Abstract
Multiple epigenetic alterations contribute to prostate cancer progression by deregulating gene expression. Epigenetic mechanisms, especially differential DNA methylation at imprinting control regions (termed DMRs), normally ensure the exclusive expression of imprinted genes from one specific parental allele. We therefore wondered to which extent imprinted genes become deregulated in prostate cancer and, if so, whether deregulation is due to altered DNA methylation at DMRs. Therefore, we selected presumptive deregulated imprinted genes from a previously conducted in silico analysis and from the literature and analyzed their expression in prostate cancer tissues by qRT-PCR. We found significantly diminished expression of PLAGL1/ZAC1, MEG3, NDN, CDKN1C, IGF2, and H19, while LIT1 was significantly overexpressed. The PPP1R9A gene, which is imprinted in selected tissues only, was strongly overexpressed, but was expressed biallelically in benign and cancerous prostatic tissues. Expression of many of these genes was strongly correlated, suggesting co-regulation, as in an imprinted gene network (IGN) reported in mice. Deregulation of the network genes also correlated with EZH2 and HOXC6 overexpression. Pyrosequencing analysis of all relevant DMRs revealed generally stable DNA methylation between benign and cancerous prostatic tissues, but frequent hypo- and hyper-methylation was observed at the H19 DMR in both benign and cancerous tissues. Re-expression of the ZAC1 transcription factor induced H19, CDKN1C and IGF2, supporting its function as a nodal regulator of the IGN. Our results indicate that a group of imprinted genes are coordinately deregulated in prostate cancers, independently of DNA methylation changes.
Keywords: imprinted genes, prostate cancer, DNA methylation, imprinted gene network, cancer epigenetics, ZAC1, pyrosequencing, differentially methylated region
Introduction
During development, imprinted genes control growth and metabolism of the fetus in order to balance its requirements with those of the mother. Defects in imprinting therefore disturb fetal growth and development, but also may elicit specific pediatric cancers.1-3 However, disturbances in the regulation of imprinted genes have also been found to contribute to the typical cancers of older ages.4-6 For instance, the imprinted IGF2 gene, which encodes an insulin-like growth factor, has been implicated in pediatric as well as adult cancers, including Wilms tumor, colorectal carcinoma, breast, and prostate cancer.6-9
The monoallelic expression of imprinted genes is achieved by the interaction of several epigenetic mechanisms, including differential DNA methylation at specific regulatory regions (DMRs), histone modifications, the action of long non-coding RNAs (many of which are themselves the products of imprinted genes) and long-range chromatin interactions facilitated by the methylation-sensitive binding of the insulator protein CTCF.10 Accordingly, disturbances in the expression of imprinted genes in cancers may be caused by genetic mechanisms such as the deletion of the sole expressed allele or by epigenetic mechanisms such as aberrant DNA methylation at the DMRs controlling the expression of individual or clustered imprinted genes.5
In prostate cancer, a large number of epigenetic alterations have been described, including increased DNA methylation at CpG-islands, decreased DNA methylation at repeat sequences as well as global changes in histone modifications and deregulation or mutation of chromatin-modifying enzymes like the H3K27 methylase EZH2.11,12 We therefore wondered to which extent imprinted genes, whose expression is particularly strongly dependent on intact epigenetic regulation, might be affected by the large-scale disturbance of epigenetic mechanisms in this tumor type. To date, however, only IGF2 has been studied in any detail.9 Relaxation of IGF2 imprinting, manifesting as its bi-allelic expression, has been found to be very common in benign as well as cancerous prostatic tissues of older men.13 Since IGF2 loss of imprinting was associated with increased IGF2 expression levels in benign regions of cancer-carrying prostates, it is thought to represent an early field change associated with or setting the stage for prostate carcinogenesis. Interestingly, in actual prostate carcinomas, IGF2 mRNA levels were found to be lower than in adjacent benign tissues and the association of loss of imprinting with increased gene expression becomes blurred.13
In order to gauge the extent of deregulation of imprinted genes in prostate cancer more generally, we previously conducted an in silico analysis of microarray gene expression studies collected in the Oncomine database (www.oncomine.org).14 Somewhat contrary to our expectations, this analysis revealed that of the 62 genes considered imprinted in humans at the time (as listed on www.geneimprint.com), only a subset of 12 genes was significantly differentially expressed between carcinoma and benign prostate tissue across multiple studies. Of note, 10 genes could not be analyzed because of a lack of studies. Most imprinted genes displaying altered expression belong to a functionally interacting and often co-regulated gene network, which had been identified by functional and bioinformatical analyses in the mouse.15 Notably, several members of the network act as transcriptional regulators of others, thereby constituting important nodes.16 For instance, the transcription factor Zac1 encoded by the Plagl1 gene directly or indirectly regulates other member genes like Cdkn1c, Igf2, and H19.15
We therefore went on to validate the result of the in silico analysis in our well characterized set of tissue samples. Specifically, we wondered whether the expression changes were correlated with each other, as one might expect in a co-regulated network. After both assumptions had been confirmed, we searched for epigenetic and other molecular mechanisms that might cause the altered expression of imprinted genes in prostate cancer. For this purpose, we searched for DNA methylation alterations at the relevant DMRs and tested the influence of DNA methylation and histone deacetylation inhibitors as well as androgen. Furthermore, we examined whether the changes in imprinted genes correlated with those in the expression of prostatic oncogenes EZH2, ERG, and HOXC6,17-21 or to clinical parameters of prostate cancer progression. Finally, we tested the ability of a presumptive nodal transcription factor, ZAC1, to restore the expression of other downregulated imprinted genes in a prostate cancer cell line.
Results
Expression of imprinted genes in benign and cancerous prostatic tissues
As described previously, an analysis of 14 microarray studies archived in the Oncomine database revealed at least close to statistically significant differences (P < 0.05) in the expression of each of the imprinted genes HYMAI, PLAGL1/ZAC1, CDKN1C, MEG3, PEG3, PEG10, SGCE, PPP1R9A, NDN, SNRPN/SNURF, INPP5F, and GNAS between benign and cancerous prostate tissues across the studies.14 According to that analysis, 9 of these genes were downregulated (HYMAI, PLAGL1/ZAC1, SGCE, PEG10, INPP5F, CDKN1C, MEG3, NDN, and PEG3), two were upregulated (PPP1R9A and GNAS) and SNRPN/SNURF was significantly upregulated in several studies, but downregulated in others.14 This latter finding may be caused by the differential expression of particular transcript variants of this complex gene.
We additionally investigated the expression of these genes in the data set of a further large and well-documented microarray study from Taylor et al.22 that had been submitted to the Oncomine database after our initial analysis had been conducted. When disregarding SNRPN/SNURF, all genes except for HYMAI showed the same trends in this data set with six genes (SGCE, PEG10, PPP1R9A, INPP5F, CDKN1C, and PEG3) again exhibiting statistically significant differences in expression (not shown).
We therefore measured the expression of ZAC1, ZAC1delta, TFPI2, SGCE, PEG10, PPP1R9A, PON2, INPP5F, INPP5Fv2, CDKN1C, KCNQ1OT1/LIT1, IGF2, H19, MEG3, NDN, SNRPN, SNURF, PEG3, and GNAS by qRT-PCR in the well-characterized set of prostate carcinoma and benign tissues from our institution. This selection was based on the following arguments and a number of preliminary experiments. HYMAI was found to be expressed at extremely low levels and was therefore excluded. Instead, two different splice variants of ZAC1 were measured, as they encode different protein isoforms of this crucial transcription factor. Three of the candidate genes (SGCE, PEG10, and PPP1R9A) are located in the same gene cluster at chromosome 7q21. We therefore included data on two additional genes from this cluster (TFPI2, PON2) that we had obtained previously.23 Note that PON2 may likely not be imprinted in humans. Two different transcript variants of INPP5F were measured, as only the INPP5Fv2 variant arises from an imprinted promoter.24 In addition to CDKN1C, the non-coding RNA LIT1 originating in the relevant imprinting control region was measured as a potential regulator. Although they had not been highlighted by the microarray analysis, the imprinted genes IGF2 and H19 were included as the best studied imprinted genes in prostate cancer up to now. In addition to MEG3, we considered determining the expression of the oppositely (paternally) imprinted gene DLK1 from the same cluster, but its expression was found to be below the level of detection in both benign and cancerous tissues (data not shown). The GNAS and SNRPN/SNURF genes produce many transcript variants due to differential promoter usage and splicing. After ascertaining or excluding the expression of several of the major variants in prostatic tissues (data not shown), we settled on one predominantly expressed GNAS transcript and on one set of primers each for SNRPN and SNURF. All expression values were adjusted to TBP mRNA, which we and others have found to constitute the most stable reference gene when comparing benign and cancerous prostate tissues.25
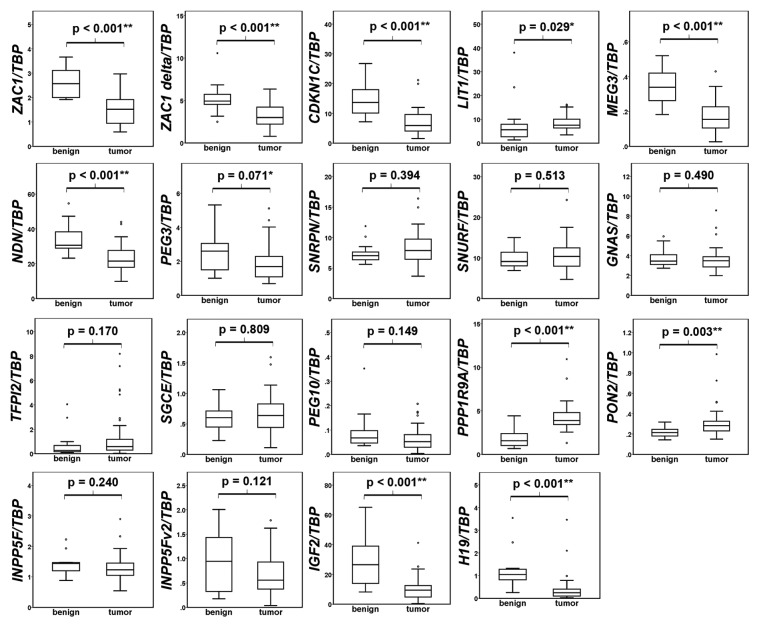
Box plot representations of the qRT-PCR results are compiled in Figure 1. In accord with the microarray studies, ZAC1 (both transcripts), CDKN1C, MEG3, and NDN were significantly downregulated and PPP1R9A was upregulated. PON2 and LIT1 were also found to be significantly upregulated in prostate cancer. According with the prediction, there was a trend for downregulation of PEG10, INPP5Fv2, and PEG3 in cancer tissues, but the changes were not statistically significant in our sample set. Expression of SGCE, SNRPN, SNURF and GNAS was similar between the benign and carcinoma samples in our set, with no trend toward decrease or increase. In accord with a previous report,13 IGF2 and H19 genes were expressed at lower levels in the cancers as compared with benign prostatic tissues.
