Abstract
Listeria monocytogenes is an intracellular, foodborne gastrointestinal pathogen that is primarily responsible for causing listeriosis or food poisoning in otherwise healthy individuals. Infections that arise during pregnancy or within immune compromised individuals are much more serious resulting in the risk of fetal termination or fetal fatality postpartum in the former and septicemia or meningitis with a 20% fatality rate in the latter. While the roles of internalin proteins and listeriolysin-O in the infection process are well characterized, the specific roles of lysine-modified phospholipids in the membrane of L. monocytogenes are not. Investigation into the lipid bilayer composition of L. monocytogenes indicated that the overall proportions of lipids, including lysylcardiolipin and lysylphosphatidylglycerol (LysPG), vary with growth temperature and growth phase. In addition, we demonstrate that LysPG formation is essential for L. monocytogenes survival in the presence of increased osmolytic stress but has no effect on bacterial adherence, invasion or survival in the presence of physiologically relevant concentrations of human neutrophil peptide (HNP-1). In the absence of LysPG synthesis, L. monocytogenes unexpectedly retained flagellum-mediated motility at 37 °C. Taken together, these findings show that LysPG formation in L. monocytogenes has broader functions in virulence and survival beyond its known role in the modification of membrane potential previously observed in other bacteria.
Keywords: aminoacyl-tRNA, lipid, Listeria, membrane, multiple peptide resistance factor, phosphatidylglycerol
Introduction
Listeria monocytogenes is a gram-positive, facultative anaerobic, intracellular foodborne pathogen that is unique in its ability to survive and replicate over a wide range of temperatures from −1 °C to 45 °C.1 The bacterium is also characterized by possession of peritrichous flagellum-mediated motility at 20 to 25 °C but not at 37 °C.2-4 While L. monocytogenes is a relatively uncommon cause of illness in the general population, infections of pregnant patients, neonates, the elderly and those with predisposing medical conditions are particularly problematic with the potential for high rates of morbidity and mortality.1 According to the Centers for Disease Control, in the USA 1651 cases of listeriosis were reported between 2009 and 2011 with a fatality rate of 21%. During infection, L. monocytogenes is known to induce its own uptake into phagocytic and non-phagocytic cells and spread from cell to cell using actin polymerization-based motility.5 The involvement of L. monocytogenes-encoded internalin proteins and listeriolysin-O in this intracellular invasion process are well documented.6 It is likely that additional mechanisms for evasion of the host immune system remain unidentified or under-characterized in the case of this bacterium including, for example, lipid aminoacylation.7
In many pathogens, changes occur in the composition of the lipid bilayer in response to various environmental stressors including those specifically encountered upon infection of the host. Modification of phosphatidylglycerol with lysine was first discovered in Staphylococcus aureus. In this bacterium, disruption of the gene encoding the enzyme responsible for this lipid modification resulted in increased sensitivity to a variety of antimicrobials, leading to the designation of multiple peptide resistance factor (MprF).8 Homologs of MprF, otherwise known as the aminoacylphosphatidylglycerol synthases (aaPGS), are encoded within the genomes of at least 204 species of bacteria. They can be found in 93 genera of gram-positive bacteria (mostly firmicutes and actinobacteria) and gram-negative bacteria (mostly proteobacteria) as well as in three species of archaea from the genus Methanosarcina.9 Members of the aaPGS family of enzymes are broadly responsible for the aminoacylation of phospholipids with amino acids including lysine, arginine, and alanine.9
Aminoacylation of phospholipids by the aaPGS enzyme family results in alterations in lipid-lipid interactions that can affect the packing, fluidity, and permeability of the cell membrane.10 For example, the presence of lysylphosphatidylglycerol (LysPG) in liposomes results in increased impermeability to rubidium ions. S. aureus membranes containing increased levels of LysPG show a combination of increased fluidity and decreased permeability to daptomycin.11,12 While the general trend of increased resistance to cationic antimicrobial peptides (CAMPs) can be identified in most microorganisms with phosphatidylglycerol modifications, findings in the literature suggest that the modifications may also confer resistance to a broader range of conditions, many of which remain unidentified to date. For example, exposure of S. aureus, Enterococcus faecalis, and Rhizobium tropici to acidic conditions causes an increase in the proportion of LysPG in the membrane.13 The formation of alanylphosphatidylglycerol (AlaPG) in Pseudomonas aeruginosa has also been linked to survival under acidic growth conditions induced by growth in sodium lactate as well as resistance to cefsulodin, the heavy metal ion Cr3+ and the cationic peptide protamine.14 The specific roles of lysine-modified phospholipids, such as cardiolipin and phosphatidylglycerol, in the membrane of L. monocytogenes are currently under-characterized with only some evidence to suggest that they may be important for defensin resistance.7 We now demonstrate that the role of lysine modification of phosphatidylglycerol within the membrane of L. monocytogenes extends beyond resistance to CAMPs into protection against osmolytic stress and regulation of the motility operon. This supports the theory that aaPGS enzymes are involved in adaptation to a broad, and currently under explored, range of environmental conditions in different microorganisms. Further characterization of the roles of these lipid modifications in different bacteria could render the aaPGS enzyme family a suitable target for development of new antimicrobial therapies for the treatment of infections by pathogens that are becoming increasingly resistant to multiple antibiotics.
Results
Membrane lipid composition changes with growth phase and temperature
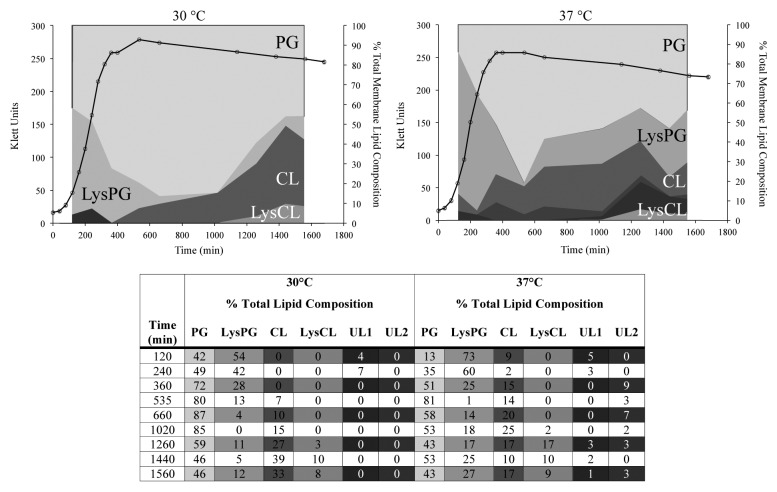
In many bacteria, changes in membrane lipid composition can be seen during progression through the growth cycle and as a response to fluctuations in growth temperature. Such modifications are often essential for the maintenance of overall membrane fluidity and vary depending on the bacterial genera and species. Studies characterizing the effect of cold shock on L. monocytogenes have shown that reduction in growth temperature from 30 °C to 6 °C is correlated with an increase in the proportion of neutral lipids and branched chain fatty acids in the cell membrane as well as a decrease in overall lipid chain length.15,16 Since a cell wall stress regulator, VirR,7 controls LysPGS production in L. monocytogenes, we decided to investigate whether there was also a detectable change in the proportion of LysPG and other lipids in the membrane when cells were grown at 30 °C as opposed to 37 °C. Differences were observed in the membrane lipid composition of L. monocytogenes grown at 37 °C in comparison to 30 °C (Fig. 1B and A, respectively). As expected from previous reports,16 the major detected components of the L. monocytogenes membrane bilayer were phosphatidylglycerol, cardiolipin and their lysine-modified forms. At both 30 °C and 37 °C, LysPG made up the highest proportion (54% and 73%, respectively) of the total lipid composition at early stages of growth while cardiolipin increased during progression into early stationary phase (Fig. 1). Following on from this, a consistent trend is found at both growth temperatures whereby the presence of LysPG peaks during the exponential phase, declines during the early exponential stage and increases again during the late stationary phase (Fig. 1). Notably, LysPG is found to make up a consistently higher proportion of the overall membrane lipids at 37 °C when compared with 30 °C. In contrast to this, cardiolipin appears later in the growth cycle at 30 °C (first detectable appearance at 535 min as opposed to 120 min) and continues to increase to an overall greater level than was detected at 37 °C (Fig. 1). Lysine-modified cardiolipin is only found during the late stationary phase regardless of growth temperature. Two unidentifiable lipids were also detected (Fig. 1). Unidentified lipid one (UL1) is present at both growth temperatures and increases slightly during exponential phase before disappearing completely at 30 °C, and reappearing in late stationary phase in cells grown at 37 °C. In contrast, UL2 is only present in the membrane of L. monocytogenes EGD-e grown at 37 °C (Fig. 1).
Figure 1. Temperature-dependent changes in the membrane lipid composition of L. monocytogenes. Growth of L. monocytogenes EGD-e in BHI in the presence of [32P] PPi is represented as a measure of optical density by the black line. Aliquots of bacterial culture were removed and used for lipid isolation and subsequent separation by TLC. The percentage of each lipid species of the total lipid composition was determined by phosphorimaging. The percent of the total lipids made up of each species is relative to the area occupied on the graph. Detected lipids include cardiolipin (CL), lysylcardiolipin (LysCL), lysylphosphatidylglycerol (LysPG), phosphatidylglycerol (PG), unidentified lipid 1 (UL1), and unidentified lipid 2 (UL2). UL2 was present in the membranes of L. monocytogenes EGD-e grown at 37 °C (B), but not at 30 °C (A).
