Bacteria may encounter a very wide variety of conditions in nature. These include changes in salinity, temperature, pH, and the presence of antibacterial molecules that have evolved either to kill them or impede their growth. In response, some bacteria have evolved modifications of their membrane lipids that improve their survival. Among these are amino acyl derivatives of phosphatidylglycerol (PG) and cardiolipin (diphosphatidylglycerol). A lysine derivative of PG (Fig. 1) was first found in Staphylococcus aureus.1,2 Other amino acids linked to PG including ornithine and alanine have been detected in bacteria.3,4 The amino acid group is transferred from aminoacyl-tRNA to PG.5,6
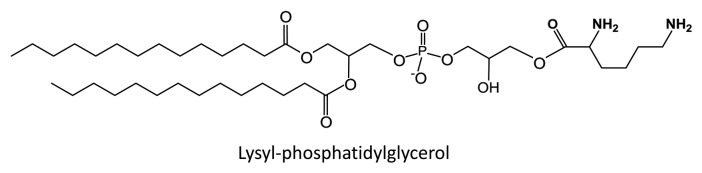
Figure 1. Lysyl-phosphatidylglycerol.
Soon after the discovery of lys-PG it was observed that the ratios of PG and lys-PG changed as the pH of the culture medium was varied. In S. aureus grown at pH 7, PG and cardiolipin were the predominant lipids; however at pH 5 there was a marked increase in lys-PG and a decrease in PG.1,2 Using artificial bilayers formed from lipids purified from bacteria, Hopfer et al. found that membranes made from positively-charged lys-PG were selective for the negatively-charged chloride ion, whereas negatively-charged membranes made from PG and cardiolipin were highly cation selective.7 Thus an increase in lys-PG at the expense of the negatively charged lipids could potentially serve to protect the cell from increased proton concentrations at lower pH.
The gene mprF (multi peptide resistance factor), which encodes aminoacyl-PG synthase, was discovered by screening a transposon insertion library of Staphylococcus xylosus C2a for sensitivity to the lantibiotic gallidermin, a polypeptide antibiotic.8 This gene conferred resistance of S. aureus to cationic proteins and polypeptides of the defensin and protegrin families, ancient and effective components of innate immunity.9 Aminoacyl-PG synthases are encoded in the genomes of a wide variety of gram-positive bacteria (mainly firmicutes and actinobacteria) and in gram-negative proteobacteria.10,11 Members of the MprF family may be specific for alanine or lysine or in some cases, both.12
The genome of Listeria monocytogenes, a gram-positive, facultatively intracellular pathogen that infects a wide variety of animal species including humans, contains an mprF ortholog and the cells are able to synthesize both lys-PG and lys-cardiolipin.13,14 This foodborne pathogen has been responsible for several large outbreaks that mainly affect the immunocompromised, the very young, the elderly and pregnant women, resulting in approximately 20% mortality.15 Thedieck et al. found that an mprF mutant of L. monocytogenes had increased sensitivity to gallidermin, but only at a relatively low concentration, 0.5 μg/ml, in liquid medium. At higher concentrations the wild-type and mutant were equally affected. The resistance conferred by MprF to the α-defensins HNP-1 and -2, were moderate and also concentration-dependent. These authors also reported that infection with an mprF mutant produced fewer bacteria after 1–2 h of infection than the wild type in Caco-2 epithelial cells, HeLa cells and the P388D1 macrophage cell line. In a mouse model of infection, growth of the mutant in the spleen was attenuated and even more so in the liver.14 Thus it appears that MprF serves as a virulence factor for this pathogen and that resistance to cationic antimicrobial peptides (CAMPs) contributes significantly to its intracellular survival and its pathogenicity. Lys-PG and lys-cardiolipin are not confined to pathogenic species of Listeria, they are also found in L. innocua, L. seeligeri, and L. welshimeri, which may encounter antimicrobial peptides in their environments.13
The findings of Dare et al.16 draw a clear distinction between Bacillus subtilis, a saprophytic non-pathogen and L. monocytogenes. It should be noted that B. subtilis has phosphatidylethanolamine as a major lipid, which is not present in L. monocytogenes. Thus the content of lys-PG was lower in B. subtilis when both were grown at 37 °C. Interestingly, the absence of MprF negatively affected the growth of B. subtilis in response to a much wider range of antibacterial substances than was the case for L. monocytogenes. These included antibiotics, membrane disrupters and a cationic surfactant. Indeed, the effects of chloroxylenol, a disrupter of membrane potential, were different for the two species; the wild-type strain of B. subtilis was more resistant than the ΔmprF mutant, but the contrary was true for L. monocytogenes. The presence of MprF (aa-PG synthase) in L. monocytogenes did confer resistance to two aminoglycosides, tobramycin and geneticin, but no effect of this mutation on resistance to these compounds was observed in the case of B. subtilis.
