Abstract
Endothelial dysfunction is common in acute and chronic organ injury. Isoflurane is a widely used halogenated volatile anesthetic during the perioperative period and protects against endothelial cell death and inflammation. In this study, we tested whether isoflurane induces endothelial ecto-5′-nucleotidase (CD73) and cytoprotective adenosine generation to protect against endothelial cell injury. Clinically relevant concentrations of isoflurane induced CD73 activity and increased adenosine generation in cultured human umbilical vein or mouse glomerular endothelial cells. Surprisingly, isoflurane-mediated induction of endothelial CD73 activity occurred within 1 hr and without synthesizing new CD73. We determined that isoflurane rapidly increased CD73 containing endothelial microparticles into the cell culture media. Indeed, microparticles isolated from isoflurane-treated endothelial cells had significantly higher CD73 activity as well as increased CD73 protein. In vivo, plasma from mice anesthetized with isoflurane had significantly higher endothelial cell-derived CD144+ CD73+ microparticles and had increased microparticle CD73 activity compared to plasma from pentobarbital-anesthetized mice. Supporting a critical role of CD73 in isoflurane-mediated endothelial protection, a selective CD73 inhibitor (APCP) prevented isoflurane-induced protection against human endothelial cell inflammation and apoptosis. In addition, isoflurane activated endothelial cells Rho kinase evidenced by myosin phosphatase target subunit-1 and myosin light chain phosphorylation. Furthermore, isoflurane-induced release of CD73 containing microparticles was significantly attenuated by a selective Rho kinase inhibitor (Y27632). Taken together, we conclude that the volatile anesthetic isoflurane causes Rho kinase-mediated release of endothelial microparticles containing preformed CD73 and increase adenosine generation to protect against endothelial apoptosis and inflammation.
Introduction
More than 1 trillion endothelial cells in human body cover the entire circulatory system and play an integral role in maintaining homeostasis of all organs [1]. Endothelial cells regulate vascular tone, angiogenesis, coagulation and release several critically important autocrine and paracrine compounds including sphingosine 1-phosphate, adenosine, nitric oxide, and prostaglandins [2], [3]. Furthermore, endothelial cells release microparticles originating from plasma membrane and these microparticles contain many biological active compounds including cell surface enzymes, membrane receptors as well as lipoproteins, RNAs and microRNAs [4]. Clinically, endothelial dysfunction contributes to acute and chronic vascular and organ injury [2], [3]. Indeed, endothelial cell death occurs frequently after ischemia and reperfusion injury, diabetes, coronary artery disease and sepsis [3], [5], [6].
Volatile anesthetic is administered to virtually all patients subjected to general anesthesia making it one of the most frequently used medications during the perioperative period [7]. Volatile anesthetics have non-anesthetic effects on the heart, vasculature and respiratory system by regulating blood pressure, heart rate, airway tone and systemic vascular resistance [8], [9]. In addition, volatile anesthetics have anti-necrotic and anti-inflammatory effects and protect against ischemia reperfusion injury of the heart, kidney and intestine [9]–[11]. Furthermore, volatile anesthetics attenuate the hyperactive systemic inflammatory response during sepsis [12].
After inhalation, volatile anesthetics are first taken up by the pulmonary circulatory system and all endothelial cells in the body are rapidly exposed. We and others previously demonstrated that volatile anesthetics protect against endothelial cell necrosis, apoptosis and inflammation due to hypoxia, lipopolysaccharide and cytokine exposure [13]–[17]. However, the detailed signaling mechanisms of volatile anesthetic-mediated endothelial protection remain incompletely understood. We recently demonstrated that one of the most widely used volatile anesthetic isoflurane (2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane) induces the expression of ecto-5′-nucleotidase (CD73) in renal proximal tubular cells [18]. Isoflurane-mediated induction of CD73 activity with subsequently increased synthesis of adenosine reduces proximal tubular necrosis, apoptosis and inflammation after renal ischemia and reperfusion injury. In this study, we tested whether isoflurane stimulates endothelial CD73 activity and generates adenosine to protect against endothelial cell death and inflammation. To our surprise, isoflurane did not induce new CD73 synthesis in endothelial cells as observed in renal proximal tubular cells. Instead, we determined that isoflurane activates endothelial cell Rho kinase to release preformed CD73 contained within endothelial microparticles to increase adenosine generation to protect endothelial apoptosis and inflammation.
Methods and Materials
Ethics Statement
All animal work was approved by Columbia University Institutional Animal Care and Use Committee.
Endothelial Cell Culture and Exposure to Isoflurane
Immortalized human umbilical vein endothelial cells (EA.hy926, American Type Culture Collection, Manassas, VA) cells were grown in high-glucose DMEM plus 10% FBS. Immortalized mouse glomerular endothelial cells (GENC) were obtained from Dr. M. Madaio (Georgia Regents University) and grown in low glucose DMEM/Ham’s F12 medium plus 10% FBS, 2 mM l-glutamine, and 10 mM HEPES [19]. Cells were plated in 6-well plates when 80% confluent and used in the experiments described below when confluent after 24 hr serum deprivation.
Endothelial cells were exposed to isoflurane (Abbott Laboratories, Chicago, IL) as described previously [13]. In brief, endothelial cells were placed in an air-tight, 37°C, humidified modular incubator chamber (Billups-Rothenberg, Del Mar, CA) and exposed to 0–2.5% (0–2 MAC) isoflurane (where 1 MAC is defined as the percent concentration in the alveolus of an inhaled anesthetic agent required to prevent 50% of subjects from moving in response to a painful stimulus when used as the sole anesthetic) mixed with 95% air+5% CO2 (carrier gas). Exposure to isoflurane lasted between 0 to 16 hr. To inhibit CD73 or Rho kinase activity, some endothelial cells were pretreated with 100 µM α,β-methylene ADP (APCP, a selective CD73 inhibitor, Sigma, St Louis, MO) or with 10 µM Y27632 (a selective Rho Kinase inhibitor, Sigma, St Louis, MO) 30 min. before isoflurane treatment [20].
Microparticle Isolation and Flow Cytometry
After Columbia University Institutional Animal Care and Use Committee approval, we anesthetized adult male C57BL/6 (Harlan, Indianapolis, IN) to 4 hr of equipotent doses of either pentobarbital or isoflurane (1.2% or ∼1 MAC) as described previously [18]. The mice were placed on a heating pad under a warming light to maintain body temperature ∼36–38°C. After 4 hr, animals were killed and plasma isolated for microparticle isolation.
Microparticles were isolated by differential centrifugation as described by Amabile et al. [21]. Endothelial cell culture medium or mouse plasma was centrifuged once at 1,000×g for 10 min to remove the cell pellet and the resulting supernatant was centrifuged at 20,000×g for 40 min. The resulting pellet containing microparticles was analyzed for CD73 activity as well as CD73 immunoblotting.
We also subjected isolated endothelial and mouse plasma microparticles to flow cytometric analysis with a Quanta SC flow cytometer (Beckman Coulter, Miami, FL). To determine the relative expression of CD73, microparticles isolated from endothelial cell culture media were stained with Annexin V (Beckman Coulter, Miami, FL) and CD73 (BD Biosciences, San Jose, CA) for 5 min at room temperature. Mouse plasma microparticles were stained with CD73 and CD144 (an endothelial cell marker, BD Biosciences, San Jose, CA) to determine the CD73 expression in endothelial microparticles.
