Abstract
Paneth cells are long-lived secretory cells that reside in the base of the crypts of Lieberkühn of the small intestine. They produce an arsenal of molecules that are involved in numerous biological processes, ranging from the control of gut microbial populations to supporting the intestinal stem cell niche. Because of these important functions, Paneth cell abnormalities are becoming implicated in a variety of disease processes. As such, it is necessary to establish parameters that will allow for the comprehensive study of Paneth cells in health and disease. In this addendum, we highlight critical design aspects involved in the study of Paneth cells and their downstream effects on the intestinal microbiota. The importance of this approach is demonstrated by our recent findings that Nod2 does not regulate mouse Paneth cell antimicrobial function, in contrast to previous reports. This work defines key issues to consider when studying Paneth cells in mouse systems.
Keywords: Nod2, Paneth cell, inflammatory bowel diseases, defensin, microbiota, background strain
Introduction
Paneth cells comprise one of the primary secretory cell lineages of the small intestinal epithelium. Located at the base of the crypts of Lieberkühn, these cells are readily identified by their prominent eosinophilic granules, which contain a diverse repertoire of secretory products.1 Examples of such molecules include antimicrobial peptides (AMPs), pro-inflammatory mediators, and signal transduction proteins. AMPs in particular are highly abundant within Paneth cell secretory granules.2 These antibiotic peptides are evolutionary ancient molecules that host organisms use to control microbes in their environment. While more than 1500 AMPs have been reported to date,3 the primary Paneth cell antimicrobials include: α-defensins (cryptdins), cryptdin-related sequence (CRS) peptides, lysozyme-P (Lyz), secreted phospholipase A2, the C-type lectins (Reg3α, Reg3γ, HIP/PAP), and angiogenin 4.4 This notable AMP diversity provides an important component of innate defense again bacteria, fungi, enveloped viruses, and protozoan organisms.
Given their potent antimicrobial activity, it follows that Paneth cells play a key role in host defense against enteric pathogens. Indeed, mouse models with various Paneth cell AMP deficiencies support this premise. For example, mice lacking functional ileal α-defensins are more susceptible to intestinal infection with Salmonella typhimurium.5 Interestingly, these mice also have profound shifts in the prominent phyla of their commensal intestinal microbiota.6 Therefore, Paneth cells not only combat pathogenic organisms, but also modulate the endogenous bacterial communities of the gut. This suggests that the physiologic importance of these cells extends beyond pathogen control, and includes the regulation of commensal host-microbial interactions at the intestinal surface.
Because the intestinal microbiota plays a key role in numerous complex medical disorders (ranging from metabolic syndromes such as obesity to inflammatory disorders such as Crohn disease), understanding the mechanisms that regulate Paneth cell function has great importance for a wide spectrum of human diseases. However, as these mechanistic studies are pursued further in animal models, it is imperative to develop a paradigm to accurately study Paneth cells in such systems. The importance of such a paradigm is demonstrated in a recent study from our laboratory, which revealed that when mouse background and husbandry conditions are strictly controlled, previously reported effects of the molecule Nod2 on Paneth cell α-defensin expression and gut microbial composition are abrogated.7 The following addendum will highlight issues necessary to consider when designing mouse studies of Paneth cells and the enteric microbiota, and detail techniques that can be used to study Paneth cells in a systematic fashion.
Regulation of Paneth Cell Antimicrobial Function
Although a comprehensive understanding of the regulation of Paneth cell antimicrobial function is still evolving, key concepts have emerged. Specifically, Paneth cell function is regulated at multiple levels that include: (1) control of Paneth cell development and maturation; (2) transcriptional regulation of AMP expression; (3) post-translational activation of AMP molecules; and (4) modulation of Paneth cell secretion. While additional genetic, epigenetic, translational, and post-translational mechanisms of Paneth cell regulation are likely to be elucidated, the stated regulatory checkpoints provide a framework for the comprehensive study of Paneth cell function.