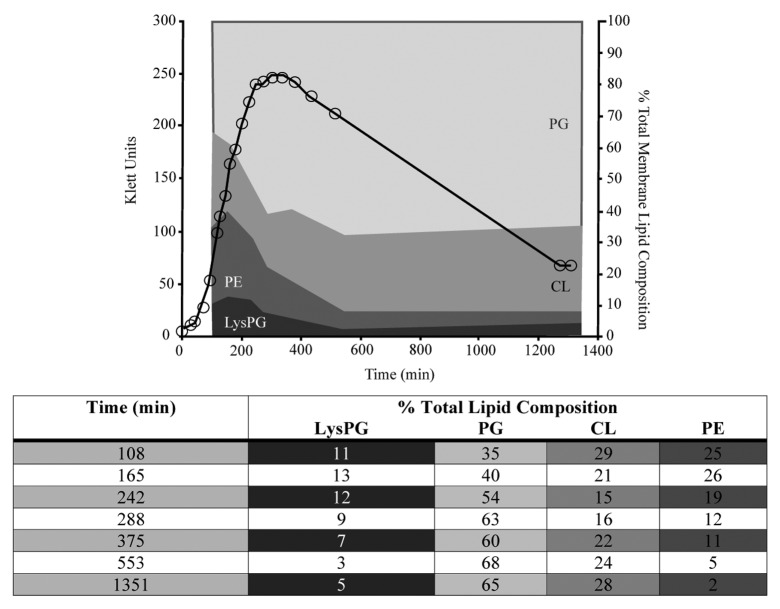
To compare the role of aaPGS in L. monocytogenes to other gram-positive bacteria, the growth phase dependence of Bacillus subtilis LysPG formation was also determined. In order to follow LysPG formation, B. subtilis 1A100 was grown in LB supplemented with [32P]-labeled PPi. Samples of culture were taken at different phases of growth and used for lipid isolation, separation and identification as described above for L. monocytogenes. Analysis of the acquired data indicated that phosphatidylglycerol is present throughout the growth cycle and is the major lipid species present in the bilayer (Fig. 2). This is consistent with the observation that this phospholipid is essential for the viability of B. subtilis.17 The proportion of phosphatidylethanolamine decreases as the growth cycle progresses, reaching levels that are approximately 13-fold lower in the stationary phase when compared with the exponential phase (Fig. 2). In the case of cardiolipin, a decrease was detected during the exponential phase followed by a subsequent recovery to approaching earlier levels during stationary phase. LysPG is present in the membrane during all phases of growth, peaking during exponential phase and declining to a steady level as cell growth rate diminishes (Fig. 2). These findings, in combination with the data acquired for L. monocytogenes, clearly indicate that growth phase is a key factor involved in determining membrane phospholipid composition.
Figure 2. The effect of growth phase progression on lipid composition in B. subtilis. The growth of B. subtilis 1A100 at 37 °C in LB in the presence of [32P] PPi, is represented by the black line. Aliquots of bacterial culture were removed and used for lipid isolation and subsequent separation by TLC. The percentage of each lipid species of the total lipid composition was determined by phosphorimaging. Each lipid species found in the membrane of B. subtilis is represented, and the proportion of the membrane it occupies is reflected in the corresponding shaded area. The shading of the rows matches that of the area represented by the lipid species on the graph. Abbreviations are as follows: lysylphosphatidylglycerol (LysPG), phosphatidylglycerol (PG), cardiolipin (CL), and phosphatidylethanolamine (PE).
Loss of LysPGS causes increased sensitivity to antimicrobials, detergents, and osmolytes
Previous studies aimed at determining the function of LysPGS have demonstrated that absence of the lysine-modified form of phosphatidylglycerol in the membrane of L. monocytogenes is associated with disease attenuation in a mouse model.7 This phenotype was attributed to an increased susceptibility to CAMPs produced by the host immune system and specific cell lines.18 However, the nature of the reported effects could be indicative of LysPG formation having other, as yet undetermined, roles on cellular physiology, prompting us to investigate the broader cellular roles of aaPGSs in gram-positive bacteria.
In order to determine the broader physiological effects of LysPG formation in B. subtilis strain 1A100, a ΔlysPGS strain was created. Growth of the ΔlysPGS strain was compared with that of the wild type strain using Biolog phenotypic microarray panels designed to test for the usage of carbon/nitrogen/sulfur sources, survival in varying pH and resistance to a range of antimicrobial agents targeting various cellular processes. Growth phenotypes were considered relevant if the tested strain reached at least double the optical density of the comparative strain in the presence of a particular compound. In conditions under which B. subtilis 1A100 grew better than the ΔlysPGS strain, LysPG was considered beneficial, rendering the organism resistant to antimicrobial effects (Table S1). A large proportion of the compounds identified as having increased activity against the ΔlysPGS strain have mechanisms of action targeting a cell wall associated process or component. These findings are consistent with the previously proposed function of LysPGS in B. subtilis, which is to provide resistance to antimicrobial agents produced by other microorganisms sharing their environmental niche.19 Surprisingly, many of the identified compounds have a neutral charge as opposed to being cationic. This differs from what would be predicted to be the case for another well-studied gram-positive organism, Staphylococcus aureus, in which formation of LysPG is dependent upon, and imparts resistance to, positively charged molecules.20,21
Seven compounds were identified whose presence resulted in better growth of the ΔlysPGS strain in comparison to the wild type strain (Table S1). At least four of these compounds exhibit similar modes of action to those for which LysPG formation imparts resistance. More specifically, they target the cell membrane or proteins that localize to this region. One hypothesis to explain why LysPG formation might enhance susceptibility to these particular compounds is that they may require cationic lipids, such as those that are lysine-modified, to interact with the membrane and exert their functions. Overall, the combination of identified compounds strongly suggests that LysPG production is critical for maintenance of the integrity of the B. subtilis lipid bilayer. In addition, growth curves performed for the B. subtilis wild type and ΔlysPGS strains in the presence of two of the identified compounds for which LysPG formation conferred resistance (azlocillin and cefsulodin), provided independent support of the reliability of the screening methodology used in this study (Fig. 3).
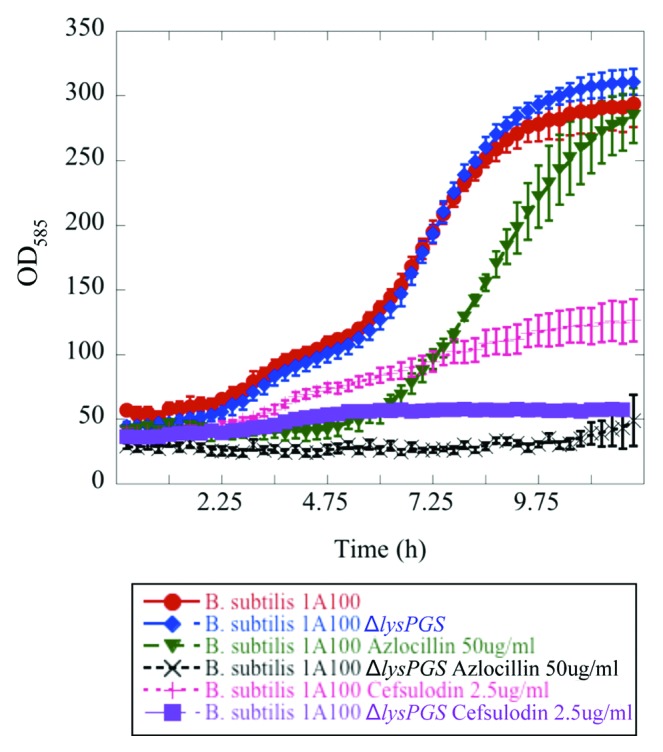
Figure 3.Bacillus subtilis strain 1A100 ΔlysPGS is susceptible to β-lactam antibiotics. The presence of LysPG in the membrane allows B. subtilis 1A100 (red) to resist the effects of the cell wall targeting, β-lactam antibiotics azlocillin (green), and cefsulodin (pink). Concentrations of azlocillin and cefsulodin were used at the minimum inhibitory concentration of the ΔlysPGS strain corresponding to 50 μg/mL and 2.5 μg/mL, respectively. The absence of LysPG in B. subtilis 1A100 ΔlysPGS does not result in a growth defect in the absence of antibiotic (blue), but causes decreased growth compared with the wild type strain in the presence of azlocillin (black) and cefsulodin (purple). Data represent averages of three independent experiments and the corresponding standard errors.
In order to understand the effects conferred on L. monocytogenes by lysine-modification of phosphatidylglycerol, the same Biolog phenotypic microarray methodology developed for B. subtilis was used. This enabled comparison of the growth of L. monocytogenes strains EDG-e (wild-type) and EDG-e ΔLysPGS. The results of this study indicated that, when grown at 37 °C, the presence of LysPG in the lipid bilayer of this bacterium conferred resistance to a series of osmolytes including sodium chloride, urea, sodium sulfate and potassium chloride (Table S2). In addition, the presence of LysPG was associated with increased resistance to the aminoglycosides geneticin (G418) and tobramycin (Table S2). Independent growth curves performed in BHI medium supplemented with various concentrations of urea and sodium chloride confirmed the role of L. monocytogenes LysPG in osmolytic protection (Table 1).
Table 1. Loss of LysPG results in impaired growth of L. monocytogenes in the presence of osmolytes.
| Growth condition | Increase in doubling time (min)a |
|---|---|
| No addition | 0.6 ± 24 |
| 4.5% NaCl | 3.4 ± 2.8 |
| 6% NaCl | 9.9 ± 5.1 |
| 6% Urea | 49.2 ± 10.5 |
aIncrease in doubling time for L. monocytogenes EGD-e ΔlysPGS compared with wild type during the logarithmic phase of growth. Values are the means of three independent experiments, errors indicate SD.