Induction of Endothelial Cell Apoptosis and Inflammation
After exposure to 2.5% isoflurane or with carrier gas for 1 hr, EA.hy9262 cells were exposed to tumor necrosis factor-alpha (TNF-α, 20 ng/ml) plus cycloheximide (10 µg/ml) for 16 hr to induce apoptosis or to TNF-α (20 ng/ml) for 6 hr to induce inflammation as described previously [18]. Apoptosis was assessed by detecting poly-(adensosine diphosphate-ribose)-polymerase (PARP) and caspase 3 fragmentations as described [22], [23]. Inflammation was assessed by measuring mRNAs encoding markers of inflammation including TNF-α, ICAM-1 and VCAM-1 (Table 1) with RT-PCR as described previously [18], [24].
Table 1. Primers used to amplify cDNAs based on published GenBank sequences for human.
| Primers | Sequence (Sense/Antisense) | Product Size (bp) | Cycle Number | Annealing Temp (°C) |
| TNF-α | 5′-CGGGACGTGGAGCTGGCCGAGGAG-3′ | 355 | 24 | 68 |
| 5′-CACCAGCTGGTTATCTCTCAGCTC-3′ | ||||
| ICAM-1 | 5′-GCAGACAGTGACCATCTACAGC-3′ | 400 | 16 | 60 |
| 5′-GCCATCCTTTAGACACTTGAGC-3′ | ||||
| VCAM-1 | 5′-TCTTGTTTGCCGAGCTAAATTA-3′ | 364 | 22 | 55 |
| 5′-TAAATGGTTTCTCTTGAACAA-3′ | ||||
| CD73 | 5′-CCA ATT CTG AGT GCA AAC AT-3′ | 315 | 23 | 62 |
| 5′-CCT CCC ACC ACG ACG TCC AC-3′ | ||||
| GAPDH | 5′-ACCACAGTCCATGCCATCAC-3′ | 450 | 15 | 65 |
| 5′-CACCACCCTGTTGCTGTAGCC-3′ |
bp, base pairs; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; ICAM-1, intercellular adhesion molecule-1; TNF-α, tumor necrosis factor-alpha; VCAM-1, vascular cell adhesion molecule-1; CD73, Ecto-5′-nucleotidase. Respective anticipated PCR product size (bp, base pairs), PCR cycle number for linear amplification, and annealing temperatures used for each primer are also provided.
Detection of Endothelial CD73 mRNA and Protein Expression
We measured mRNA encoding human CD73 after isoflurane treatment as described [25] with primers listed in Table 1. In addition, EA.hy9262 cell lysates were collected for immunoblotting analyses of CD73 (Santa Cruz Biotechnologies, Santa Cruz, CA) and β-actin (internal protein loading control, Sigma, St Louis, MO) as described previously after isoflurane treatment [25].
HPLC to Measure Endothelial Cell Adenosine Generation
EA.hy9262 or mouse glomerular endothelial cell culture media were collected after isoflurane treatment and assayed for adenosine by HPLC as described [18]. Adenosine was quantified on a C18 reversed-phase column with a binary low-pressure gradient elution system with a UV detector set to 254 nm as described [26]. Adenosine deaminase activity and adenosine uptake were inhibited with 10 µM erythro-9-(2-hydroxy-3-nonly)adenine (EHNA) and 10 µM dipyridamole, respectively.
Endothelial Cell Lysate or Microparticle CD73 Activity Assay
CD73 activity was measured by tracking the conversion of AMP to adenosine with or without 100 µM APCP using a modified protocol according to Gelain et al. [27].
Rho Kinase Activity Assay and Phospho-myosin Light Chain Immunoblotting
After 30 min treatment with 2.5% isoflurane or with carrier gas, we measured EA.hy9262 cell Rho kinase activity with a commercial assay that measures myosin phosphatase target subunit-1 (MYPT-1) Threonine residue 696 phosphorylation (Millipore, Billerica, MA). We also assessed Rho kinase activation by detecting myosin light chain (MLC) Serine-19 phosphorylation. EA.hy9262 cell lysates were probed with anti-phosphor-MLC 2 antibody as well as total-MLC 2 antibody (1∶10,000 anti-MLC20 (:1000, Cell Signaling Technology, Danvers, MA) and subjected to immunoblotting as described [18].
Statistical Analysis
The data were analyzed with Student’s t-test when comparing means between 2 groups or with one way analysis of variance plus TUKEY’s post hoc multiple comparison test to compare mean values across multiple treatment groups. All data are expressed throughout the text as means ± SEM.
Results
Isoflurane Increases Adenosine Generation in Cultured Endothelial Cells
We first determined whether isoflurane treatment increases adenosine generation in endothelial cells. Human umbilical vein (EA.hy9262) or mouse glomerular endothelial cells (GENC) were treated with 0–2.5% isoflurane for 6 hr and we measured adenosine levels in cell culture media with HPLC as described previously [18]. We determined that isoflurane treatment significantly increased adenosine levels in human and mouse endothelial cell culture media when compared with carrier gas-treated (room air plus 5% CO2) cells (Figure 1A).
Figure 1. Isoflurane increases adenosine generation in cultured endothelial cells.
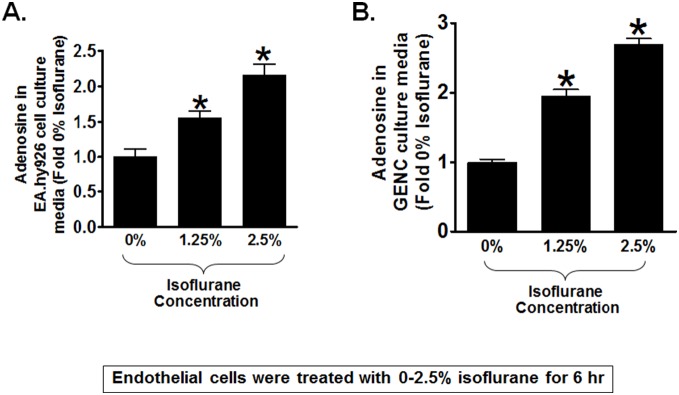
Adenosine in media from human umbilical vein endothelial cells (EA.hy926, A) or mouse glomerular endothelial cells (GENC, B) measured with high pressure liquid chromatography. Isoflurane treatment (0–2.5%) for 6 hr increased adenosine concentrations when compared to carrier gas-treated controls (N = 5). Data are presented as means ± SEM. *P<0.05 vs. carrier gas-treated controls. Error bars represent 1 SEM.