Paneth cell development and maturation
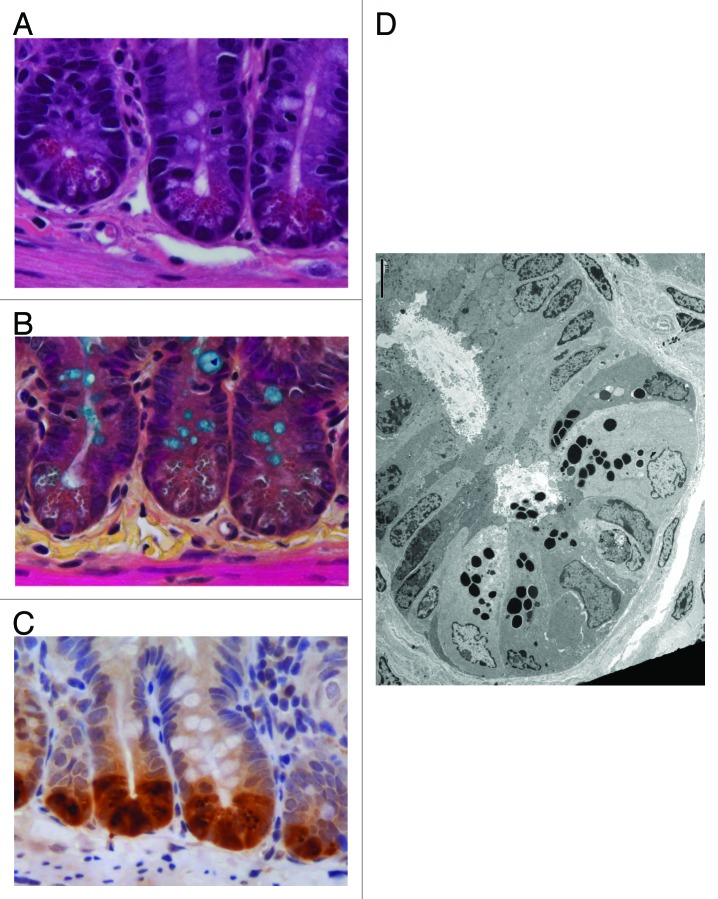
The ability of Paneth cells to control gut microbes is dependent upon their proper development and maturation. For example, mice with reduced Paneth cell numbers demonstrate increased gut bacterial translocation.8 Moreover, mice hypomorphic for the autophagy protein Atg16l1 possess Paneth cells with highly disorganized granules and altered secretion.9 This results in aberrant Lyz in the mucus layer of the small intestine. As shown in Figure 1, various methods can be used to assess Paneth cell number and morphology. Paneth cells are typically enumerated on a per-crypt basis, though we have utilized flow cytometry to quantify Lyz+ Paneth cells in epithelial cell preparations of the entire ileum, in order to minimize the bias of counting Paneth cells from only selected high-power fields.7,10 Furthermore, the morphology of Paneth cell granules can be assessed using transmission electron microscopy (Fig. 1D). This technique can also be combined with immunogold staining to assess the localization of specific AMPs to Paneth cell granules.11
Figure 1. Microscopic evaluation of mouse Paneth cells. (A) Hematoxylin and eosin (640×) staining of small intestinal tissue reveals Paneth cell secretory granules by their eosinophilic coloration. (B) Alternatively, these subcellular structures can be discerned by phloxine-tartrazine staining (640 × ). In this image, goblet cells are co-visualized using Alcian Blue. (C) Immunohistochemical recognition of epithelial-specific expression of Paneth cell products, such as lysozyme, can also be used to visualize Paneth cells (640×). (D) Transmission electron micrograph of the fine structure of Paneth cells is aided by the electron-dense nature of the Paneth cell secretory granules. Scale bar is 5 μm. Images in panels (B-C) provided by Dekaney C, UNC.
Transcriptional regulation of AMP expression
Numerous challenges exist to the study of AMP transcription. AMPs represent an extremely diverse class of molecules, in which various peptide subsets are subject to distinct regulatory mechanisms. However, because there are defined AMP groups that comprise the primary Paneth cell antimicrobials (see introduction), a rational approach to evaluate AMP transcription is via quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) of selected groups and molecules. For AMP groups that contain numerous, diverse peptides (such as the α-defensins and CRS peptides), primers have been generated that amplify the entire class of molecules.7,12,13 In addition, primers specific for representative molecules of the primary AMP classes have been reported.7 Therefore, the combination of group- and molecule-specific qRT-PCR can provide a general overview of AMP transcriptional activity. This technique is limited by the number of AMPs that can be assessed in a given study. To overcome this, transcript profiling of Paneth cell-specific RNA isolated via laser capture microdissection has been described.9
Post-translational activation of AMP molecules
Numerous AMPs require proteolytic processing prior to activation. This is well established for the α-defensins. In mice, matrix metalloproteinase 7 (Mmp7, or matrilysin) cleaves α-defensin pro-peptides into their mature, active form.5 Acid urea-PAGE (AU-PAGE) represents a useful technique for characterizing processed α-defensins, as these molecules are known to migrate to a distinct α-defensin zone on AU-PAGE gels (Fig. 2A). Mice that are deficient for Mmp7 possess no discernable bands in this region.14 When coupled with mass spectrometry, AU-PAGE can be used to identify the specific α-defensin isoforms present in a given sample.10 Moreover, the α-defensin zone of an AU-PAGE gel can be excised, overlaid onto an agarose plate of confluent bacteria, and assessed for zones of bacterial growth inhibition.15 This allows for the evaluation of functional changes in the antimicrobial activity of α-defensins extracted from multiple samples (Fig. 2B).