Figure 1. Expression of imprinted genes in prostate benign and cancer tissues. The mRNA expression of the indicated imprinted genes relative to TBP in benign (n = 13) and tumor (n = 45) prostate tissues was assessed by qRT-PCR and is represented as boxplots. Mann-Whitney-U test was used to evaluate the differences between the two groups. The significance (p) is shown above the bracket in each individual panel (* P < 0.05, ** P < 0.01).
Correlations among imprinted genes expression in prostate cancer
In order to investigate whether the postulated imprinted gene network also exists in prostate cancer tissue, we calculated the correlations between all investigated genes. Indeed, the expression of each two genes from the group comprising ZAC1, INPP5Fv2, CDKN1C, MEG3, NDN, PEG3, H19, and IGF2 correlated positively with each other and all expressions except for PEG3 correlated negatively with PPP1R9A expression (Table 1; Fig. 2A). These results suggest the coordinate regulation of these genes, whereby PPP1R9A is overexpressed and the other genes are more or less strongly downregulated in prostate cancer tissues. In order to test whether the expression of ZAC1 may predict changed expression of the other genes, we split the tumor samples in two groups by ZAC1 expression below or above the median value and then used Mann-Whitney-U test to evaluate differential expression of the other imprinted genes. Indeed, the expression of CDKN1C, MEG3, NDN, PEG3, SGCE, INPP5F, INPP5Fv2, H19, and IGF2 genes was significantly lower in the tumor samples with lower ZAC1 expression (Fig. S1). When according groups were formed based on the median value of other imprinted genes, Mann-Whitney-U test likewise yielded significant differences in the expression of most other correlated imprinted genes (Table S4). This result suggests that the expression changes of these genes occur in a coordinate manner in the same set of tumor samples.
Table 1. Spearman correlation coefficients of the expression of each two of the indicated imprinted genes.
| Spearman rho | ZAC1 | ZAC1 delta | SGCE | PEG10 | PPP1R9A | PON2 | INPP5F | INPP5Fv2 | CDKN1C | LIT1 | MEG3 | NDN | PEG3 | SNRPN | SNURF | GNAS | TFPI2 | H19 | IGF2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZAC1 | 1,000 | ||||||||||||||||||
| ZAC1 delta | .638** | 1,000 | |||||||||||||||||
| SGCE | .244 | .214 | 1,000 | ||||||||||||||||
| PEG10 | .367* | .258 | .600** | 1,000 | |||||||||||||||
| PPP1R9A | -.377* | -.227 | -.436** | -.575** | 1,000 | ||||||||||||||
| PON2 | .116 | -.222 | .214 | -.216 | .226 | 1,000 | |||||||||||||
| INPP5F | .324* | .285 | .223 | .148 | .069 | .174 | 1,000 | ||||||||||||
| INPP5Fv2 | .636** | .361* | .553** | .476** | -.383** | .192 | .209 | 1,000 | |||||||||||
| CDKN1C | .551** | .457** | .566** | .651** | -.448** | -.130 | .470** | .584** | 1,000 | ||||||||||
| LIT1 | .019 | .112 | .163 | .025 | .175 | -.080 | .096 | .123 | .362* | 1,000 | |||||||||
| MEG3 | .544** | .614** | .288 | .493** | -.311* | -.131 | .335* | .520** | .589** | .307* | 1,000 | ||||||||
| NDN | .554** | .493** | .558** | .477** | -.416** | .090 | .226 | .648** | .618** | .290 | .620** | 1,000 | |||||||
| PEG3 | .483** | .457** | .260 | .358* | -.053 | -.074 | .517** | .471** | .623** | .429** | .564** | .572** | 1,000 | ||||||
| SNRPN | -.050 | -.330* | -.120 | -.215 | .336* | .339* | -.094 | .103 | -.161 | .034 | -.025 | .086 | .007 | 1,000 | |||||
| SNURF | -.190 | -.459** | .054 | -.216 | .272 | .479** | -.075 | .097 | -.140 | .111 | -.155 | .139 | .034 | .878** | 1,000 | ||||
| GNAS | -.045 | -.028 | -.026 | -.206 | .211 | .205 | .045 | -.021 | -.040 | .202 | .023 | .266 | .219 | .414** | .562** | 1,000 | |||
| TFPI2 | .077 | .167 | .439** | .025 | .091 | .312* | -.018 | .199 | .037 | -.029 | .007 | .175 | -.183 | .072 | .080 | -.179 | 1,000 | ||
| H19 | .519** | .438** | .433** | .430** | -.424** | -.097 | .251 | .448** | .479** | .120 | .407** | .669** | .326* | -.107 | -.109 | .071 | .199 | 1,000 | |
| IGF2 | .506** | .532** | .362* | .471** | -.458** | -.182 | .219 | .512** | .552** | .074 | .602** | .735** | .368* | -.008 | -.107 | -.016 | .149 | .700** | 1,000 |
The correlation was calculated with SPSS using expression data from 45 prostate cancer tissues (*P < 0.05, **P < 0.01).
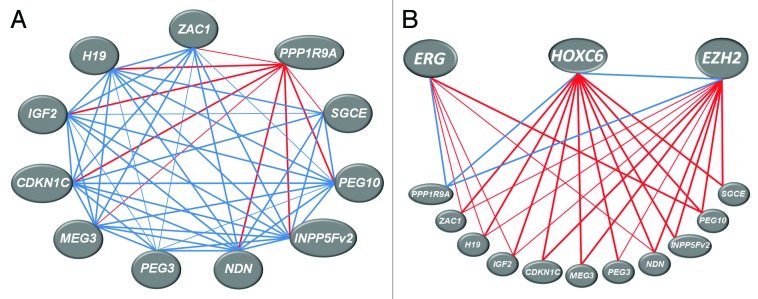
Figure 2. Representation of the significant correlations among expression of imprinted genes (A) and between imprinted genes and EZH2, ERG and HOXC6 genes expression (B) in prostate cancer tissues. Spearman correlation analysis was performed in SPSS software using gene expression data (obtained by qRT-PCR) for 45 prostate cancers. Thicker lines represent correlations with P < 0.01, thinner lines –correlations with P < 0.05; positive correlations are shown in blue, negative ones - in red. For exact values see Table 1.
Co-expression of imprinted genes could be due to their close proximity and co-regulation within a cluster. While the above co-regulated genes are located in several different clusters, the expression of several of the genes from the same cluster like SNRPN and SNURF, H19 and IGF2, PEG10 and SGCE, and PON2 and PPP1R9A likewise correlated positively with each other. The latter four genes all belong to the same cluster at chromosome 7q21, but correlate only pair-wise to each other, which is plausible as PON2 and PPP1R9A are upregulated in cancer tissues, and the others are not. This finding suggests that the mechanism causing deregulation does not affect the entire cluster. A significant positive, albeit weak correlation (ρ = 0.362, P < 0.05) was found between the overall downregulated gene CDKN1C and the overall upregulated LIT1 gene situated both at 11p15.5. This peculiar correlation results from regulation of both genes in the same direction in a subset of the tumor samples. Overexpression of the non-coding RNAs H19 and LIT1 in cancer is often associated with downregulation of the IGF2 and CDKN1C genes located in the respective clusters.26-28 According to our analysis, unexpectedly, the expression of these ncRNA/protein-coding gene pairs was positively correlated in prostate cancer tissues.
Correlation between imprinted gene expression and clinical parameters of prostate cancer progression
In order to test whether the aberrant expression of imprinted genes is associated with prostate cancer progression we analyzed their correlations to clinical parameters of the disease including tumor stage, lymph node metastasis, Gleason score and biochemical recurrence. The expression of the well-characterized prostatic oncogenes ERG, EZH2, and HOXC6 were included in this analysis as positive controls. In accord with previous observations by us and others,20,29 increasing HOXC6 expression correlated best with adverse parameters of prostate cancer. Only a limited number of statistically significant associations were found for the imprinted genes (Fig. S2). Most prominently, decreased expression of ZAC1 and its splice form ZAC1 delta correlated significantly with tumor stage and recurrence, hinting at a role in prostate cancer progression. The overall low correlation between expression of imprinted genes and clinical parameters, however, suggests that their deregulation is a general phenomenon in prostate cancer that is not restricted to particular stages or grades of the disease.