Isoflurane Transiently Increases CD73 Activity in Cultured Endothelial Cells
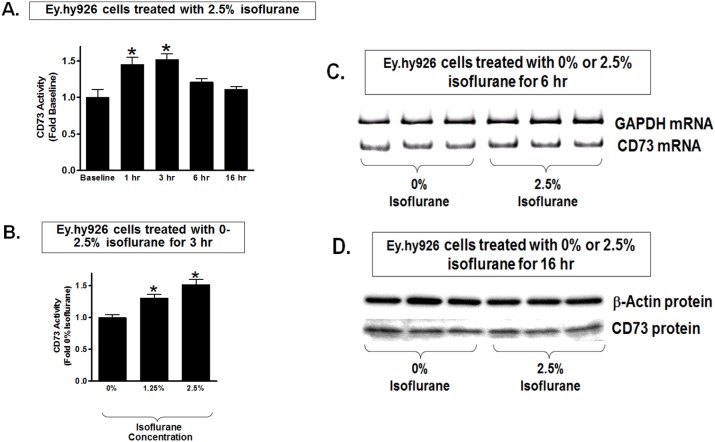
The next set of experiments determined whether isoflurane stimulates CD73 activity to increase adenosine generation in cultured endothelial cells. EA.hy9262 cells had significantly increased cell surface CD73 activity (conversion of AMP to adenosine) 1 to 3 hr after treatment with 2.5% isoflurane (Figure 2A). We also observed increased CD73 activity in EA.hy9262 cells with 1.25–2.5% isoflurane treatment for 3 hr (Figure 2B). Surprisingly, CD73 activity subsequently decreased with longer duration of isoflurane treatment (Figure 2A). Furthermore, transient increase in CD73 activity in EA.hy9262 cells did not require the induction of new CD73 synthesis as CD73 mRNA (Figure 2C) and protein (Figure 2D) did not increase with isoflurane treatment in these cells. Therefore, our studies show that isoflurane transiently increases CD73 activity without inducing new CD73 synthesis in cultured endothelial cells.
Figure 2. Isoflurane transiently increases CD73 activity without changing CD73 synthesis in cultured endothelial cells.
A. Human umbilical vein endothelial (EA.hy926) cells treated with 2.5% isoflurane showed a significant but transient induction of CD73 activity. CD73 activity peaked at 3 hr and then decreased to near baseline at 6–16 hr after isoflurane treatment (N = 6–8). B. Isoflurane treatment for 3 hr caused dose-dependent increase in CD73 activity in EA.hy926 cells compared to carrier gas-treated cells (N = 4–5). Data are presented as means ± SEM. *P<0.05 vs. CD73 activity measured at baseline (A) or in cells treated with 0% isoflurane (B). C and D. Representative images for CD73 mRNA (RT-PCR) and protein (immunoblotting) expression in EA.hy926 cells. EA.hy926 cells were treated with carrier gas or with 2.5% isoflurane for 6 hr (C) or for 16 hr (D). Isoflurane treatment did not increase CD73 mRNA or protein expression in EA.hy926 cells. Representative of 3–4 experiments.
Isoflurane Treatment Release Endothelial Cells Microparticles Containing CD73
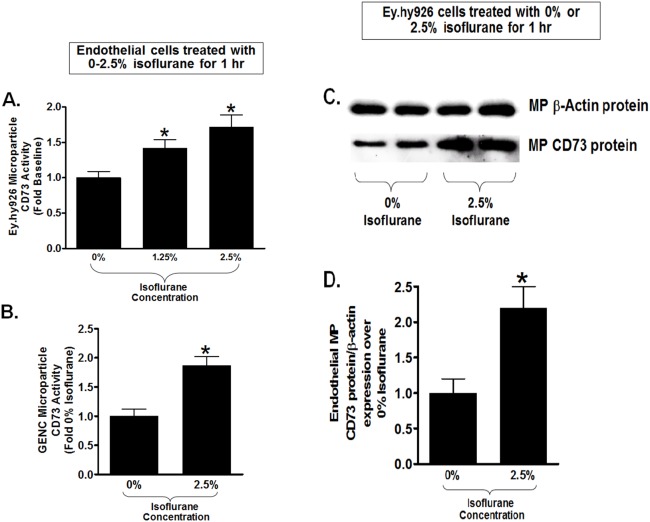
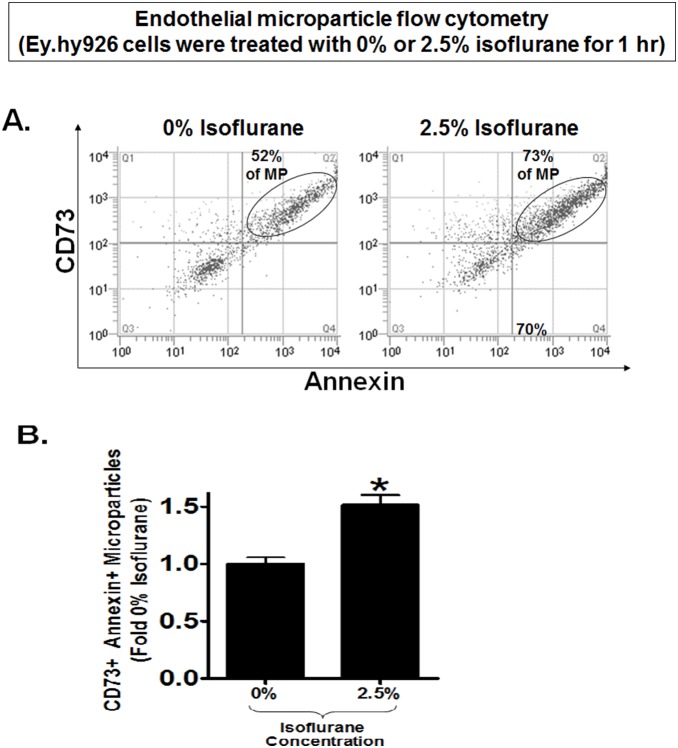
Since isoflurane transiently increases cell plasma membrane CD73 activity without inducing new CD73 synthesis in cultured endothelial cells, we tested whether isoflurane induces the release of plasma membrane microparticles containing CD73. Indeed, microparticles isolated from cell culture media of EA.hy9262 cells treated with 1.25 or 2.5% isoflurane for 1 hr had significantly increased CD73 activity compared to microparticles isolated from carrier gas-treated cells (Figure 3A). Isoflurane (2.5%) treatment for 1 hr also increased CD73 activity in microparticles isolated from GENC media (Figure 3B). Finally, microparticles from endothelial cells treated with 2.5% isoflurane had significantly increased CD73 protein expression compared to microparticle isolated from carrier gas-treated endothelial cells (Figure 3C and 3D). Flow cytometric analyses confirmed that endothelial cells treated with 2.5% isoflurane for 1 hr had significantly (∼50%) higher CD73+ Annexin V+ microparticles compared to microparticles isolated from carrier gas-treated endothelial cells (Figure 4).
Figure 3. Isoflurane releases CD73 containing microparticles in cultured endothelial cells.
A. Human umbilical vein endothelial (EA.hy926) cells were treated with 0–2.5% isoflurane for 1 hr and endothelial cell culture media microparticles (MP) were isolated and assayed for CD73 activity. Isoflurane caused a significant increase in human endothelial cell microparticle CD73 activity (N = 5–8). B. Microparticles isolated from mouse glomerular endothelial cells (GENC) treated with 2.5% isoflurane for 1 hr also had higher CD73 activity compared to carrier gas-treated cells (N = 4–6). C and D. Representative CD73 immunoblotting images (C) and band intensity quantifications (D) from microparticles isolated from EA.hy926 cells. Beta-actin protein expression was also quantified to normalize lane loading. Isoflurane treatment (2.5% for 1 hr) significantly increased CD73 protein expression in EA.hy926 cell microparticles compared to carrier gas-treated cells. *P<0.05 vs. carrier gas group. Error bars represent 1 SEM.