Figure 2. Protein expression and functional analysis of mouse Paneth cell α-defensins. (A) 300 μg of acid-extracted and dialyzed ileal protein from individual C57BL/6 (lanes 2–5) and 129/SvEv (lanes 6–9) mice are separated using acid urea-PAGE. These are run alongside a sample of recombinant α-defensin 4 (lane 1) and stained with Coomassie brilliant blue. From the α-defensin region of the gel (denoted by a box), note that C57BL/6 and 129/SvEv mice express dissimilar sets of these peptides. The identity of the isoforms associated with each band can be ascertained using mass spectrometric analysis. In this study, the following peptides were identified by mass spectrometry: 1-Defa5; 2-Defa24, Defa27; 3-Defa20, Defa21; 4-Defa2; 5-Defa22; 6-Defa5; 7-Defa2/18v, Defa11, Defa16, Defa21; 8-Defa18, Defa25; and 9-Defa4. (B) In this gel overlay assay, the α-defensin region of an unstained AU-PAGE gel is allowed to incubate for 3 h at 37 °C on a layer of media-poor agarose laden with mid-log phase bacterial cells. Overnight bacterial growth of these plates with a media-rich agarose overlay reveals zones of bacterial inhibition associated with α-defensin bands of the AU-PAGE gel. This study demonstrated that C57BL/6 mice express α-defensins that are dramatically more bactericidal against the colitogenic E. coli strain NC101 than the α-defensins expressed by 129/SvEv mice. (Gulati et al., ref. 10).
Paneth cell secretion
Numerous stimuli, including bacteria, bacterial products, and cholinergic agonists, are able to induce Paneth cell exocytosis.2 This process is associated with a biphasic increase in cytosolic Ca2+,16 which is regulated by a Ca2+-activated potassium channel known as Kcnn4.17 These mechanisms have been elucidated by stimulating isolated small intestinal crypts with various microbial or pharmacological agents. However, while these crypt secretion assays provide important mechanistic insight into Paneth cell exocytosis, they do have limitations. Specifically, they do not recapitulate the biological milieu of the in vivo small intestine. This is critical, because after secretion, the luminal environment of the host can modulate the microbicidal activity of AMPs. For instance, secreted AMPs are concentrated within the surface mucus layer of the small intestine, focusing antimicrobial activity to this compartment.18 Moreover, luminal factors such as the ionic microenvironment, redox state, and even intestinal transit can modulate AMP microbicidal activity.19-21 Therefore, studies that examine the in vivo effects of AMP dysfunction provide key information regarding global Paneth cell antimicrobial activity. Examples of such investigations include evaluating the composition of the commensal gut microbiota and the assessment of host susceptibility to enteric pathogens.
Paneth Cells and Crohn Disease
As described above, the ability of Paneth cells to modulate the gut microbiota suggests an important role for these cells in a broad spectrum of human diseases. In particular, Crohn disease (CD) is a chronic inflammatory condition of the gut that is believed to develop as a result of dysregulated immune responses to the indigenous intestinal microbiota.22 Intriguingly, the most common site of intestinal involvement in CD is the distal ileum, where Paneth cell abundance is greatest.23 Numerous CD risk alleles have also been associated with Paneth cell dysfunction. For example, TCF-4 is a Wnt pathway transcription factor that regulates Paneth cell development and α-defensin expression.24 Polymorphisms in the promoter region of the TCF-4 gene are associated with small intestinal CD,25 and patients with CD have reduced expression and binding activity of this transcription factor.26 Additional CD risk alleles that have been associated with Paneth cell abnormalities include NOD2, ATG16L1, XBP1, KCNN4, AGR2, and IRGM.9,27-31 These lines of evidence suggest that Paneth cell dysfunction may play an important role in the pathogenesis of CD.
