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) were searched for randomized clinical trials, until December 2013, that compared any of approved BP-DES and BMS. Efficacy endpoints were target-vessel revascularization (TVR), target-lesion revascularization (TLR) and in-stent late loss (ISLL). Safety endpoints were death, myocardial infarction (MI), definite stent thrombosis (DST). The meta-analysis included 7 RCTs with 2,409 patients. As compared with BMS, there was a significantly reduced TVR (OR [95% CI] = 0.37 [0.28–0.50]), ISLL (OR [95% CI] = −0.41 [−0.48–0.34]) and TLR (OR [95% CI] = 0.38 [0.27–0.52]) in BP-DES patients. However, there were no difference for safety outcomes between BP-DES and BMS.
Conclusions
BP-DES is more effective in reducing ISLL, TVR and TLR, as safe as standard BMS with regard to death, ST and MI. Further large RCTs with long-term follow-up are warranted to better define the relative merits of BP-DES.
Introduction
The development of bare-metal stents (BMS) represents a considerable advance over balloon angioplasty in preventing restenosis by attenuating early arterial recoil and contraction. However, 15% to 20% of patients required ≥1 repeat revascularization procedure within the 6 to 12 months after BMS implantation [1]. Polymer based drug-eluting stents (DES) are currently widely used to reduce restenosis and the need for repeat revascularization, representing a major advance for percutaneous coronary intervention (PCI). [2] However, well publicized concerns raises with the long-term safety of stent thrombosis (ST) [3].
At present, great efforts have been prompted to develop alternative stents with biodegradable polymers (BP) for drug delivery, which degrade over time, and therefore hope to provide comparable long term safety to BMS while maintaining the early antirestenosis of DES. Previous studies have shown biodegradable polymer drug-eluting stents (BP-DES) is a safe and efficacious alternative to conventional durable polymer DES [4], [5], [6]. However, uncertainty exists regarding the relative performance of BP-DES vs. BMS.
Methods
Established methods [7] were used in compliance with the PRISMA statement for reporting systematic reviews and meta-analyses in health care interventions [8].
Search Strategy
We searched Embase, PubMed, and Cochrane Central Register of Controlled Trials (CENTRAL) for studies on BP-DES until December 2013. The search strategy was formulated as the AND-combination of terms 1) Polymer 2) Stent, in Randomized controlled trials (RCTs). There was no language restriction for the search.
References of meta-analyses, review articles, and original studies identified by the electronic searches were manually checked for additional trials. For studies that did not report outcomes of interest, efforts to contact authors were performed to obtain further details. Internet-based sources of information on the results of clinical trials in cardiology www.theheart.org, www.cardiosource.com/clinicaltrials, www.clinicaltrialresults.com, and www.tctmd.com) were also searched. In addition, we searched conference abstracts of the following societies: American College of Cardiology, Transcatheter Cardiovascular Therapeutics, American Heart Association, European Society of Cardiology, Society of Cardiovascular Angiography and Intervention and Euro-PCR.
Selection Criteria
Inclusion criteria were: 1. Human studies related to PCI. 2. RCTs. 3. BMS as control. Exclusion criteria were: 1. Non-RCT; 2. Sub-study of the RCT. Two authors (Yangguang Yin and Yao Zhang) independently assessed trial bias risk and extracted data.
Data Extraction and Synthesis
Efficacy outcomes were target-lesion revascularization (TLR), target-vessel revascularization (TVR) and in-stent late loss (ISLL). Safety outcomes were death, myocardial infarction (MI) and stent thrombosis (ST). Stent thrombosis was defined as Academic Research Consortium (ARC) [9]. TLR or TVR defined as any revascularization procedure involving the target lesion or vessel owing to luminal re-narrowing in the presence of symptoms or objective signs of ischemia, respectively.
Quality Assessment
The CONSORT 2010 Statement, as a standard for the quality control assessment, was applied to evaluate the quality of the studies included. For each evaluation criterion of the CONSORT 2010 Statement, we assigned ‘Adequate’, ‘Not Adequate’, or ‘Unclear’ to evaluate the quality of the 7 RCTs included. The following criteria were used: Adequate indicated low bias and completely fulfilled quality standards with the least bias; Unclear indicated a lack of information or bias uncertainty; and Not Adequate was assigned if the criteria were completely unfulfilled or there was a high likelihood of bias. If a trial completely fulfilled at least six quality standards of the 10 inclusion/exclusion criteria, it was considered to be of high quality. Two reviewers (Yangguang Yin and Yao Zhang) independently evaluated and cross-checked the quality and assessed the bias of the literatures.
Statistical Methods
All statistical tests were performed using the Cochrane Collaboration’s Revman5.2.6.
The chi-square test was used to examine differences in categorical variables, such as the frequencies, A P value<.05 was considered statistically significant. Summary estimate includes odds ratio (OR), Standard Mean Difference, (SMD) and its 95% confidence intervals (CI) were used as summary statistics in forest plot.
Heterogeneity among studies was determined by the Chi-square-based Q test and the I2 statistics. A p value less than 0.05 for the Q test together with an I2 value greater than 50% was considered a measure of severe heterogeneity. Therefore, the pooled OR estimate of each study was calculated using the fixed-effect model (the Mantel–Haenszel method); otherwise, the random-effects model (the DerSimonian and Laird method) was used. The Potential publication bias for each of the pooled study groups was assessed with a funnel plot. A two-tailed test was used to assess the funnel plot asymmetry; the significance was set at p<.05 level.