Figure 4. Flow cytometric analyses of endothelial cell culture media microparticles.
A. Representative flow cytometric analyses of microparticles isolated from EA.hy926 endothelial cell culture media. EA.hy926 cells were treated with 2.5% isoflurane or with carrier gas for 1 hr and isolated microparticles were incubated with CD73 antibody and Annexin V. B. EA.hy926 cells treated with 2.5% isoflurane for 1 hr had significantly higher CD73+ Annexin V+ microparticles compared to microparticles isolated from carrier gas-treated endothelial cells (N = 5). *P<0.05 vs. carrier gas group. Error bars represent 1 SEM.
Isoflurane Increases CD73 Containing Endothelial-derived Microparticles in Mouse Plasma
We next determined whether isoflurane anesthesia increases endothelial cell-derived CD73 containing microparticles in plasma of mice anesthetized with isoflurane. Figure 5A shows that plasma of mice anesthetized with 1.2% isoflurane for 3 hr had significantly increased CD144 (an endothelial cell marker)+ CD73+ microparticles compared to microparticles isolated from mice anesthetized with equi-anesthetic dose of pentobarbital. Furthermore, plasma microparticles isolated from mice anesthetized with isoflurane had significantly higher CD73 activity when compared to CD73 activity in plasma microparticles isolated from pentobarbital-anesthetized mice (Figure 5B).
Figure 5. Isoflurane increases CD73 containing endothelial cell-derived microparticles in mouse plasma.
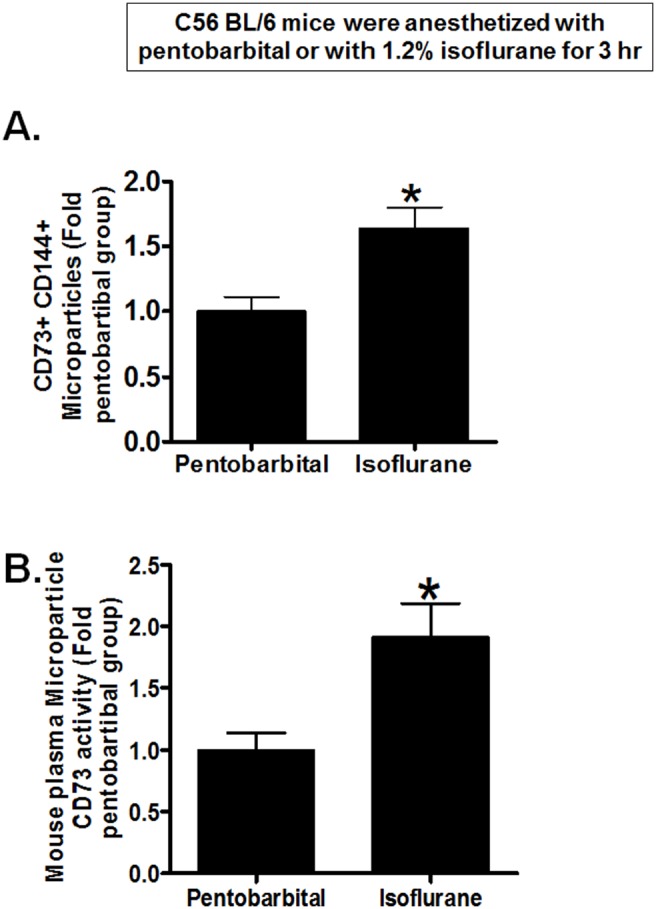
Mouse plasma microparticles (MP) were isolated after 1.2% isoflurane or equi-anesthetic dose of pentobarbital anesthesia for 3 hr. A. Mice anesthetized with isoflurane had significantly increased CD144 (endothelial cell marker)+ CD73+ plasma microparticles compared to mice anesthetized with pentobarbital (N = 4). B. Plasma microparticles isolated from mice anesthetized with isoflurane had significantly higher CD73 activity when compared to CD73 activity in microparticles isolated from pentobarbital-anesthetized mice (N = 5). *P<0.05 vs. carrier gas group. Error bars represent 1 SEM.
Isoflurane Reduces Endothelial Cell Apoptosis and Inflammation via Induction of Endothelial Microparticle CD73
We then tested whether CD73 is critical for isoflurane-mediated protection against endothelial apoptosis and inflammation. EA.hy9262 cells pretreated with carrier gas underwent apoptosis with robust PARP and caspase-3 fragmentation (Figure 6A) after TNF-α and cycloheximide treatment for 16 hr. In contrast, EA.hy9262 cells pretreated with 2.5% isoflurane for 1 hr had reduced apoptotic death indicated by decreased PARP and caspase-3 fragmentation. Supporting a critical role of CD73 activation in isoflurane-mediated protection against endothelial cell apoptosis, cells pretreated with a selective CD73 inhibitor APCP (100 µM) were not protected against endothelial apoptosis with isoflurane treatment (Figure 6A). Endothelial cells treated with TNF-a for 6 hr showed induction of several pro-inflammatory mRNAs including TNF-α, ICAM-1 and VCAM-1. Isoflurane pretreatment reduced the upregulation of these pro-inflammatory mRNAs. Again supporting a critical role of endothelial CD73 in isoflurane-mediated reduction in inflammation, endothelial cells pretreated with a selective CD73 inhibitor APCP (100 µM) were not protected against endothelial inflammation with isoflurane treatment (Figure 6B).
Figure 6. Isoflurane reduces endothelial cell apoptosis and inflammation via CD73.