Since L. monocytogenes is exposed to detergents during invasion of the human gastrointestinal tract,22 the importance of LysPG for bile survival was tested in comparison to a Δbsh positive control strain, which is compromised in its ability to breakdown bile in the surrounding media.23 The absence of LysPG in the membrane was demonstrated to have a potentially adverse impact on the ability of L. monocytogenes to survive in the presence of bile in comparison to the wild type strain (Fig. 4). This finding is consistent with a possible role for LysPG in gastrointestinal tract survival.
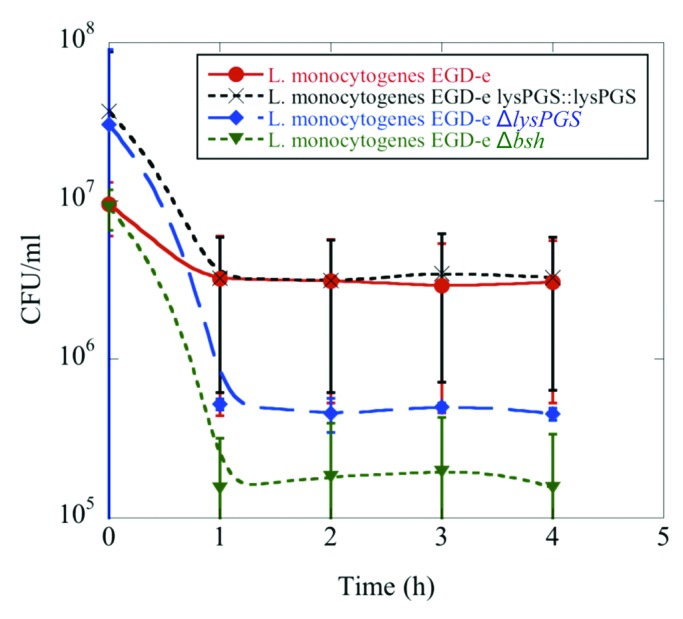
Figure 4. LysPG loss results in decreased survival of L. monocytogenes in the presence of bile. Colony forming units/mL remain the same for L. monocytogenes EGD-e (red) and L. monocytogenes EGD-e ΔlysPGS::lysPGS (black) after incubation in 24% bile over the course of 4 h, while the survival rate of L. monocytogenes EGD-e ΔlysPGS (blue) decreases as does that of positive control, L. monocytogenes EGD-e Δbsh (green). Data represent averages of three independent experiments and the corresponding standard errors.
LysPG formation does not play a role in RC-1 or HNP-1 defensin resistance
In order to further investigate the hypothesis that the role of LysPG in L. monocytogenes virulence is not solely based upon charge-mediated repulsion of CAMPs produced by the host immune system, susceptibility of the ΔlysPGS strain to retrocyclin-1 (RC-1) and human neutrophil peptide-1 (HNP-1) was tested at physiologically relevant concentrations. Results demonstrate that, at typical physiological levels, RC-1 and HNP-1 exposure causes no significant difference in the proliferation of the ΔlysPGS strain in comparison to the wild type (Fig. 5). This apparent lack of effect on proliferation in the case of HNP-1 contradicts previous studies that suggest LysPG formation is critical for CAMP resistance and that this underlies the decrease in virulence of a ΔlysPGS strain in a mouse model.19
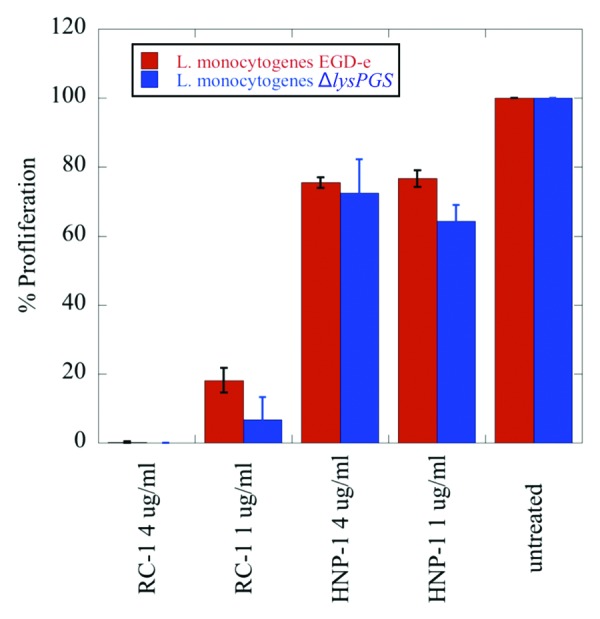
Figure 5. LysPG formation does not play a role in L. monocytogenes EGD-e resistance to the defensins RC-1 and HNP-1. No significant difference is observed in the percent proliferation of the ΔlysPGS strain (blue) in comparison to the wt strain (red) when incubated for 15 min in the presence of varying concentrations of RC-1 or HNP-1. Data was normalized to bacterial counts resulting from the incubation of both strains in DMEM alone, which was set to 100% proliferation. Calculated P values for the tested concentrations of RC-1 and HNP-1 are not less than 0.05, indicating no statistically significant difference between the percent proliferation of the two strains incubated in these conditions.
Loss of LysPGs causes no significant effect on protein localization, bacterial adhesion, or bacterial internalization
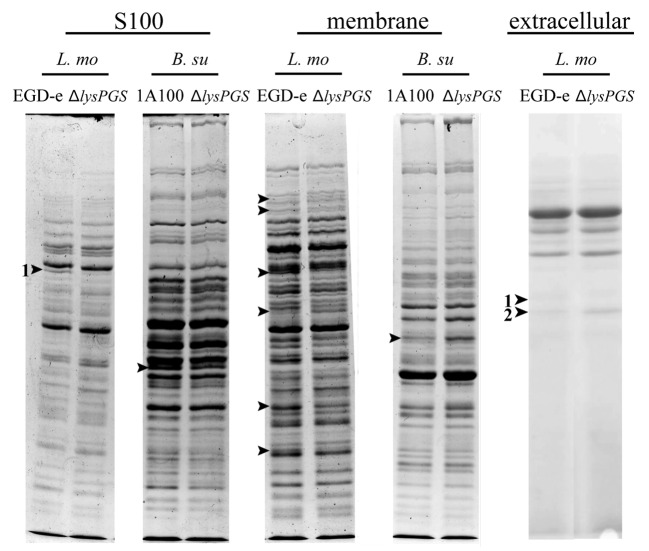
In order to further understand both the cellular role of modified phosphatidylglycerol and the phenotypic effects of lysPGS deletion in L. monocytogenes, changes in protein localization were investigated in the wild type and the deletion strain. Many eukaryotic and prokaryotic proteins are known to require localization to lipid rafts in order to function correctly.24,25 In addition, some proteins require a specific lipid content to be present in the membrane either to localize to this position or to function efficiently.24,26-28 For example, in S. aureus, loss of LysPG prevents localization of the CAMP-responsive two component sensing system SaeS and results in reduction in the overall levels of proteins involved in glycerolipid metabolism, cell wall metabolism and transportation.29 In this study, we attempted to identify protein localization changes in L. monocytogenes EGD-e ΔlysPGS by comparison of the protein profiles of cytoplasmic, cell membrane and extracellular fractions isolated from cultures grown at 30 °C and 37 °C in BHI medium to the late log phase. As a control, protein localization changes were also investigated in the B. subtilis ΔlysPGS strain, although this was only cultured at 37 °C in LB medium. In both cases, proteins that were detected as being either present or absent from the ΔlysPGS strain in comparison to the wild-type strain were determined by SDS PAGE analysis (Fig. 6) and identified by mass spectrometry of extracted bands. As a control, for each protein band that was excised, the same region where the protein was absent was excised from either the wild type or the ΔlysPGS strain as appropriate. As such, only proteins that were identified by mass spectrometry to be in the sample lane but not in the corresponding control were taken as positive results. No attempts were made to quantify the differences in protein levels. As shown in Tables 2 and 3, only one protein of interest was identified in the L. monocytogenes ΔlysPGS strain that was not found in the corresponding wild type control at 37 °C. This protein was localized to the non-cytoplasmic secreted fraction and was identified as flagellin. However, subsequent investigation using immunoblotting against the flagellin encoded by the flaA gene indicated that there were no detectable differences in localization of flagellin between the wild-type and the ΔlysPGS strain at either 37 °C or 30 °C (data not shown). This suggested that identification of this protein in the secreted fraction of the ΔlysPGS strain could be an artifact resulting from flagella shearing during sample preparation. To determine whether or not this was the case, further studies were performed to investigate the role of LysPG formation in the motility of L. monocytogenes (see below). No significant changes were identified in the protein profiles of the cytoplasmic, membrane or cell wall fractions of L. monocytogenes or in any of the B. subtilis fractions.
Figure 6. Changes in protein localization correlated with LysPG loss in B. subtilis and L. monocytogenes. Arrows indicate bands that were sent for identification by mass spectrometry and numbers indicate positive identifications in comparison to the appropriate control. Strains are abbreviated as follows: L. mo, L. monocytogenes; EGD-e, L. monocytogenes EGD-e; ΔlysPGS, L. monocytogenes EGD-e ΔlysPGS; B. su, B. subtilis; 1A100, B. subtilis 1A100; ΔlysPGS, B. subtilis 1A100 ΔlysPGS. Numbered excised bands were subsequently analyzed as shown in Table 2 (S100 band 1) and Table 3 (extracellular bands 1 and 2).