Analysis of epigenetic regulation of imprinted DMRs
In order to investigate whether disturbances in DNA methylation at the DMRs or other local control sequences were responsible for the deregulation of imprinted genes we assessed their DNA methylation status by bisulfite pyrosequencing of DNA from the prostate tissue set. For each of the regions ZAC1 DMR (at chromosome 6q24), 7q21 DMR1, 7q21 DMR2, KvDMR (11p15.1), MEG3 DMR (14q32), NDN DMR (15q11.2), H19 DMR (11p15.5), and CDKN1C promoter (11p15.1), methylation at 4‒7 CpG positions (depending on the assay) was studied. Statistical analyses were performed for the methylation value averaged across all CpG sites as well as for each single CpG site of each sequence.
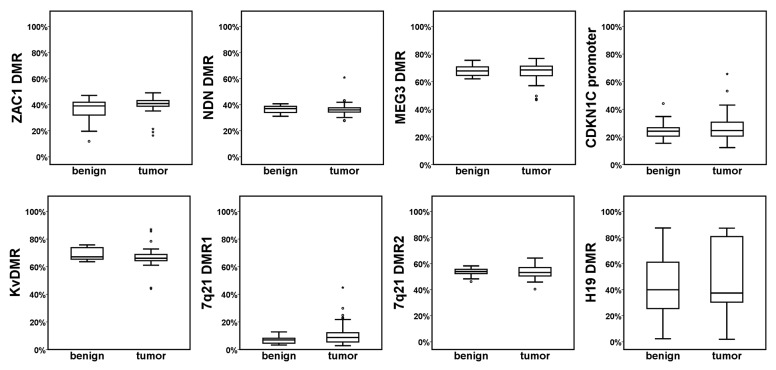
Mean methylation across all CpGs did not significantly differ between benign and tumor tissues for any of the analyzed DMRs (Fig. 3). Mean methylation of ZAC1 DMR, KvDMR, 7q21 DMR2, MEG3 DMR and NDN DMR in both carcinoma and benign tissues ranged between 35‒70%, which is compatible with the 50% expected for ideal imprinted DMRs. In single cases from either sample group the average methylation of the ZAC1 DMR was diminished to less than 20%, which could be ascribed to allelic loss. In the 7q21 cluster, we studied two regions, since the DMR1 region reported by others30 to be located within the actual DMR exhibited only approximately 10% methylation in prostatic tissues, implying that this region is rather adjacent to than part of the actual DMR. Nevertheless, methylation at either region was conserved in the cancer tissues.
Figure 3. Methylation of imprinted DMRs in benign and tumor prostatic tissues. DNA methylation (%) of the indicated imprinted DMRs in benign and tumor prostatic tissues was quantitated by bisulfite pyrosequencing. The boxplots represent the mean methylation values of all assessed CpG positions for the indicated region for benign or tumor samples. Mann-Whitney-U test was used to evaluate the differences between the two sample groups; none of the differences was significant at P < 0.05. Note the exceptionally large variation in the H19 DMR.
To confirm that beside the level of DNA methylation its pattern too was preserved in cancer tissues, we exemplary examined by bisulfite sequencing individual alleles of NDN DMR from three cancer samples heterozygous for this region (Fig. S3).
The studied region in the CDKN1C promoter contains four CpG positions situated between -714 and -701 relative to the transcription start site; its hypermethylation in lung cancer was reported to correlate with lower CDKN1C expression.31 While its average methylation (~25%) was similar in the benign and carcinoma prostate tissues, methylation exceeded 40% in a few cases, including one benign sample.
Methylation of the H19 DMR differed substantially from that of all other investigated sequences. Although no significant differences were detected between benign and cancerous prostatic tissues, methylation of this sequence was highly variable in both types of tissues (Fig. 3). However, this variation was not continuous, but almost all samples could be assigned to three clear-cut groups. About one third of the tissues showed methylation levels between 30% and 45%, fitting the expected methylation level of a normal imprinted DMR, another third exhibited less than 20% methylation, and the remaining third were more than 80% methylated (Fig. S4A). This suggests that both loss and gain of methylation occur at the H19 DMR in benign as well as cancerous prostates (see discussion section).
Among all these sites, only two single CpG positions showed significant differences between benign and cancer tissues, namely CpG2 from the MEG3 DMR exhibited generally lower methylation than the other CpGs and consistently lower methylation in carcinoma tissues (Mann-Whitney-U test: P = 0.002). CpG6 from the NDN DMR region was significantly less methylated in tumor tissues than in benign tissues (Mann-Whitney-U test: P = 0.014).
To further test to which extent epigenetic modifications may influence the expression of the candidate imprinted genes, we treated three prostate cancer cell lines with the DNA methylation inhibitor 5-aza-2'-deoxycytidine (5-aza) and the pan-histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA). The effects of these treatments were quite disparate among the three cell lines (Fig. S5), which may be explained by different sensitivity to the respective inhibitors as well as differences in the basic expression level and acquired epigenetic status at the analyzed genes. Nevertheless, generally, treatment with 5-aza strongly induced the expression of the ncRNA genes MEG3 and H19, and more modestly ZAC1, PEG3 and CDKN1C expression. Treatment with SAHA resulted in the strong induction of PEG10, NDN and MEG3 but, interestingly, in downregulation of SGCE, SNURF and SNRPN expression (Fig. S5). These results confirm that imprinted genes remain in part regulated by DNA methylation and histone acetylation in prostate cancer cell lines, but that these mechanisms are neither uniformly relevant across the group of genes nor across the cell lines.
Correlation between DNA methylation of DMRs and imprinted gene expression
Although the average methylation levels of all analyzed DMRs remained steady in benign and tumor tissues, we wondered whether smaller or less frequent changes in the average DMR methylation or that of single CpG positions may influence the expression of imprinted genes in the respective locus. Correlation analysis showed that the mean methylation of 7q21 DMR1 correlated negatively to the expression of SGCE and PEG10, but positively to PPP1R9A expression (all significant correlations are compiled in Table 2). Furthermore, CDKN1C expression was negatively correlated with the mean methylation of its promoter. Correlations between gene expression and methylation of single CpG sites were more frequently observed, but were mostly rather weak. Stronger correlations were found only between the MEG3 DMR CpG2 methylation and MEG3 expression (ρ = 0.535, P < 0.001) and with 7q21 DMR2 CpG3 methylation and PPP1R9A expression (ρ = 0.427, P = 0.003).
Table 2. Spearman correlation between DMR methylation and imprinted gene expression.
| DMR Methylation | Gene expression | rho | P | n |
|---|---|---|---|---|
| 7q21 DMR1 | SGCE | - 0.491 | 0.001 | 46 |
| PEG10 | - 0.423 | 0.003 | 46 | |
| PPP1R9A | 0.559 | 0.000 | 46 | |
| 7q21 DMR1 CpG4 | PON2 | 0.356 | 0.015 | 46 |
| 7q21 DMR1 CpG6 | PON2 | 0.330 | 0.025 | 46 |
| 7q21 DMR2 CpG3 | PEG10 | - 0.292 | 0.047 | 47 |
| PPP1R9A | 0.427 | 0.003 | 47 | |
| 7q21 DMR2 CpG4 | PPP1R9A | 0.328 | 0.024 | 47 |
| 7q21 DMR2 CpG5 | PPP1R9A | 0.368 | 0.011 | 47 |
| ZAC1 DMR CpG6 | ZAC1 | - 0.328 | 0.030 | 44 |
| NDN DMR CpG7 | NDN | - 0.314 | 0.032 | 47 |
| MEG3 DMR CpG2 | MEG3 | 0.535 | 0.000 | 47 |
| CDKN1C promoter | CDKN1C | - 0.310 | 0.034 | 47 |
| CDKN1C promoter CpG4 | IGF2 | - 0.357 | 0.014 | 47 |
| LIT1 | - 0.293 | 0.046 | 47 | |
| H19 DMR CpG4 | IGF2 | - 0.294 | 0.050 | 45 |
| H19 DMR CpG7 | IGF2 | - 0.308 | 0.040 | 45 |
Spearman correlation coefficient (rho) was used to evaluate the correlation between the DNA methylation of each individual CpG position or the mean of all CpG positions in the assesed DMRs with the expression of the local imprinted genes in prostate cancer tissues. Only significant correlations are included in the table (*P < 0.05; **P < 0.01). In the cases where the mean methylation of all CpGs correlated significantly with gene expression, it also did for most of the single CpG positions in that region and is therefore not explicitly shown.
The H19 DMR again constituted an exception in this regard. Its methylation status did not correlate to H19 or IGF2 expression in the tumors in general, but the samples in the low methylation group exhibited higher levels of both H19 and IGF2 expression among both benign and tumor tissues (Fig. S4B and C).
Taken together, nevertheless, this data suggests that altered methylation at DMRs is a minor factor in the deregulation of imprinted genes in prostate cancer.
Biallelic expression of PPP1R9A in benign and cancerous prostates
In the mouse, Pon2 and Ppp1r9a are reported to be biallelically expressed in embryonic cells and the placenta.32 In humans, according to geneimprint.com, PON2 is likely to be expressed biallelically generally. PPP1R9A is thought to be imprinted in a tissue-specific fashion, as a study examining human PPP1R9A expression in several embryonic tissues and in the placenta reported monoallelic expression in skeletal muscle, but not other tissues.33 No publication on the state of its imprinting in the prostate has been available to date.
In order to monitor if loss of imprinting (LOI) might have caused the upregulated PPP1R9A expression in the tumor tissues, we examined its allele-specific expression. Pyrosequencing analysis exploiting a SNP in the PPP1R9A mRNA sequence revealed that all heterozygous tissue samples (17/47 tumor and 5/13 benign) exhibited biallelic PPP1R9A expression (Fig. 4). Thus, PPP1R9A is not imprinted in the human prostate and its increased expression is not caused by LOI.