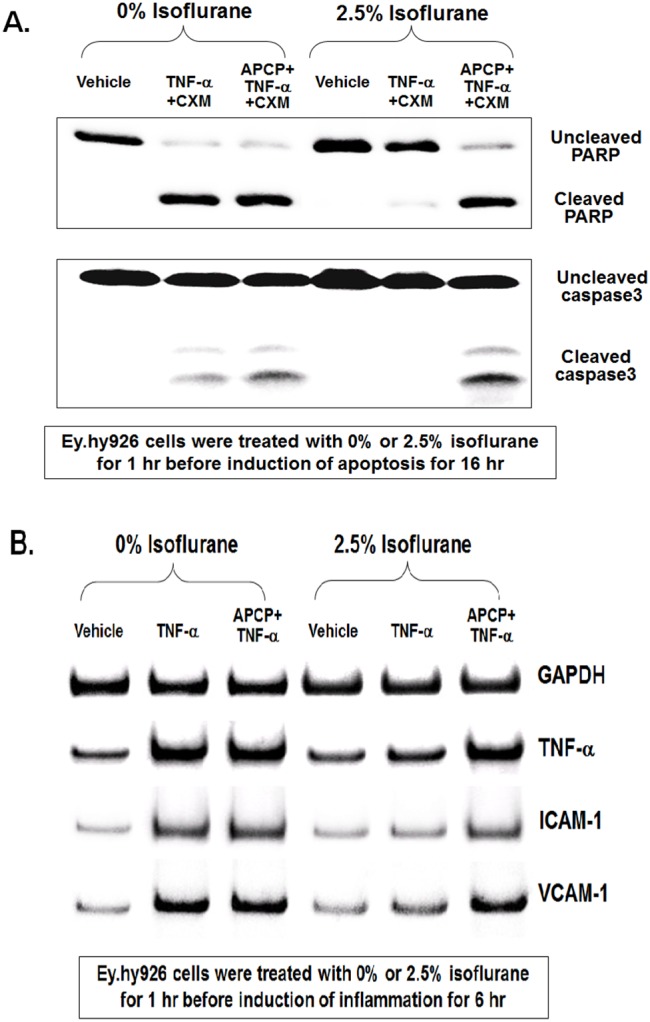
A. Representative immunoblot of poly(adenosine diphosphate-ribose) polymerase (PARP) and caspase-3 fragmentation (N = 4) as indices of EA.hy926 endothelial cell apoptosis induced by TNF-α (20 ng/ml) and cycloheximide (CHX; 10 µg/ml) treatment for 16 hr. EA.hy926 cells treated with TNF-α and cycloheximide for 16 hr had robust PARP and caspase-3 fragmentation. In contrast, EA.hy926 cells treated with 2.5% isoflurane for 1 hr before the induction of apoptosis isoflurane showed reduced apoptotic death indicated by decreased PARP and caspase-3 fragmentation. Supporting a critical role of CD73 in isoflurane-mediated protection against EA.hy926 cell apoptosis, cells pretreated with a selective CD73 inhibitor APCP (100 µM) were not protected against endothelial apoptosis with isoflurane pretreatment. B. Representative gel images of pro-inflammatory mRNA expression (VCAM-1, ICAM-2 and TNF-a) in EA.hy926 endothelial cells treated with TNF-α (20 ng/ml) for 6 hr. EA.hy926 cells treated with TNF-α had increased mRNA encoding markers of inflammation. In contrast, EA.hy926 cells treated with 2.5% isoflurane for 1 hr before TNF-α treatment had reduced inflammatory mRNA expression. Again, supporting a critical role of CD73 in isoflurane-mediated protection against EA.hy926 cell inflammation, cells pretreated with a selective CD73 inhibitor APCP were not protected against endothelial inflammation with isoflurane pretreatment.
Isoflurane-mediated Activation of Rho Kinase Mediates the Release of Endothelial Microparticles Containing CD73
Since previous studies showed that Rho kinase activation increases endothelial microparticle generation [28]–[30], we tested the hypothesis that isoflurane activates endothelial Rho kinase to release endothelial microparticles containing CD73. Figure 7A shows a significant increase in Rho kinase activity (detected by MYPT-1 phosphorylation) in EA.hy9262 cells treated with 2.5% isoflurane for 30 min. compared to carrier gas. Furthermore, Figure 7B shows increased p-MLC immunoreactivity in EA.hy9262 cells treated with 2.5% isoflurane for 30 min. Total MLC immunoreactivity did not change with isoflurane treatment. In addition, we were able to block isoflurane-mediated MLC phosphorylation with a selective inhibitor of Rho kinase (Y27632, data not shown). Finally, Figure 8 shows that a selective Rho kinase inhibitor Y27632 prevented isoflurane-mediated increases in microparticle CD73 activity in EA.hy9262 cells supporting a critical role for Rho kinase in isoflurane-mediated release of CD73 containing endothelial microparticles.
Figure 7. Isoflurane stimulates Rho kinase activity in human endothelial cells.
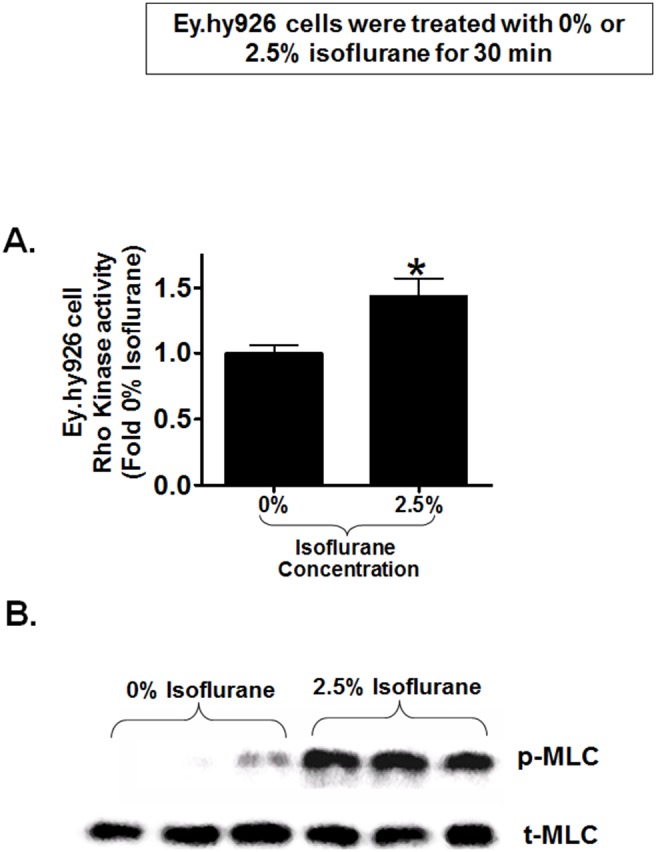
A. Rho kinase activity in human endothelial (EA.hy9262) cells was measured by detecting myosin phosphatase target protein-1 phosphorylation after treatment with 2.5% isoflurane or with carrier gas for 30 min. Isoflurane significantly increased Rho kinase activity in EA.hy9262 cells compared to carrier gas-treated cells (N = 5). *P<0.05 vs. carrier gas group. Error bars represent 1 SEM. B. EA.hy9262 endothelial Rho kinase activity was also assessed by detecting myosin light chain (MLC) phosphorylation in EA.hy9262 cells with immunoblotting. MLC phosphorylation increased in EA.hy9262 cells treated with 2.5% isoflurane for 30 min compared to carrier gas-treated cells. Total MLC immunoreactivity did not change with isoflurane treatment. Representative of 2 experiments performed in triplicate.
Figure 8. Isoflurane-mediated activation of Rho kinase mediates the release of CD73 containing endothelial microparticles.
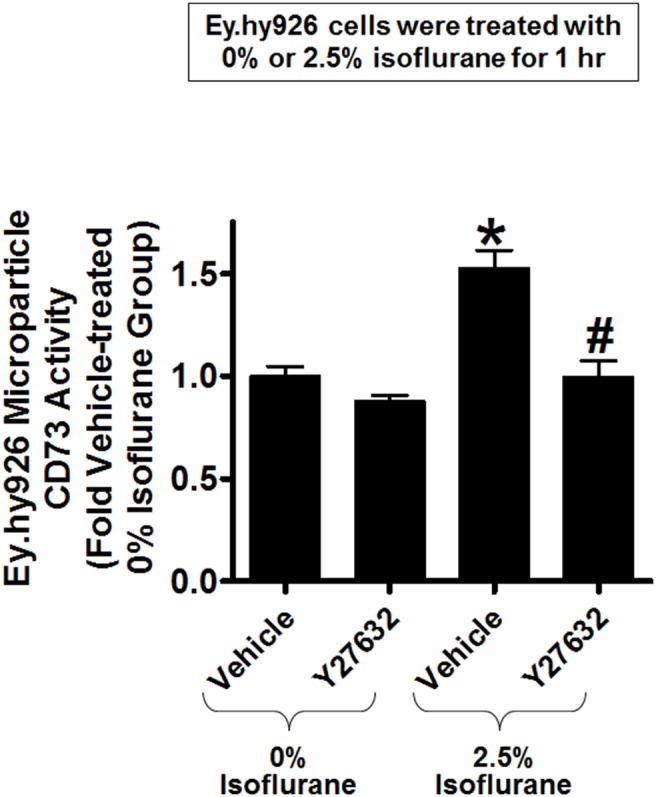
Human endothelial (EA.hy9262) cells were treated with 2.5% isoflurane or with carrier gas for 1 hr. Some cells were pretreated with a selective Rho kinase inhibitor Y27632 for 30 min. before isoflurane or carrier gas treatment. Y27632 prevented the isoflurane-mediated increase in microparticle CD73 activity in EA.hy9262 cells (N = 4). *P<0.05 vs. carrier gas group treated with vehicle. #P<0.05 vs. isoflurane group treated with vehicle. Error bars represent 1 SEM.
