Abstract
We sought to determine whether a low fermentable substrate diet (LFSD) decreases abdominal pain frequency in children with irritable bowel syndrome (IBS) and to identify potential microbial factors related to diet efficacy. Pain symptoms, stooling characteristics, breath hydrogen and methane, whole intestinal transit time, stool microbiome, and metabolite composition were collected and/or documented in eight children with IBS at baseline and during one week of an LFSD intervention. Pain frequency (P < 0.05), pain severity (P < 0.05), and pain-related interference with activities (P < 0.05) decreased in the subjects while on the LFSD. Responders vs. non-responders: four children (50%) were identified as responders (>50% decrease in abdominal pain frequency while on the LFSD). There were no differences between responders and non-responders with respect to hydrogen production, methane production, stooling characteristics, or gut transit time. Responders were characterized by increased pre-LFSD abundance of bacterial taxa belonging to the genera Sporobacter (P < 0.05) and Subdoligranulum (P < 0.02) and decreased abundance of taxa belonging to Bacteroides (P < 0.05) relative to non-responders. In parallel, stool metabolites differed between responders and non-responders and were associated with differences in microbiome composition. These pilot study results suggest that an LFSD may be effective in decreasing GI symptoms in children with IBS. Microbial factors such as gut microbiome composition and stool metabolites while on the diet may relate to LFSD efficacy.
Keywords: FODMAPs, abdominal pain, irritable bowel syndrome, metabolites, microbiome, pediatric
Introduction
Childhood irritable bowel syndrome (IBS) is a common functional gastrointestinal (GI) disorder, affecting up to 19% of school-aged children.1 As in adults, childhood IBS is believed to be multifactorial, with different factors (e.g., diet, visceral hyperalgesia, and gut microbiome composition) potentially playing more of a role in one individual with IBS as compared with another.2 We recently reported that as a group, children with IBS have a different gut microbiome composition as compared with healthy controls.3 Whether these differences impact the success of treatments or interventions is unknown.
In adults with IBS, multisubstrate carbohydrate elimination diets, which are believed to decrease gut microbial fermentation, have demonstrated efficacy in reducing GI symptoms.4-6 These include limiting overall carbohydrate intake and/or limiting intake of specific carbohydrates believed to be poorly digested, fermentable, and osmotic (low fermentable substrate diet, LFSD).4-6 Proposed mechanisms for efficacy have included decreased microbial hydrogen production7 and decreased stool output, resulting in improved stool form primarily in adults with IBS-diarrhea predominant.8 However, not all those given an LFSD benefit. To date, identifying the subpopulation in which these dietary interventions will be most successful has not been accomplished.9
To our knowledge, a comprehensive LFSD has not been formally investigated in children with IBS. We hypothesized that, similar to adults with IBS, an LFSD would be effective in children. Therefore, we undertook a pilot study to evaluate the potential efficacy of such a diet in reducing abdominal pain frequency in children with IBS. In addition, we sought to examine microbial factors that might be related to whether the diet improves IBS symptoms and thereby gain insight into potential baseline factors that may help predict a successful response to an LFSD.
Results
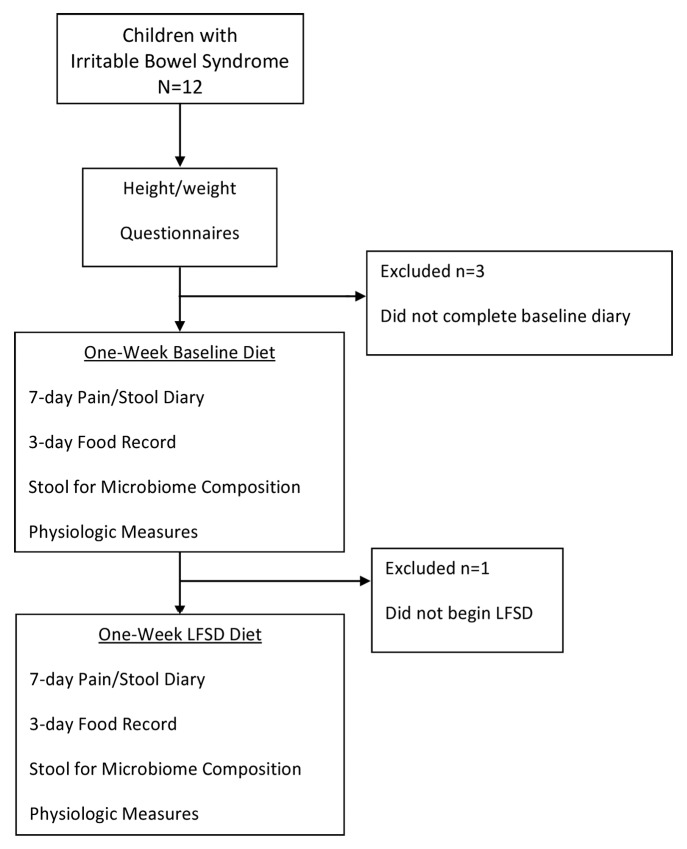
Twelve children with pediatric Rome III-defined IBS were enrolled in a pilot study, of whom eight completed the study (Fig. 1). No adverse events were encountered during the study. Mean age in those completing the trial was 10.9 ± 3.6 y (range 7–16). There were 4 boys and 4 girls. Race and ethnicity were as follows: 6 Caucasians, 1 Asian, and 1 Hispanic. Mean BMI% was 50.0 ± 30.6. Based on stool form classifications, six of the children were IBS-constipation predominant (IBS-C), one was IBS-diarrhea predominant (IBS-D), and one was IBS-mixed type (IBS-M). Of the four responders, three had IBS-C, and one IBS-D. Four of eight subjects were classified as responders to the diet. Responders and non-responders did not differ with respect to age, height, weight, BMI, sex, or race and/or ethnicity (data not shown).
Figure 1. Trial flow sheet. LFSD, low fermentable substrate diet.
Several dietary changes occurred during the LFSD, including a decrease in the number of highly fermentable substrate foods eaten per day (Table S1). However, we did not identify differences in dietary intake between responders and non-responders, either at baseline or during the LSFD (Table S2).