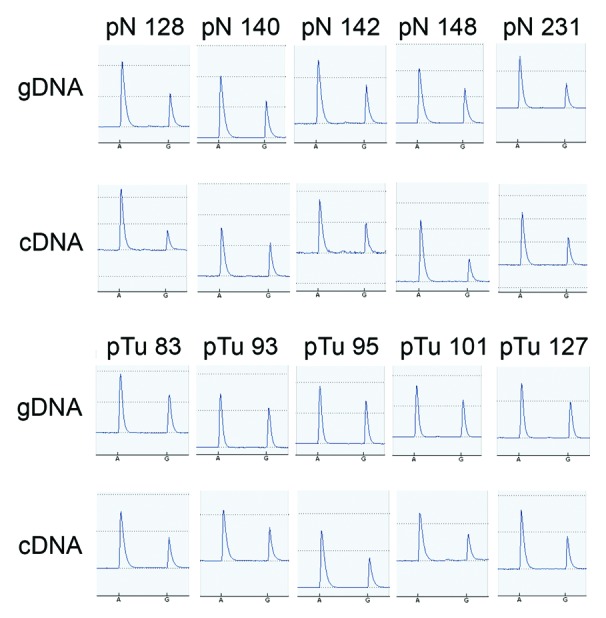
Figure 4. Biallelic expression of PPP1R9A in prostate benign and cancer tissues. Heterozygosity at the SNP rs854544 in the coding sequence of PPP1R9A was determined by SNP pyrosequencing analysis using genomic DNA (gDNA). Biallelic expression of the gene is evident from the presence of two peaks for the polymorphism A/G, as examined by pyrosequencing analysis of PCR products using cDNA as a template from benign (“pN”s in upper panel) and tumor (“pTu”s in lower panel) heterozygous samples.
Relation of imprinted genes expression to prostatic oncogenes
We were curious how the imprinted genes expression changes might be related to the expression of important prostatic oncogenes like ERG, EZH2 and HOXC6. In line with expectations and as reported previously elsewhere for our sample set,29,34,35 expression of these three genes was highly significantly increased in the prostate cancer tissues. In particular, ERG was strongly overexpressed in about half of the samples (22/45), a finding typical of unselected prostate cancer cohorts. Interestingly, HOXC6 and EZH2 expression correlated well with each other but not with that of ERG (Fig. 2B). The relation of imprinted gene expression to oncogene expression was investigated by Spearman correlation as well as by Mann-Whitney-U test following stratification into ‘low’ and ‘high’ expression groups (see Methods). By either analysis, expression of HOXC6 and EZH2 appeared related to the expression of the majority of genes from the imprinted gene network (Figs. S6 and S7), whereas ERG expression was related to only a few (Fig. S8). Notably, the expression of all three oncogenes was positively correlated with that of PPP1R9A, whereas the correlations with all other imprinted genes were negative. Taken together, these data suggest that altered expression of imprinted genes is related to the overexpression of EZH2 and HOXC6 in prostate cancer.
Influence of androgens on imprinted genes expression
Aberrant androgen signaling is another common characteristic of prostate carcinogenesis. Since many imprinted genes participate in growth and differentiation signaling pathways, we wondered whether they might be regulated by androgens in prostate cancer cells. We therefore measured gene expression following treatment of the androgen-sensitive LNCaP prostate cancer cell line with the synthetic androgen R1881. While the known AR target gene KLK3 (PSA) was significantly induced by androgen, most of the assessed imprinted genes were not significantly influenced by androgen signaling, apart from CDKN1C and NDN, which were slightly downregulated after 24 h of androgen treatment (Fig. 5). Thus, individual imprinted genes may be sensitive to androgen signaling, but androgens do not seem to constitute a major factor affecting their expression in prostate cancer.
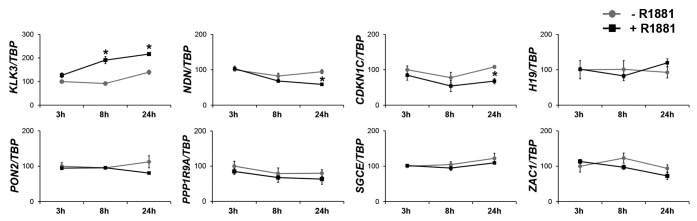
Figure 5. Influence of androgens on imprinted genes expression. Expression of the indicated imprinted genes was measured relative to TBP mRNA by qRT-PCR in LNCaP cells treated for 3, 8 or 24 h with 10 nM synthetic androgen R1881 (black boxes) or with solvent only (gray circles). Treatments were performed in biological duplicates and qRT-PCR was performed in duplicate for each sample, whereby less than 10% variation between duplicates was accepted. The data points with error bars represent mean values from the biological duplicates normalized to the expression of the untreated control at 3 h that was set to 100%. Standard deviation is presented as error bars. *Significant changes (P < 0.05) as calculated by T-Test.
Induction of imprinted genes by ZAC1 overexpression
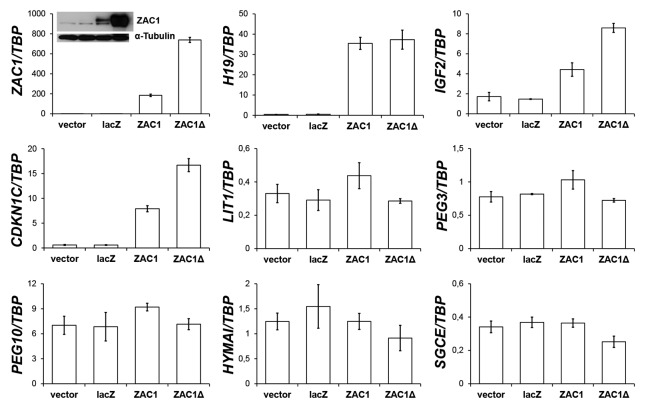
The transcription factor Zac1 encoded by the Plagl1 gene has been described as a strong node in the mouse imprinted gene network.15 Since most of our candidate genes belong to this network and their expression strongly correlated with that of ZAC1, their downregulation could be a consequence of decreased ZAC1 expression. In order to determine whether the transcription factor is able to induce the expression of other imprinted genes in prostate carcinoma cells, we overexpressed human ZAC1 and its shorter protein isoform ZAC1delta in the 22Rv1 prostate carcinoma cell line, which like most other prostate cancer cell lines (except for PC3) expresses low levels of ZAC1 (Fig. S9). Note that expression constructs for ZAC1 and ZAC1delta generated different amounts of ZAC1 mRNA and protein (Fig. 6). As predicted, overexpression of ZAC1 strongly induced expression of H19, CDKN1C and IGF2, but did not significantly affect the expression of PEG10, PEG3, LIT1, SGCE or HYMAI (Fig. 6). Analysis by immunoblotting showed that the product of the CDKN1C gene, p57KIP2 became also strongly induced (not shown).
Figure 6. Induction of imprinted genes by transient ZAC1 overexpression. mRNA expression of the indicated imprinted genes was measured by qRT-PCR in 22Rv1 cells transfected for 48 h with an expression vector containing cDNA encoding the full-length ZAC1 protein (ZAC1) or the shorter ZAC1 protein isoform (ZAC1Δ). As controls, the empty vector (vector) or the same vector containing the lacZ gene (lacZ) were employed. Transfections were performed in duplicates, PCR for each of which was performed in duplicates. The data presented are mean values from the biological duplicates (bars) of the mRNA expression relative to TBP including standard deviation (error bars). Notably, transfection of the short ZAC1Δ form resulted in higher ZAC1 protein amounts than transfection of the full-length ZAC1 form, as assessed with western blot analysis (insert in the first panel). α-Tubulin was detected as a loading control. The protein samples were loaded in the same sequence as shown in the panel
Discussion
Although deregulation of various imprinted genes has been implicated in several cancer types, there is little information on their expression and function in prostate cancer with most available data concerning IGF2. An in silico analysis of published microarray studies suggested that a significant, but limited number of the more than 60 genes considered as imprinted in humans might be deregulated at the level of mRNA expression in prostate cancer.14 The present experimental study suggests that many of the genes spotted by the in silico analysis are indeed deregulated in the expected direction. Notably, the data for five genes were fully consistent between the in silico and experimental analyses, namely PLAGL1/ZAC1, PPP1R9A, CDKN1C, MEG3 and NDN. For three further genes (PEG10, INPP5F and PEG3), qRT-PCR measurements revealed a tendency toward the result expected from the in silico analysis. There was no gene with truly discrepant results. Conversely, the in silico analysis may have missed deregulation of some imprinted genes in prostate cancer. One reason is that not all imprinted genes were well represented on the array platforms (e.g., 10 genes could not be considered because they were represented in only a single microarray study or none at all). A critical point is why the in silico analysis missed IGF2 and H19, which were clearly downregulated in the present qRT-PCR analysis in keeping with other reports.13 We can trace the discrepancy to the heterogeneity of the microarray results, i.e., studies variously reporting up- or downregulation, which prevented the downregulation of these genes to become significant across all studies. Thus, with due caution, an in silico analysis of microarray studies is a suitable tool for identifying a core set of imprinted genes deregulated in a particular cancer.