The Role of NOD2 in Paneth Cell Function
Of the risk alleles associated with CD, polymorphisms of the NOD2 gene correlate strongest with disease risk.32 However, despite this powerful association, the mechanism by which NOD2 regulates intestinal inflammation remains unclear. Interestingly, NOD2 is highly expressed in Paneth cells,33 suggesting that this molecule may modulate intestinal inflammation by regulating Paneth cell antimicrobial function. This concept has been supported by studies demonstrating that NOD2 regulates α-defensin expression in patients with CD,27 though this work has been subject to some debate.34 Additional mouse studies have shown that Nod2−/− mice exhibit significantly lower ileal mRNA expression of specific α-defensin isoforms (Defa4 and Defa-rs10) than their WT counterparts.35 Given this intriguing finding, we sought to comprehensively characterize Paneth cell function in Nod2−/− mice.7 Based on previously reported data, we hypothesized that Nod2−/− mice would have impaired Paneth cell antimicrobial function.
To test our hypothesis, we utilized the study design framework described above, based on evaluating Paneth cells at multiple regulatory checkpoints. Unexpectedly, we found that mouse Paneth cell antimicrobial function is not dependent upon intact Nod2 signaling. This conclusion is based on a series of key findings. First, we showed that Nod2 does not regulate Paneth cell development, as evidenced by similar numbers of Paneth cells in WT and Nod2−/− mice. Second, aside from a modest reduction in the CRS1C class, we observed no significant differences in transcript levels of the major mouse Paneth cell AMP groups between WT and Nod2−/− mice. This included the α-defensins, which were assessed using global cryptdin primers, as well as those specific for Defa3, Defa5, and Defa20. Third, there were no defects in the post-translational processing of α-defensins in Nod2−/− mice. Specifically, our AU-PAGE analysis showed identical α-defensin peptide profiles between experimental groups, including equivalent bactericidal activity of these molecules against commensal and pathogenic bacterial strains. These cumulative data demonstrate that the biosynthesis of functional Paneth cell α-defensins is not impaired in Nod2−/− mice.
To ensure that we were not overlooking a biologically relevant defect in Paneth cell secretion, our final experiments examined the fecal microbiota of WT and Nod2−/− mice using 454-sequencing of the bacterial 16S rRNA gene. Indeed, impaired Paneth cell secretory responses in Nod2−/− mice have been reported in previous studies.36 Our results, however, showed no differences in fecal microbial composition between the two experimental groups. We have since generated data demonstrating that ileal-adherent bacterial communities are also similar between WT and Nod2−/− mice (Fig. 3A). Moreover, we have found that Nod2−/− mice do not display increased susceptibility to Listeria monocytogenes infection in vivo (Fig. 3B), in contrast to previous reports.35 These studies suggest that, while Nod2 may regulate Paneth cell secretion at some level, the loss of such regulation does not lead to profound changes of the gut microbiota or increased susceptibility to specific enteric pathogens. Nevertheless, it remains possible that Nod2 may play additional roles in Paneth cells, beyond the regulation of AMP production and antimicrobial function.
Figure 3. Nod2 does not affect host responses to intestinal commensal or pathogenic bacteria. (A) We have previously reported that co-housed littermates of wild-type and Nod2−/− mice do not differ significantly in the composition of fecal microbiota.7 Here, we present the analysis of 454 sequences of 16S rRNA from ileal mucosal samples. Principal Coordinate Analysis (PCoA) is shown using the first two coordinates of PCoA based on weighted UniFrac dissimilarity of these sequences. Circles indicate Nod2−/− (KO) mice, while squares represent wild-type (WT) mice. Each cage is depicted with a unique color with the cage numbers displayed next to each symbol. Similar to what we observed with stool samples from our previous analysis, we noted that genotype did not influence the composition of the ileal mucosally-adherent bacteria. (B) To determine whether Nod2 affected host invasion by intestinal pathogens, we exposed WT and KO mice to Listeria monocytogenes by oral gavage. After 72 h of colonization, bacterial translocation was evaluated from homogenized liver and spleen samples. Over the course of colonization, no significant difference in weight loss was observed between the two groups (left panel). Moreover, we noted that Nod2 status did not affect the ability of L. monocytogenes to translocate and colonize host liver and spleen tissue (right panel).
The Influence of Mouse Background Strain on Paneth Cell Function
Our data demonstrate that mouse Paneth cell antimicrobial function is independent of Nod2. We speculate that these results differ from earlier work due to the variability of mouse background utilized in the previous study. In our investigations, WT and Nod2−/− littermates were derived by breeding heterozygous Nod2+/− mice that were on a C57BL/6 (B6) background. In contrast, the originally reported Nod2−/− mice were constructed by injecting genetically manipulated 129S1/Sv-derived W9.5 embryonic stem cells into B6 blastocysts.35 No clear backcrossing of these animals to a homogenous background was described. Therefore, it is possible that the ostensible differences in α-defensin expression previously attributed to Nod2-deficiency could, in fact, be due to variations in mouse strain between experimental groups.
