Abstract
Global incidence rates for inflammatory bowel disease (IBD) have gradually risen over the past 20 years. Genome-wide association studies (GWAS) have identified over 160 genetic loci associated with IBD; however, inherited factors only account for a partial contribution to the disease risk. We have recently shown that urban airborne particulate matter (PM) ingested via contaminated food can alter gut microbiome and immune function under normal and inflammatory conditions. In this addendum, we will discuss how PM can modify the gut microbial form and function, provide evidence on changes seen in intestinal barrier, and suggest a working hypothesis of how pollutants affect the gastrointestinal tract. The significance of the work presented could lead to identifying airborne pollutants as potential risk factors and thus provide better patient care management.
Keywords: air pollution, environment, cytokines, gut microbiota, mucosal inflammation, intestinal permeability, short chain fatty acids
Introduction
The world is seeing a dramatic rise in population growth in urban areas. As urban populations grow, the quality of the environment, and especially urban air pollution, will play an increasingly important role in public health. It has become clear that the disease burden due to urban air pollution is on the rise. Though research on airborne pollutants has mostly focused on cardiovascular and respiratory effects, emerging evidence suggests air pollutants are also having adverse effects on the gastrointestinal tract. Epidemiological studies have revealed an association between exposure to air pollution and different gastrointestinal diseases including inflammatory bowel disease (IBD),1,2 appendicitis,3 irritable bowel syndrome,4 and enteric infections in infants.5
Components of Air Pollution
Air pollution is a heterogeneous mixture of a number of substances, including gases (e.g., carbon dioxide, carbon monoxide, ozone, nitric oxide, and sulfur dioxide), volatile organic compounds (e.g., benzene), and particulate matter.6 Particulate matter (PM) is a key pollutant in airborne pollution that has been associated with many adverse health conditions. Most airborne PM pollutants are derived from combustion of fossil fuels (e.g., from car exhaust) and industrial effluents. The major components of PM are pollen, sulfates, nitrates, organic carbon, mineral dust, polycyclic aromatic hydrocarbons (PAH), metals, ions, and biological components (microbial particles, lipopolysaccharide, and spores).7 These particles are defined according to their aerodynamic diameter as either fine particles of a diameter smaller than 2.5 μm (PM2.5) or coarse with a diameter smaller than 10 μm (PM10).7 The overall composition of air pollution depends upon the local sources; these include fossil fuel combustion from cars, home furnaces, and factories, as well as livestock emissions. The total pollution exposure of an individual will be determined not only by where they live but also where they travel and where they work. Two components of air pollution that have been extensively investigated for biological effects include ozone and particulate matter (PM). Studies have shown that exposure to ozone results in an increase in cellular permeability and a breakdown of tight junctions, while increased PM exposure is linked with increased cardiovascular mortality.8,9 It is also likely that other components of air pollution yet to be studied may also have detrimental effects on human physiology.
Exposure of the Gut to Particulate Matter
The gastrointestinal tract is exposed to high concentrations of pollutant PM. Human studies have shown mucociliary transport of inhaled PM are quickly cleared from the lungs and into the intestine.10 Furthermore, pollutant PM contaminates both our food and water supply in significant amounts and, hence, account for additional oral route exposure.11,12 It has been estimated that 1012–1014 particles are ingested per day by an individual on a typical Western diet, with an estimated mucosal uptake of ~1% (109–1012 per day).13,14 As such, understanding what role PM might be playing in the recent rise of gastrointestinal diseases, particularly the rise in incidences of IBD, is a relevant point of study.