Results
Study Selection
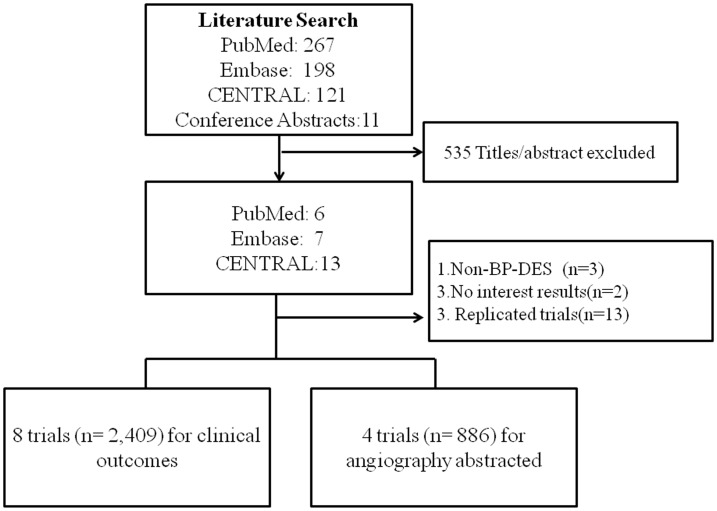
We identified 7 RCTs that satisfied our inclusion criteria (Figure 1). [10], [11], [12], [13], [14], [15], [16], [17] Additional follow-up data on safety and efficacy were available for PAINT trial [11]. The STEALTH trial 5 years and EUCATAX 2 years updated studies are just abstracts without strict peer review and sufficient outcomes data, and therefore excluded [18], [19].
Figure 1. Flow diagram of the review process.
Altogether, 7 trials (n = 2,409) were finally analyzed to compare the clinical outcomes with 1,307 and 1,102 allocated to the BP-DES and BMS, respectively. Four trials were used for angiography evaluation of ISLL. For 3-arm PAINT trial, ISLL data was abstracted to compare BP-DES (sirolimus or paclitaxel arm) to BMS, respectively.
Baseline Characteristics
The baseline characteristics are described in Table 1. Mean lesion length was 21.01±9.33 mm in the BP-DES group as compared to 20.09±8.84 mm in the BMS group. Mean vessel size was 2.94±0.45 mm in BP-DES and 2.94±0.45 mm in BMS (Table 2). Mean stent size was 3.09±0.38 mm in BP-DES and 3.08±0.63 mm in BMS. Mean stent length was 21.2±7.35 mm in the BP-DES group as compared to 20.39±6.82 mm in the BMS group (Table 3). The target vessel was 33.3% patients with RAD, 30.4% with LCX and 35.8% with LAD in the BP-DES group, as compared to 26.7% patients with RAD, 32.2% with LCX and 40.5% with LAD in the BMS group. Mean age was similar in the two groups (62.9±10.03 vs. 63.1±10.14). Men represented 73.9% of the BP-DES and 77.8% of the BMS population. There were 21.1% patients with diabetes in the BP-DES group and 17.9% in the BMS group. Mean dual anti-platelet duration was similar in the 2 groups (7.3 vs. 6.9 months).
Table 1. Main characteristics of the included studies.
| No. | Trial | FU (m) | sample | drugs | Male (%) | Age (Year+SD) | DAPT (m) | Diabets% | Admission Diagnosis | ||||||||
| DP | BMS | DP | BMS | DP | BMS | DP | BMS | DP | BMS | DP | BMS | ||||||
| 1 | PAINT | 36 | 217 | 57 | P/S | bare | 64.1 | 66.7 | 59.9 | 10.4 | 58.5 | 9.6 | 12 | 12 | 31.8 | 26.3 | CAD |
| 2 | EUROSTAR II | 8 | 152 | 151 | paclitaxel | bare | 74.3 | 68.9 | 64.9 | 9.2 | 66.2 | 9.4 | 6 | 6 | 26.3 | 22.5 | CAD |
| 3 | STEALTH | 6 | 80 | 40 | biolimus | bare | 60 | 82.5 | 62.2 | 10.1 | 61.1 | 9.4 | 3 | 3 | 26.6 | 22.5 | CAD |
| 4 | COMFOR-AMI | 12 | 575 | 582 | biolimus | bare | 80.5 | 78.2 | 60.7 | 11.6 | 60.4 | 11.9 | 12 | 12 | 14.6 | 15.5 | STIMI |
| 5 | EUCATAX | 12 | 211 | 211 | Paclitaxel | bare | 83.4 | 79.1 | 63.8 | 10.2 | 64.7 | 12.2 | 6 | 3 | 23.2 | 16.1 | CAD |
| 6 | CORACTO | 24 | 45 | 46 | sirolimus | bare | 69.6 | 82.2 | 64.7 | 9.9 | 64.8 | 8.9 | 6 | 6 | 21.7 | 22.2 | CTO |
| 7 | FUTURE 1 | 12 | 27 | 15 | Everolimus | bare | 85.2 | 86.7 | 64.2 | 8.8 | 65.6 | 9.6 | 6 | 6 | 3.7 | 0 | CAD |
Table 2. Vessel Size and Lesion Length of the included studies.