Ample evidence exists supporting the impact of mouse background strain on Paneth cell function. Strain-specific cryptdin alleles were identified by restriction fragment length polymorphism analysis in early studies analyzing the chromosomal alignment of the mouse cryptdin gene.37 Varying AU-PAGE patterns of α-defensin migration were subsequently identified, suggesting a distinct profile of α-defensins in B6 mice.14 Our lab has recently reported an extensive analysis of Paneth cell function in B6 and 129/SvEv (129) mice.10 Interestingly, the two α-defensins previously shown to be diminished in Nod2−/− mice (Defa4 and Defa-rs10) were not detected in B6 mice, but strongly expressed in 129 animals. This is consistent with reports indicating that neither of these genes is present in the B6 genome assembly.38 The detection of these transcripts in the original Nod2−/− mice solidifies the notion that such mice were on a mixed background containing a non-B6 component at the time of analysis. Future work in our laboratory is planned to examine Nod2−/− mice on a 129/SvEv background, to further define the effects of background strain on Paneth cell function in this model.
Environmental Influences on Gut Microbial Composition
The advent of high-throughput, deep sequencing of the bacterial 16S rRNA gene has allowed for the comprehensive profiling of intestinal bacterial communities in mammalian hosts. Mouse systems, in particular, offer the ability to study the effects of various factors on gut microbial composition in a highly controlled fashion. This is in contrast to human studies, which are limited by the immense genetic and environmental variation inherent to such investigations. However, there are key environmental variables that must be controlled in mouse studies as well. This is highlighted by our findings described above, which demonstrated that WT and Nod2−/− mice have similar gut bacterial communities when littermates are cohoused for the duration of study.7
The results of our work diverge from studies that have found a strong influence of Nod2 on gut microbial composition.39-41 However, in many of these investigations, WT and Nod2−/− mice were obtained from colonies that were maintained independently from one another. While such studies do offer initial insight into the impact of Nod2 on gut microbial composition, they are limited in their ability to control for confounders such as cage- and litter-effects on the intestinal microbiota. Indeed, studies that have meticulously controlled for such factors demonstrate similar findings to our work. For example, a recent report by Robertson et al. using F2 littermate controls showed that Nod1 and Nod2 signaling does not alter the composition of intestinal bacterial communities.42 Similarly, Ubeda et al. demonstrated that MyD88- and TLR-deficient mice developed divergent intestinal microbial profiles relative to WT mice when bred in isolation; however, these differences were lost when WT and KO littermates were housed together prior to analysis.43
It appears, then, that additional environmental factors influence the structure of the intestinal microbiota, even in highly controlled inbred mouse studies. Previous work has demonstrated that maternal transmission,44 time after exposure,45 and cage microenvironment46 can have a significant impact on gut microbial composition. In our analyses, we found that the cage mice were housed in had a stronger influence on intestinal microbial composition than Nod2 signaling.7 However, it should be noted that our design involved housing mice of the same litter within the same cage, and hence it is not possible to distinguish cage and litter effects in our analysis. Regardless, these findings suggest that the number of cages (and perhaps litters) used in mouse microbiota studies should be a key consideration in experimental design, as well as downstream statistical analyses.
Conclusions and Future Directions
In this addendum, we have attempted to highlight critical design issues involved in the study of Paneth cells and the enteric microbiota. Specifically, we emphasize that comprehensive evaluation of Paneth cells can be achieved through multiple levels of investigation, including examination of development, AMP biosynthesis (including transcription, translation, and post-translational processing), secretion, and downstream effects on the host in vivo. Moreover, it is essential to ensure that mouse background is strictly controlled, as this can have a profound impact on Paneth cell antimicrobial function. Finally, studies that analyze gut microbial composition should include a priori consideration of cage and litter numbers, as well as appropriate treatment of such confounders in downstream statistical analyses.