We have previously noted that most of the significantly deregulated imprinted genes from the in silico analysis belong to an extended transcriptional network of strongly linked imprinted and other genes (Imprinted Gene Network, IGN) identified by bioinformatics meta-analysis of microarray data from multiple mouse tissues.15 It has been hypothesized that any of the identified imprinted gene “hubs” in the IGN may represent the sum of smaller, distinct networks present in different tissues.36 Accordingly, co-expression and functional association of imprinted genes has been found in several different tissues and particular cell populations.16,37-39 Among the potential mechanisms reported so far are direct transcriptional interactions of the transcription factor Zac1 with other imprinted genes, physical association of the Igf2-H19 imprinting control region (ICR) with other imprinted domains and a direct regulatory function of the ncRNA H19.15,16,40
We observed strong correlations between the expression of several IGN genes in prostate cancer. Interestingly, four of the five genes consistently highlighted by the in silico and experimental approach (PLAGL1/ZAC1, CDKN1C, MEG3 and NDN) are located in the same sub-network.15 These four genes are all coordinately downregulated in prostate cancer, whereas the PPP1R9A gene was the only one upregulated, with its expression inversely correlated with that of the others. Since we found PPP1R9A to be biallelically expressed both in benign and cancer prostatic tissues, its overexpression is not due to changes in its imprinting. Nonetheless, the excellent, but inverse correlation of its expression with that of the imprinted genes in the network suggests that it may actually represent another network component. Unfortunately, very little is known about the regulation of this gene yet, but we note that its expression was strongly positively correlated with that of prostatic oncogenes. PPP1R9A encodes a regulatory subunit of protein phosphatase 1, designated neurabin-I for its best characterized function in neurons. It certainly deserves further investigation in prostate cancer. Taken together, we conclude that evidence for an imprinted gene network is also detectable in the human prostate and at least a sub-network is deregulated in prostate cancer.
The ZAC1, CDKN1C, MEG3, and NDN genes undergoing the most pronounced changes in expression are each located on different chromosomes. It is therefore unlikely that their highly coordinated decreased expression is caused by allelic losses, the more so as chromosomes 11, 14 and 15 are rarely subject to deletions in prostate cancer. ZAC1 is located on chromosome 6q which is affected by losses at some frequency, but these losses occur mostly in other segments such as 6q15, where MAP3K7 is a tumor suppressor candidate.41 Accordingly, we observed a few cases with exceptionally low methylation at the ZAC1 DMR, which could reflect allelic loss, but stable methylation in all other samples. Genetic changes are more likely to contribute to altered expression of genes in the 7q21 imprinted gene cluster, as this region is gained or amplified in a moderate fraction of prostate cancers and the long arm of chromosome 7 undergoes complex rearrangements in some cases.42 However, the regular overexpression of PPP1R9A is difficult to explain by chromosomal alterations. Rather, since both PON2 and PPP1R9A, which are biallelically expressed, are upregulated it is likely that the underlying mechanism is independent of imprinting and affects the distant part of the 7q21 imprinted gene cluster that is presumably not imprinted in adult human tissues.
Our working hypothesis had been that changes in the expression of imprinted genes might be a consequence of global epigenetic deregulation during prostate cancer progression. Obviously, this speculation is not substantiated by our data. For one, the combined results of the in silico and qRT-PCR analyses strongly suggest that the changes in expression concern a specific subset of imprinted genes. Moreover, there were only limited changes in DNA methylation at the investigated DMRs or promoter regions that appeared overall unrelated to the changes in expression. Of note, the tissue samples used had been found in several previous studies to display all the alterations of DNA methylation characteristic of prostate carcinoma.43,44 We conclude, therefore, that imprinted gene methylation remains remarkably stable in prostate cancers despite multiple disturbances in epigenetic mechanisms.
A notable exception from this general conclusion concerns the IGF2/H19 locus. We found frequent gain or loss of methylation at the H19 DMR in both benign and cancerous prostatic tissues resulting in approximately three different methylation states (80%, 40%, and 10%). These could be best explained by assuming that the highest level represents paternal-like methylation and the lowest level maternal-like methylation. As chromosomal losses at 11p15.5 are rare in prostate cancer and the changes were also observed in benign tissues, these changes most likely reflect epigenetic alterations, i.e., increased or decreased methylation in one allele, rather than allele losses. A study in bladder cancer also found frequent changes in DNA methylation of the H19 DMR resulting in similar methylation states. Importantly, these did not correlate with the presence of LOI as measured by biallelic expression.45 In prostate carcinoma, Bhusari et al.13 and Paradowska et al.46 have also reported changes in the methylation of the H19/IGF2 DMRs. The former study observed lower methylation of the DMR0 (located in the IGF2 gene) in tumors with lower IGF2 expression. They did not report methylation levels for the H19 DMR in tumors, but showed LOI occurring in benign prostate tissues independent of methylation changes at this site. In accord with our proposition, mean methylation was around 50% in benign tissues. The latter study reported high methylation (>80%) of the H19 DMR in benign prostate hyperplasia and decreases to 41% in some prostate cancers, but gene expression was not studied. Taken together, our study adds further observations indicating complex changes in imprinting, DNA methylation and expression at the IGF2/H19 locus over the course of prostatic carcinogenesis, as in other cancer types.47 Importantly, our data suggest that this region is singularly affected among all imprinted genes implying that the mechanisms responsible for the changes at the IGF2/H19 locus are specific to this region.
Considering the above arguments, the most likely explanation for the overall changes in imprinted gene expression is a coordinate deregulation of sub-nets of the imprinted gene network in prostate cancer. Since this deregulation does not appear to represent a simple consequence of global epigenetic disturbances in prostate cancer, further work will be required to identify the primary cause of this deregulation. In this respect, its close correlation with overexpression of EZH2 and HOXC6 suggests that the deregulation may occur concomitantly with the activation of these oncogenes or represent one facet of their pleiotropic effects. This is plausible, as EZH2 is known to interact with many histone-modifying enzymes, including BMI1 and EED, which are indispensable for the establishment of the chromatin modifications at imprinted loci.48-50 However, only the Igf2 and Peg3 imprinted genes have been unequivocally identified as direct targets of Ezh2 in murine cells,48 and in human studies investigating the global effects of EZH2 knockdown only the expression of CDKN1C,51 TFPI2,51 and SNRPN17 was changed. Instead, EZH2 targets many transcription factors that might mediate its effects indirectly.
Among the imprinted genes we studied, only MEG3 was shown to be a direct target of HOXC6 by means of ChIP-chip analysis20 whereas in knockout and overexpression models, the expression of NDN, PEG10, TFPI2, Snrpn, and Igf2 was changed.20 Both a transcriptional activator and repressor, HOXC6 activates some target genes and represses others. Unfortunately, the mechanism by which it achieves gene repression is unknown.52 Since HOXC6 transcriptionally affects many genes from the WNT, NOTCH and BMP pathways as well as IGF/PI3K signaling (reviewed in ref. 53), imprinted genes like NDN, PEG10, TFPI2, Snrpn and Igf2 regulated by HOXC6, but not bound by the factor are presumably indirectly affected by experimental manipulation of HOXC6. Interestingly, the HOXC8 gene, a direct HOXC6 target,20 is co-induced with HOXC6 in prostate cancer and is a regulator of ZAC1.19,54-56 Thus, HOXC6 may influence ZAC1 expression and thereby the imprinted gene network through HOXC8. HOXC8 is also known to modulate androgen signaling in prostate cancer.57 Although several imprinted genes modulate androgen response,58,59 our data do not suggest that the deregulation of imprinted genes is caused by the distorted androgen response in prostate cancer, as none of the investigated genes appeared to be strongly and significantly regulated by androgens in LNCaP cells.
While the primary cause of the imprinted gene network deregulation remains thus to be identified, our results provide evidence that deregulation might be propagated in parts of the network via the downregulation of the transcription factor ZAC1. In the mouse, Zac1 has been demonstrated to regulate transcription of several imprinted genes directly or indirectly.15 In our study, decreased ZAC1 expression correlated particularly well with that of all imprinted genes undergoing significant changes in expression. Moreover, restoration of ZAC1 in 22Rv1 cells induced several imprinted genes. Particularly strong induction was observed for CDKN1C, an established direct target gene of ZAC1, leading to accumulation of its product, p57KIP2. Interestingly, p57KIP2 has been found to inhibit carcinogenesis in the mouse prostate60 and to regulate senescence in human prostatic cell lines.61 Thus, ZAC1 might be a nodal regulator of the imprinted gene network in human prostatic cells as well.
Finally, our study did not address the function of the imprinted genes deregulated in prostate cancer. In general, imprinted genes regulate various aspects of fetal and trophoblast development, endocrine function, growth, tissue homeostasis, metabolism and postnatal behavior.62
Many of these genes were found to be co-expressed in somatic stem cells.38 It is perhaps not surprising, but could certainly be pertinent that the subset of imprinted genes identified here is involved particularly in the control of growth and apoptosis (reviewed in refs. 14 and 62). For instance, several imprinted genes were reported to be upregulated upon growth arrest in embryonic fibroblasts in a coordinate manner.63 Restoration of the expression of individual genes, e.g., CDKN1C, or restoration of normal regulation, e.g., by ZAC1 or at an upstream regulatory step, might therefore lead to growth arrest in prostate carcinoma cells. These ideas are currently being investigated in our lab.