| No. | Published | Trial | inclusion | Stent Platform | drugs | Vessel Size (mm+SD) | Lesion Length (mm+SD) | ||||||||
| DP | BMS | DP | BMS | DP | BMS | DP | BMS | ||||||||
| 1 | Lemos 2012 | PAINT | de novo, native, 2.5–3.5 mm;single stent ≤29 mm | SS | SS | paclitaxel | bare | 3.1 | 0.4 | 3.1 | 0.4 | NA | NA | NA | NA |
| SS | SS | Sirolimus | bare | 3.1 | 0.3 | 3.1 | 0.4 | 21.8 | 4.8 | 22.5 | 5 | ||||
| 2 | Silber 2011 | EUROSTAR II | de novo, native, 2.5–3.5 mm;leision ≤24 mm | CC | SS | paclitaxel | bare | 2.74 | 0.51 | 2.73 | 0.48 | 15.12 | 7.58 | 15.16 | 7.69 |
| 3 | Grube 2005 | STEALTH | de novo, native, 2.75–4.0 mm | SS | SS | biolimus | bare | 2.95 | 0.4 | 2.97 | 0.42 | 15.37 | 4.64 | 13.75 | 3.77 |
| 4 | Räber 2012 | COMFOR-AMI | STIMI | SS | SS | biolimus | bare | 3.04 | 0.47 | 3.01 | 0.46 | 18.19 | 9.73 | 17.77 | 9.57 |
| 5 | Rodriguez 2011 | EUCATAX | de novo, 70% ≤stenosis | SS | SS | Paclitaxel | bare | 2.75 | 0.5 | 2.85 | 0.5 | 16.2 | 6.1 | 15.6 | 6.3 |
| 6 | Reifart 2010 | CORACTO | native, CTO, 2.5–4.5 mm | SS | SS | sirolimus | bare | 2.7 | 0.51 | 2.8 | 0.63 | 39.4 | 23.1 | 35.8 | 20.7 |
| 7 | Grube 2004 | FUTURE 1 | de novo, 2.75–4.0 mm;leision ≤18 mm | SS | SS | Everolimus | bare | 3.1 | 0.47 | 2.96 | 0.43 | NA | NA | NA | NA |
Table 3. Target Vessel, Stent Length and Stent Diameter of the included studies.
| No. | Published | Target Vessel | Stent Length | Stent Diameter | |||||||||||||
| drugs | RCA | LCX | LAD | (mm+SD) | (mm+SD) | ||||||||||||
| DP | BMS | DP | BMS | DP | BMS | DP | BMS | DP | BMS | DP | BMS | ||||||
| 1 | Lemos 2012 | paclitaxel | bare | 33.3 | 15.8 | 44.1 | 57.9 | 22.5 | 26.3 | 22.5 | 5.5 | 22.5 | 5 | 3.1 | 0.4 | 3.1 | 0.4 |
| Sirolimus | bare | 25.5 | 15.8 | 56.6 | 57.9 | 17.9 | 26.3 | 21.8 | 4.8 | 22.5 | 5 | 3.1 | 0.3 | 3.1 | 0.4 | ||
| 2 | Silber 2011 | paclitaxel | bare | 36 | 31.3 | 23.8 | 27 | 39 | 40.5 | 16.98 | 6.74 | 17.01 | 8.29 | NA | NA | NA | NA |
| 3 | Grube 2005 | biolimus | bare | 34.1 | 27.5 | 37.8 | 30 | 28 | 42.5 | 19.03 | 8.76 | 16.23 | 5.53 | NA | NA | NA | NA |
| 4 | Räber 2012 | biolimus | bare | 45.9 | 44.6 | 14.3 | 15.5 | 39.3 | 39.6 | 25.2 | 12.7 | 24.1 | 12.3 | 3.2 | 0.4 | 3.2 | 1.1 |
| 5 | Rodriguez 2011 | Paclitaxel | bare | 17.6 | 25.1 | 18.5 | 23.8 | 62.8 | 48.5 | 21.7 | 5.6 | 20 | 4.8 | 2.96 | 0.4 | 2.93 | 0.6 |
| 6 | Reifart 2010 | sirolimus | bare | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 7 | Grube 2004 | Everolimus | bare | 41 | 27 | 18 | 13 | 41 | 60 | NA | NA | NA | NA | NA | NA | NA | NA |
Safety Endpoints
Death
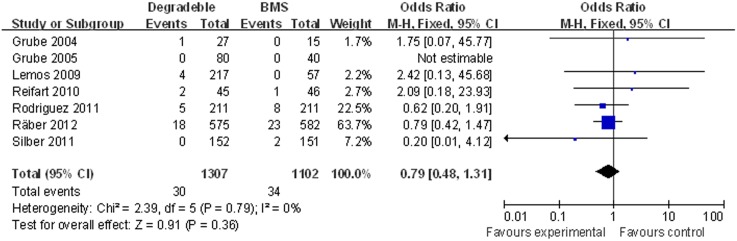
There was no significant difference in the rate of death with BP-DES as compared with BMS: 2.29% (30/1,307) in the BP-DES group and 3.09% (34 of 1,102) in the BMS group (OR [95% CI] = 0.79 [0.48–1.31]) (Figure 2).
Figure 2. Individual and summary odds ratios for death in patients treated with BP-DES vs. BMS.
Myocardial infarction
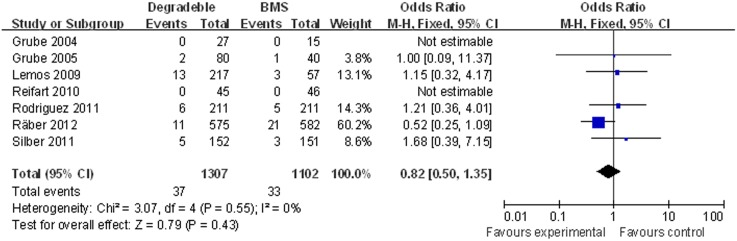
There was no significant difference in the rate of MI with BP-DES as compared with BMS: 2.83% (37/1,307) in the BP-DES group and 2.99% (33/1,102) in the BMS group (OR [95% CI] = 0.82 [050–1.35]) (Figure 3).