Table 2. Comparison of the cytosolic protein profile of L. monocytogenes EGD-e ΔlysPGS and the wild-type strain.
| Sample | emPAI | Description | Molecular weight (kDa) |
|---|---|---|---|
| 1 | 13.11 (10.47) | Acetolactate synthase | 61.673 |
| 1 | 2.61 (16.18) | Chaperonin GroEL | 57.332 |
| 1 | 0.49 (3.19) | Catalase | 55.854 |
| 1 | (1.49) | Prolyl-tRNA synthetase | 63.313 |
| Control | 6.57 (4.26) | Acetolactate synthase | 61.673 |
| Control | 2.23 (7.80) | Chaperonin GroEL | 57.332 |
| Control | (1.36) | Catalase | 55.854 |
| Control | (1.90) | Prolyl-tRNA synthetase | 63.313 |
| Control | (1.58) | Lysyl-tRNA synthetase | 57.402 |
Table 3. Comparison of the secreted protein profile of L. monocytogenes EGD-e ΔlysPGS and the wild-type strain.
| Sample | emPAI | Description | Molecular weight (kDa) |
|---|---|---|---|
| 1 | 0.68 | Flagellin | 30.483 |
| 1Control | 0.11 | Flagellin | 30.483 |
| 2 | 12.39 | Flagellin | 30.483 |
| 2 | 2.31 | UDP-N-acetylglucosamine 1-carboxyvinyltransferase | 29.04 |
| 2Control | 2.67 | UDP-N-acetylglucosamine 1-carboxyvinyltransferase | 29.04 |
| 2Control | 0.73 | Cell wall binding protein | 28.718 |
In the absence of noticeable changes in protein localization, differential fluorescence staining was used to investigate the effect of LysPG loss on the ability of L. monocytogenes to adhere to and be internalized by Caco-2 cells. L. monocytogenes EGD-e, L. monocytogenes EGD-e ΔlysPGS, and L. monocytogenes EGD-e ΔlysPGS::lysPGS were grown to mid-exponential phase corresponding to an OD600 of 0.8, washed with PBS, and diluted in Eagle’s minimal essential media (MEM) to a multiplicity of infection (MOI) of 5 bacteria per Caco-2 cell. Following fixation with paraformaldehyde, bacteria were detected using two rounds of immunofluorescent labeling, one performed prior to Caco-2 cell lysis and a second following lysis. Caco-2 cell nuclei were stained with DAPI. External bacteria labeled with Alexa568 tagged secondary antibody were counted and subtracted from the total (labeled with Alexa488 tagged secondary antibody) to determine the number of internalized bacteria per Caco-2 cell. At an MOI of 5, which allowed visualization of individual bacteria, no significant difference was seen in the adherence or internalization of the ΔlysPGS strain compared with the wild-type strain (Fig. 7).
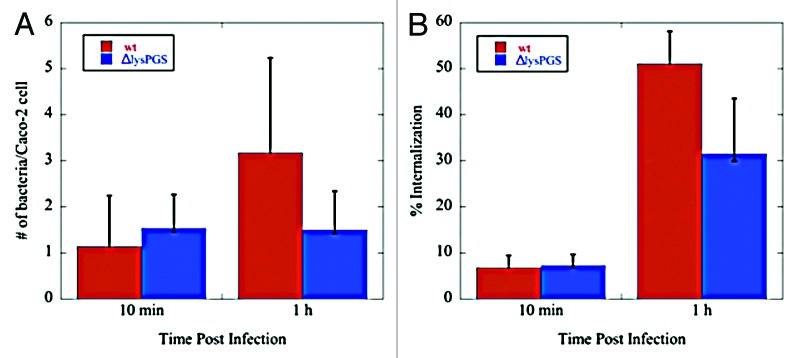
Figure 7. Loss of lysPGS does not affect L. monocytogenes adherence to or internalization by Caco-2 cells. Graphs show the number of bacteria associated per Caco-2 cell (A) or the percent of the total bacteria internalized (B) for the L. monocytogenes EGD-e (wt) or L. monocytogenes EGD-e ΔlysPGS (ΔlysPGS) after 10 min and 1 h following infection.
Listeria monocytogenes ΔlysPGS maintains motility at 37 °C
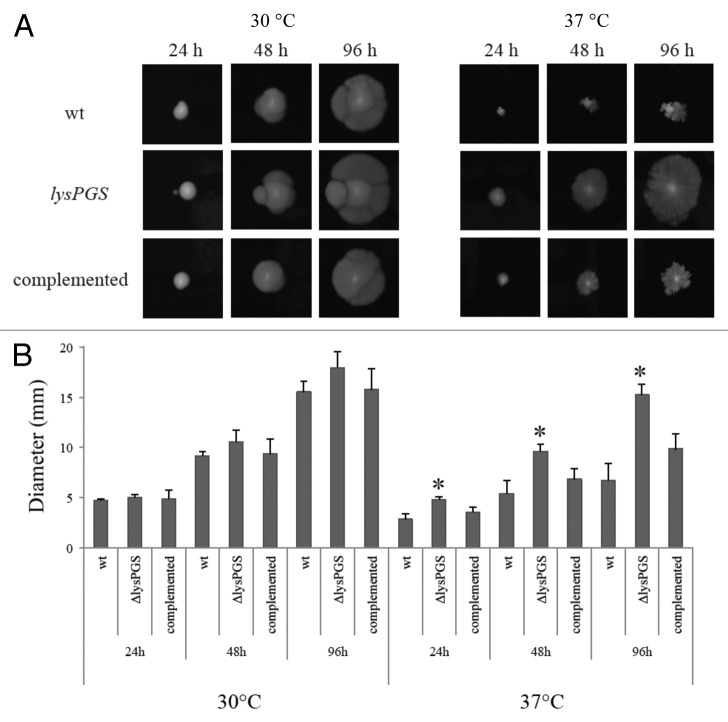
Stringent control of flagellar-mediated motility is essential in L. monocytogenes. While motility in the host is advantageous for navigation of the gastrointestinal tract and host-cell targeting, secretion of flagellin or the presence of a flagellum triggers the inflammasome and can result in subsequent clearance by the immune system.30,31 In L. monocytogenes, flagellar motility is thermo-regulated by the action of an anti-repressor protein called GmaR, which is also an O-linked N-acetylglucosamine transferase responsible for posttranslational modification of flagellin with up to six molecules of β-O-GlcNAc.32 GmaR becomes unstable at the non-permissive growth temperature (37 °C) and dissociates from the motility operon repressor, MogR, thus resulting in downregulation of motility.33-35 Investigation using immunoblotting against the flagellin-encoding flaA gene indicated that there were no detectable differences in localization of flagellin between the wild-type and the ΔlysPGS strain at either 37 °C or 30 °C. However, flagellin was detected to exhibit altered gel mobility in the ΔlysPGS strain compared with the wild-type strain indicating a potential difference in the O-acetylation, polymerization, or activity state of the protein (data not shown). For this reason, the motility status of the wild-type and the ΔlysPGS strain were investigated at 37 °C and 30 °C. At 30 °C both strains were found to be equally motile, as reflected in equal colony diameters. However at 37 °C ΔlysPGS maintains motility while the wild-type and the ΔlysPGS complemented strain do not (Fig. 8).
Figure 8. Loss of LysPG in the membrane allows L. monocytogenes EGD-e to remain motile at 37 °C. Colony diameters are similar for all three strains when grown at 30 °C. At 37 °C the L. monocytogenes EGD-e ΔlysPGS colony diameter is consistently larger than the colony diameters of both the L. monocytogenes EGD-e (wt) and L. monocytogenes EGD-e ΔlysPGS::lysPGS (complemented) strain (A). The assay was repeated in triplicate and plated on BHI agar in duplicate. Diameter averages are represented in graphical form with statistically relevant (two tailed t test values of P < 0.001) differences indicated by an asterisk (B).
Discussion
Lysine modification of membrane phospholipids exerts widely varied physiological effects on different bacteria
Prior to this study, it had been concluded that the primary role of modification of acidic phospholipids with positively charged amino acids is grounded within the provision of resistance to cationic antimicrobial peptides (CAMPs).7,8,19,36 The enzymes responsible for this modification activity, aaPGSs, have been extensively studied in S. aureus where LysPG formation is responsible for resistance to CAMPs, particularly daptomycin in methicillin resistant strains. In addition, S. aureus LysPG has been determined to have a role in membrane localization of several proteins as well as aiding the initiation of DNA replication.26,36,37 One of the proteins that is dependent upon LysPG formation for its localization to the membrane, SaeS, is a member of a two-component sensing system that directly detects cationic peptides in the bacterial environment.20,38,39 Neither B. subtilis nor L. monocytogenes possess such a system for lysPGS regulation, with expression thought to be constitutive and uncontrolled in the former and regulated by a general cell wall stress two-component sensing system, VirRS, in the latter.7 Results of investigations into the effects of lysine-modification of phospholipids in B. subtilis and L. monocytogenes presented in this study indicate that they have a broader role within the maintenance of cell wall integrity than was determined to be the case in S. aureus.