Materials and Methods
Tissue samples
The set of prostate carcinoma and benign tissues from cancer-carrying prostates was obtained from patients treated at the Dept. of Urology by radical prostatectomy in the period 1997–2002 and has been characterized for multiple clinical and molecular features as described elsewhere.43,64,65 In brief, immediately after surgical removal, prostates were sectioned by an experienced pathologist. Tumor specimens were only collected when tumors were grossly apparent and could be unequivocally identified by their characteristic yellow or orange-yellow color and the matched tumor-free region was macroscopically free of tumor burden. Representative samples of 3 mm maximal diameter of tumor and tumor-free tissue specimens were collected, immediately snap frozen in liquid nitrogen and stored at -80 °C. Macroscopic separation between tumor and non-tumorous tissues was histologically verified by analyzing tissue specimens immediately adjacent to the specimens collected for analysis. Histologic diagnoses and Gleason grading of tumors was performed according to the TNM guidelines of the UICC from 1997. In brief, 20 cancers were staged pT2 and 25 were staged pT3 or pT4. A Gleason score <7 was assigned to 13 tumors, 26 were assigned a Gleason score of 7, and 6 tumors had a score >7. Lymph node metastases were found in 11 cases; distal metastases were detectable in none of the patients at the time of surgery. Eighteen patients of the 45, for whom follow-up data was available, experienced a biochemical recurrence, as defined by a serum PSA level of >0.2 ng/mL in two consecutive measurements. Patient consent was obtained and the study was approved by the Ethics Committee of the Medical Faculty of the Heinrich Heine University.
DNA and RNA extraction
High molecular RNA and DNA was extracted from aliquots of powdered tissues of prostatic benign and cancer tissue specimens as elsewhere described.43
Cell lines and treatment
The prostate carcinoma cell lines LNCaP, 22Rv1 and PC-3 were cultured in RPMI-1640 (Gibco Life Technologies), supplemented with 10% fetal calf serum (FCS) and penicillin (100 U/mL)/streptomycin (100 µg/mL). For investigation of androgen effects, LNCaP cells were treated for 3, 8 or 24 h with or without 10 nM of the synthetic androgen R1881 (Sigma) in RPMI-1640 supplemented with 10% charcoal-stripped (steroid-free) fetal bovine serum (cFBS, Biowest). For the investigation of effects of epigenetic inhibitors, LNCaP, 22Rv1 and PC3 cells were incubated with 2 µM of 5-aza-2'-deoxycytidine (Sigma) for 72 h with daily medium changes or with 5 µM suberoylanilide hydroxamic acid (SAHA, Cayman Chem. Company) for 24 h. The treatments were performed in duplicates.
Transfection experiments
The plasmids pcDNA4/TO, pcDNA4/TO.lacZ, pcDNA4/TO.ZAC1 (isoform 1) or pcDNA4/TO.ZAC1delta were transfected into 22Rv1 cells using X-tremeGENE 9 DNA transfection reagent (Roche) and cells were harvested 48 h later. The transfections were performed in triplicates.
Quantitative RT-PCR
Synthesis of cDNA (cDNA) was performed according to the manufacturer’s protocol using the QuantiTect reverse transcription kit (Qiagen). Real-time RT-PCR for each sample was performed in technical duplicates on an ABI 7900 HT PCR System (Applied Biosystems) using QuantiTect SybrGreen PCR Kit (Qiagen). Experimental variation for the quantity of PCR product among duplicates was accepted when below 10%. The PCR conditions were as follows: polymerase activation at 95° C for 15 min, followed by 35 amplification cycles consisting of denaturation at 94° C for 15 s, annealing at the specific for each primer set temperature for 30 s, and extension at 72° C for 30 s. Primer sequences with the respective annealing temperature and product size are given in Table S1. The expression of ZAC1 transcript forms in tumor cells was assessed with primers ‘ZAC1 F1+R1’ and ‘ZAC1 delta F2+R2’. The NCBI nomenclature of the transcripts amplified with these primer pairs is also given in Table S1. In ZAC1 overexpression experiments, ZAC1 expression was measured with the primer pair ‘ZAC1 F2+R2’ that assesses all ZAC1 transcript variants.
Relative expression was calculated by the standard curve method using a dilution series with cDNA of a cell line or normal tissue which strongly expressed the gene of interest. The mRNA expression was normalized to that of the housekeeping TBP gene for each sample.
Immunoblot analysis
Cells were lysed in RIPA-like buffer containing 5 M NaCl, 1% NP-40, 0.5% DOC, 0.1% SDS, 1 mM EDTA, 50 mM Tris, pH 8.0, and freshly added 10 µL/mL protease inhibitor cocktail (Sigma). Ten μg of protein was resolved in a 12% sodium dodecyl sulfate-PAGE minigel and transferred onto Immobilon-P membrane (Millipore). Membranes were probed with primary antibody against ZAC1 (H-253, #sc22811, Santa Cruz Biotechnology) at 4 °C overnight, washed extensively with 0.1% Tween-20 in Tris-buffered saline and incubated with secondary antibody conjugated with horseradish peroxidase. α-Tubulin was detected as a loading control using a specific antibody (B512, #T5168, Sigma). The signals were visualized with enhanced luminescence (WesternBrightTM Quantum, Advansta).
Bisulfite conversion
Genomic DNA (1 µg) was treated with sodium bisulfite to convert unmethylated cytosines to uracils with the EZ DNA Methylation Gold Kit (Zymo Research) according to the manufacturer’s instructions.
Pyrosequencing analysis
Quantitative measurement of methylation at specific CpG sites in the following regions- ZAC1 DMR, 7q21 DMR1 and DMR2, MEG3 DMR, KvDMR, H19 DMR, NDN DMR and CDKN1C promoter (for details see Table S2) was performed by pyrosequencing on a Biotage PyroMark Q24 instrument using PyroMark Gold Q24 reagents (Qiagen). The data was analyzed with PyroMark Q24 version 2.0 software.
To generate the products to be sequenced, bisulfite-converted DNA was amplified by PCR as follows. Each 50 μl PCR reaction contained 1× Coralload Concentrate (including 1.5 mM MgCl2), 10 μmol dNTPs, 20 pmol of each amplification primer (one of which was biotinylated) (for primer details see Table S2), 4 U HotStarTaq DNA Polymerase, and 2.5 μl bisulfite-converted DNA. Thermocycling conditions included: initial denaturation at 95 °C for 15 min, 45 cycles of: denaturation at 95° C for 30 s, annealing at the specific temperature for each primer set for 30 s, extension at 72° C for 30 s; and a final extension at 72° C for 10 min. After confirmation of product size on a 2%-agarose gel, PCR products were prepared for pyrosequencing.
The data was analyzed and the average methylation for each CpG position or for all assessed CpG sites among all samples in the benign or tumor tissue groups was used for quantitation.
Analysis for heterozygosity
Heterozygosity at the SNP rs3743340 in the NDN gene promoter and at the SNP rs854544 in the coding sequence of PPP1R9A was determined by pyrosequencing analysis of PCR products, as described above (for details see Table S2), using DNA from all benign and tumor prostatic tissues as template. To assess monoallelic vs. biallelic expression of PPP1R9A the same assay was applied to cDNA of all confirmed heterozygotic samples.
Bisulfite sequencing
Following conformation of heterozygosity at the SNP rs3743340 in the NDN gene (see above), PCR products of the NDN DMR region (conditions as used for methylation analysis) using bisulfite converted DNA from three tumor samples were cloned into the pCR4.Topo vector for sequencing (Invitrogen) and transformed into competent ONE Shot E. coli cells (Invitrogen). Individual plasmids were isolated from ampicillin-resistant bacterial clones and single alleles were sequenced by standard techniques.
Statistics
Spearman’s rank correlation coefficient (ρ) was used to estimate the dependence among the expression of the analyzed imprinted genes or between each imprinted gene and the EZH2, HOXC6 and ERG oncogenes. Furthermore, it was applied to study the correlation between the expression of the assessed genes and the methylation levels of the corresponding DMRs in the prostatic tumor tissues.
Mann-Whitney-U test was used to analyze gene expression in the tissue samples grouped according to the following criteria: benign vs. tumor, T-stage: pT2 vs. pT3+pT4, Gleason sum: GS < 7 vs. 7 and 7 vs. >7, local lymph node metastasis: no vs. yes, biochemical recurrence: no vs. yes, expression of each EZH2, ERG, and HOXC6 genes: “low” vs. “high.” The stratification into ‘low’ and ‘high’ oncogene expression groups was performed according to the expression cut-off values corresponding to: for EZH2 gene, the maximum expression of benign tissues; for ERG, the median expression of the tumor tissues; and for HOXC6, the first quartile of the expression of tumor tissues. ZAC1 expression groups were defined as below vs. above the median. In an analogous fashion, such groups were created for all other imprinted genes.
H19 DMR methylation groups used for visualization were defined by <30% vs. 35–45% vs. >80% methylation and were not used for statistical tests due to small size of groups. The sizes of all compared groups are detailed in Table S3. All statistical analyses were conducted with IBM SPSS 20 software.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Prof Rainer Engers for tissue selection, to Dr Christian Arsov for advice on acquiring the follow-up data and to Christiane Hader for tissue extractions. This study was financially supported by the Wilhelm-Sander-Foundation.