Figure 3. Individual and summary odds ratios for myocardial infarction in patients treated with BP-DES vs. BMS.
Definite stent thrombosis
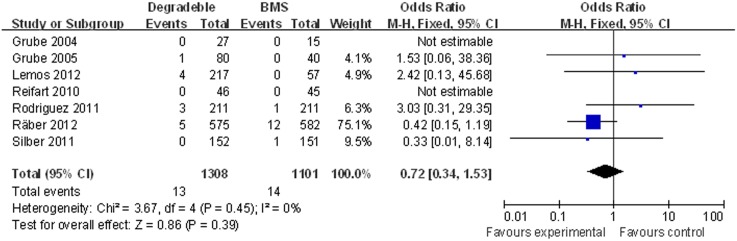
Seven studies (2,409 patients) with mean follow-up 10.5 months were included to compare the ST between BP-DES vs. BMS. There was no significant difference in the rate of total DST with BP-DES as compared with BMS: 0.99% (13/1,308) in the BP-DES group and 1.27% (14/1,101) in the BMS group (OR [95% CI] = 0.72 [0.34–1.53]) (Figure 4).
Figure 4. Individual and summary odds ratios for definite stent thrombosis (DST) in patients treated with BP-DES vs. BMS.
The meta-analysis did not showed a significant decreased late DST in patients treated with BP-DES (0.38%, 5/1,308) as compared to patients receiving BMS (0.18%, 2/1,101) (OR [95% CI] = 0.57 [0.23–1.40]) (Figures S1).
There was no significant difference in the rate of early DST with BP-DES as compared with BMS: 0.54% (7/1,308) in the BP-DES group and 1.59% (12/1,101) in the BMS group (OR [95% CI] = 1.19 [0.30–4.72]) (Figures S2).
Efficacy Endpoints
Target lesion revascularisation
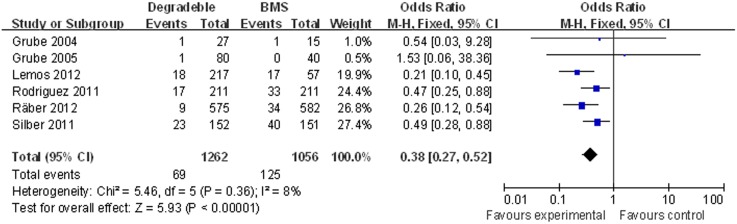
Six studies with 2,318 patients were included. The meta-analysis showed a significant decreased TLR in patients treated with BP-DES (5.47%, 69/1,262) as compared to patients receiving BMS (11.84%, 125/1,056) (OR [95% CI] = 0.38 [0.27–0.52]) (Figure 5).
Figure 5. Individual and summary odds ratios for TLR in patients treated with BP-DES vs. BMS.
Target vessel revascularisation
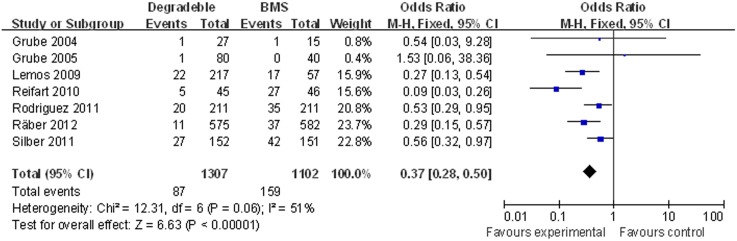
Seven studies (2,409 patients) with mean follow-up 10.5 months were included. The meta-analysis showed a significant decreased TVR in patients treated with BP-DES (6.66%, 87/1,307) as compared to patients receiving BMS (14.43%, 159/1,102) (OR [95% CI] = 0.37 [0.28–0.50]) (Figure 6).
Figure 6. Individual and summary odds ratios for TVR in patients treated with BP-DES vs. BMS.
In-stent late loss
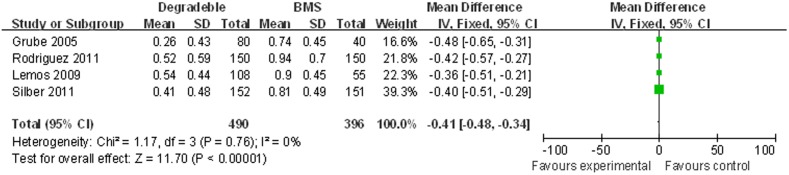
We included 886 patients with mean follow-up 7 months. ISLL significantly decreased in BP-DES group (0.43±0.49 mm) compared to BMS group (0.85 mm±0.52). (SMD [95% CI] = −0.41 [–0.48; –0.34]), when paclitaxel arm data of PAINT trial was used as BP-DES group. (Figure 7).
Figure 7. Standardized mean difference (SMD) for ISLL in patients treated with BP-DES vs. BMS.
The results were confirmed when sirolimus arm data of PAINT trial was used as BP-DES group (0.38±0.48 mm), comparing to BMS group (0.85 mm±0.52). (SMD [95% CI] = −0.46 [–0.53; –0.39]). (Figures S3).
Sensitivity and subgroup analyses
Sensitivity analysis was performed by removing each of the studies one at a time, which did not detected any influence of any single study on the overall results.