Pain symptoms
In the total population of those who completed the trial, the number of abdominal pain episodes, mean and maximum pain severity, and pain limiting activities decreased significantly on the LFSD (Table 1). Pain frequency did not differ between responders and non-responders during the baseline period (11.2 ± 6.7 episodes vs. 11.8 ± 7.0, P = 0.9). During the LFSD period, responders had a lower pain frequency than non-responders, but the difference was not statistically significant (2.5 ± 3.3 episodes vs. 10.0 ± 7.6, P = 0.12).
Table 1. Pain characteristics and stooling frequency during baseline and low fermentable substrate diet (LFSD) periods.
| Symptom | Baseline | LFSD | P value |
|---|---|---|---|
| Pain episodes/week | 11.5 ± 6.3* | 6.3 ± 6.8 | 0.02 |
| Mean pain throughout the week | 1.8 ± 1.1 | 0.8 ± 0.7 | 0.02 |
| Maximum pain severity of worst episode | 5.8 ± 2.5 | 3.9 ± 2.9 | 0.04 |
| Number of pain episodes interfering with activity | 9.9 ± 7.9 | 6.3 ± 7.2 | 0.05 |
| Number of bowel movements | 5.8 ± 2.6 | 3.5 ± 2.4 | 0.11 |
Mean ± SD
Stooling characteristics and transit time
In the entire cohort, there was an overall trend toward having fewer bowel movements without a change in mean stool form while on the LFSD (Table 1). We did not detect statistically significant differences between responders and non-responders with respect to the frequency of bowel movements/week at baseline (5.75 ± 2.2 vs. 5.75 ± 3.3, P = 0.9) or during the LFSD (2.75 ± 2.2 vs. 4.25 ± 2.6, P = 0.7).
Whole intestinal transit time in hours (43.1 ± 29.2 vs. 48.0 ± 31.8) did not change significantly between baseline and the LFSD when examined in the entire cohort. Similarly, we did not identify significant differences in whole intestinal transit time between responders and non-responders at baseline (37.3 ± 28.1 vs. 47.6 ± 33.5 h, P = 0.69) or during the LFSD (53.0 ± 35.6 vs. 44.2 ± 33.6 h, P = 0.63).
Hydrogen and methane production
We did not detect differences in hydrogen production (area under the curve) in the overall cohort between the baseline and LFSD periods (16572 ± 7929 vs. 17943 ± 6561 ppm*min, respectively). At baseline there was no difference between responders and non-responders (18997 ± 9627 vs. 14146 ± 6190 ppm*min, respectively) in hydrogen production. During the LFSD, responders produced less hydrogen than did non-responders (14462 ± 6980 vs. 21424 ± 4406 ppm*min, respectively, P = 0.14), but this did not reach statistical significance.
In the overall cohort there was a numerical but non-significant decrease in methane production (area under the curve) between baseline and LFSD periods (4761 ± 10479 ppm*min. vs. 2701 ± 3443, respectively). We did not identify differences between responders and non-responders in methane production at baseline (7933 ± 15119 vs. 1589 ± 897 ppm*min, respectively) or during the LFSD (4052 ± 4633 vs. 1351 ± 1154 ppm*min, respectively).
Microbial communities and associated metabolites
Stool bacterial community richness is defined as the number of unique operational taxonomic units (OTUs) detected per sample. Diversity is defined as the distribution of these species among the community. Richness varied, with samples containing between 99 and 169 OTUs. Baseline and LFSD samples did not differ in the average number of OTUs (Table S2). Neither OTU richness nor related α-diversity metrics (e.g., Shannon H' and Simpson 1/D) differed from baseline to LFSD periods (Table S2).
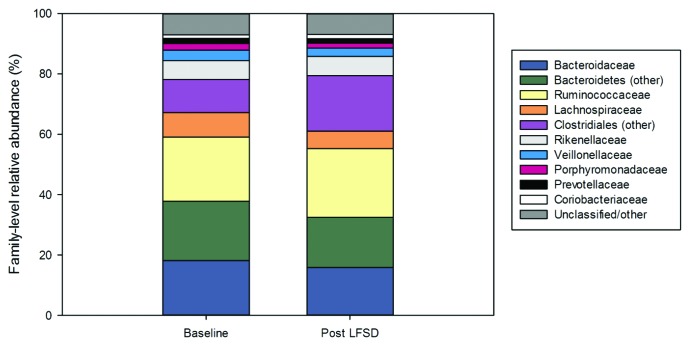
Baseline stool communities were composed, in large part, of taxa belonging to the Bacteroidaceae, Ruminococcaceae, Lachnospiraceae, and related orders. Within the entire cohort, the distribution of these taxa did not change significantly after exposure to the LFSD; however, overall trends toward increased abundances of members of the Clostridiales and decreased abundance of Bacteroidetes were observed following the LFSD intervention (Fig. 2).
Figure 2. The gut microbiome (family-level) at baseline and during the low fermentable substrate diet by relative abundance. LFSD, low fermentable substrate diet.
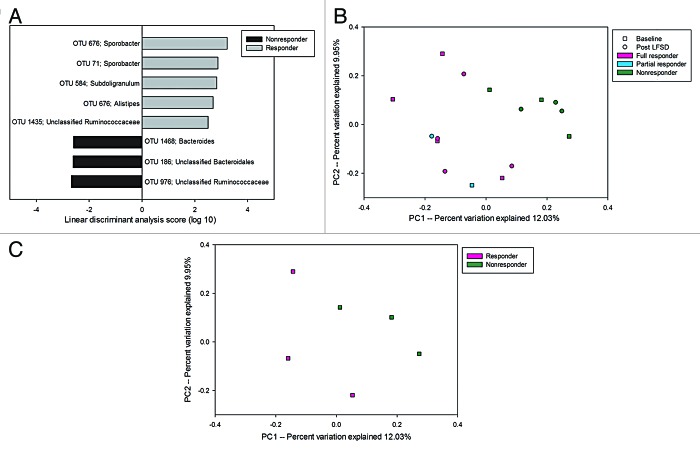
In contrast to the comparison of baseline vs. LFSD periods for the entire cohort, there was significantly greater OTU richness and diversity at baseline within the stool communities of responders compared with non-responders (Table S3). Among responders, LEfSe analysis identified significantly increased abundances of OTUs belonging to the family Ruminococcaceae (Fig. 3A). Specifically, these included members of the genera Sporobacter and Subdoligranulum. Non-responders were characterized by significantly increased abundance of two OTUs belonging to the class Bacteroidales and an OTU representing members of the family Ruminococcaceae (Fig. 3A). Furthermore, the baseline microbiomes of responders and non-responders were fairly well separated by OTU composition as seen on principal components analysis (Fig. 3B). This was even more striking when using only those children with IBS-C (Fig. 3C). Responder microbial communities were enriched in OTUs resembling Phascolarctobacterium faecium when we limited our analysis to IBS-C subjects only.