Glossary
Abbreviations:
- 5-aza
5-aza-2'-deoxycytidine
- CpG
cytosine-phosphate-guanosine
- DMR
differentially methylated region
- GS
Gleason sum/score
- H3K27me3
Histone 3, lysine 27 trimethylated, ICR, imprinting control region
- IGN
imprinted gene network
- LOI
loss of imprinting
- ncRNA
non-coding RNA
- PRC2
Polycomb Repressive Complex 2
- PSA
prostate specific antigen
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- SAHA
suberoylanilide hydroxamic acid
- TNM
Tumor Node Metastasis
- UICC
Union for International Cancer Control
References
- 1.Arima T, Kamikihara T, Hayashida T, Kato K, Inoue T, Shirayoshi Y, Oshimura M, Soejima H, Mukai T, Wake N. ZAC, LIT1 (KCNQ1OT1) and p57KIP2 (CDKN1C) are in an imprinted gene network that may play a role in Beckwith-Wiedemann syndrome. Nucleic Acids Res. 2005;33:2650–60. doi: 10.1093/nar/gki555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dekel B, Metsuyanim S, Schmidt-Ott KM, Fridman E, Jacob-Hirsch J, Simon A, Pinthus J, Mor Y, Barasch J, Amariglio N, et al. Multiple imprinted and stemness genes provide a link between normal and tumor progenitor cells of the developing human kidney. Cancer Res. 2006;66:6040–9. doi: 10.1158/0008-5472.CAN-05-4528. [DOI] [PubMed] [Google Scholar]
- 3.Jacob KJ, Robinson WP, Lefebvre L. Beckwith-Wiedemann and Silver-Russell syndromes: opposite developmental imbalances in imprinted regulators of placental function and embryonic growth. Clin Genet. 2013;84:326–34. doi: 10.1111/cge.12143. [DOI] [PubMed] [Google Scholar]
- 4.Jelinic P, Shaw P. Loss of imprinting and cancer. J Pathol. 2007;211:261–8. doi: 10.1002/path.2116. [DOI] [PubMed] [Google Scholar]
- 5.Uribe-Lewis S, Woodfine K, Stojic L, Murrell A. Molecular mechanisms of genomic imprinting and clinical implications for cancer. Expert Rev Mol Med. 2011;13:e2. doi: 10.1017/S1462399410001717. [DOI] [PubMed] [Google Scholar]
- 6.Murrell A. Genomic imprinting and cancer: from primordial germ cells to somatic cells. ScientificWorldJournal. 2006;6:1888–910. doi: 10.1100/tsw.2006.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuo QS, Yan R, Feng DX, Zhao R, Chen C, Jiang YM, Cruz-Correa M, Casson AG, Kang XD, Han F, et al. Loss of imprinting and abnormal expression of the insulin-like growth factor 2 gene in gastric cancer. Mol Carcinog. 2011;50:390–6. doi: 10.1002/mc.20731. [DOI] [PubMed] [Google Scholar]
- 8.Ito Y, Koessler T, Ibrahim AE, Rai S, Vowler SL, Abu-Amero S, Silva AL, Maia AT, Huddleston JE, Uribe-Lewis S, et al. Somatically acquired hypomethylation of IGF2 in breast and colorectal cancer. Hum Mol Genet. 2008;17:2633–43. doi: 10.1093/hmg/ddn163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui H. Loss of imprinting of IGF2 as an epigenetic marker for the risk of human cancer. Dis Markers. 2007;23:105–12. doi: 10.1155/2007/363464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferguson-Smith AC. Genomic imprinting: the emergence of an epigenetic paradigm. Nat Rev Genet. 2011;12:565–75. doi: 10.1038/nrg3032. [DOI] [PubMed] [Google Scholar]
- 11.Jerónimo C, Bastian PJ, Bjartell A, Carbone GM, Catto JW, Clark SJ, Henrique R, Nelson WG, Shariat SF. Epigenetics in prostate cancer: biologic and clinical relevance. Eur Urol. 2011;60:753–66. doi: 10.1016/j.eururo.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 12.Nelson WG, Yegnasubramanian S, Agoston AT, Bastian PJ, Lee BH, Nakayama M, De Marzo AM. Abnormal DNA methylation, epigenetics, and prostate cancer. Front Biosci. 2007;12:4254–66. doi: 10.2741/2385. [DOI] [PubMed] [Google Scholar]
- 13.Bhusari S, Yang B, Kueck J, Huang W, Jarrard DF. Insulin-like growth factor-2 (IGF2) loss of imprinting marks a field defect within human prostates containing cancer. Prostate. 2011;71:1621–30. doi: 10.1002/pros.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribarska T, Bastian KM, Koch A, Schulz WA. Specific changes in the expression of imprinted genes in prostate cancer--implications for cancer progression and epigenetic regulation. Asian J Androl. 2012;14:436–50. doi: 10.1038/aja.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varrault A, Gueydan C, Delalbre A, Bellmann A, Houssami S, Aknin C, Severac D, Chotard L, Kahli M, Le Digarcher A, et al. Zac1 regulates an imprinted gene network critically involved in the control of embryonic growth. Dev Cell. 2006;11:711–22. doi: 10.1016/j.devcel.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Gabory A, Ripoche MA, Le Digarcher A, Watrin F, Ziyyat A, Forné T, Jammes H, Ainscough JF, Surani MA, Journot L, et al. H19 acts as a trans regulator of the imprinted gene network controlling growth in mice. Development. 2009;136:3413–21. doi: 10.1242/dev.036061. [DOI] [PubMed] [Google Scholar]
- 17.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–9. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 18.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–8. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 19.Miller GJ, Miller HL, van Bokhoven A, Lambert JR, Werahera PN, Schirripa O, Lucia MS, Nordeen SK. Aberrant HOXC expression accompanies the malignant phenotype in human prostate. Cancer Res. 2003;63:5879–88. [PubMed] [Google Scholar]
- 20.McCabe CD, Spyropoulos DD, Martin D, Moreno CS. Genome-wide analysis of the homeobox C6 transcriptional network in prostate cancer. Cancer Res. 2008;68:1988–96. doi: 10.1158/0008-5472.CAN-07-5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu K, Wu ZJ, Groner AC, He HH, Cai C, Lis RT, Wu X, Stack EC, Loda M, Liu T, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science. 2012;338:1465–9. doi: 10.1126/science.1227604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribarska T, Ingenwerth M, Goering W, Engers R, Schulz WA. Epigenetic inactivation of the placentally imprinted tumor suppressor gene TFPI2 in prostate carcinoma. Cancer Genomics Proteomics. 2010;7:51–60. [PubMed] [Google Scholar]
- 24.Choi JD, Underkoffler LA, Wood AJ, Collins JN, Williams PT, Golden JA, Schuster EF, Jr., Loomes KM, Oakey RJ. A novel variant of Inpp5f is imprinted in brain, and its expression is correlated with differential methylation of an internal CpG island. Mol Cell Biol. 2005;25:5514–22. doi: 10.1128/MCB.25.13.5514-5522.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt U, Fuessel S, Koch R, Baretton GB, Lohse A, Tomasetti S, Unversucht S, Froehner M, Wirth MP, Meye A. Quantitative multi-gene expression profiling of primary prostate cancer. Prostate. 2006;66:1521–34. doi: 10.1002/pros.20490. [DOI] [PubMed] [Google Scholar]
- 26.Wilkin F, Paquette J, Ledru E, Hamelin C, Pollak M, Deal CL. H19 sense and antisense transgenes modify insulin-like growth factor-II mRNA levels. Eur J Biochem. 2000;267:4020–7. doi: 10.1046/j.1432-1327.2000.01438.x. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann MJ, Florl AR, Seifert HH, Schulz WA. Multiple mechanisms downregulate CDKN1C in human bladder cancer. Int J Cancer. 2005;114:406–13. doi: 10.1002/ijc.20749. [DOI] [PubMed] [Google Scholar]
- 28.Fitzpatrick GV, Soloway PD, Higgins MJ. Regional loss of imprinting and growth deficiency in mice with a targeted deletion of KvDMR1. Nat Genet. 2002;32:426–31. doi: 10.1038/ng988. [DOI] [PubMed] [Google Scholar]
- 29.Vinarskaja A, Yamanaka M, Ingenwerth M, Schulz WA. DNA Methylation and the HOXC6 Paradox in Prostate Cancer. Cancers (Basel) 2011;3:3714–25. doi: 10.3390/cancers3043714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bourque DK, Avila L, Peñaherrera M, von Dadelszen P, Robinson WP. Decreased placental methylation at the H19/IGF2 imprinting control region is associated with normotensive intrauterine growth restriction but not preeclampsia. Placenta. 2010;31:197–202. doi: 10.1016/j.placenta.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Pateras IS, Apostolopoulou K, Koutsami M, Evangelou K, Tsantoulis P, Liloglou T, Nikolaidis G, Sigala F, Kittas C, Field JK, et al. Downregulation of the KIP family members p27(KIP1) and p57(KIP2) by SKP2 and the role of methylation in p57(KIP2) inactivation in nonsmall cell lung cancer. Int J Cancer. 2006;119:2546–56. doi: 10.1002/ijc.22214. [DOI] [PubMed] [Google Scholar]
- 32.Monk D, Wagschal A, Arnaud P, Müller PS, Parker-Katiraee L, Bourc’his D, Scherer SW, Feil R, Stanier P, Moore GE. Comparative analysis of human chromosome 7q21 and mouse proximal chromosome 6 reveals a placental-specific imprinted gene, TFPI2/Tfpi2, which requires EHMT2 and EED for allelic-silencing. Genome Res. 2008;18:1270–81. doi: 10.1101/gr.077115.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakabayashi K, Makino S, Minagawa S, Smith AC, Bamforth JS, Stanier P, Preece M, Parker-Katiraee L, Paton T, Oshimura M, et al. Genomic imprinting of PPP1R9A encoding neurabin I in skeletal muscle and extra-embryonic tissues. J Med Genet. 2004;41:601–8. doi: 10.1136/jmg.2003.014142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffmann MJ, Engers R, Florl AR, Otte AP, Muller M, Schulz WA. Expression changes in EZH2, but not in BMI-1, SIRT1, DNMT1 or DNMT3B are associated with DNA methylation changes in prostate cancer. Cancer Biol Ther. 2007;6:1403–12. doi: 10.4161/cbt.6.9.4542. [DOI] [PubMed] [Google Scholar]