With regard to ISLL, the overall results in favor of BP-DES were confirmed when paclitaxel-eluting BP-DES were analyzed separately: BP-DES group (0.49±0.50 mm) compared to BMS group (0.88 mm±0.55). (SMD [95% CI] = –0.39 [–0.47; –0.32]). (Figures S4).
Subgroup analysis of outcomes between BMS and BP-BES, as well as biodegradable limus- and sirolimus eluting stents are performed, which confirmed that BP-DES is more effective in reducing ISLL, TVR and TLR, as safe as standard BMS with regard to death, ST and MI. (Figures S5–S21).
Discussion
This is the first meta-analysis that directly compared outcomes between BP-DES and BMS. The main finding is that patients allocated to BP-DES showed significantly less ISLL, TVR and TLR, with comparable MI, death, DST to those treated with BMS.
Drug-eluting stents (DES) with durable polymer coating rapidly transformed the practice of percutaneous coronary intervention (PCI), by significantly reducing rates of restenosis in comparison with bare-metal stents (BMS). [20], [21] However, residual polymer in the coronary milieu induces inflammatory response at the vessel-wall and then contributes to late thrombotic stent as well as late neointimal overgrowth [22], [23].
Degradable-polymer DES has been developed by providing similar controlled drug release with subsequent degradation of the polymer in 3–9 months and, therefore, appears to be a promising solution to overcome this problem [4], [5]. However, at least 2 benchmarks, efficacy and safety, should be considered when appraising the results of BP-DES. First, they should demonstrate comparable, if not superior, safety results compared with BMS and DES. Second, the BP-DES should also reduce the incidence of revascularization compared with BMS, and be shown to be at least noninferior in regard to contemporary DES [6]. At present, clinical data have accumulated to support the use of biodegradable polymer stents to be a safe and efficacious alternative to conventional durable polymer DES [24], [25].
Several RCTs have been investigated to compare outcomes of BP-DES vs. BMS. FUTURE I was the first prospective, single-blind, randomized trial to evaluate the safety and efficacy of everolimus-eluting stents (EES), coated with a bio-absorbable polymer, comparing with BMS [17]. In this initial clinical experience, BP-DES demonstrated a safe and efficacious method to reduce in-stent neointimal hyperplasia and restenosis. In PAINT trial, Lemos et al tested 2 novel DES, covered with a biodegradable-polymer carrier and releasing paclitaxel or sirolimus, which were compared against a bare metal stent [10]. They found both BD-DES were effective in reducing neointimal hyperplasia and 1-year re-intervention, compared to BMS. The COMFORTABLE AMI [14] is the largest RCTs (1161 patients) to date, comparing outcomes of BP-DES vs. BMS. This study showed that the use of biolimus-eluting stents with a biodegradable polymer resulted in a lower rate of the composite of major adverse cardiac events at 1 year among patients with ST-elevation myocardial infarction undergoing primary PCI. However, all those trials comparing BP-DES vs. BMS were not powerful to reveal potential differences in low frequency events including MI, death and ST, and therefore their relative efficacy and safety remains undetermined.
The findings of the current study are novel and important for at least 2 reasons.
First, our study directly addressed the comparison of outcomes between BP-DES and BMS. We didn’t show a significant difference in death, DST and MI for BP-DES, as compared to BMS. These results should be explained carefully: 1. It is our opinion that an analysis with 2,409 patients was unable to detect a significant advantage may be interpreted as a limited difference between BP-DES and BMS. 2. The low incidence of death, DST and MI has made investigating the difference of these outcomes difficult. Further large RCTs with long-term follow-up are warranted to better define the relative merits of BP-DES.
Recently, 2 large scale network studies have showed BP-DES were associated with significantly lower rates of cardiac death/MI, MI, and ST than BMS [24], [25]. In our opinion, these different results should be noted and could be explained with following reasons: 1. Network analysis is a different statistic method with our meta-analysis, which allows for indirect comparisons of stents not in any of the individual trials (comparison of stent A vs. C by using trials comparing A vs. B and B vs. C), and may include more trials. 2. We included 7 trials of direct comparison of BP-DES vs. BMS for data abstraction. However, Palmerini et al [25] only included 1 trial (COMFORTABLE AMI) in their analysis, indicating that most of their results about BP-DES vs. BMS are based on indirect comparison. 3. Duration of antiplatelet therapy in patients treated with BD-DES and BMS differed across trials and therefore represented a confounding factor in these 2 network analysis.
Secondary, we for the first time reported an improved anti-restenotic efficacy of BP-DES vs. BMS with lower ISLL in 8 months, as well as a significant reduction of BP-DES in both TLR and TVR. This finding is supported by the results of previous network meta-analysis regarding to TVR [24], [25]. Thus, similar findings with different trials further clarified the efficacy profile of BP-DES, as compare to BMS.
DES had revolutionized the practice of interventional cardiology and been implanted in the majority of PCI procedures over the past decades. However, BMS are still used especially in patients with AMI, high bleeding risk or large coronary vessel (>3.0 mm). Thus, the findings of a reduced ISLL, TVR and TLR, as well as non-inferior safety outcomes with BP-DES as compared to BMS in our meta-analysis are clinically significant and indicating a safe and efficacious alternative to conventional BMS.
Multiple studies have shown improved safety and efficacy of second generation everolimus-eluting stent than early-generation sirolimus-eluting, paclitaxel-eluting stents, [26], [27], [28] and therefore, representing the standard care to which new stent designs should be compared. [29] Also, previous studies have also proved superior outcomes with EES when compared to BMS. [30], [31], [32] However, there is only FUTURE I trial comparing the safety and efficacy of biodegradable polymer everolimus-eluting stents (BP-EES) and BMS. This study indicated that BP-EES with biodegradable polymer could be a safe and efficacious method to reduce in-stent neointimal hyperplasia and restenosis. [17].