Phenotypic microarray based screening to search for conditions under which LysPG alters B. subtilis physiology identified many compounds that target various aspects of the cell wall in terms of both its biosynthesis and integrity. Many of the compounds identified originate from, or are derivatives of, compounds isolated from plants, fungi and other bacteria. This directly correlates with the previous proposed function of LysPGS in the environmental adaptation of B. subtilis.19 It was also noted that many of the identified compounds inhibit the activities of a multitude of B. subtilis proteins that are thought to be associated with lipid rafts.24 This may indicate that LysPG formation is integral for the association of such proteins, or merely that reorganization of the bilayer upon loss of LysPG causes a breakdown in membrane permeability thereby affecting functionality of these proteins. Studies attempting to identify the distribution of LysPG in the membrane have shown that it is organized in a spiral pattern,40 supporting the latter notion; however, detection of lipid localization can vary based on the technique utilized so the former cannot formally be ruled out.41
Formation of LysPG in L. monocytogenes, previously thought to provide resistance to CAMPs, confers resistance to only a few compounds tested in the phenotypic microarray screen including several osmolytes and two antimicrobial compounds. Survival in the presence of bile, which contains many membrane-disrupting compounds, was also tested since L. monocytogenes traverses the colon in order to establish an infection in humans.42 Loss of LysPG potentially resulted in some demonstration of decreased survival in the presence of bile in comparison to the wild- type strain, but was not diminished to the extent of the L. monocytogenes EGD-e Δbsh strain that lacks bile salt hydrolase, an activity known to play a direct role in bile acid survival.43 These findings indicate that, although LysPG formation in L. monocytogenes EGD-e provides resistance to a different set of compounds than in B. subtilis, both organisms require LysPG for maintenance of cell wall integrity in the presence of membrane disrupting antimicrobial agents.
To determine if these observed phenotypic differences between B. subtilis and L. monocytogenes were dependent on variations in lipid composition, the membrane content was analyzed during various phases of growth. Lipid composition was found to vary greatly between the two species. Specifically, phosphatidylethanolamine was identified only in B. subtilis and LysPG was found to be present at consistently higher levels in L. monocytogenes. Similar to earlier findings, the levels of cardiolipin increased as L. monocytogenes entered stationary phase, which was not observed in B. subtilis. This suggests that when there is no LysPG made its place in the cell membrane is occupied by other lipids, presumably PG and cardiolipin. The substantial differences in the degree of modified lipid concentration, reaching up to 13% in B. subtilis and 73% in L. monocytogenes, may account for the poor overlap between the phenotypic microarray data sets presented here and the phenotypes identified previously in studies of LysPG in S. aureus.29,36 Taken together, these findings suggest that the roles of amino acid modifications to cell membrane phospholipids may vary depending on the bacterial genera and species and the particular niches and stressors that they are required to adapt to.
LysPG and LysCL in L. monocytogenes are involved in regulation of motility repression
Previous studies of lysPGS in L. monocytogenes EGD-e showed that strains lacking the lipid modification activity were attenuated in virulence.7 The ΔlysPGS strain exhibited a decrease in internalization previously attributed to increased susceptibility to CAMPs produced by the tested cell lines.44-46 This increase in CAMP susceptibility was not observed at the physiological concentrations of the CAMP HNP-1 used in this study. Due to the absence of corroborating data for the previously proposed role of LysPG in L. monocytogenes from our phenotypic microarray screen or defensin survival studies and, in light of temperature-dependent changes in lipid composition, protein localization was examined. Flagellin was the only protein found to vary in the extracellular fraction of the ΔlysPGS strain when compared with the wild-type strain. However, there was no detectable difference in the level or localization of flagellin in the cellular protein fractions isolated from the L. monocytogenes EGD-e and EGD-e ΔlysPGS.
The ΔlysPGS strain was found to retain motility at 37 °C indicating that the identified flagellin forms functional flagella. At 37 °C the motility operon within L. monocytogenes is usually repressed in a thermo-dependent manner by degradation of the MogR anti-repressor, GmaR, whose expression is regulated by an orphan response regulator DegU.33,34,47 Strains of L. monocytogenes lacking both DegU and MogR still repress motility in a temperature-dependent fashion and this finding, combined with ΔlysPGS retention of motility at 37 °C, indicates that formation of LysPG and LysCL may contribute to motility repression. The findings presented in this study suggest that LysPG and LysCL do not affect flagellin localization but that they could either be necessary for adequate regulation of the membrane potential used to drive the flagellar motor or for assembly of a motor protein required for motility. In previous studies utilizing liposomes consisting solely of either unmodified phosphatidylglycerol or LysPG the membrane potential was measured as −60 mV and +60 mV, respectively.11 However, there was no change in membrane potential in strains of S. aureus that exhibited resistance to daptomycin and increased levels of phosphatidylglycerol in their membranes compared with susceptible isolates.48 This again supports the suggestion that the effects of these modified lipids depend upon the bacterial genera.
LysPGS formation in L. monocytogenes has a broad role in maintaining membrane integrity that extends beyond modification of membrane potential
Membrane lipid content is known to change depending on growth phase, growth temperature and environmental conditions in order to preserve membrane properties and maintain specific cellular processes. In the case of L. monocytogenes, lipid composition varies when grown at 30 °C vs. 37 °C. In addition, loss of LysPG and LysCL allows the ΔlysPGS strain to retain motility at 37 °C. This is the first instance of motility regulation being linked to acidic phospholipid neutralization. Such a linkage may contribute to an explanation for the decreased virulence of L. monocytogenes EGD-e ΔlysPGS that was previously observed in mice since flagellin is a known trigger of the inflammasome, which promotes bacterial clearance through host cell apoptosis.49 Further characterization of multiple members of the aaPGS family of enzymes across several bacterial genera and species may enable the design of novel therapeutic agents that can be used to treat infections by pathogens that are becoming increasingly resistant to antibiotics.
Materials and Methods
Bacterial strains, plasmids, and growth conditions
L. monocytogenes EGD-e, L. monocytogenes EGD-e ΔlysPGS, and L. monocytogenes EGD-e ΔlysPGS::lysPGS50 were graciously provided by Professor T Chakraborty (Justus-Liebeig University). L. monocytogenes EGD-e Δbsh was graciously provided by Professor Pascale Cossart (Institut Pasteur). The parental B. subtilis 1A100 strain was obtained from the Bacillus Stock Center (Ohio State University). A marker-less deletion of lysPGS in this strain was constructed by Campbell integration of an I-SceI cut site using regions flanking lysPGS.
The region immediately upstream of, and including a portion of, lysPGS was amplified by polymerase chain reaction (PCR) using B. subtilis 1A100 genomic DNA and primers I-SceI FF EcoRI and I-SceI FR XhoI (Table S3) to yield a 1356 bp product. The downstream region of lysPGS was amplified in a similar manner using the primer pair I-SceI BF XhoI and I-SceI BR EcoRI to yield a 1300 bp product. Both PCR products were integrated into EcoRI-linearized vector pE5916 using the In-Fusion (Clontech) system of homologous recombination following the protocol provided by the supplier. The recombinant plasmids were transformed into a recA+strain of Escherichia coli, TG1, and selected for on ampicillin (100 μg/mL) LB agar plates. Primer PE5916 integration was used to sequence constructs to ensure directional integration of both PCR products.
Plasmids were then transformed into B. subtilis 1A100 using induced natural competency detailed in a protocol modified by K Fredrick (Ohio State University), originally published by D Dubnau (New Jersey Medical School). Campbell integration of the introduced plasmid via homologous recombination in a single region results in the whole plasmid, containing a I-SceI cut site, being incorporated in the B. subtilis 1A100 genome. Transformants were selected by plating on spectinomycin (100 μg/mL) LB agar. Chromosomal integration of the plasmid into the genome was confirmed by PCR amplification using the primers I-SceI genomic integration and I-SceI FF EcoRI (Table S1). Several strains positive for plasmid integration were then transformed with plasmid pSS4432 carrying the sequence encoding I-SceI, using the aforementioned method of natural transformation. Transformants were selected on LB agar containing both spectinomycin (100 μg/mL) and kanamycin (25 μg/mL). Colonies resulting from this selection were streaked for isolation three times on kanamycin LB agar plates prior to patching on both spectinomycin and kanamycin. Loss of spectinomycin resistance indicated that a double strand break had been introduced and subsequent DNA repair had either removed the plasmid leaving behind a non-functional truncated portion of lysPGS or had been repaired so that full-length lysPGS remained. Sixteen of these colonies were streaked three times on LB without selection to promote the loss of pSS4432, and after the third passage colonies were patched on LB containing spectinomycin, and LB containing kanamycin to ensure the loss of both markers had taken place. Loss of LysPG synthetic activity upon deletion of lysPGS was confirmed by TLC analysis of isolated membrane lipids (data not shown).
All L. monocytogenes strains were grown in Brain Heart Infusion (BHI) medium or on BHI agar plates. Unless otherwise specified, isolated colonies were used to start 21 h cultures grown at 37 °C for subsequent experiments. All B. subtilis strains were grown in LB, or on LB agar plates. Isolated colonies were used to start 16 h cultures grown at 37 °C for subsequent experiments. For phenotypic microarray (PM) analyses both L. monocytogenes and B. subtilis strains were streaked out from glycerol stocks for isolation of single colonies on Biolog Universal Growth media (BUG) + 5% sheep blood.
Biolog phenotypic microarray
Phenotype Microarray (PM) panels 1–20, 100× Redox Dye F, and 1.2× inoculation fluids (IF) 0a and 10b were all obtained from Biolog. Chemicals used to prepare the PM additives were obtained from Sigma. Growth phenotypes of B. subtilis 1A100 and B. subtilis 1A100 ΔlysPGS were determined for Biolog PM1–20 plates when grown at 37 °C for 48 h by following the B. subtilis protocol supplied by Biolog. Additives and preparation of inoculum for L. monocytogenes were identical to those for B. subtilis. Growth phenotypes of L. monocytogenes EGD-e, L. monocytogenes EGD-e ΔlysPGS, and L. monocytogenes EGD-e ΔlysPGS::lysPGS were determined for Biolog PM1-PM20 when grown at 37 °C for 48 h by following the Listeria protocol supplied by the company. Strains were compared in a pairwise manner. Phenotypes exhibiting growth of one strain 2-fold over that of the other strain in one concentration and demonstrating a consistent trend across all three concentrations of particular substances, when applicable, were considered significant.