- 35.Schulz WA, Ingenwerth M, Djuidje CE, Hader C, Rahnenführer J, Engers R. Changes in cortical cytoskeletal and extracellular matrix gene expression in prostate cancer are related to oncogenic ERG deregulation. BMC Cancer. 2010;10:505. doi: 10.1186/1471-2407-10-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smits G, Kelsey G. Imprinting weaves its web. Dev Cell. 2006;11:598–9. doi: 10.1016/j.devcel.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Sandhu KS, Shi C, Sjölinder M, Zhao Z, Göndör A, Liu L, Tiwari VK, Guibert S, Emilsson L, Imreh MP, et al. Nonallelic transvection of multiple imprinted loci is organized by the H19 imprinting control region during germline development. Genes Dev. 2009;23:2598–603. doi: 10.1101/gad.552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berg JS, Lin KK, Sonnet C, Boles NC, Weksberg DC, Nguyen H, Holt LJ, Rickwood D, Daly RJ, Goodell MA. Imprinted genes that regulate early mammalian growth are coexpressed in somatic stem cells. PLoS One. 2011;6:e26410. doi: 10.1371/journal.pone.0026410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lui JC, Finkielstain GP, Barnes KM, Baron J. An imprinted gene network that controls mammalian somatic growth is down-regulated during postnatal growth deceleration in multiple organs. Am J Physiol Regul Integr Comp Physiol. 2008;295:R189–96. doi: 10.1152/ajpregu.00182.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Z, Tavoosidana G, Sjölinder M, Göndör A, Mariano P, Wang S, Kanduri C, Lezcano M, Sandhu KS, Singh U, et al. Circular chromosome conformation capture (4C) uncovers extensive networks of epigenetically regulated intra- and interchromosomal interactions. Nat Genet. 2006;38:1341–7. doi: 10.1038/ng1891. [DOI] [PubMed] [Google Scholar]
- 41.Liu W, Chang BL, Cramer S, Koty PP, Li T, Sun J, Turner AR, Von Kap-Herr C, Bobby P, Rao J, et al. Deletion of a small consensus region at 6q15, including the MAP3K7 gene, is significantly associated with high-grade prostate cancers. Clin Cancer Res. 2007;13:5028–33. doi: 10.1158/1078-0432.CCR-07-0300. [DOI] [PubMed] [Google Scholar]
- 42.Bachmann N, Haeusler J, Luedeke M, Kuefer R, Perner S, Assum G, Paiss T, Hoegel J, Vogel W, Maier C. Expression changes of CAV1 and EZH2, located on 7q31 approximately q36, are rarely related to genomic alterations in primary prostate carcinoma. Cancer Genet Cytogenet. 2008;182:103–10. doi: 10.1016/j.cancergencyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Florl AR, Steinhoff C, Müller M, Seifert HH, Hader C, Engers R, Ackermann R, Schulz WA. Coordinate hypermethylation at specific genes in prostate carcinoma precedes LINE-1 hypomethylation. Br J Cancer. 2004;91:985–94. doi: 10.1038/sj.bjc.6602030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulz WA, Alexa A, Jung V, Hader C, Hoffmann MJ, Yamanaka M, Fritzsche S, Wlazlinski A, Müller M, Lengauer T, et al. Factor interaction analysis for chromosome 8 and DNA methylation alterations highlights innate immune response suppression and cytoskeletal changes in prostate cancer. Mol Cancer. 2007;6:14. doi: 10.1186/1476-4598-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Byun HM, Wong HL, Birnstein EA, Wolff EM, Liang G, Yang AS. Examination of IGF2 and H19 loss of imprinting in bladder cancer. Cancer Res. 2007;67:10753–8. doi: 10.1158/0008-5472.CAN-07-0329. [DOI] [PubMed] [Google Scholar]
- 46.Paradowska A, Fenic I, Konrad L, Sturm K, Wagenlehner F, Weidner W, Steger K. Aberrant epigenetic modifications in the CTCF binding domain of the IGF2/H19 gene in prostate cancer compared with benign prostate hyperplasia. Int J Oncol. 2009;35:87–96. doi: 10.3892/ijo_00000316. [DOI] [PubMed] [Google Scholar]
- 47.Leick MB, Shoff CJ, Wang EC, Congress JL, Gallicano GI. Loss of imprinting of IGF2 and the epigenetic progenitor model of cancer. Am J Stem Cells. 2012;1:59–74. [PMC free article] [PubMed] [Google Scholar]
- 48.Sher F, Boddeke E, Olah M, Copray S. Dynamic changes in Ezh2 gene occupancy underlie its involvement in neural stem cell self-renewal and differentiation towards oligodendrocytes. PLoS One. 2012;7:e40399. doi: 10.1371/journal.pone.0040399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu HA, Bernstein E. Partners in imprinting: noncoding RNA and polycomb group proteins. Dev Cell. 2008;15:637–8. doi: 10.1016/j.devcel.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 50.Zacharek SJ, Fillmore CM, Lau AN, Gludish DW, Chou A, Ho JW, Zamponi R, Gazit R, Bock C, Jäger N, et al. Lung stem cell self-renewal relies on BMI1-dependent control of expression at imprinted loci. Cell Stem Cell. 2011;9:272–81. doi: 10.1016/j.stem.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 2006;20:1123–36. doi: 10.1101/gad.381706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramachandran S, Liu P, Young AN, Yin-Goen Q, Lim SD, Laycock N, Amin MB, Carney JK, Marshall FF, Petros JA, et al. Loss of HOXC6 expression induces apoptosis in prostate cancer cells. Oncogene. 2005;24:188–98. doi: 10.1038/sj.onc.1207906. [DOI] [PubMed] [Google Scholar]
- 53.Moreno CS. The Sex-determining region Y-box 4 and homeobox C6 transcriptional networks in prostate cancer progression: crosstalk with the Wnt, Notch, and PI3K pathways. Am J Pathol. 2010;176:518–27. doi: 10.2353/ajpath.2010.090657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lei H, Juan AH, Kim MS, Ruddle FH. Identification of a Hoxc8-regulated transcriptional network in mouse embryo fibroblast cells. Proc Natl Acad Sci U S A. 2006;103:10305–9. doi: 10.1073/pnas.0603552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lei H, Wang H, Juan AH, Ruddle FH. The identification of Hoxc8 target genes. Proc Natl Acad Sci U S A. 2005;102:2420–4. doi: 10.1073/pnas.0409700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waltregny D, Alami Y, Clausse N, de Leval J, Castronovo V. Overexpression of the homeobox gene HOXC8 in human prostate cancer correlates with loss of tumor differentiation. Prostate. 2002;50:162–9. doi: 10.1002/pros.10045. [DOI] [PubMed] [Google Scholar]
- 57.Axlund SD, Lambert JR, Nordeen SK. HOXC8 inhibits androgen receptor signaling in human prostate cancer cells by inhibiting SRC-3 recruitment to direct androgen target genes. Mol Cancer Res. 2010;8:1643–55. doi: 10.1158/1541-7786.MCR-10-0111. [DOI] [PubMed] [Google Scholar]
- 58.Ulrix W, Swinnen JV, Heyns W, Verhoeven G. Androgens down-regulate the expression of the human homologue of paternally expressed gene-3 in the prostatic adenocarcinoma cell line LNCaP. Mol Cell Endocrinol. 1999;155:69–76. doi: 10.1016/S0303-7207(99)00113-6. [DOI] [PubMed] [Google Scholar]
- 59.Gonit M, Zhang J, Salazar Md, Cui H, Shatnawi A, Trumbly R, Ratnam M. Hormone depletion-insensitivity of prostate cancer cells is supported by the AR without binding to classical response elements. Mol Endocrinol. 2011;25:621–34. doi: 10.1210/me.2010-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin RJ, Lho Y, Wang Y, Ao M, Revelo MP, Hayward SW, Wills ML, Logan SK, Zhang P, Matusik RJ. Down-regulation of p57Kip2 induces prostate cancer in the mouse. Cancer Res. 2008;68:3601–8. doi: 10.1158/0008-5472.CAN-08-0073. [DOI] [PubMed] [Google Scholar]
- 61.Schwarze SR, Shi Y, Fu VX, Watson PA, Jarrard DF. Role of cyclin-dependent kinase inhibitors in the growth arrest at senescence in human prostate epithelial and uroepithelial cells. Oncogene. 2001;20:8184–92. doi: 10.1038/sj.onc.1205049. [DOI] [PubMed] [Google Scholar]
- 62.Charalambous M, da Rocha ST, Ferguson-Smith AC. Genomic imprinting, growth control and the allocation of nutritional resources: consequences for postnatal life. Curr Opin Endocrinol Diabetes Obes. 2007;14:3–12. doi: 10.1097/MED.0b013e328013daa2. [DOI] [PubMed] [Google Scholar]
- 63.Hayashida T, Eversole-Cire P, Jones PA, Sasaki H. Imprinted genes are up-regulated by growth arrest in embryonic fibroblasts. J Biochem. 1997;122:901–3. doi: 10.1093/oxfordjournals.jbchem.a021850. [DOI] [PubMed] [Google Scholar]
- 64.Hornstein M, Hoffmann MJ, Alexa A, Yamanaka M, Müller M, Jung V, Rahnenführer J, Schulz WA. Protein phosphatase and TRAIL receptor genes as new candidate tumor genes on chromosome 8p in prostate cancer. Cancer Genomics Proteomics. 2008;5:123–36. [PubMed] [Google Scholar]
- 65.Schulz WA, Elo JP, Florl AR, Pennanen S, Santourlidis S, Engers R, Buchardt M, Seifert HH, Visakorpi T. Genomewide DNA hypomethylation is associated with alterations on chromosome 8 in prostate carcinoma. Genes Chromosomes Cancer. 2002;35:58–65. doi: 10.1002/gcc.10092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.