Biolimus is the limus analogue with the highest lipophilicity used for drug elution on currently available stent platforms. [33] Theoretically, the increased lipophilicity of the drug biolimus may provide a more rapid and homogeneous drug distribution, potentially leading to a more potent anti-inflammatory and antithrombotic local effect. In fact, previous study reported that BP-BES, as compared to PP-EES, showed similar stent coverage and apposition as assessed by OCT at 6–8 months. [34] Furthermore, meta-analysis and clinical trials proved that BP-BES are as safe and efficacious as the current standard of a thin-strut EES with a durable biocompatible polymer. [35], [36], [37], [38] So, BP-BES may also be an alternative standard choice for comparing the stents safety and efficacy.
We compared the outcomes of BMS with BP-BES, as well as biodegradable limus- and sirolimus eluting stents. All the subgroup analysis support our conclusions that BP-DES is more effective in reducing ISLL, TVR and TLR, as safe as standard BMS with regard to death, ST and MI.
Limitations
1. The limitations of the meta-analytical approach are well known and documented. [39] 2. We didn’t have data for all trials at each time period; therefore, this limited comparison of rates across time within a specific end point. 3. Inclusion criteria were not equivalent across the included trials, however, reflects the broadly inclusive nature of the included patient population. 4. Our meta-analysis might be un-powerful to detect the difference of low incidence events such as MI, death and ST. 5. A major limitation is absence of comparisons with DES like everolimus eluting ones, which represent the standard of care for PCI.
Conclusions
BP-DES is more effective in reducing ISLL, TVR and TLR, as well as comparable with BMS in regard to death, ST and MI. Further large RCTs with long-term follow-up are warranted to better define the relative merits of BP-DES.
Supporting Information
Individual and summary odds ratios for late definite stent thrombosis (DST) in patients treated with BP-DES vs. BMS.
(JPG)
Individual and summary odds ratios for early definite stent thrombosis (DST) in patients treated with BP-DES vs. BMS.
(JPG)
Standardized mean difference (SMD) for ISLL in patients treated with BP-DES vs. BMS.
(JPG)
Standardized mean difference (SMD) for ISLL in patients treated with paclitaxel-eluting BP-DES vs. BMS.
(JPG)
Individual and summary odds ratios for death in patients treated with BP-BES vs. BMS.
(JPG)
Individual and summary odds ratios for myocardial infarction in patients treated with BP-BES vs. BMS.
(JPG)
Individual and summary odds ratios for TLR in patients treated with BP-BES vs. BMS.
(JPG)
Individual and summary odds ratios for TVR in patients treated with BP-BES vs. BMS.
(JPG)
Individual and summary odds ratios for early definite stent thrombosis in patients treated with BP-BES vs. BMS.
(JPG)
Individual and summary odds ratios for late definite stent thrombosis (DST) in patients treated with BP-BES vs. BMS.
(JPG)
Individual and summary odds ratios for death in patients treated with BP-limus eluting stents vs. BMS.
(JPG)
Individual and summary odds ratios for myocardial infarction in patients treated with BP-limus eluting stents vs. BMS.
(JPG)
Individual and summary odds ratios for TLR in patients treated with BP-limus eluting stents vs. BMS.
(JPG)
Individual and summary odds ratios for TVR in patients treated with BP-limus eluting stents vs. BMS.
(JPG)
Individual and summary odds ratios for early definite stent thrombosis in patients treated with BP-limus eluting stents vs. BMS.
(JPG)
Individual and summary odds ratios for late definite stent thrombosis in patients treated with BP-limus eluting stents vs. BMS.
(JPG)
Standardized mean difference (SMD) for ISLL in patients treated with BP-limus eluting stents vs. BMS.
(JPG)
Individual and summary odds ratios for death in patients treated with BP-SES vs. BMS.
(JPG)
Individual and summary odds ratios for myocardial infarction in patients treated with BP-SES vs. BMS.
(JPG)
Individual and summary odds ratios for TVR in patients treated with BP-SES vs. BMS.
(JPG)
Individual and summary odds ratios for late define stent thrombosis in patients treated with BP-SES vs. BMS.
(JPG)
Protocols of dual anti-platelet therapy (DAPT).
(DOCX)
PRISMA 2009 Checklist.
(DOC)
Funding Statement
This study was supported by grants from National Natural Science Foundation of China (81070168 and 81370213) and Third Military Medical University (2010XLC28). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, et al. (1994) A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med 331: 496–501. [DOI] [PubMed] [Google Scholar]
- 2. Bangalore S, Kumar S, Fusaro M, Amoroso N, Attubato MJ, et al. (2012) Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation 125: 2873–2891. [DOI] [PubMed] [Google Scholar]
- 3. Camenzind E, Steg PG, Wijns W (2007) Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation 115: 1440–1455 discussion 1455. [DOI] [PubMed] [Google Scholar]
- 4. Stefanini GG BR, Serruys PW, de Waha A, Meier B, Massberg S, et al. (2012) Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J 33: 1214–1222. [DOI] [PubMed] [Google Scholar]
- 6. Garg S, Bourantas C, Serruys PW (2013) New concepts in the design of drug-eluting coronary stents. Nat Rev Cardiol 10: 248–260. [DOI] [PubMed] [Google Scholar]
- 7.The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration website, Available: http://www.cochrane.org/resources/handbook. Accessed 2011 March.