Figure 3. (A) LEfSe analysis differences of baseline microbial composition between responders and non-responders at the OTU level. Positive linear discriminant analysis (LDA) scores represent OTUs that were enriched in responders, while negative LDA scores indicate OTUs that were enriched in non-responders. (B) Microbiomes of children with IBS who are responders segregate from that of non-responders to a low fermentable substrate diet. A partial responder (reduction in pain frequency >30% but not 50%) is identified. (C) Responder vs. non-responder segregation is particularly seen when only comparing those with IBS with constipation (IBS-C) predominance.
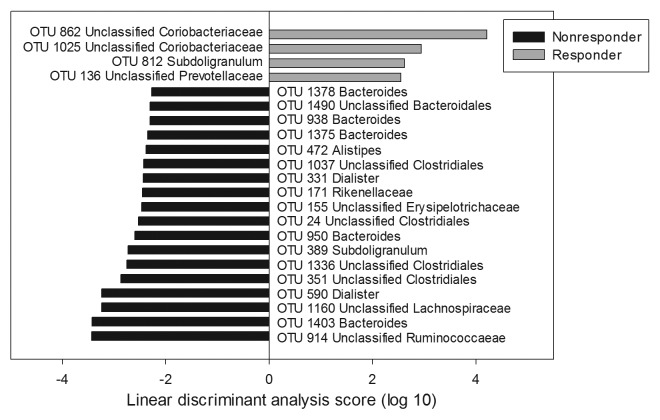
Differences between responders and non-responders in community composition were also detected following the LFSD intervention (Fig. 4). The responder community was found to be enriched in OTUs resembling Subdoligranulum, members of the Prevotellaceae, and unclassified Coriobacteriaceae. Notably, it was also depleted in Dialister, a genus belonging to the family Veillonellaceae. In contrast, the non-responder community was enriched with an Acetivibrio cellulolyticus-like OTU (OTU 914, unclassified Ruminococcaceae) and multiple Bacteroides- and Dialister-like OTUs. On average, Bacteroides accounted for 21% of the non-responder communities, whereas Bacteroides only accounted for 10% of the sequences recovered from the responder communities. Likewise, at the genus-level, Dialister accounted for nearly 5% of non-responder communities, but only 0.15% of the sequences recovered from responders.
Figure 4. LEfSe analysis of differences in microbial composition during the low fermentable substrate diet between responders and non-responders at the OTU level. Positive linear discriminant analysis (LDA) scores represent OTUs that were enriched in responders, while negative LDA scores indicate OTUs that were enriched in non-responders.
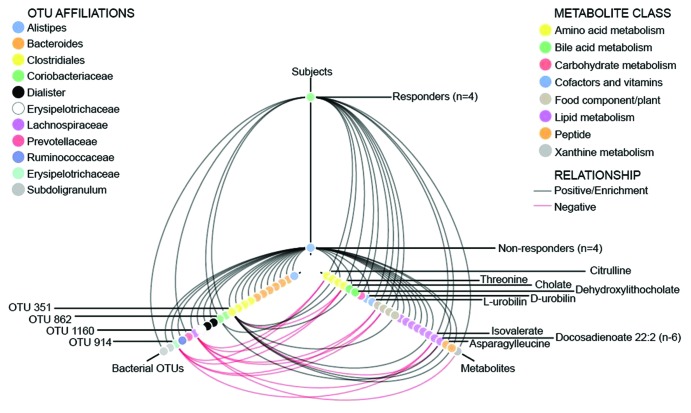
To identify microbial-metabolite changes associated with responders to the LFSD, a tripartite network analysis was performed to identify fecal metabolites that correlated with microbiome differences between responders and non-responders (Fig. 5). Ten fecal metabolites (Table S3) differed during the LFSD vs. baseline when reviewing the entire cohort. At baseline, 22 fecal metabolites differed at baseline between responders and non-responders (Table S4). During the LFSD, 39 fecal metabolites differed between responders and non-responders (Table S5). Metabolites such as L-urobilin were more strongly associated with responders while metabolites such as cholate were more strongly associated with non-responders (Fig. 5).
Figure 5. Hive plot showing tripartite associations among microbial taxa, metabolites, and clinical response following a low fermentable substrate dietary intervention. Responders were defined as those with a ≥50% decrease in abdominal pain frequency during the intervention. Red lines indicate a negative correlation (OTU vs. metabolite) or relative depletion (OTU or metabolite vs. subjects), while black lines indicate positive correlations or enrichment. OTUs and metabolites are color coded by taxonomic affiliation or metabolite class, and OTUs and metabolites of specific interest are highlighted with labels. Positive correlations are shown with respect to subject correlations though the inverse (negative correlation) is present in the other subject group (responders vs. non-responders).
Discussion
To our knowledge, this is the first pediatric study to evaluate the use of a comprehensive LFSD in childhood IBS. As found in adult studies, our pilot results suggest that this type of dietary intervention decreases abdominal pain frequency and severity in pediatric subjects with IBS. We found microbial factors such as baseline and dietary intervention stool microbiome composition, and subsequent stool metabolite associations differed between responders and non-responders. Our preliminary observations should prompt further investigation of an LFSD as a dietary intervention to ameliorate the symptoms in this population.