Two clear phenotypes of the mprF deletion mutant in L. monocytogenes were increased sensitivity to the osmolytes urea, sodium chloride, potassium chloride and sodium sulfate, and decreased survival in the presence of bile salts. High osmotic pressure is encountered by L. monocytogenes during its passage through the cytosol and bile salts may present a barrier during passage through the intestinal tract. Notably, L. monocytogenes expresses a bile salt hydrolase, an enzyme that confers resistance to bile salts.17 The absence of this activity had a greater effect on survival in the presence of bile salts than the loss of MprF.
In wild-type L. monocytogenes motility is greatly decreased at 37 °C compared with 30 °C. However, this was not the case for the ΔmprF strain which retained motility at 37 °C. The effects of the presence of lys-PG on the complex thermoregulation of L. monocytogenes motility through the MogR antirepressor, GmaR18,19 require further study.
Most of the effects of lys-PG can be attributed to its ion selectivity and its general effects on membrane permeability. It is surprising that the present study finds no effects on sensitivity to RC-1, a θ-defensin, and HNP-1, an α-defensin. As noted above, previous studies have shown that there is a concentration-dependent effect of loss of lys-PG on resistance to HNP-1.14 Furthermore, whereas these authors noted a significant decrease in the mprF mutant in Caco-2 cells at 1 h after infection, Dare et al. did not find the differences to be significant.
The expression of several important virulence factors of L. monocytogenes is under the control of PrfA, the expression of which is also highly regulated. In contrast, the expression of mprF is under the control of the two-component regulatory system VirR/VirS.20 The differences noted between the present results of Dare et al.16 and those reported by Thedieck et al. could result from the ways the bacteria are grown and handled prior to infection. Cells grown and processed under different conditions could show considerable variation in the regulation of expression of genes under the control of a two-component system.
So, where are we? What appeared to be a simple lipid modification that conferred resistance to widespread CAMPs and ultimately the ability to infect and flourish in cells that produce these peptides, may have wider ramifications such as the control of thermoregulated motility and resistance to bile salts and high osmotic strength. L. monocytogenes and other important human and domestic animal pathogens require constant surveillance, thus it is critical to increase our understanding of the defenses they have evolved against host and environmental compounds that can help to contain them.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Houtsmuller UMT, van Deenen LLM. On the amino acid esters of phosphatidyl glycerol from bacteria. Biochim Biophys Acta. 1965;106:564–76. doi: 10.1016/0005-2760(65)90072-X. [DOI] [PubMed] [Google Scholar]
- 2.Houtsmuller UMT, Van Deenen LLM. On the accumulation of amino acid derivates of phosphatidylglycerol in bacteria. Biochim Biophys Acta. 1964;84:96–8. doi: 10.1016/0926-6542(64)90106-4. [DOI] [PubMed] [Google Scholar]
- 3.Houtsmuller UMT, van Deenen LLM. Identification of a bacterial phospholipid as an O-ornithine ester of phosphatidyl glycerol. Biochim Biophys Acta. 1963;70:211–3. doi: 10.1016/0006-3002(63)90743-1. [DOI] [PubMed] [Google Scholar]
- 4.Macfarlane MG. Characterization of lipoamino-acids as O-amino-acid esters of phosphatidylglycerol. Nature. 1962;196:136–8. doi: 10.1038/196136a0. [DOI] [Google Scholar]
- 5.Lennarz WJ, Nesbitt JA, Reiss J. Biosynthesis of Lysyl Phosphatidylglycerol. Fed Proc. 1966;25:521. [Google Scholar]