- 8. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, et al. (2007) Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 115: 2344–2351. [DOI] [PubMed] [Google Scholar]
- 10. Lemos PA, Moulin B, Perin MA, Oliveira LA, Arruda JA, et al. (2009) Randomized evaluation of two drug-eluting stents with identical metallic platform and biodegradable polymer but different agents (paclitaxel or sirolimus) compared against bare stents: 1-year results of the PAINT trial. Catheter Cardiovasc Interv 74: 665–673. [DOI] [PubMed] [Google Scholar]
- 11. Lemos PA, Moulin B, Perin MA, Oliveira LA, Arruda JA, et al. (2012) Late clinical outcomes after implantation of drug-eluting stents coated with biodegradable polymers: 3-year follow-up of the PAINT randomised trial. EuroIntervention 8: 117–119. [DOI] [PubMed] [Google Scholar]
- 12. Silber S, Gutierrez-Chico JL, Behrens S, Witzenbichler B, Wiemer M, et al. (2011) Effect of paclitaxel elution from reservoirs with bioabsorbable polymer compared to a bare metal stent for the elective percutaneous treatment of de novo coronary stenosis: the EUROSTAR-II randomised clinical trial. EuroIntervention 7: 64–73. [DOI] [PubMed] [Google Scholar]
- 13. Grube E, Hauptmann KE, Buellesfeld L, Lim V, Abizaid A (2005) Six-month results of a randomized study to evaluate safety and efficacy of a Biolimus A9 eluting stent with a biodegradable polymer coating. EuroIntervention 1: 53–57. [PubMed] [Google Scholar]
- 14. Raber L, Kelbaek H, Ostojic M, Baumbach A, Heg D, et al. (2012) Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA 308: 777–787. [DOI] [PubMed] [Google Scholar]
- 15. Rodriguez AE, Vigo CF, Delacasa A, Mieres J, Fernandez-Pereira C, et al. (2011) Efficacy and safety of a double-coated paclitaxel-eluting coronary stent: the EUCATAX trial. Catheter Cardiovasc Interv 77: 335–342. [DOI] [PubMed] [Google Scholar]
- 16. Reifart N, Hauptmann KE, Rabe A, Enayat D, Giokoglu K (2010) Short and long term comparison (24 months) of an alternative sirolimus-coated stent with bioabsorbable polymer and a bare metal stent of similar design in chronic coronary occlusions: the CORACTO trial. EuroIntervention 6: 356–360. [DOI] [PubMed] [Google Scholar]
- 17. Grube E, Sonoda S, Ikeno F, Honda Y, Kar S, et al. (2004) Six- and twelve-month results from first human experience using everolimus-eluting stents with bioabsorbable polymer. Circulation 109: 2168–2171. [DOI] [PubMed] [Google Scholar]
- 18.Ricardo Costa RM, Alexandre Abizaid, Karl E Hauptmann, Eberhard Grube (2011) STEALTH I: 5-year Follow-up from a Prospective Randomized Study of Biolimus A9-Eluting Stent with a Biodegradable Polymer Coating vs a Bare Metal Stent. JACC 58/20/Suppl B.
- 19.Rodriguez-Granillo AM MJ, Fernandez-Pereira C, Delacasa A, Vigo CF, Risau G, et al. (2013) Two-year reports of efficacy and safety end points from the randomised, multicentre and controlled Eucatax trial. Eurointervention website, Available: http://www.pcronline.com/eurointervention/M_issue/156. Accessed 2013.
- 20. Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, et al. (2004) A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med 350: 221–231. [DOI] [PubMed] [Google Scholar]
- 21. Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, et al. (2003) Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 349: 1315–1323. [DOI] [PubMed] [Google Scholar]
- 22. Virmani R, Guagliumi G, Farb A, Musumeci G, Grieco N, et al. (2004) Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation 109: 701–705. [DOI] [PubMed] [Google Scholar]
- 23. Finn AV, Kolodgie FD, Harnek J, Guerrero LJ, Acampado E, et al. (2005) Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation 112: 270–278. [DOI] [PubMed] [Google Scholar]
- 24. Bangalore S, Toklu B, Amoroso N, Fusaro M, Kumar S, et al. (2013) Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis. BMJ 347: f6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Palmerini T, Biondi-Zoccai G, Della Riva D, Mariani A, Sabate M, et al. (2014) Clinical Outcomes with Bioabsorbable Polymer-based versus Durable Polymer-based Drug-Eluting Stents and Bare Metal Stents: Evidence from a Comprehensive Network Meta-analysis. J Am Coll Cardiol 63: 299–307. [DOI] [PubMed] [Google Scholar]
- 26. Stone GW, Rizvi A, Sudhir K, Newman W, Applegate RJ, et al. (2011) Randomized comparison of everolimus- and paclitaxel-eluting stents: 2-year follow-up from the SPIRIT IV trial. J Am Coll Cardiol 58: 19–25. [DOI] [PubMed] [Google Scholar]
- 27. Smits PC, Kedhi E, Royaards KJ, Joesoef KS, Wassing J, et al. (2011) 2-year follow-up of a randomized controlled trial of everolimus- and paclitaxel-eluting stents for coronary revascularization in daily practice: COMPARE. J Am Coll Cardiol 58: 11–18. [DOI] [PubMed] [Google Scholar]
- 28. Palmerini T, Biondi-Zoccai G, Della Riva D, et al. (2012) Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet 379: 1393–402. [DOI] [PubMed] [Google Scholar]
- 29. Ormiston JA, Webster MWI (2012) Stent thrombosis: has the firestorm been extinguished? Lancet 379: 1368–69. [DOI] [PubMed] [Google Scholar]