When attempting to identify factors that may account for LFSD efficacy, previous studies in adults have identified non-compliance as a factor.10 One of the strengths of this pilot study is confirming compliance through the capture of nutrient intake during a habitual week and during the intervention period. We observed decreases in specific carbohydrates and reduction in intake of high FODMAPs (fermentable oligosaccharides disaccharides monosaccharides and polyols)5 containing foods in the studied cohort. However, we also found several dietary changes beyond carbohydrate intake with children appearing to have reduced intake in several areas, including total calories. Nevertheless, we did not identify differences in consumption of nutrients or the number of fermentable items between responders and non-responders during the LFSD period, suggesting that dietary intake alone did not account for the marked differences in pain response between the two groups.
With respect to gaining insight into potential mechanisms possibly explaining the response to the dietary intervention, our study results suggest, as others have postulated, that the gut microbiota are a modifying factor with respect to the effect of dietary interventions in functional bowel disorders such as IBS.9 A subset of children with IBS responded more robustly to the diet (responders). Though demographic and clinical parameters did not differentiate responders from non-responders, responders appeared to have a different microbiome composition at baseline and during the LFSD. In line with this observation, the microbiological metabolic response as reflected by fecal metabolites differed between the two groups prior to and while on the LFSD.
Recently, reduced abundances of luminal bifidobacteria were identified in adults with IBS after starting an LFSD, though comprehensive evaluation of microbiome composition-based differences between those who did and did not respond to the LFSD at baseline was not undertaken.8 We found that the responder microbial community was enriched in OTUs resembling Sporobacter at baseline and when we limited our analysis to IBS-C subjects only, Phascolarctobacterium faecium. Members of the genus Sporobacter are described as strict anaerobes and are known to degrade aromatic compounds and produce short chain fatty acids.11 Phascolarctobacterium faecium is also a strict a
naerobe and is known to produce propionate.12 In contrast, non-responders were found to be enriched in Bacteroides and Bacteroidales which are known to have an extremely wide range of saccharolytic options.13 We hypothesize that the trend toward an increased amount of hydrogen production while on the LFSD in non-responders suggests that continued fermentation occurred in this sub-group despite the dietary changes.
Previous studies in adults with IBS have suggested that the fecal profile of short chain fatty acids may influence abdominal pain symptoms,14 though differences were not seen in short chain fatty acid production between controls and those placed on a 4-week LFSD.8 In contrast, we identified a relative increase in isovalerate in responders vs. non-responders during the LFSD. Fructooligosaccharide supplementation has been shown to decrease isovalerate production in 16 patients who had undergone ileal pouch-anal anastomoses.15 This was attributed to increased protein fermentation.15 During the LFSD intervention, responders showed an enrichment of bacteria belonging to the Coriobacteriaceae. The best matches to these in the NCBI nr database were Adlercreutzia equolifaciens (95% similarity) and Gordonibacter pamelaeae (also sharing ~95% similarity with the 16S rRNA gene), both of which are known to be involved in protein metabolism. A recent metabolomics-based study reported altered amino acid concentrations in the stool of subjects with IBS relative to healthy controls.16 Moreover, amino acid metabolism has been shown to differ in in vitro and animal experiments based on varying levels of fermentable carbohydrates.16 Different protein metabolizers and decreased protein fermentation observed among the responders suggest a mechanism that may have contributed to the positive LFSD response and account for differences in dipeptide (e.g., lysylleucine and asparagylleucine) and amino acid metabolite associations (e.g., threonine and citrulline) found between responders and non-responders. Further evaluations, potentially including more comprehensive metagenomics (e.g., whole genome sequencing), may help elucidate the role of proteases within the microbiome.
The differing abundances of Sporobacter, Subdoligranulum, and Phascolarctobacterium between the responders and non-responders at baseline suggest that these taxa may have the potential to serve as biomarkers to predict the likelihood of success for LFSD intervention. In the future this may help minimize the likelihood of starting an intervention that is unlikely to benefit children with IBS. Baseline bacterial gene count and species composition were recently found to significantly influence the effect of a dietary intervention in obese individuals: obese individuals with a low gene count were more responsive to the intervention as compared with obese individuals with a high gene count.17 Given the size of our cohort, additional data are needed to confirm whether baseline microbial composition will determine LFSD efficacy in children with IBS.
During the LFSD intervention, the non-responders continued to be enriched with Bacteroides-like OTUs and showed increased abundances of an Acetivibrio cellulolyticus-like OTU (OTU 914), a Dorea-like OTU (OTU 1160), and Dialister-like OTUs (OTUs 590 and 331). Both Acetivibrio cellulolyticus and Dorea formicigenerans are known H2 producers18,19 and may have contributed to the trend toward enhanced breath H2 production observed in the non-responders after LFSD intervention. Previous studies in adults with IBS have demonstrated decreases in breath H2 production paralleling improved symptoms with dietary interventions.20,21 However, to our knowledge these previous studies did not compare hydrogen production of those who improved while on the dietary intervention vs. those who did not. In addition, OTU 914 and 1160 are negatively correlated with multiple metabolites (e.g., citrulline and threonine) that were enriched in responders (Fig. 5).
Dialister sp. are common gut commensals, but their role in intestinal disease and abdominal pain remains unclear. Joossens et al. reported decreased abundances of Dialister invisus in subjects with Crohn disease relative to unaffected controls.22 In contrast, however, Jeffery et al. reported increased abundances of Dialister-like OTUs in adult IBS subjects.22,23 The persistence of Dialister and other members of the Veilonellaceae may have contributed to the lack of response to the LFSD intervention among the non-responder subjects. This speculation awaits further studies.