Importantly, air pollution is not the only inhaled environmental exposure that has been associated with gastrointestinal disease. Smoking is an example of an inhaled exposure that has been shown to influence the susceptibility of developing IBD.15-18 Animal model studies suggest that smoking may mediate its effects through alterations of intestinal microbiota.19
Epidemiologic Associations between Air Pollution and Gut Disease
Epidemiologic data are beginning to show that air pollution has important health effects on the gastrointestinal tract. Long-term exposure to higher concentrations of nitrogen dioxide and PM were associated with an increased risk of early-onset Crohn disease.1 Among Crohn disease patients, the risk associated with air pollution increased linearly across concentration levels suggesting a dose response effect.1 Additionally, a second study demonstrated that a rise in total measured air pollutants was associated with an increased risk of hospitalizations for IBD.2 Short-term exposure to air pollution has also been associated with gastrointestinal diseases. A single city study demonstrated an association between ozone exposure and appendicitis.20 A replication study conducted in 12 cities across Canada confirmed the association between ozone and appendicitis. Moreover, this study showed that higher levels of ozone exposure shifted the course of appendicitis toward a perforating phenotype.3 A multi-city study showed that an acute rise in air pollution was associated with increased emergency department visits for non-specific acute abdominal pain in young women.4 Another multi-city study demonstrated that infants were more likely to visit emergency departments for gastroenteritis when carbon dioxide levels were acutely elevated.5
However, important limitations of epidemiologic air pollution studies should be considered, including that facts that air pollution exposures are generally based on regional estimates, not personal monitoring; gastrointestinal diseases are identified based on administrative databases that may contain misclassification errors; false positive associations can occur when multiple air pollutants are considered; and the possibility of bias due to confounding factors. As more biological studies are performed, a clearer picture of the effects of particular air pollutants on gastrointestinal function is bound to emerge.
Ingested Particulate Matter Alters the Gut Microbiome
There are over 1014 microbes in the human gastrointestinal tract and they encode for over 100-fold more unique genes than our own genome.21 Recent evidence has implicated a dysfunctional gut microbiome in the development of several disorders, including diabetes, obesity, metabolic disorders, and IBD.22 As such, understanding the factors that cause alterations in the gut microbes is important to understand disease pathogenesis. Despite the well-studied effects of environmental pollutants on various health conditions, little is known on how air pollution impacts the gut microbiome. Using a human gastrointestinal simulator, Van de Wiele et al. illustrated that human intestinal bacteria metabolised inorganic arsenic from contaminated soils into various toxic species.23 In another experiment, gut microbes were also shown to transform PAHs into compounds that exhibit estrogenic properties in the body.23 These results highlight that gut microbes are engaged in bioactivation of inorganic compounds, which in turn may contribute to disease risk development and perpetuation of chronic diseases.
In our recent work, we showed that ingested pollutant particles altered gut microbiota composition and function.24 IL-10−/− mice (whose colitis development depends on the gut microbiota) given particulate matter in their chow showed significant changes in the relative amounts of Bacteroidetes, Firmicutes, and Verrucomicrobia. These changes in microbial abundance correlated with changes in short chain fatty acid production, in that mice fed the particles had increased concentrations of the branched chain fatty acids isobutyrate and isovalerate in the cecum. Isobutyrate and isovalerate in human stool originate from the degradation of amino acids valine, leucine, or isoleucine,25 and the finding of an increase in these compounds indicates a shift from a carbohydrate to a protein fermentation environment, indicating changes in microbial composition. In addition, exposure of the gut to particulate matter resulted in a decreased concentration of butyrate. This is significant in that butyrate is an essential fatty acid for colonocytes and mucosal immune cells, and a depletion in butyrate is commonly associated with a decrease in barrier function and increased susceptibility to mucosal inflammation.26 In a similar study, mice exposed to polychlorinated biphenyls (PCBs) from contaminated foods showed a substantial shift in abundance and composition of the gut microbiome.27 The mechanism underlying this PM-induced shift in microbial composition remains to be shown; however, taken together, these results suggest that ingested environmental pollutants can significantly alter both gut microbe composition and also metabolic processes. These alterations can have detrimental effects on not only the digestion process occurring in the lumen, but also on the structure and function of the intestinal mucosa.