Discussion
Overactive inflammatory response is a detrimental complication of surgery and perioperative surgical infections. Anti-inflammatory and cytoprotective effects of volatile inhalational anesthetics are well recognized [31], [32]. However, the mechanisms mediating the protective effects of volatile anesthetics have not been fully elucidated. Our findings suggest that volatile anesthetics, at least in part, attenuate endothelial inflammation and apoptosis by releasing CD73 containing endothelial microparticles. We found that clinically relevant concentrations of isoflurane (1.25–2.5%) increased adenosine generation and rapidly increased endothelial CD73 activity in cultured human umbilical vein endothelial and mouse glomerular endothelial cells. We were surprised to discover that isoflurane-mediated induction of CD73 activity was transient and occurred without any changes in CD73 expression. We determined that isoflurane-mediated induction of CD73 activity was due to the release of preformed CD73 contained in endothelial plasma membrane microparticles. CD73 activity was critical for isoflurane-mediated protection against endothelial apoptosis and inflammation. Finally, the mechanisms of isoflurane-mediated release of CD73 containing microparticles are mediated by activation of endothelial Rho kinase.
In addition to its analgesic and anesthetic properties, volatile anesthetics have non-anesthetic effects in virtually every cell type. Importantly, volatile anesthetics protect against cell death and inflammation in several key organs including the heart, brain, kidney and intestine. For example, several clinically utilized volatile anesthetics including isoflurane precondition the heart against ischemia and reperfusion injury [11]. We have previously demonstrated that volatile anesthetics including isoflurane display anti-necrotic and anti-inflammatory effects in renal proximal tubule cells in vivo and in vitro [5], [12], [13]. Harnessing these non-anesthetic properties of volatile anesthetics may have important clinical implications for critically ill patients anesthetized in the OR and sedated in the ICU.
Previous studies have shown that isoflurane anesthesia in mice and rats attenuates lipopolysaccharide induced endothelial inflammation [16], [17]. Furthermore, isoflurane inhalation after LPS injection in rats in vivo attenuates systemic cytokine (IL-1β and IL-6) upregulation and well as lung injury [33], [34]. We previously showed that volatile anesthetic isoflurane protect against human endothelial apoptosis and inflammation [13], [35]. Consistent with our findings, others have also shown that isoflurane treatment attenuated lipopolysaccharide or TNF-α induced endothelial cell inflammation and death in vitro [14], [36]. Although the robust anti-inflammatory and protective effects of isoflurane have been demonstrated in vivo and in vitro, the detained cytoprotective mechanisms remained elusive.
We recently showed that isoflurane protected against renal tubular necrosis, apoptosis and inflammation by direct induction of CD73 enzyme and activity leading to enhanced adenosine generation [18]. CD73 is a well-known anti-inflammatory and anti-ischemic enzyme. Mice deficient in CD73 have increased tissue and vascular inflammation and have a higher mortality rate after ischemia and reperfusion injury and sepsis [37], [38] [39]. Moreover, enhanced CD73 activity protects against intestinal, cardiac and renal ischemia reperfusion injury [37], [38], [40]. Cell surface CD73 catalyzes the hydrolysis of AMP to adenosine and is a critical step in extracellular adenosine generation [41]. Extracellular adenosine regulates diverse and important physiological effects including cardiac inotropy and chronotropy, vascular tone and kidney glomerular filtration rate. Furthermore, adenosine protects against tissue injury and inflammation after ischemia and reperfusion or sepsis. Adenosine acts via activation of 4 G-protein coupled purinergic receptors [A1, A2a, A2b and A3 adenosine receptors] [41], [42]. In particular, activation of A1, A2a or A2bARs protects against ischemia reperfusion injury in the kidney, heart, liver and brain [43], [44]. Unlike findings in renal proximal tubular cells where CD73 synthesis was increased after isoflurane treatment, isoflurane increases adenosine generation in endothelial cells by releasing preformed CD73 contained in endothelial plasma membrane microparticles without synthesizing new CD73 enzyme [18]. The exact subtype(s) of adenosine receptor(s) involved in endothelial protection by isoflurane-mediated adenosine generation remains to be elucidated.
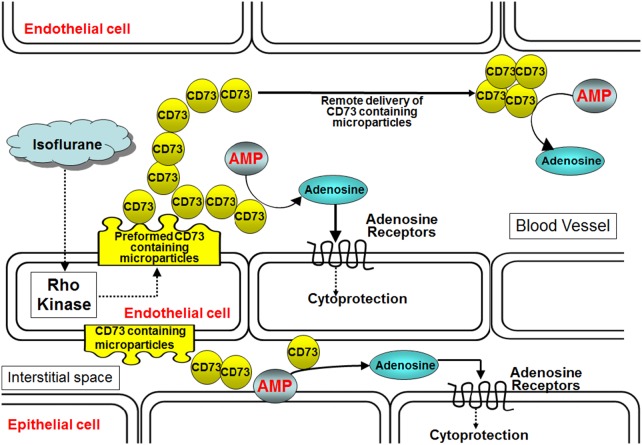
In this study, we demonstrate that isoflurane rapidly released endothelial microparticles containing preformed CD73 in cultured endothelial cells as well as in plasma of mice. Indeed, CD73 was directly responsible for isoflurane-mediated endothelial cell protection. Plasma membrane microparticles are phospholipid microvesicles of submicron (0.1 to 1.0 µm) fragments that originate from plasma membrane blebbing and are subsequently shed [4], [45]. Microparticles play an important role in the transfer of materials between cells. Furthermore, they are critical in transferring signaling information to cells close by or far away. Plasma microparticles are elevated in several pathological conditions including vascular thrombosis, hyperlipidemia, diabetes, chronic renal dysfunction and cancer [4], [28], [46]. Here we show that endothelial microparticles could also have a cytoprotective and beneficial role. We propose that isoflurane treatment propagates the systemic release of endothelial microparticles containing active CD73 that function as cytoprotective messengers by generating adenosine. Isoflurane-mediated endothelial microparticle generation may prevent damage and favor vascular repair by preventing endothelial apoptosis and inflammation. Furthermore, microparticle-mediated delivery of CD73 allows adenosine formation in different cell types (e.g., epithelial cell). Finally, microparticle generation allows remote delivery of CD73 away from the originating endothelial cell. Our findings imply that isoflurane-mediated generation of CD73 containing microparticles in one organ (e.g., lung) may travel to distant locations (e.g., kidney, liver) to produce multi-organ anti-inflammatory effects (Figure 9).