Whether differences identified in heme degradation pathways (responders having higher association with L-urobilin), bile acids (responders having higher association with dehydroxylithocholate) and lipid metabolism (responders having higher association with docosadienoate) truly relate to the clinical response seen is unknown. We note that diet has been shown to alter microbiota composition and heme oxygenase 1 expression in infants24 and, more specifically, that oligosaccharides have been found to induce heme oxygenase-1 expression in macrophages and inhibit inflammation in a murine colitis model.25 We speculate that dietary changes can in fact influence microbiota urobilin production, though further work is needed in this area. The differences seen may lend further support to underlying differences in microbiome composition being present between the two responder groups as urobilin production is dependent on microbial degradation pathways and specifically production of L-urobilin is associated with different microbiota than that of D-urobilin.26 In parallel, gut microbiota have been associated with regulation of intestinal absorption and metabolism of fatty acids27 and bile acids,28 while a Bacteroides predominance, which was found in the Non-responder group, has been associated with Western diets composed of proteins and fats.29
The length of time needed for a dietary intervention to be beneficial likely depends on the mechanism of action by which consumption of a food causes symptoms.9 With respect to carbohydrate intolerance, symptoms consistent with those seen in IBS (e.g., bloating and abdominal pain) have been seen after a single challenge, suggesting that elimination of poorly tolerated carbohydrates may lead to rapid benefits.30 Studies in adults with IBS have reported differences in symptoms in as little as two days of a high or low fermentable substrate diet intervention.21 Moreover, gut microbiome changes have been identified within 24 h of a dietary shift.29 Thus, the literature supports our use of one week on the LFSD for this initial pilot study. Further, identification of responders vs. non-responders and physiologic changes (e.g., breath H2 production and microbiome composition) suggest that a week on the LFSD was adequate. Nonetheless, given the chronic nature and symptom variability of IBS, future long-term studies evaluating the relationships between duration of the LFSD and both symptom and microbiome responses are needed.
Previous interventions using a low carbohydrate diet have often focused on adults with diarrhea-predominant IBS.4,6 In our cohort, the majority of children had a constipation-predominant subtype; and in line with this, the majority of responders were more likely to have a constipation-predominant subtype. These preliminary data suggest that LFSD efficacy might not be limited to the diarrhea-predominant subtype. Though whole intestinal transit time did not change while on the diet, we note that children had a trend toward fewer bowel movements. We hypothesize this may be related to decreased overall intake of calories during the dietary intervention, with future, long-term studies needed to help delineate this further.
There are limitations to our study. First, due to its nature as a pilot study, there is a relatively small sample size. Larger sample sizes are needed in future studies to assess the findings. Second, the intervention was not placebo-controlled. However, lack of a control may have been ameliorated, in part, by the instructions to the participants that they would be taught one of two potentially efficacious diets, despite all being taught how to follow the same LFSD. Based on our pilot study, a placebo-controlled trial is warranted.
In conclusion, LFSD appears to hold promise as a therapy for a subset of children with IBS. Our results suggest that gut microbial factors may play a central role in the efficacy of LFSD. Future studies should include comprehensive gut microbial composition analysis and metabolomics to clarify the role of the GI microbiota in the clinical responses to an LFSD.
Patients and Methods
Ethics statement
All study procedures were approved by the Baylor College of Medicine Institutional Review Board with written informed consent obtained from the parents and assent from the children.
Pediatric subject evaluation and enrollment
Children (7–17 y of age) with IBS were eligible for participation. Children were identified from pediatric practices by screening referrals to tertiary pediatric gastroenterology care for chronic abdominal pain and via newsletters and internet advertisements to the community. Parents and children were further screened by phone for inclusion and exclusion criteria and to establish current symptoms prior to enrollment. Exclusion criteria included the use of antibiotics or probiotics within 3 mo. The study was registered under clinicaltrials.gov (# NCT01018498). Participants completed a one-time visit at Texas Children’s Hospital during which their height, weight, and race/ethnicity were captured. Children and parents completed a pediatric Rome III GI symptom questionnaire to ensure IBS criteria were met.1 Study recruitment began July 1, 2009, and the study ended on September 1, 2011.
Dietary intervention
Children and their parents met with a registered research dietitian (A.R.M.) during the visit at Texas Children’s Hospital. They were taught how to keep a complete food diary (including quantity and composition of each food). Children and their parents were then told they would be asked to begin one of two diets as determined by the dietitian. However, all subjects were taught how to follow the same LFSD based on the low FODMAPs approach.5 Participants were provided sample menus and a table detailing foods to avoid and foods allowed on the diet. Furthermore, participants were given the dietitian’s contact information to answer any questions during the intervention period.
Subjects then completed a one-week period in which they continued their own standard habitual (baseline) diet, followed by one week during which they followed the LFSD (Fig. 1). The participants completed a three-day food record during the baseline period and again during the dietary intervention period. The Nutrition Data System for Research (University of Minnesota) version 2010 was used to analyze the food records. The dietitian also manually counted the number of foods that were high in FODMAPs content within the baseline and LFSD period.
Pain and stool diary
The primary outcome of the study was abdominal pain frequency (number of pain episodes). Responders were defined as having a ≥50% decrease in pain frequency in response to the LFSD as recommended for outcomes in IBS studies.31 Related secondary outcomes included abdominal pain severity and abdominal pain interference of activities. In order to measure these outcomes, children kept a one-week pain and stool diary as described previously during both the baseline and LFSD periods.32,33 Abdominal pain ratings were made 3 times per day (awakening, after lunch, and evening) during each one-week period. Pain ratings were recorded directly into the database via a dedicated touchtone telephone line. The child rated the pain using a validated 0–10 scale for measuring abdominal pain in children with 0 being “no pain at all” and 10 representing the “worst pain you can imagine.”33 The maximum level of pain was defined as the greatest pain intensity recorded during the one-week period. Mean pain was defined as the average pain rating throughout the entire week. Every stool passed during the one-week period, including those submitted for analysis (see below) were characterized by the children using the validated modified pediatric Bristol stool form chart.32,34 Children were subtyped based on stool form as having IBS-constipation (hard ≥5% and loose ≤25% of the time), IBS-diarrhea (hard ≤25% and loose ≥25%), IBS-mixed (hard or loose 25%), or IBS-untyped (does not meet criteria for IBS-C, IBS-D, or IBS-M).35
Physiologic measures
On the last day of both the baseline and LFSD periods, children were asked to capture up to 14 hourly breath samples. Samples were collected using a standard collection kit (Kidsampler System®, Quintron Instrument Co) and analyzed for hydrogen, methane, and carbon dioxide using gas chromatography (MicroLyzer® Model SC, QuintTron Instrument Company).36,37 Concentrations of hydrogen and methane were normalized to the concentration of carbon dioxide, while carbon dioxide production rates were calculated based on body weight.38 An area under the curve was calculated based on the hydrogen and methane concentrations over time. If necessary, the number of hours was normalized between the baseline and dietary intervention periods to ensure an equal number of hours analyzed between the two periods for each subject.