Confirmation of selected Biolog phenotypes
The fungus-derived antimicrobial agents, azlocillin and cefsulodin, were chosen to confirm phenotypes exhibited by B. subtilis 1A100 ΔlysPGS. Concentrated stocks were made of the two antibiotics which were diluted to 2× the final concentration being tested in IF-10b. Sixteen hour cultures of B. subtilis 1A100 and B. subtilis 1A100 ΔlysPGS were diluted to a final OD600 of 0.1 in IF-10b containing 2× Redox Dye F. To inoculate the plate 50 μL of the 2× concentrated antibiotic was added to 50 μL of bacterial cell suspension containing tetrazolium dye. Growth of the strains at 37 °C in the presence of varying concentrations of cefsulodin or azlocillin over a period of 24 h was monitored using an Omnilog plate reader. The concentrations of antibiotics shown are indicative of the inhibitory concentration that reduced the growth of the ΔlysPGS strain to 50% that of the wild-type strain (IC50).
The osmolytes urea and NaCl were chosen to confirm Biolog results and characterize osmolyte sensitivity for L. monocytogenes EGD-e ΔlysPGS over a larger range of concentrations. Concentrated stocks (20% w/v) of urea or salt were made in autoclaved BHI, filter sterilized, and stored at room temperature. Dilutions of the 20% stocks were made in sterile BHI to create a range of concentrations from 1% to 16%. Fifty milliliters of the 2× concentrated osmolytes were added to the wells of a Biolog 96-well microtiter plate. Twenty-one hours bacterial cultures grown in BHI were diluted to a final OD600 of 0.1 in BHI containing 2× Redox Dye F (Biolog). The 96-well microtiter plate containing the osmolyte dilutions was inoculated by adding 50 μL of the appropriate bacterial cell suspension. Plates were incubated at 37 °C over a period of 24 h in the Omnilog plate reader, with growth monitored as a function of respiration every 15 min.
L. monocytogenes bile survival assay
Overnight 5 mL BHI cultures were used to inoculate 25 mL of BHI supplemented with 24% sodium cholenate (Sigma Aldrich) to a final concentration of 1.5 × 107 colony forming units (CFU)/mL. These calculations were performed by measuring the optical density of the overnight cultures and the assumption that a spectrophotometer reading of 1 at OD600 is equal to 1.0 × 109 CFU/mL of L. monocytogenes. Immediately following inoculation, samples of cell culture were removed, serially diluted, and plated on BHI agar for enumeration. Cultures were grown at 37 °C in a shaking incubator set to 250 rotations per minute (rpm). Serial dilutions (10−1 to10−4) were performed in 25% Ringer solution with 100 μL samples taken from each culture every hour for 4 h. The two highest serial dilutions were plated on BHI agar in duplicate using 100 μL of the diluted cell suspension. Plates were incubated at 37 °C for 1 to 2 d until colonies were 0.5 mm in diameter at which point they were used to calculate the average CFU/mL.
L. monocytogenes defensin kill curve
L. monocytogenes EGD-e and isogenic ΔlysPGS strains were grown overnight with shaking at 37 °C in BHI. The following day, bacteria were diluted 1/10 and were grown at 37 °C until they reached mid- exponential phase (OD600 of 0.7–0.8). One milliliter of culture was washed in warm phosphate buffered saline (PBS). Bacterial suspensions were diluted in Dulbecco’s modified Eagle medium (DMEM) to a final concentration of 106 CFU/mL in bovine serum albumin (BSA)-coated eppendorf tubes. The defensins, RC-1 (a gift from Dr Robert I Lehrer, Department of Medicine, David Geffen School of Medicine at UCLA) and HNP-1 (a gift from Dr Wuyuan Lu, Institute of Human Virology, Department of Biochemistry and Molecular Biology, University of Maryland School of Medicine), were diluted to a final concentration of 4 μg/mL or 1 μg/mL. Tubes were incubated for 15 min in the presence of 5% CO2 at 37 °C and then washed twice in 1 mL PBS. Bacteria were diluted and plated in duplicate on BHI agar. Plates were incubated at 37 °C for 48 h prior to enumeration. Results are expressed as percent proliferation of each strain compared with an untreated control normalized to 100% (corresponding to 1.0 × 106 cell/mL).
Lipid composition determination
The relative compositions of the main phospholipids of L. monocytogenes EGD-e and B. subtilis 1A100 were monitored as described.51 Briefly, 100 μL of an overnight culture (OD600 of 3 or 5 for L. monocytogenes EGD-e and B. subtilis 1A100, respectively) was used to inoculate 30 mL of media supplemented with 2 × 106 cpm/mL of [32P] PPi. The resulting cultures were incubated at 30 °C or 37 °C in a shaking incubator set to 250 rpm. Growth rate was monitored by measuring turbidity with a Klett colorimeter. At various times during incubation an aliquot (150 Klett units) was removed, pelleted and stored at −20 °C. Lipids were extracted from each sample using a modified Bligh and Dyer method as described.51 Dried lipids were resuspended in 50 μL of chloroform: methanol (2:1 v/v) and 4 μL of each sample was analyzed on a silica gel HLF TLC plate (Analtech). TLCs were developed in chloroform: methanol: water (14:6:1 v/v/v). The relative abundances of phosphatidylglycerol (PG), phosphatidylethanolamine (PE), cardiolipin (CL), LysPG, and LysCL were determined by phosphorimaging using a Storm phosphorimager (Molecular Dynamics). PG, PE, and CL were identified by comigration with commercially available standards (Sigma).
Isolation and analysis of protein fractions
Overnight cultures of the L. monocytogenes EGD-e strains were diluted to 1/100 OD600 in 50 mL of BHI in a 250 mL flask. Cultures of B. subtilis 1A100 were grown and diluted in the same manner in 50 mL of LB in a 500 mL flask. All strains were grown at 37 °C and the three L. monocytogenes strains were also grown at 30 °C in a shaking incubator (250 rpm). Cultures were harvested during late exponential phase (L. monocytogenes OD600 of 1.5 and B. subtilis OD600 of 2.0). Secreted proteins, cellular extracts, cell membrane proteins and cell wall proteins were isolated following a combination of the previously published protocols.52,53 Briefly, bacterial cultures were pelleted at 6200 × g for 15 min. The supernatant was transferred to a 50 mL Falcon tube and the pellet was washed with 1 mL of sucrose wash buffer (SWB, 10 mM TRIS-HCl pH 6.9, 10 mM MgCl2, and 500 mM sucrose) prior to centrifugation for 15 min at 6200 × g. The wash was removed from the re-pelleted cells and the cell pellet was stored at −80 °C. Forty milliliters of supernatant from each culture was filter sterilized using a 0.22 μM syringe filter into a 50 mL Falcon tube. Secreted proteins were isolated by trichloroacetic acid (TCA) precipitation. TCA was added to 10% of the final volume of filtered supernatant and tubes were incubated at 4 °C for 2 h to 12 h. Proteins were pelleted following incubation by centrifugation at 6200 × g and washed once in ice cold acetone. The acetone was removed and pellets were allowed to dry inverted for 1 h prior to re-suspension in 200 μL of 1M TRIS-HCl pH 8.8 and storage at −20 °C. Protoplasts were generated by incubation of the cell pellet in 0.1 mL SWB containing 10 mg/mL lysozyme (Merck) and 2500 U/mL mutanolysin (Sigma) for 2 h at 37 °C. The cell wall proteins were then isolated by centrifugation at 6200 × g for 15 min at 4 °C and subsequent removal of the supernatant. Pelleted protoplasts were washed in 1 mL SWB and re-suspended in 200 μL protoplast lysis buffer (PLB, 100 mM TRIS-HCl pH 7.5, 10 mM MgCl2, 100 mM NaCl, and 362 U benzonase nuclease [Sigma]). Lysis occurred during three freeze–thaw cycles and the cytoplasmic and membrane fractions were isolated following centrifugation at 16 000 × g for 10 min at 4 °C. The resulting supernatant consisted of the cytoplasmic protein fraction and the pellet represented cell membrane proteins. Cell membrane proteins were re-suspended in 0.1 mL Tris-EDTA (TE) buffer (10 mM TRIS-HCl pH 7.9 and 1 mM EDTA Na2). All protein fractions were stored at −20 °C and analyzed by 12% SDS PAGE. Differences in the protein profiles were excised from the gel and sent for mass spectrometry (MS) analysis. Controls consisted of removal of gel from the identical location in both the wild-type and mutant protein profiles and removal of any proteins appearing in both MS data sets.
Immunodetection of FlaA
Seven cm gels were used for protein transfer to nitrocellulose membrane using a dry blot apparatus and current of 43 mA/cm2 for 1 h. Membranes were probed using a 1/500 fold dilution of rabbit α–L. monocytogenes flagellin antibodies (Fisher Scientific), or a 1/100 fold dilution of mouse α–p60 antibodies (a gift from Professor T Chakraborty). p60 detection was used as a localization control as it is a protein that was also identified by mass spectrometry, it is expressed during the stationary phase of growth and is localized to the peptidoglycan layer. Alkaline phosphatase conjugated α-rabbit or α-mouse antibodies, respectively, were used at a 1/100 000 fold dilution along with a chemiluminescence detection kit to visualize the proteins.