Numerous challenges remain regarding the study of Paneth cells in health and disease. In particular, the lack of in vitro systems to study these cells has made it difficult to elucidate mechanistic pathways that control Paneth cell development and function. Interestingly, recent work using ex vivo crypt culture systems may offer an innovative approach to overcome these obstacles.11 Indeed, a recent study using cultured small intestinal enteroids supports our findings of similar Paneth cell numbers in WT and Nod2−/− mice.47 In vivo, Paneth cell studies have been limited by the lack of a Paneth cell-specific knockout model. However, a system for this was recently published using transgenic mice in which an α-defensin promoter drives expression a Cre recombinase, allowing for gene deletion specifically in Paneth cells.48 As these technologies become more readily available in the future, understanding precisely how Paneth cells influence gut microbial structure and intestinal stem cell function will become possible. Such insight may offer new approaches to the treatment of complex medical diseases, focusing on manipulation of Paneth cell function as a novel therapeutic modality.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
The authors wish to thank Dr Christopher Dekaney (University of North Carolina at Chapel Hill) for his critical review of this manuscript. Dr Dekaney also provided histology images used in this addendum. We also thank Dr Andre Ouellette (University of Southern California) for kindly providing the recombinant α-defensin 4 described in our studies. Finally, we acknowledge the services provided by the Histology and Microbiome Cores at the UNC Center for Gastrointestinal Biology and Disease. This work was funded by a Career Development Award from the Children’s Digestive Health and Nutrition Foundation/Crohn’s and Colitis Foundation of America (A.S.G.) and NIH grants K08 DK09517 (A.S.G.), K01 DK092330 (I.M.C.), R01 DK53347 (R.B.S.), P40 RR018603 (R.B.S.), and DK34987 (Robert S Sandler, PI).
Glossary
Abbreviations:
- 129
129/SvEv
- AMP
antimicrobial peptide
- AU-PAGE
acid urea-polyacrylamide gel electrophoresis
- B6
C57BL/6
- CD
Crohn’s disease
- CRS
cryptdin-related sequence
- H&E
hematoxylin and eosin
- IHC
immunohistochemistry
- LCM
laser capture microdissection
- Lyz
lysozyme
- Mmp7
matrix metalloproteinase 7
- Nod2
nucleotide-binding, oligomerization domain 2
- qRT-PCR
quantitative reverse-transcriptase polymerase chain reaction
- TEM
transmission electron microscopy
- TLR
toll-like receptor
- WT
wild-type
References
- 1.Ouellette AJ. Paneth cell α-defensins in enteric innate immunity. Cell Mol Life Sci. 2011;68:2215–29. doi: 10.1007/s00018-011-0714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–8. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 3.Wang G, Li X, Wang Z. APD2: the updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 2009;37:D933–7. doi: 10.1093/nar/gkn823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356–68. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 5.Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, López-Boado YS, Stratman JL, Hultgren SJ, Matrisian LM, Parks WC. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–7. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 6.Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjöberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shanahan MT, Carroll IM, Grossniklaus E, White A, von Furstenberg RJ, Barner R, Fodor AA, Henning SJ, Sartor RB, Gulati AS. Mouse Paneth cell antimicrobial function is independent of Nod2. Gut. 2013 doi: 10.1136/gutjnl-2012-304190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci U S A. 2008;105:20858–63. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–63. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gulati AS, Shanahan MT, Arthur JC, Grossniklaus E, von Furstenberg RJ, Kreuk L, Henning SJ, Jobin C, Sartor RB. Mouse background strain profoundly influences Paneth cell function and intestinal microbial composition. PLoS One. 2012;7:e32403. doi: 10.1371/journal.pone.0032403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouellette AJ. Paneth cells and innate mucosal immunity. Curr Opin Gastroenterol. 2010;26:547–53. doi: 10.1097/MOG.0b013e32833dccde. [DOI] [PubMed] [Google Scholar]