- 6.Lennarz WJ, Bonsen PPM, van Deenen LL. Substrate specificity of O-L-lysylphosphatidylglycerol synthetase. Enzymatic studies on the structure of O-L-lysylphosphatidylglycerol. Biochemistry. 1967;6:2307–12. doi: 10.1021/bi00860a005. [DOI] [PubMed] [Google Scholar]
- 7.Hopfer U, Lehninger AL, Lennarz WJ. The effect of the polar moiety of lipids on the ion permeability of bilayer membranes. J Membr Biol. 1970;2:41–58. doi: 10.1007/BF01869849. [DOI] [PubMed] [Google Scholar]
- 8.Peschel A, Jack RW, Otto M, Collins LV, Staubitz P, Nicholson G, Kalbacher H, Nieuwenhuizen WF, Jung G, Tarkowski A, et al. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J Exp Med. 2001;193:1067–76. doi: 10.1084/jem.193.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peschel A, Sahl HG. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol. 2006;4:529–36. doi: 10.1038/nrmicro1441. [DOI] [PubMed] [Google Scholar]
- 10.Roy H. Tuning the properties of the bacterial membrane with aminoacylated phosphatidylglycerol. IUBMB Life. 2009;61:940–53. doi: 10.1002/iub.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy H, Ibba M. Broad range amino acid specificity of RNA-dependent lipid remodeling by multiple peptide resistance factors. J Biol Chem. 2009;284:29677–83. doi: 10.1074/jbc.M109.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roy H, Ibba M. RNA-dependent lipid remodeling by bacterial multiple peptide resistance factors. Proc Natl Acad Sci U S A. 2008;105:4667–72. doi: 10.1073/pnas.0800006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer W, Leopold K. Polar lipids of four Listeria species containing L-lysylcardiolipin, a novel lipid structure, and other unique phospholipids. Int J Syst Bacteriol. 1999;49:653–62. doi: 10.1099/00207713-49-2-653. [DOI] [PubMed] [Google Scholar]
- 14.Thedieck K, Hain T, Mohamed W, Tindall BJ, Nimtz M, Chakraborty T, Wehland J, Jänsch L. The MprF protein is required for lysinylation of phospholipids in listerial membranes and confers resistance to cationic antimicrobial peptides (CAMPs) on Listeria monocytogenes. Mol Microbiol. 2006;62:1325–39. doi: 10.1111/j.1365-2958.2006.05452.x. [DOI] [PubMed] [Google Scholar]
- 15.Lorber B. Listeriosis. In: Goldfine H, Shen H, eds. Listeria monocytogenes: Pathogenesis and host response New York: Springer; 2007;13-31. [Google Scholar]
- 16.Dare K, Shepherd J, Roy H, Seveau S, Ibba M. LysPGS formation in Listeria monocytogenes has broad roles in maintaining membrane integrity beyond antimicrobial peptide resistance. Virulence. 2014;5:534–46. doi: 10.4161/viru.28359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Begley M, Sleator RD, Gahan CGM, Hill C. Contribution of three bile-associated loci, bsh, pva, and btlB, to gastrointestinal persistence and bile tolerance of Listeria monocytogenes. Infect Immun. 2005;73:894–904. doi: 10.1128/IAI.73.2.894-904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamp HD, Higgins DE. Transcriptional and post-transcriptional regulation of the GmaR antirepressor governs temperature-dependent control of flagellar motility in Listeria monocytogenes. Mol Microbiol. 2009;74:421–35. doi: 10.1111/j.1365-2958.2009.06874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamp HD, Higgins DE. A protein thermometer controls temperature-dependent transcription of flagellar motility genes in Listeria monocytogenes. PLoS Pathog. 2011;7:e1002153. doi: 10.1371/journal.ppat.1002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandin P, Fsihi H, Dussurget O, Vergassola M, Milohanic E, Toledo-Arana A, Lasa I, Johansson J, Cossart P. VirR, a response regulator critical for Listeria monocytogenes virulence. Mol Microbiol. 2005;57:1367–80. doi: 10.1111/j.1365-2958.2005.04776.x. [DOI] [PubMed] [Google Scholar]