Particulate Matter Increases Intestinal Permeability
At the interface between the external luminal environment and the internal body proper lies the highly regulated epithelial monolayer that not only serves to absorb nutrients and water, but also provides important barrier and immune surveillance mechanisms.28 Changes in intestinal barrier have been associated with a number of diseases including IBD and celiac disease.29 In our study we found that upon ingestion of particulate matter, there was an increased small intestinal permeability that was accompanied with an inflammatory response. Analysis of lactulose/mannitol excretion in urine revealed that pollutant PM increased permeability compared with the control group. Mutlu et al. showed that decreased epithelial barrier caused by particulate matter was associated with rearrangement of the epithelial tight junction proteins.30 They further showed that particulate matter-induced barrier disruptions were linked to free radical oxygen species (ROS) that were generated by epithelial cells. These observations suggest that either directly or indirectly, particulate matter contributes to increased gut permeability. As such, gut exposure to airborne pollutant may play a pathogenic role in contributing to gastrointestinal disorders through disruptions in the epithelial barrier.
Increases in gut permeability have been linked with intestinal inflammation in several animal models and in human diseases.29 In the IL-10−/− mouse, ingested particulate matter both increased gut permeability and exacerbated colonic inflammation.24 Past studies have demonstrated that ingested particulate matter reduces colonic contractility due to neuronal impairment rather than muscular mechanisms.4 As such, colonic mucosal immune cells will have prolonged exposure to particulate matter. In addition, several studies have demonstrated in vitro exposure of immune cells to particulate matter to result in an enhanced pro-inflammatory cytokine secretion, due in part to increased levels of oxidative stress and activation of MyD88.31-35
In addition to gastrointestinal disease, inflammation has been shown to have a strong association with many chronic diseases seen in western countries, including asthma, type 1 and 2 diabetes, and atherosclerosis.36,37 Type 1 diabetes is associated with type 1 interferon production and alterations in T cell signaling, suggesting an autoimmune response, while type 2 diabetes is associated with enhanced inflammatory responses in adipose tissue and insulin resistance.38 Obesity and atherosclerosis have also been associated with chronic inflammation as evidenced by increased levels of serologic markers such as C-reactive protein (CRP).36,37 Thus, a particulate matter-induced increase in gut permeability may have a significant contribution to enhanced levels of systemic inflammation, due to an unrestrained influx of microbial products from the gut into the systemic circulation.
Model of Particulate Matter Induction of Intestinal and Systemic Inflammation
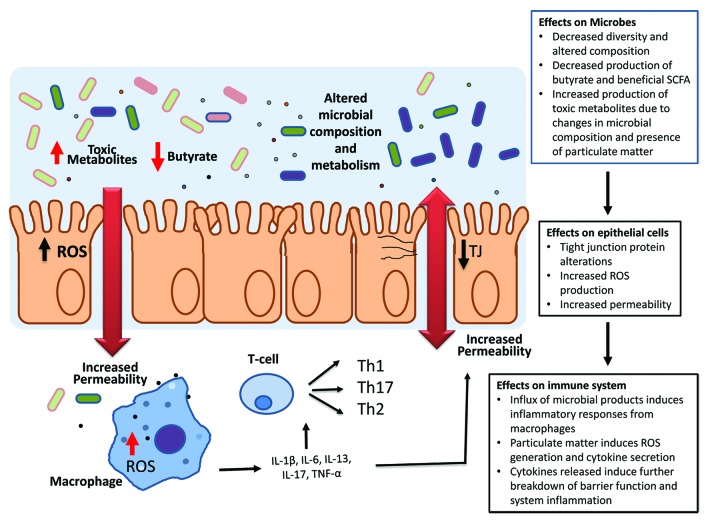
As epidemiological evidence mounts for ingested pollutant PM having a role in gastrointestinal disorders, dissecting the mechanisms behind such alterations will provide insight into how particulate matter can trigger disease onset or reoccurrence. Based on the research evidence provided, we would propose the following mechanism (Fig. 1).