- 12.Darmoul D, Ouellette AJ. Positional specificity of defensin gene expression reveals Paneth cell heterogeneity in mouse small intestine. Am J Physiol. 1996;271:G68–74. doi: 10.1152/ajpgi.1996.271.1.G68. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson J, Pütsep K, Chu H, Kays RJ, Bevins CL, Andersson M. Regional variations in Paneth cell antimicrobial peptide expression along the mouse intestinal tract. BMC Immunol. 2008;9:37. doi: 10.1186/1471-2172-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shirafuji Y, Tanabe H, Satchell DP, Henschen-Edman A, Wilson CL, Ouellette AJ. Structural determinants of procryptdin recognition and cleavage by matrix metalloproteinase-7. J Biol Chem. 2003;278:7910–9. doi: 10.1074/jbc.M210600200. [DOI] [PubMed] [Google Scholar]
- 15.Lehrer RI, Rosenman M, Harwig SS, Jackson R, Eisenhauer P. Ultrasensitive assays for endogenous antimicrobial polypeptides. J Immunol Methods. 1991;137:167–73. doi: 10.1016/0022-1759(91)90021-7. [DOI] [PubMed] [Google Scholar]
- 16.Satoh Y, Habara Y, Ono K, Kanno T. Carbamylcholine- and catecholamine-induced intracellular calcium dynamics of epithelial cells in mouse ileal crypts. Gastroenterology. 1995;108:1345–56. doi: 10.1016/0016-5085(95)90681-9. [DOI] [PubMed] [Google Scholar]
- 17.Ayabe T, Wulff H, Darmoul D, Cahalan MD, Chandy KG, Ouellette AJ. Modulation of mouse Paneth cell alpha-defensin secretion by mIKCa1, a Ca2+-activated, intermediate conductance potassium channel. J Biol Chem. 2002;277:3793–800. doi: 10.1074/jbc.M107507200. [DOI] [PubMed] [Google Scholar]
- 18.Meyer-Hoffert U, Hornef MW, Henriques-Normark B, Axelsson LG, Midtvedt T, Pütsep K, Andersson M. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut. 2008;57:764–71. doi: 10.1136/gut.2007.141481. [DOI] [PubMed] [Google Scholar]
- 19.Masuda K, Sakai N, Nakamura K, Yoshioka S, Ayabe T. Bactericidal activity of mouse α-defensin cryptdin-4 predominantly affects noncommensal bacteria. J Innate Immun. 2011;3:315–26. doi: 10.1159/000322037. [DOI] [PubMed] [Google Scholar]
- 20.Lehrer RI, Ganz T, Szklarek D, Selsted ME. Modulation of the in vitro candidacidal activity of human neutrophil defensins by target cell metabolism and divalent cations. J Clin Invest. 1988;81:1829–35. doi: 10.1172/JCI113527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Lisle RC. Altered transit and bacterial overgrowth in the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G104–11. doi: 10.1152/ajpgi.00548.2006. [DOI] [PubMed] [Google Scholar]
- 22.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–94. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 23.Keshav S. Paneth cells: leukocyte-like mediators of innate immunity in the intestine. J Leukoc Biol. 2006;80:500–8. doi: 10.1189/jlb.1005556. [DOI] [PubMed] [Google Scholar]
- 24.van Es JH, Jay P, Gregorieff A, van Gijn ME, Jonkheer S, Hatzis P, Thiele A, van den Born M, Begthel H, Brabletz T, et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol. 2005;7:381–6. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 25.Koslowski MJ, Kübler I, Chamaillard M, Schaeffeler E, Reinisch W, Wang G, Beisner J, Teml A, Peyrin-Biroulet L, Winter S, et al. Genetic variants of Wnt transcription factor TCF-4 (TCF7L2) putative promoter region are associated with small intestinal Crohn’s disease. PLoS One. 2009;4:e4496. doi: 10.1371/journal.pone.0004496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wehkamp J, Wang G, Kübler I, Nuding S, Gregorieff A, Schnabel A, Kays RJ, Fellermann K, Burk O, Schwab M, et al. The Paneth cell alpha-defensin deficiency of ileal Crohn’s disease is linked to Wnt/Tcf-4. J Immunol. 2007;179:3109–18. doi: 10.4049/jimmunol.179.5.3109. [DOI] [PubMed] [Google Scholar]
- 27.Wehkamp J, Harder J, Weichenthal M, Schwab M, Schäffeler E, Schlee M, Herrlinger KR, Stallmach A, Noack F, Fritz P, et al. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53:1658–64. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–56. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simms LA, Doecke JD, Roberts RL, Fowler EV, Zhao ZZ, McGuckin MA, Huang N, Hayward NK, Webb PM, Whiteman DC, et al. KCNN4 gene variant is associated with ileal Crohn’s Disease in the Australian and New Zealand population. Am J Gastroenterol. 2010;105:2209–17. doi: 10.1038/ajg.2010.161. [DOI] [PubMed] [Google Scholar]
- 30.Zheng W, Rosenstiel P, Huse K, Sina C, Valentonyte R, Mah N, Zeitlmann L, Grosse J, Ruf N, Nürnberg P, et al. Evaluation of AGR2 and AGR3 as candidate genes for inflammatory bowel disease. Genes Immun. 2006;7:11–8. doi: 10.1038/sj.gene.6364263. [DOI] [PubMed] [Google Scholar]