The subject also swallowed a small pill of carmine red. All stools were collected for the next 2 d, and subjects were instructed to record the first presence of reddish discoloration in their stools. The time from ingestion of the carmine red to visualization in the stool defined whole gut transit time.
Stool microbiome composition determination
DNA extraction, bacterial 16S rRNA gene amplification, and 454 sequencing of 16S rRNA gene libraries were performed at the Texas Children’s Microbiome Center as previously described.3 In brief, DNA was extracted from stool samples using a commercial DNA extraction kit (MO-BIO PowerSoil® DNA Isolation Kit, MO-BIO Laboratories) following the modified protocols described by the Human Microbiome Project.39 Individual sequence libraries were generated using bar-coded primers targeting the V3-V5 region of the 16S rRNA gene.3 Following emulsion PCR, the PCR products were pooled and sequenced on the GS-FLX platform (454 Life Sciences/Roche).
The pooled sequence data were quality-filtered and parsed by barcode using the QIIME software package v. 1.3.0,40 as implemented in the Genboree Microbiome Toolbench.41 Sequences that had lengths shorter than 200 bp, had average quality scores <20, harbored ambiguous base calls, or had mismatches to their barcode or sequencing primer were excluded from downstream analysis. The quality-filtered sequences were assigned to operational taxonomic units (OTUs, sequences that share ≥97% similarity) using Cd-Hit.42 Sequences were screened for chimeras using the ChimeraSlayer algorithm,43 and all potential chimeras were excluded from downstream analysis. Taxonomic identities were assigned to the remaining pool of sequences using the Ribosomal Database Project Classifier (Release 10).44 BLAST searches of NCBI’s GenBank nr database were used to provide supplemental identity information for particular OTUs of interest.
An average of 2145 high-quality 16S rRNA gene sequences were generated per stool sample (range: 1013–4125). Given the variation in library size and the potential for differences in sequencing depth to bias the calculation of diversity metrics, each library was randomly sub-sampled to contain 1013 sequences. All results presented here are based on these sub-sampled libraries. Alpha diversity metrics, including OTU richness, Shannon H', and the reciprocal Simpson index (1/D) were calculated using QIIME.
Full sequence libraries were deposited in the NCBI Sequence Read Archive under project accession number SRP018824.
Stool metabolite composition measurements
As previously described, all stool samples were sent to Metabolon, Inc., for quantification of metabolites.45 For sample preparation, water was added to a volume of 5 μL/mg of tissue and subjected to homogenization by rapid shaking. Fecal pellets were first lyophilized then resuspended in water (20 μL/mg of dried sample) before homogenization. Following homogenization, 100 μL of the fecal suspensions were used for extraction. Samples were prepared using the automated MicroLab STAR system (Hamilton Company). A recovery standard was added before the first step in the extraction process for quality control (QC) purposes. Sample extraction was conducted using an aqueous methanol extraction process to remove the protein fraction while allowing maximum recovery of small molecules. The resulting extract was divided into 4 fractions: 1 for analysis by ultra-performance liquid chromatography tandem mass spectroscopy (UPLC/MS/MS2; positive mode), 1 for UPLC/MS/MS2 (negative mode), 1 for gas chromatography mass spectroscopy (GC/MS), and 1 for backup. Samples were placed briefly on a TurboVap (Zymark) to remove the organic solvent. Each sample was then frozen and dried under vacuum and prepared to run on either the UPLC/MS/MS2 or GC/MS instruments.
Samples were processed as described previously.46,47 For quality assurance (QA)/QC, additional samples were included with each day's analysis. These samples included extracts of a pool of well-characterized human plasma, extracts of a pool created from a small aliquot of the experimental samples, and process blanks. QC samples were spaced evenly among the injections, and all experimental samples were randomly distributed throughout the run. A selection of QC compounds was added to every sample for chromatographic alignment, including those under test.
Raw data were extracted, peak-identified, and QC processed using Metabolon's hardware and software.45 Metabolon maintains a library based on authenticated standards that contain the retention time/index (RI), mass to charge ratio (m/z), and chromatographic data (including MS/MS spectral data) on all molecules present in the library. Furthermore, biochemical identifications are based on 3 criteria: retention index within a narrow RI window of the proposed identification, nominal mass match to the library +/− 0.2 amu, and the MS/MS forward and reverse scores between the experimental data and authentic standards. The MS/MS scores are based on a comparison of the ions present in the experimental spectrum to the ions present in the library spectrum. While there may be similarities between these molecules based on one of these factors, the use of all 3 data points can be used to distinguish and differentiate biochemicals. More than 2400 commercially available purified standard compounds have been acquired and registered into LIMS for distribution to both the LC and GC platforms for determination of their analytical characteristics.
Statistical analysis
Those who completed the LFSD trial were included for all subsequent statistical analyses. IBM Statistics (version 20) was used for comparisons between baseline and LFSD periods within subjects using paired Wilcoxon (continuous variables) or McNemar tests (categorical variables). Comparisons between responders and non-responders were done using Chi-square testing for categorical variables and Mann-Whitney U non-parametric testing for continuous variables.
Differences in stool microbiome baseline and post-diet community composition, as well as differences in composition between responders and non-responders, were evaluated using Mann-Whitney tests and principal components analysis of unweighted Unifrac distances, as calculated in QIIME. The LEfSe software package48 was also used to identify differentially abundant features and to evaluate the statistical significance of metagenomic biomarkers. LEfSe allowed for characterization between biological conditions taking into account effect size and statistical differences through the usage of unpaired Wilcoxon rank-sum testing.48 Unless otherwise described, data are presented as the mean ± standard deviation.
The differences in stool metabolites seen comparing baseline to post-LFSD fecal metabolites in the entire cohort was evaluated using paired t tests. Differences in stool metabolites between responders and non-responders at baseline and during the LFSD were evaluated using repeated measures ANOVA.
P values <0.05 were considered to represent significant differences for all analyses, including those involving the stool microbiome and metabolites. Given that this was a pilot study limited by sample size, multiple testing corrections were not performed.