Figure 9. Proposed mechanisms of isoflurane-mediated endothelial CD73 generation.
We hypothesize that isoflurane via Rho kinase activation releases endothelial microparticles containing CD73. Increased endothelial microparticle CD73 converts AMP to adenosine which produces cytoprotective effects on neighboring endothelial cells or renal epithelial cells via activation of adenosine receptors. Furthermore, we hypothesize that remote delivery of CD73 containing microparticles may provide systemic anti-inflammatory effects of isoflurane anesthesia. Abbreviations: AMP = adenosine monophosphate, CD73 = ecto-5′-nucleotidase.
We also demonstrate an important role for Rho kinase activation in isoflurane-mediated CD73 containing endothelial microparticle release. We show in this study that isoflurane-mediated endothelial microparticle release and induction of CD73 activity were significantly attenuated by a selective Rho kinase inhibitor. In many cell types including endothelial cells, Rho kinase regulates cytoskeleton architecture, migration and growth [47], [48]. Previous studies suggest that volatile anesthetics including isoflurane activate Rho kinase and promote Rho A function. In primary neuronal cultures as well as rat glioma C6 cell line, Rho A was activated after exposure to ∼1 MAC isoflurane [49], [50]. Evidence for Rho kinase-mediated endothelial microparticle formation and release also provided by Burger et al [28]. They showed that angiotensin II-mediated microparticle formation is mediated by Rho kinase pathways targeted to lipid rafts. In addition, thrombin induces endothelial microparticle generation via Rho kinase activation [29]. Finally, endothelial microparticles released with TNF-α treatment in human coronary artery endothelial cells are suppressed by a specific Rho kinase inhibitor (Y-27632) [30].
In summary, we demonstrate that a commonly utilized volatile anesthetic isoflurane rapidly increases endothelial cell adenosine generation via releasing microparticles containing preformed CD73. Release of CD73 and subsequent adenosine generation may result in cellular protection in neighboring and remote endothelial and epithelial cells via activation of adenosine receptors. Our current findings in endothelial cells differ considerably from findings in renal tubular epithelial cells as increased adenosine generation occurred without the induction of new CD73 synthesis. Taken together, our current study provides additional mechanistic insight into the mechanism of isoflurane-mediated endothelial cell protection.
Acknowledgments
We appreciate the technical assistance provided by Sang Won Park, Ph.D.
Funding Statement
This work was supported in part by Department of Anesthesiology, Columbia University and by National Institutes of Health grant R01 GM-067081. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jaffe EA (1987) Cell biology of endothelial cells. Hum Pathol 18: 234–239. [DOI] [PubMed] [Google Scholar]
- 2. Higashi Y, Kihara Y, Noma K (2012) Endothelial dysfunction and hypertension in aging. Hypertens Res 35: 1039–1047. [DOI] [PubMed] [Google Scholar]
- 3. Higashi Y, Noma K, Yoshizumi M, Kihara Y (2009) Endothelial function and oxidative stress in cardiovascular diseases. Circ J 73: 411–418. [DOI] [PubMed] [Google Scholar]
- 4. Burger D, Schock S, Thompson CS, Montezano AC, Hakim AM, et al. (2013) Microparticles: biomarkers and beyond. Clin Sci (Lond) 124: 423–441. [DOI] [PubMed] [Google Scholar]
- 5. Seal JB, Gewertz BL (2005) Vascular dysfunction in ischemia-reperfusion injury. Ann Vasc Surg 19: 572–584. [DOI] [PubMed] [Google Scholar]
- 6. Van Hoozen BE, Albertson TE (1999) Endothelial cell dysfunction: a potential new approach for the treatment of sepsis. Crit Care Med 27: 2836–2838. [DOI] [PubMed] [Google Scholar]
- 7.Cullen KA, Hall MJ, Golosinskiy A (2009) Ambulatory surgery in the United States, 2006. Natl Health Stat Report 1–25. [PubMed]
- 8. Hofbauer R, Kaye AD, Kapiotis S, Frass M (1999) The immune system and the effects of non-volatile anesthetics on neutrophil transmigration through endothelial cell monolayers. Curr Pharm Des 5: 1015–1027. [PubMed] [Google Scholar]
- 9. Landoni G, Fochi O, Tritapepe L, Guarracino F, Belloni I, et al. (2009) Cardiac protection by volatile anesthetics. A review. Minerva Anestesiol 75: 269–273. [PubMed] [Google Scholar]
- 10. Kim M, Kim M, Kim N, D'Agati VD, Emala CW Sr, et al. (2007) Isoflurane mediates protection from renal ischemia-reperfusion injury via sphingosine kinase and sphingosine-1-phosphate-dependent pathways. Am J Physiol Renal Physiol 293: F1827–F1835. [DOI] [PubMed] [Google Scholar]
- 11.Kim M, Park SW, Kim M, D'Agati VD, Lee HT (2011) Isoflurane protects against intestinal ischemia-reperfusion injury and multi-organ dysfunction via transforming growth factor-beta1. Ann Surg In Press. [DOI] [PMC free article] [PubMed]
- 12. Lee HT, Emala CW, Joo JD, Kim M (2007) Isoflurane improves survival and protects against renal and hepatic injury in murine septic peritonitis. Shock 27: 373–379. [DOI] [PubMed] [Google Scholar]
- 13. Bakar AM, Park SW, Kim M, Lee HT (2012) Isoflurane Protects Against Human Endothelial Cell Apoptosis by Inducing Sphingosine Kinase-1 via ERK MAPK. Int J Mol Sci 13: 977–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Klaver MJ, Buckingham MG, Rich GF (2003) Isoflurane pretreatment has immediate and delayed protective effects against cytokine-induced injury in endothelial and vascular smooth muscle cells. Anesthesiology 99: 896–903. [DOI] [PubMed] [Google Scholar]
- 15. Mobert J, Zahler S, Becker BF, Conzen PF (1999) Inhibition of neutrophil activation by volatile anesthetics decreases adhesion to cultured human endothelial cells. Anesthesiology 90: 1372–1381. [DOI] [PubMed] [Google Scholar]
- 16.Hayes JK, Havaleshko DM, Plachinta RV, Rich GF (2004) Isoflurane pretreatment supports hemodynamics and leukocyte rolling velocities in rat mesentery during lipopolysaccharide-induced inflammation. Anesth Analg 98: 999–1006, table. [DOI] [PubMed]
- 17. Plachinta RV, Hayes JK, Cerilli LA, Rich GF (2003) Isoflurane pretreatment inhibits lipopolysaccharide-induced inflammation in rats. Anesthesiology 98: 89–95. [DOI] [PubMed] [Google Scholar]
- 18. Kim M, Ham A, Kim JY, Brown KM, D'Agati VD, et al. (2013) The volatile anesthetic isoflurane induces ecto-5′-nucleotidase (CD73) to protect against renal ischemia and reperfusion injury. Kidney Int 84: 90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akis N, Madaio MP (2004) Isolation, culture, and characterization of endothelial cells from mouse glomeruli. Kidney Int 65: 2223–2227. [DOI] [PubMed] [Google Scholar]