- 31.Liu B, Gulati AS, Cantillana V, Henry SC, Schmidt EA, Daniell X, Grossniklaus E, Schoenborn AA, Sartor RB, Taylor GA. Irgm1-deficient mice exhibit Paneth cell abnormalities and increased susceptibility to acute intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2013;305:G573–84. doi: 10.1152/ajpgi.00071.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, et al. International IBD Genetics Consortium (IIBDGC) Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–24. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogura Y, Lala S, Xin W, Smith E, Dowds TA, Chen FF, Zimmermann E, Tretiakova M, Cho JH, Hart J, et al. Expression of NOD2 in Paneth cells: a possible link to Crohn’s ileitis. Gut. 2003;52:1591–7. doi: 10.1136/gut.52.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simms LA, Doecke JD, Walsh MD, Huang N, Fowler EV, Radford-Smith GL. Reduced alpha-defensin expression is associated with inflammation and not NOD2 mutation status in ileal Crohn’s disease. Gut. 2008;57:903–10. doi: 10.1136/gut.2007.142588. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–4. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 36.Petnicki-Ocwieja T, Hrncir T, Liu YJ, Biswas A, Hudcovic T, Tlaskalova-Hogenova H, Kobayashi KS. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci U S A. 2009;106:15813–8. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouellette AJ, Pravtcheva D, Ruddle FH, James M. Localization of the cryptdin locus on mouse chromosome 8. Genomics. 1989;5:233–9. doi: 10.1016/0888-7543(89)90051-7. [DOI] [PubMed] [Google Scholar]
- 38.Shanahan MT, Tanabe H, Ouellette AJ. Strain-specific polymorphisms in Paneth cell α-defensins of C57BL/6 mice and evidence of vestigial myeloid α-defensin pseudogenes. Infect Immun. 2011;79:459–73. doi: 10.1128/IAI.00996-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rehman A, Sina C, Gavrilova O, Häsler R, Ott S, Baines JF, Schreiber S, Rosenstiel P. Nod2 is essential for temporal development of intestinal microbial communities. Gut. 2011;60:1354–62. doi: 10.1136/gut.2010.216259. [DOI] [PubMed] [Google Scholar]
- 40.Mondot S, Barreau F, Al Nabhani Z, Dussaillant M, Le Roux K, Doré J, Leclerc M, Hugot JP, Lepage P. Altered gut microbiota composition in immune-impaired Nod2(-/-) mice. Gut. 2012;61:634–5. doi: 10.1136/gutjnl-2011-300478. [DOI] [PubMed] [Google Scholar]
- 41.Couturier-Maillard A, Secher T, Rehman A, Normand S, De Arcangelis A, Haesler R, Huot L, Grandjean T, Bressenot A, Delanoye-Crespin A, et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest. 2013;123:700–11. doi: 10.1172/JCI62236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robertson SJ, Zhou JY, Geddes K, Rubino SJ, Cho JH, Girardin SE, Philpott DJ. Nod1 and Nod2 signaling does not alter the composition of intestinal bacterial communities at homeostasis. Gut Microbes. 2013;4:222–31. doi: 10.4161/gmic.24373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ubeda C, Lipuma L, Gobourne A, Viale A, Leiner I, Equinda M, Khanin R, Pamer EG. Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of TLR-deficient mice. J Exp Med. 2012;209:1445–56. doi: 10.1084/jem.20120504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friswell MK, Gika H, Stratford IJ, Theodoridis G, Telfer B, Wilson ID, McBain AJ. Site and strain-specific variation in gut microbiota profiles and metabolism in experimental mice. PLoS One. 2010;5:e8584. doi: 10.1371/journal.pone.0008584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gillilland MG, 3rd, Erb-Downward JR, Bassis CM, Shen MC, Toews GB, Young VB, Huffnagle GB. Ecological succession of bacterial communities during conventionalization of germ-free mice. Appl Environ Microbiol. 2012;78:2359–66. doi: 10.1128/AEM.05239-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCafferty J, Mühlbauer M, Gharaibeh RZ, Arthur JC, Perez-Chanona E, Sha W, Jobin C, Fodor AA. Stochastic changes over time and not founder effects drive cage effects in microbial community assembly in a mouse model. ISME J. 2013;7:2116–25. doi: 10.1038/ismej.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Limbergen J, Geddes K, Henderson P, Russell RK, Drummond HE, Satsangi J, Griffiths AM, Philpott DJ, Wilson DC. Paneth cell marker CD24 in NOD2 knockout organoids and in inflammatory bowel disease (IBD) Gut. 2013;••• doi: 10.1136/gutjnl-2013-305077. [DOI] [PubMed] [Google Scholar]
- 48.Adolph TE, Tomczak MF, Niederreiter L, Ko HJ, Böck J, Martinez-Naves E, Glickman JN, Tschurtschenthaler M, Hartwig J, Hosomi S, et al. Paneth cells as a site of origin for intestinal inflammation. Nature. 2013;503:272–6. doi: 10.1038/nature12599. [DOI] [PMC free article] [PubMed] [Google Scholar]