Subject-metabolite-microbiome visualization
Subject-OTU-metabolite relationships were visualized using a hive plot approach.49 OTUs that differed significantly between responders and non-responders, as determined by LEfSe analysis (above), were included in the plot, as were those metabolites which differed significantly between responders and non-responders following the LFSD intervention (Table S5). Potential relationships between OTUs and metabolites were evaluated using an all vs. all Spearman correlation of OTUs and metabolites, followed by Benjamini-Hochberg corrections for multiple comparisons in R.50 Those OTUs and metabolites which were found to share significant correlations (q < 0.10) are highlighted in Figure 5.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
Funding was provided by the NASPGHAN Foundation/Nestle Young Investigator Development Award to B.P.C. This study also was supported in part by R01 NR05337 (R.J.S.) and UH3 DK083990 (J.V.) from the National Institutes of Health (R.J.S.), the Daffy’s Foundation (R.J.S.), American College of Gastroenterology Clinical Research Award (B.P.C.), the USDA/ARS under Cooperative Agreement No. 6250-51000-043 (R.J.S.), and P30 DK56338 which funds the Texas Medical Center Digestive Disease Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work is a publication of the University of Washington and the USDA/ARS Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, and Texas Children's Hospital. The contents do not necessarily reflect the views or policies of the USDA, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. We thank Jane Muir, Jacqueline Barett, and Dr Peter Gibson for their guidance with the dietary intervention, Yoni Samocha, Sonia Singh, and Linda Cao for their research assistance, and Yue Shang and Jessica Runge from the Texas Children’s Microbiome Center for their assistance in sample processing and sequencing.
References
- 1.Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, Walker LS. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527–37. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McOmber ME, Shulman RJ. Recurrent abdominal pain and irritable bowel syndrome in children. Curr Opin Pediatr. 2007;19:581–5. doi: 10.1097/MOP.0b013e3282bf6ddc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saulnier DM, Riehle K, Mistretta TA, Diaz MA, Mandal D, Raza S, Weidler EM, Qin X, Coarfa C, Milosavljevic A, et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology. 2011;141:1782–91. doi: 10.1053/j.gastro.2011.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austin GL, Dalton CB, Hu Y, Morris CB, Hankins J, Weinland SR, Westman EC, Yancy WS, Jr., Drossman DA. A very low-carbohydrate diet improves symptoms and quality of life in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7:706–8, e1. doi: 10.1016/j.cgh.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J Gastroenterol Hepatol. 2010;25:252–8. doi: 10.1111/j.1440-1746.2009.06149.x. [DOI] [PubMed] [Google Scholar]
- 6.Staudacher HM, Whelan K, Irving PM, Lomer MC. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J Hum Nutr Diet. 2011;24:487–95. doi: 10.1111/j.1365-277X.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 7.Ong DK, Mitchell SB, Barrett JS, Shepherd SJ, Irving PM, Biesiekierski JR, Smith S, Gibson PR, Muir JG. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1366–73. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 8.Staudacher HM, Lomer MC, Anderson JL, Barrett JS, Muir JG, Irving PM, Whelan K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr. 2012;142:1510–8. doi: 10.3945/jn.112.159285. [DOI] [PubMed] [Google Scholar]
- 9.Gibson PR, Shepherd SJ. Food choice as a key management strategy for functional gastrointestinal symptoms. Am J Gastroenterol. 2012;107:657–66, quiz 667. doi: 10.1038/ajg.2012.49. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd SJ, Gibson PR. Fructose malabsorption and symptoms of irritable bowel syndrome: guidelines for effective dietary management. J Am Diet Assoc. 2006;106:1631–9. doi: 10.1016/j.jada.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Grech-Mora IFM-L, Ollivier B, Rimbault A, Presnier G, Garcia J-L, Garnier-Sillam E. Isolation and characterization of Sporobacter termitidis gen. nov., sp. nov., from the digestive tract of the wood-feeding termite Nasutitermes lujae. Int J Syst Bacteriol. 1996;46:512–8. doi: 10.1099/00207713-46-2-512. [DOI] [Google Scholar]
- 12.Del Dot TOR, Stackebrandt E. Phascolarctobacterium faecium gen. nov., sp. nov., a novel taxon of the Spormusa group of bacteria. Syst Appl Microbiol. 1993;16:380–4. doi: 10.1016/S0723-2020(11)80269-9. [DOI] [Google Scholar]
- 13.Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3:289–306. doi: 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512–9, e114-5. doi: 10.1111/j.1365-2982.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- 15.Alles MS, Katan MB, Salemans JM, Van Laere KM, Gerichhausen MJ, Rozendaal MJ, Nagengast FM. Bacterial fermentation of fructooligosaccharides and resistant starch in patients with an ileal pouch-anal anastomosis. Am J Clin Nutr. 1997;66:1286–92. doi: 10.1093/ajcn/66.5.1286. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen HS, Holtug K, Mortensen PB. Degradation of amino acids to short-chain fatty acids in humans. An in vitro study. Scand J Gastroenterol. 1988;23:178–82. doi: 10.3109/00365528809103964. [DOI] [PubMed] [Google Scholar]
- 17.Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, et al. ANR MicroObes consortium Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–8. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 18.Taras D, Simmering R, Collins MD, Lawson PA, Blaut M. Reclassification of Eubacterium formicigenerans Holdeman and Moore 1974 as Dorea formicigenerans gen. nov., comb. nov., and description of Dorea longicatena sp. nov., isolated from human faeces. Int J Syst Evol Microbiol. 2002;52:423–8. doi: 10.1099/00207713-52-2-423. [DOI] [PubMed] [Google Scholar]