L. monocytogenes invasion assay
Caco-2 cells were grown in Eagle’s minimal essential media (MEM, Invitrogen) supplemented with 20% heat-inactivated fetal bovine serum, 10 units penicillin/streptomycin, 0.01 mM non-essential amino acids solution (Invitrogen), and 1 mM sodium pyruvate solution. Cells were used at passage 2–6 and prepared prior to infection as described.54,55 Glass coverslips were coated with rat tail collagen prior to seeding Caco-2 cells at 1.0 × 105 cells/well in 24-well cell culture plates. Overnight cultures of the bacterial strains were diluted to an OD600 of 0.1 in 5 mL of BHI and grown to mid-exponential phase (OD600 of 0.8). Bacteria were washed three times with phosphate-buffered saline (PBS) and re-suspended in 1 mL PBS before dilution in MEM to an MOI of 5 bacteria/Caco-2 cell. The bacterial suspensions were added to the Caco-2 cells in duplicate and the plates then centrifuged at 1500 rpm for 2 min. Bacterial infection took place at 37 °C either for 5 min or 1 h in an incubator supplemented with 5% CO2. Caco-2 cells were washed thoroughly with 1 mL PBS, repeated four times before being fixed and fluorescently labeled as described54 with the following exceptions. After incubation with secondary antibody Alexa568 the Caco-2 cells were washed twice with 1 mL of PBS and permeabilized using 0.2% Triton X-100 for 5 min. Immediately following permeabilization, a second labeling step was performed to label total bacteria using the same polyclonal α–L. monocytogenes primary antibody and a secondary antibody labeled with Alexa488. Images were acquired in a similar manner to that described.54 Modifications were as follows: 50 sets of images were acquired for each condition and bacterial and cell counts were performed visually. Results are expressed as the number of bacteria per Caco-2 cell calculated by dividing the total bacteria by the number of Caco-2 cells, determined by counting DAPI stained nuclei. The percent internalization was calculated using the following formula:
L. monocytogenes motility assay
Motility was measured as described.56 Briefly, 2× BHI media containing 0.2% w/v activated charcoal was autoclaved and filter sterilized before being combined with an equal volume of 0.6% w/v media grade agar. The resulting BHI plates with 0.3% w/v agar were inoculated by stabbing a needle used to pick up isolated colonies directly into the agar; isolated colonies originated from BHI agar plates incubated for 24 h at 30 °C or 37 °C. The experiment was performed in duplicate, and plated in triplicate. Plates were incubated at 30 °C and 37 °C and diameters were measured after 24, 48, and 96 h.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Professors Chakraborty, Cossart, Lehrer, and Lu for their kind gifts of materials used in this study. This work was supported by National Institutes of Health Grant GM65183.
References
- 1.Ramaswamy V, Cresence VM, Rejitha JS, Lekshmi MU, Dharsana KS, Prasad SP, Vijila HM. Listeria--review of epidemiology and pathogenesis. J Microbiol Immunol Infect. 2007;40:4–13. [PubMed] [Google Scholar]
- 2.Gründling A, Burrack LS, Bouwer HG, Higgins DE. Listeria monocytogenes regulates flagellar motility gene expression through MogR, a transcriptional repressor required for virulence. Proc Natl Acad Sci U S A. 2004;101:12318–23. doi: 10.1073/pnas.0404924101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peel M, Donachie W, Shaw A. Temperature-dependent expression of flagella of Listeria monocytogenes studied by electron microscopy, SDS-PAGE and western blotting. J Gen Microbiol. 1988;134:2171–8. doi: 10.1099/00221287-134-8-2171. [DOI] [PubMed] [Google Scholar]
- 4.Way SS, Thompson LJ, Lopes JE, Hajjar AM, Kollmann TR, Freitag NE, Wilson CB. Characterization of flagellin expression and its role in Listeria monocytogenes infection and immunity. Cell Microbiol. 2004;6:235–42. doi: 10.1046/j.1462-5822.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 5.Tilney LG, Portnoy DA. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sleator RD, Watson D, Hill C, Gahan CG. The interaction between Listeria monocytogenes and the host gastrointestinal tract. Microbiology. 2009;155:2463–75. doi: 10.1099/mic.0.030205-0. [DOI] [PubMed] [Google Scholar]
- 7.Thedieck K, Hain T, Mohamed W, Tindall BJ, Nimtz M, Chakraborty T, Wehland J, Jänsch L. The MprF protein is required for lysinylation of phospholipids in listerial membranes and confers resistance to cationic antimicrobial peptides (CAMPs) on Listeria monocytogenes. Mol Microbiol. 2006;62:1325–39. doi: 10.1111/j.1365-2958.2006.05452.x. [DOI] [PubMed] [Google Scholar]
- 8.Peschel A, Jack RW, Otto M, Collins LV, Staubitz P, Nicholson G, Kalbacher H, Nieuwenhuizen WF, Jung G, Tarkowski A, et al. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J Exp Med. 2001;193:1067–76. doi: 10.1084/jem.193.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy H, Ibba M. Broad range amino acid specificity of RNA-dependent lipid remodeling by multiple peptide resistance factors. J Biol Chem. 2009;284:29677–83. doi: 10.1074/jbc.M109.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy H. Tuning the properties of the bacterial membrane with aminoacylated phosphatidylglycerol. IUBMB Life. 2009;61:940–53. doi: 10.1002/iub.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haest CW, de Gier J, den Kamp JA OP, Bartels P, van Deenen LL. Chages in permeability of Staphylococcus aureus and derived liposomes with varying lipid composition. Biochim Biophys Acta. 1972;255:720–33. doi: 10.1016/0005-2736(72)90385-9. [DOI] [PubMed] [Google Scholar]
- 12.Jones T, Yeaman MR, Sakoulas G, Yang SJ, Proctor RA, Sahl HG, Schrenzel J, Xiong YQ, Bayer AS. Failures in clinical treatment of Staphylococcus aureus Infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. Antimicrob Agents Chemother. 2008;52:269–78. doi: 10.1128/AAC.00719-07. AAC.00719-07 [pii] 10.1128/AAC.00719-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vinuesa P, Neumann-Silkow F, Pacios-Bras C, Spaink HP, Martínez-Romero E, Werner D. Genetic analysis of a pH-regulated operon from Rhizobium tropici CIAT899 involved in acid tolerance and nodulation competitiveness. Mol Plant Microbe Interact. 2003;16:159–68. doi: 10.1094/MPMI.2003.16.2.159. [DOI] [PubMed] [Google Scholar]
- 14.Klein S, Lorenzo C, Hoffmann S, Walther JM, Storbeck S, Piekarski T, Tindall BJ, Wray V, Nimtz M, Moser J. Adaptation of Pseudomonas aeruginosa to various conditions includes tRNA-dependent formation of alanyl-phosphatidylglycerol. Mol Microbiol. 2009;71:551–65. doi: 10.1111/j.1365-2958.2008.06562.x. [DOI] [PubMed] [Google Scholar]
- 15.Mastronicolis S, German J, Megoulas N, Petrou E, Foka P, Smith G. Influence of cold shock on the fatty-acid composition of different lipid classes of the food-borne pathogen Listeria monocytogenes. Food Microbiol. 1998;15:299–306. doi: 10.1006/fmic.1997.0170. [DOI] [Google Scholar]
- 16.Mastronicolis SK, Boura A, Karaliota A, Magiatis P, Arvanitis N, Litos C, Tsakirakis A, Paraskevas P, Moustaka H, Heropoulos G. Effect of cold temperature on the composition of different lipid classes of the foodborne pathogen Listeria monocytogenes: focus on neutral lipids. Food Microbiol. 2006;23:184–94. doi: 10.1016/j.fm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Salzberg LI, Helmann JD. Phenotypic and transcriptomic characterization of Bacillus subtilis mutants with grossly altered membrane composition. J Bacteriol. 2008;190:7797–807. doi: 10.1128/JB.00720-08. JB.00720-08 [pii] 10.1128/JB.00720-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peschel A, Sahl HG. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol. 2006;4:529–36. doi: 10.1038/nrmicro1441. [DOI] [PubMed] [Google Scholar]
- 19.Staubitz P, Peschel A. MprF-mediated lysinylation of phospholipids in Bacillus subtilis--protection against bacteriocins in terrestrial habitats? Microbiology. 2002;148:3331–2. doi: 10.1099/00221287-148-11-3331. [DOI] [PubMed] [Google Scholar]
- 20.Li M, Cha DJ, Lai Y, Villaruz AE, Sturdevant DE, Otto M. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol Microbiol. 2007;66:1136–47. doi: 10.1111/j.1365-2958.2007.05986.x. [DOI] [PubMed] [Google Scholar]
- 21.Kristian SA, Dürr M, Van Strijp JA, Neumeister B, Peschel A. MprF-mediated lysinylation of phospholipids in Staphylococcus aureus leads to protection against oxygen-independent neutrophil killing. Infect Immun. 2003;71:546–9. doi: 10.1128/IAI.71.1.546-549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29:625–51. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Dussurget O, Cabanes D, Dehoux P, Lecuit M, Buchrieser C, Glaser P, Cossart P, European Listeria Genome Consortium Listeria monocytogenes bile salt hydrolase is a PrfA-regulated virulence factor involved in the intestinal and hepatic phases of listeriosis. Mol Microbiol. 2002;45:1095–106. doi: 10.1046/j.1365-2958.2002.03080.x. [DOI] [PubMed] [Google Scholar]