- 20. Park SW, Kim M, Brown KM, D'Agati VD, Lee HT (2012) Inhibition of sphingosine 1-phosphate receptor 2 protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 23: 266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amabile N, Guerin AP, Leroyer A, Mallat Z, Nguyen C, et al. (2005) Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol 16: 3381–3388. [DOI] [PubMed] [Google Scholar]
- 22. Kim M, Chen SW, Park SW, Kim M, D'Agati VD, et al. (2009) Kidney-specific reconstitution of the A1 adenosine receptor in A1 adenosine receptor knockout mice reduces renal ischemia-reperfusion injury. Kidney Int 75: 809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim M, Park SW, Kim M, Chen SW, Gerthoffer WT, et al.. (2010) Selective Renal Over-Expression of Human Heat Shock Protein 27 Reduces Renal Ischemia-Reperfusion Injury in Mice. Am J Physiol Renal Physiol. [DOI] [PMC free article] [PubMed]
- 24. Lee HT, Kim M, Kim M, Kim N, Billings Iv FT, et al. (2007) Isoflurane protects against renal ischemia and reperfusion injury and modulates leukocyte infiltration in mice. Am J Physiol Renal Physiol 293: F713–F722. [DOI] [PubMed] [Google Scholar]
- 25. Kim M, Kim M, Park SW, Pitson SM, Lee HT (2010) Isoflurane protects human kidney proximal tubule cells against necrosis via sphingosine kinase and sphingosine-1-phosphate generation. Am J Nephrol 31: 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Delabar U, Kloor D, Luippold G, Muhlbauer B (1999) Simultaneous determination of adenosine, S-adenosylhomocysteine and S-adenosylmethionine in biological samples using solid-phase extraction and high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 724: 231–238. [DOI] [PubMed] [Google Scholar]
- 27. Gelain DP, de Souza LF, Bernard EA (2003) Extracellular purines from cells of seminiferous tubules. Mol Cell Biochem 245: 1–9. [DOI] [PubMed] [Google Scholar]
- 28. Burger D, Montezano AC, Nishigaki N, He Y, Carter A, et al. (2011) Endothelial microparticle formation by angiotensin II is mediated via Ang II receptor type I/NADPH oxidase/Rho kinase pathways targeted to lipid rafts. Arterioscler Thromb Vasc Biol 31: 1898–1907. [DOI] [PubMed] [Google Scholar]
- 29. Sapet C, Simoncini S, Loriod B, Puthier D, Sampol J, et al. (2006) Thrombin-induced endothelial microparticle generation: identification of a novel pathway involving ROCK-II activation by caspase-2. Blood 108: 1868–1876. [DOI] [PubMed] [Google Scholar]
- 30. Tramontano AF, O'Leary J, Black AD, Muniyappa R, Cutaia MV, et al. (2004) Statin decreases endothelial microparticle release from human coronary artery endothelial cells: implication for the Rho-kinase pathway. Biochem Biophys Res Commun 320: 34–38. [DOI] [PubMed] [Google Scholar]
- 31. Fuentes JM, Talamini MA, Fulton WB, Hanly EJ, Aurora AR, et al. (2006) General anesthesia delays the inflammatory response and increases survival for mice with endotoxic shock. Clin Vaccine Immunol 13: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reutershan J, Chang D, Hayes JK, Ley K (2006) Protective effects of isoflurane pretreatment in endotoxin-induced lung injury. Anesthesiology 104: 511–517. [DOI] [PubMed] [Google Scholar]
- 33. Hofstetter C, Flondor M, Boost KA, Koehler P, Bosmann M, et al. (2005) Muhl H (2005) A brief exposure to isoflurane (50 s) significantly impacts on plasma cytokine levels in endotoxemic rats. Int Immunopharmacol 5: 1519–1522. [DOI] [PubMed] [Google Scholar]
- 34. Flondor M, Hofstetter C, Boost KA, Betz C, Homann M, et al. (2008) Isoflurane inhalation after induction of endotoxemia in rats attenuates the systemic cytokine response. Eur Surg Res 40: 1–6. [DOI] [PubMed] [Google Scholar]
- 35. Lee HT, Kim M, Jan M, Emala CW (2006) Anti-inflammatory and anti-necrotic effects of the volatile anesthetic sevoflurane in kidney proximal tubule cells. Am J Physiol Renal Physiol 291: F67–F78. [DOI] [PubMed] [Google Scholar]
- 36. de Klaver MJ, Manning L, Palmer LA, Rich GF (2002) Isoflurane pretreatment inhibits cytokine-induced cell death in cultured rat smooth muscle cells and human endothelial cells. Anesthesiology 97: 24–32. [DOI] [PubMed] [Google Scholar]
- 37. Grenz A, Zhang H, Eckle T, Mittelbronn M, Wehrmann M, et al. (2007) Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol 18: 833–845. [DOI] [PubMed] [Google Scholar]
- 38. Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, et al. (2007) Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation 115: 1581–1590. [DOI] [PubMed] [Google Scholar]
- 39. Hasko G, Csoka B, Koscso B, Chandra R, Pacher P, et al. (2011) Ecto-5′-nucleotidase (CD73) decreases mortality and organ injury in sepsis. J Immunol 187: 4256–4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hart ML, Henn M, Kohler D, Kloor D, Mittelbronn M, et al. (2008) Role of extracellular nucleotide phosphohydrolysis in intestinal ischemia-reperfusion injury. FASEB J 22: 2784–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Colgan SP, Eltzschig HK, Eckle T, Thompson LF (2006) Physiological roles for ecto-5′-nucleotidase (CD73). Purinergic Signal 2: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Strohmeier GR, Lencer WI, Patapoff TW, Thompson LF, Carlson SL, et al. (1997) Surface expression, polarization, and functional significance of CD73 in human intestinal epithelia. J Clin Invest 99: 2588–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Linden J (2006) New insights into the regulation of inflammation by adenosine. J Clin Invest 116: 1835–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hasko G, Linden J, Cronstein B, Pacher P (2008) Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 7: 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. gnat-George F, Boulanger CM (2011) The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol 31: 27–33. [DOI] [PubMed] [Google Scholar]
- 46. Rautou PE, Vion AC, Amabile N, Chironi G, Simon A, et al. (2011) Microparticles, vascular function, and atherothrombosis. Circ Res 109: 593–606. [DOI] [PubMed] [Google Scholar]
- 47. Zhou Q, Liao JK (2009) Rho kinase: an important mediator of atherosclerosis and vascular disease. Curr Pharm Des 15: 3108–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Budzyn K, Sobey CG (2007) Vascular rho kinases and their potential therapeutic applications. Curr Opin Drug Discov Devel 10: 590–596. [PubMed] [Google Scholar]
- 49. Lemkuil BP, Head BP, Pearn ML, Patel HH, Drummond JC, et al. (2011) Isoflurane neurotoxicity is mediated by p75NTR-RhoA activation and actin depolymerization. Anesthesiology 114: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tas PW, Gambaryan S, Roewer N (2007) Volatile anesthetics affect the morphology of rat glioma C6 cells via RhoA, ERK, and Akt activation. J Cell Biochem 102: 368–376. [DOI] [PubMed] [Google Scholar]