- 19.Laube VM, Martin SM, SM LVaM Conversion of cellulose to methane and carbon dioxide by triculture of Acetivibrio cellulolyticus, Desulfovibrio sp, and Methanosarcina barkeri. Appl Environ Microbiol. 1981;42:413–20. doi: 10.1128/aem.42.3.413-420.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dear KL, Elia M, Hunter JO. Do interventions which reduce colonic bacterial fermentation improve symptoms of irritable bowel syndrome? Dig Dis Sci. 2005;50:758–66. doi: 10.1007/s10620-005-2570-4. [DOI] [PubMed] [Google Scholar]
- 21.Ong DK, Mitchell SB, Barrett JS, Shepherd SJ, Irving PM, Biesiekierski JR, Smith S, Gibson PR, Muir JG. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1366–73. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 22.Joossens M, Huys G, Cnockaert M, De Preter V, Verbeke K, Rutgeerts P, Vandamme P, Vermeire S. Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut. 2011;60:631–7. doi: 10.1136/gut.2010.223263. [DOI] [PubMed] [Google Scholar]
- 23.Jeffery IB, O’Toole PW, Öhman L, Claesson MJ, Deane J, Quigley EM, Simrén M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- 24.Carlisle EM, Poroyko V, Caplan MS, Alverdy J, Morowitz MJ, Liu D. Murine gut microbiota and transcriptome are diet dependent. Ann Surg. 2013;257:287–94. doi: 10.1097/SLA.0b013e318262a6a6. [DOI] [PubMed] [Google Scholar]
- 25.Higashimura Y, Naito Y, Takagi T, Mizushima K, Hirai Y, Harusato A, Ohnogi H, Yamaji R, Inui H, Nakano Y, et al. Oligosaccharides from agar inhibit murine intestinal inflammation through the induction of heme oxygenase-1 expression. J Gastroenterol. 2013;48:897–909. doi: 10.1007/s00535-012-0719-4. [DOI] [PubMed] [Google Scholar]
- 26.McHardy IH, Goudarzi M, Tong M, Ruegger PM, Schwager E, Weger JR, Graeber TG, Sonnenburg JL, Horvath S, Huttenhower C, et al. Integrative analysis of the microbiome and metabolome of the human intestinal mucosal surface reveals exquisite inter-relationships. Microbiome. 2013;1:17. doi: 10.1186/2049-2618-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semova I, Carten JD, Stombaugh J, Mackey LC, Knight R, Farber SA, Rawls JF. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe. 2012;12:277–88. doi: 10.1016/j.chom.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids, and gut microbiota: Unraveling a complex relationship. Gut Microbes. 2013;4:4. doi: 10.4161/gmic.25723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomara RE, Halata MS, Newman LJ, Bostwick HE, Berezin SH, Cukaj L, See MC, Medow MS. Fructose intolerance in children presenting with abdominal pain. J Pediatr Gastroenterol Nutr. 2008;47:303–8. doi: 10.1097/MPG.0b013e318166cbe4. [DOI] [PubMed] [Google Scholar]
- 31.Irvine EJ, Whitehead WE, Chey WD, Matsueda K, Shaw M, Talley NJ, Veldhuyzen van Zanten SJ, Design of Treatment Trials Committee Design of treatment trials for functional gastrointestinal disorders. Gastroenterology. 2006;130:1538–51. doi: 10.1053/j.gastro.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 32.Chumpitazi BP, Lane MM, Czyzewski DI, Weidler EM, Swank PR, Shulman RJ. Creation and initial evaluation of a Stool Form Scale for children. J Pediatr. 2010;157:594–7. doi: 10.1016/j.jpeds.2010.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shulman RJ, Eakin MN, Jarrett M, Czyzewski DI, Zeltzer LK. Characteristics of pain and stooling in children with recurrent abdominal pain. J Pediatr Gastroenterol Nutr. 2007;44:203–8. doi: 10.1097/01.mpg.0000243437.39710.c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lane MM, Czyzewski DI, Chumpitazi BP, Shulman RJ. Reliability and validity of a modified Bristol Stool Form Scale for children. J Pediatr. 2011;159:437–41, e1. doi: 10.1016/j.jpeds.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 36.Shulman RJ, Kerzner B, Sloan HR, Boutton TW, Wong WW, Nichols BL, Klein PD. Absorption and oxidation of glucose polymers of different lengths in young infants. Pediatr Res. 1986;20:740–3. doi: 10.1203/00006450-198608000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Shulman RJ, Wong WW, Irving CS, Nichols BL, Klein PD. Utilization of dietary cereal by young infants. J Pediatr. 1983;103:23–8. doi: 10.1016/S0022-3476(83)80769-0. [DOI] [PubMed] [Google Scholar]
- 38.Niu HC, Schoeller DA, Klein PD. Improved gas chromatographic quantitation of breath hydrogen by normalization to respiratory carbon dioxide. J Lab Clin Med. 1979;94:755–63. [PubMed] [Google Scholar]
- 39.Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–14. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riehle K, Coarfa C, Jackson A, Ma J, Tandon A, Paithankar S, Raghuraman S, Mistretta TA, Saulnier D, Raza S, et al. The Genboree Microbiome Toolset and the analysis of 16S rRNA microbial sequences. BMC Bioinformatics. 2012;13(Suppl 13):S11. doi: 10.1186/1471-2105-13-S13-S11. [abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22:1658–9. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- 43.Haas BJ, Gevers D, Earl AM, Feldgarden M, Ward DV, Giannoukos G, Ciulla D, Tabbaa D, Highlander SK, Sodergren E, et al. Human Microbiome Consortium Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011;21:494–504. doi: 10.1101/gr.112730.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–7. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montrose DC, Zhou XK, Kopelovich L, Yantiss RK, Karoly ED, Subbaramaiah K, Dannenberg AJ. Metabolic profiling, a noninvasive approach for the detection of experimental colorectal neoplasia. Cancer Prev Res (Phila) 2012;5:1358–67. doi: 10.1158/1940-6207.CAPR-12-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem. 2009;81:6656–67. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- 47.Reitman ZJ, Jin G, Karoly ED, Spasojevic I, Yang J, Kinzler KW, He Y, Bigner DD, Vogelstein B, Yan H. Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc Natl Acad Sci U S A. 2011;108:3270–5. doi: 10.1073/pnas.1019393108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krzywinski M, Birol I, Jones SJ, Marra MA. Hive plots--rational approach to visualizing networks. Brief Bioinform. 2012;13:627–44. doi: 10.1093/bib/bbr069. [DOI] [PubMed] [Google Scholar]
- 50.R Development Core Team. A language and environment for statistical computing. Vienna, Australia: R Foundation for Statistical Computing, 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.