- 24.López D, Kolter R. Functional microdomains in bacterial membranes. Genes Dev. 2010;24:1893–902. doi: 10.1101/gad.1945010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kowalski MP, Pier GB. Localization of cystic fibrosis transmembrane conductance regulator to lipid rafts of epithelial cells is required for Pseudomonas aeruginosa-induced cellular activation. J Immunol. 2004;172:418–25. doi: 10.4049/jimmunol.172.1.418. [DOI] [PubMed] [Google Scholar]
- 26.Ichihashi N, Kurokawa K, Matsuo M, Kaito C, Sekimizu K. Inhibitory effects of basic or neutral phospholipid on acidic phospholipid-mediated dissociation of adenine nucleotide bound to DnaA protein, the initiator of chromosomal DNA replication. J Biol Chem. 2003;278:28778–86. doi: 10.1074/jbc.M212202200. [DOI] [PubMed] [Google Scholar]
- 27.Arias-Cartin R, Grimaldi S, Arnoux P, Guigliarelli B, Magalon A. Cardiolipin binding in bacterial respiratory complexes: structural and functional implications. Biochim Biophys Acta. 2012;1817:1937–49. doi: 10.1016/j.bbabio.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Dowhan W, Bogdanov M. Lipid-protein interactions as determinants of membrane protein structure and function. Biochem Soc Trans. 2011;39:767–74. doi: 10.1042/BST0390767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sievers S, Ernst CM, Geiger T, Hecker M, Wolz C, Becher D, Peschel A. Changing the phospholipid composition of Staphylococcus aureus causes distinct changes in membrane proteome and membrane-sensory regulators. Proteomics. 2010;10:1685–93. doi: 10.1002/pmic.200900772. [DOI] [PubMed] [Google Scholar]
- 30.Witte CE, Archer KA, Rae CS, Sauer JD, Woodward JJ, Portnoy DA. Innate immune pathways triggered by Listeria monocytogenes and their role in the induction of cell-mediated immunity. Adv Immunol. 2012;113:135–56. doi: 10.1016/B978-0-12-394590-7.00002-6. [DOI] [PubMed] [Google Scholar]
- 31.Sauer JD, Pereyre S, Archer KA, Burke TP, Hanson B, Lauer P, Portnoy DA. Listeria monocytogenes engineered to activate the Nlrc4 inflammasome are severely attenuated and are poor inducers of protective immunity. Proc Natl Acad Sci U S A. 2011;108:12419–24. doi: 10.1073/pnas.1019041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen A, Kamp HD, Gründling A, Higgins DE. A bifunctional O-GlcNAc transferase governs flagellar motility through anti-repression. Genes Dev. 2006;20:3283–95. doi: 10.1101/gad.1492606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamp HD, Higgins DE. A protein thermometer controls temperature-dependent transcription of flagellar motility genes in Listeria monocytogenes. PLoS Pathog. 2011;7:e1002153. doi: 10.1371/journal.ppat.1002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamp HD, Higgins DE. Transcriptional and post-transcriptional regulation of the GmaR antirepressor governs temperature-dependent control of flagellar motility in Listeria monocytogenes. Mol Microbiol. 2009;74:421–35. doi: 10.1111/j.1365-2958.2009.06874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen A, Higgins DE. The MogR transcriptional repressor regulates nonhierarchal expression of flagellar motility genes and virulence in Listeria monocytogenes. PLoS Pathog. 2006;2:e30. doi: 10.1371/journal.ppat.0020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staubitz P, Neumann H, Schneider T, Wiedemann I, Peschel A. MprF-mediated biosynthesis of lysylphosphatidylglycerol, an important determinant in staphylococcal defensin resistance. FEMS Microbiol Lett. 2004;231:67–71. doi: 10.1016/S0378-1097(03)00921-2. [DOI] [PubMed] [Google Scholar]
- 37.Friedman L, Alder JD, Silverman JA. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob Agents Chemother. 2006;50:2137–45. doi: 10.1128/AAC.00039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Otto M. Bacterial sensing of antimicrobial peptides. Contrib Microbiol. 2009;16:136–49. doi: 10.1159/000219377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang SJ, Bayer AS, Mishra NN, Meehl M, Ledala N, Yeaman MR, Xiong YQ, Cheung AL. The Staphylococcus aureus two-component regulatory system, GraRS, senses and confers resistance to selected cationic antimicrobial peptides. Infect Immun. 2012;80:74–81. doi: 10.1128/IAI.05669-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barák I, Muchová K, Wilkinson AJ, O’Toole PJ, Pavlendová N. Lipid spirals in Bacillus subtilis and their role in cell division. Mol Microbiol. 2008;68:1315–27. doi: 10.1111/j.1365-2958.2008.06236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hachmann AB, Angert ER, Helmann JD. Genetic analysis of factors affecting susceptibility of Bacillus subtilis to daptomycin. Antimicrob Agents Chemother. 2009;53:1598–609. doi: 10.1128/AAC.01329-08. AAC.01329-08 [pii] 10.1128/AAC.01329-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gahan CG, Hill C. Gastrointestinal phase of Listeria monocytogenes infection. J Appl Microbiol. 2005;98:1345–53. doi: 10.1111/j.1365-2672.2005.02559.x. [DOI] [PubMed] [Google Scholar]
- 43.Begley M, Sleator RD, Gahan CG, Hill C. Contribution of three bile-associated loci, bsh, pva, and btlB, to gastrointestinal persistence and bile tolerance of Listeria monocytogenes. Infect Immun. 2005;73:894–904. doi: 10.1128/IAI.73.2.894-904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogle CK, Noel JG, Guo X, Wells DA, Valente JF, Ogle JD, Alexander JW. The ability of endotoxin-stimulated enterocytes to produce bactericidal factors. Crit Care Med. 2002;30:428–34. doi: 10.1097/00003246-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 45.Wehkamp J, Schwind B, Herrlinger KR, Baxmann S, Schmidt K, Duchrow M, Wohlschläger C, Feller AC, Stange EF, Fellermann K. Innate immunity and colonic inflammation: enhanced expression of epithelial alpha-defensins. Dig Dis Sci. 2002;47:1349–55. doi: 10.1023/A:1015334917273. [DOI] [PubMed] [Google Scholar]
- 46.O’Neil DA, Porter EM, Elewaut D, Anderson GM, Eckmann L, Ganz T, Kagnoff MF. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 1999;163:6718–24. [PubMed] [Google Scholar]
- 47.Gueriri I, Cyncynatus C, Dubrac S, Arana AT, Dussurget O, Msadek T. The DegU orphan response regulator of Listeria monocytogenes autorepresses its own synthesis and is required for bacterial motility, virulence and biofilm formation. Microbiology. 2008;154:2251–64. doi: 10.1099/mic.0.2008/017590-0. [DOI] [PubMed] [Google Scholar]
- 48.Mishra NN, Yang SJ, Sawa A, Rubio A, Nast CC, Yeaman MR, Bayer AS. Analysis of cell membrane characteristics of in vitro-selected daptomycin-resistant strains of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53:2312–8. doi: 10.1128/AAC.01682-08. AAC.01682-08 [pii] 10.1128/AAC.01682-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu J, Fernandes-Alnemri T, Alnemri ES. Involvement of the AIM2, NLRC4, and NLRP3 inflammasomes in caspase-1 activation by Listeria monocytogenes. J Clin Immunol. 2010;30:693–702. doi: 10.1007/s10875-010-9425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thedieck K, Hain T, Mohamed W, Tindall BJ, Nimtz M, Chakraborty T, Wehland J, Jänsch L. The MprF protein is required for lysinylation of phospholipids in listerial membranes and confers resistance to cationic antimicrobial peptides (CAMPs) on Listeria monocytogenes. Mol Microbiol. 2006;62:1325–39. doi: 10.1111/j.1365-2958.2006.05452.x. [DOI] [PubMed] [Google Scholar]
- 51.Roy H, Ibba M. Monitoring Lys-tRNA(Lys) phosphatidylglycerol transferase activity. Methods. 2008;44:164–9. doi: 10.1016/j.ymeth.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jonquières R, Bierne H, Fiedler F, Gounon P, Cossart P. Interaction between the protein InlB of Listeria monocytogenes and lipoteichoic acid: a novel mechanism of protein association at the surface of gram-positive bacteria. Mol Microbiol. 1999;34:902–14. doi: 10.1046/j.1365-2958.1999.01652.x. [DOI] [PubMed] [Google Scholar]
- 53.Monk IR, Cook GM, Monk BC, Bremer PJ. Morphotypic conversion in Listeria monocytogenes biofilm formation: biological significance of rough colony isolates. Appl Environ Microbiol. 2004;70:6686–94. doi: 10.1128/AEM.70.11.6686-6694.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vadia S, Arnett E, Haghighat AC, Wilson-Kubalek EM, Tweten RK, Seveau S. The pore-forming toxin listeriolysin O mediates a novel entry pathway of L. monocytogenes into human hepatocytes. PLoS Pathog. 2011;7:e1002356. doi: 10.1371/journal.ppat.1002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arnett E, Lehrer RI, Pratikhya P, Lu W, Seveau S. Defensins enable macrophages to inhibit the intracellular proliferation of Listeria monocytogenes. Cell Microbiol. 2011;13:635–51. doi: 10.1111/j.1462-5822.2010.01563.x. [DOI] [PubMed] [Google Scholar]
- 56.Shetron-Rama LM, Mueller K, Bravo JM, Bouwer HG, Way SS, Freitag NE. Isolation of Listeria monocytogenes mutants with high-level in vitro expression of host cytosol-induced gene products. Mol Microbiol. 2003;48:1537–51. doi: 10.1046/j.1365-2958.2003.03534.x. [DOI] [PubMed] [Google Scholar]
- 57.Ishihama Y, Oda Y, Tabata T, Sato T, Nagasu T, Rappsilber J, Mann M. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol Cell Proteomics. 2005;4:1265–72. doi: 10.1074/mcp.M500061-MCP200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.