- 30.Omar A, Torguson R, Kitabata H, Pendyala LK, Loh JP, et al. (2014) Long-Term safety and efficacy of second-generation everolimus-eluting stents compared to other limus-eluting stents and bare metal stents in patients with acute coronary syndrome. Catheter Cardiovasc Interv. Wiley online library website, Available: http://onlinelibrary.wiley.com/doi/10.1002/ccd.25469/pdf Accessed 2014 Mar 12. [DOI] [PubMed]
- 31. Sabaté M, Brugaletta S, Cequier A (2014) The EXAMINATION trial: 2-year results from a multicenter randomized controlled trial. JACC Cardiovasc Interv 7: 64–71. [DOI] [PubMed] [Google Scholar]
- 32. Valgimigli M, Tebaldi M, Borghesi M, Vranckx P, Campo G, et al. (2014) Two-year outcomes after first- or second-generation drug-eluting or bare-metal stent implantation in all-comer patients undergoing percutaneous coronary intervention: a pre-specified analysis from the PRODIGY study. JACC Cardiovasc Interv 7: 20–8. [DOI] [PubMed] [Google Scholar]
- 33. Davi G, Patrono C (2007) Platelet activation and atherothrombosis. N Engl J Med 357: 2482–2494. [DOI] [PubMed] [Google Scholar]
- 34. Tada T, Kastrati A, Byrne RA, Schuster T, Cuni R, et al. (2014) Randomized comparison of biolimus-eluting stents with biodegradable polymer versus everolimus-eluting stents with permanent polymer coatings assessed by optical coherence tomography. Int J Cardiovasc Imaging 30: 495–504. [DOI] [PubMed] [Google Scholar]
- 35.Zhang YJ, Zhu LL, Bourantas CV, Iqbal J, Dong SJ, et al. (2014) The impact of everolimus versus other rapamycin derivative-eluting stents on clinical outcomes in patients with coronary artery disease: A meta-analysis of 16 randomized trials. J Cardiol. Sciencedirect website, Available: http://www.sciencedirect.com/science/article/pii/S0914508714000252. Accessed 2014 Feb 20. [DOI] [PubMed]
- 36. Smits PC, Hofma S, Togni M, Vázquez N, Valdés M, et al. (2013) Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent (COMPARE II): a randomised, controlled, non-inferiority trial. Lancet 381: 651–60. [DOI] [PubMed] [Google Scholar]
- 37. Natsuaki M, Kozuma K, Morimoto T, Kadota K, Muramatsu T, et al. (2013) Biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent: a randomized, controlled, noninferiority trial. J Am Coll Cardiol 62: 181–90. [DOI] [PubMed] [Google Scholar]
- 38. Separham A, Sohrabi B, Aslanabadi N, Ghaffari S (2011) The twelve-month outcome of biolimus eluting stent with biodegradable polymer compared with an everolimus eluting stent with durable polymer. J Cardiovasc Thorac Res 3: 113–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Individual and summary odds ratios for late definite stent thrombosis (DST) in patients treated with BP-DES vs. BMS.
(JPG)
Individual and summary odds ratios for early definite stent thrombosis (DST) in patients treated with BP-DES vs. BMS.
(JPG)
Standardized mean difference (SMD) for ISLL in patients treated with BP-DES vs. BMS.
(JPG)
Standardized mean difference (SMD) for ISLL in patients treated with paclitaxel-eluting BP-DES vs. BMS.
(JPG)
Individual and summary odds ratios for death in patients treated with BP-BES vs. BMS.
(JPG)
Individual and summary odds ratios for myocardial infarction in patients treated with BP-BES vs. BMS.
(JPG)
Individual and summary odds ratios for TLR in patients treated with BP-BES vs. BMS.
(JPG)
Individual and summary odds ratios for TVR in patients treated with BP-BES vs. BMS.
(JPG)
Individual and summary odds ratios for early definite stent thrombosis in patients treated with BP-BES vs. BMS.
(JPG)
Individual and summary odds ratios for late definite stent thrombosis (DST) in patients treated with BP-BES vs. BMS.
(JPG)
Individual and summary odds ratios for death in patients treated with BP-limus eluting stents vs. BMS.
(JPG)
Individual and summary odds ratios for myocardial infarction in patients treated with BP-limus eluting stents vs. BMS.
(JPG)
Individual and summary odds ratios for TLR in patients treated with BP-limus eluting stents vs. BMS.
(JPG)
Individual and summary odds ratios for TVR in patients treated with BP-limus eluting stents vs. BMS.
(JPG)
Individual and summary odds ratios for early definite stent thrombosis in patients treated with BP-limus eluting stents vs. BMS.
(JPG)
Individual and summary odds ratios for late definite stent thrombosis in patients treated with BP-limus eluting stents vs. BMS.
(JPG)
Standardized mean difference (SMD) for ISLL in patients treated with BP-limus eluting stents vs. BMS.
(JPG)
Individual and summary odds ratios for death in patients treated with BP-SES vs. BMS.
(JPG)
Individual and summary odds ratios for myocardial infarction in patients treated with BP-SES vs. BMS.
(JPG)
Individual and summary odds ratios for TVR in patients treated with BP-SES vs. BMS.
(JPG)
Individual and summary odds ratios for late define stent thrombosis in patients treated with BP-SES vs. BMS.
(JPG)
Protocols of dual anti-platelet therapy (DAPT).
(DOCX)
PRISMA 2009 Checklist.
(DOC)