Figure 1. Proposed model of particulate matter induction of intestinal and systemic inflammation. Particles will have both direct effects on the gut epithelial cells and be metabolized by resident gut microbes into toxic metabolites. PM-induced changes in epithelial tight junctions through ROS production increase gut permeability allowing for an influx of microbial products and particulate matter into the lamina propria and increased interactions with immune cells. This induces a pro-inflammatory response from resident immune cells which will further increase gut permeability and alter the luminal environment of the gut to allow for the growth of particular microbial strains more suited to survival in an inflammatory environment. Select microbial groups metabolize particulate matter into toxic metabolites which can directly influence microbial survival and epithelial function. The altered microbial community subsequently leads to changes in metabolic processes within the host whereby a decrease in short chain fatty acid production occurs along with enhanced production of non-beneficial metabolites.
Ingested particulate matter enters the gastrointestinal tract either through the mucociliary clearance of inhaled airborne pollutants from the lungs and/or dietary intake of contaminated food and drinking water. Once in the intestinal lumen, the particles will have both direct effects on the gut epithelial cells and be metabolized by resident gut microbes into toxic metabolites. PM-induced changes in epithelial tight junctions through ROS production will increase gut permeability allowing for an influx of microbial products and particulate matter into the lamina propria, and increased interactions with immune cells. This microbial influx induces a pro-inflammatory response from resident macrophage and dendritic cells, which can drive systemic inflammation, further worsen gut permeability, and alter the luminal environment of the gut to allow for the growth of particular microbial strains more suited to survival in an inflammatory environment. Further, select microbial groups may also metabolize some particulate matter into toxic metabolites, which can also directly influence microbial survival and epithelial function. The altered microbial community subsequently leads to changes in metabolic processes within the host whereby a decrease in short chain fatty acid production occurs, along with enhanced production of non-beneficial metabolites. Decreased butyrate production by gut microbes affects hosts homeostasis by increasing susceptibility to intestinal permeability and mucosal inflammation.
Conclusion
Together, our study in IL-10−/− mice, in conjunction with previous experimental and epidemiological observations, strongly suggests that ingested particulate matter could trigger and accelerate the development of gastrointestinal inflammatory diseases, particularly in genetically susceptible individuals. This can occur through a combination of factors, including increased gut permeability, decreased colonic motility and clearance, and altered gut microbial composition and metabolic function.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- 1.Kaplan GG, Hubbard J, Korzenik J, Sands BE, Panaccione R, Ghosh S, Wheeler AJ, Villeneuve PJ. The inflammatory bowel diseases and ambient air pollution: a novel association. Am J Gastroenterol. 2010;105:2412–9. doi: 10.1038/ajg.2010.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ananthakrishnan AN, McGinley EL, Binion DG, Saeian K. Ambient air pollution correlates with hospitalizations for inflammatory bowel disease: an ecologic analysis. Inflamm Bowel Dis. 2011;17:1138–45. doi: 10.1002/ibd.21455. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan GG, Tanyingoh D, Dixon E, Johnson M, Wheeler AJ, Myers RP, Bertazzon S, Saini V, Madsen K, Ghosh S, et al. Ambient ozone concentrations and the risk of perforated and nonperforated appendicitis: a multicity case-crossover study. Environ Health Perspect. 2013;121:939–43. doi: 10.1289/ehp.1206085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan GG, Szyszkowicz M, Fichna J, Rowe BH, Porada E, Vincent R, Madsen K, Ghosh S, Storr M. Non-specific abdominal pain and air pollution: a novel association. PLoS One. 2012;7:e47669. doi: 10.1371/journal.pone.0047669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orazzo F, Nespoli L, Ito K, Tassinari D, Giardina D, Funis M, Cecchi A, Trapani C, Forgeschi G, Vignini M, et al. Air pollution, aeroallergens, and emergency room visits for acute respiratory diseases and gastroenteric disorders among young children in six Italian cities. Environ Health Perspect. 2009;117:1780–5. doi: 10.1289/ehp.0900599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Goldberg MS, Villeneuve PJ. A systematic review of the relation between long-term exposure to ambient air pollution and chronic diseases. Rev Environ Health. 2008;23:243–97. doi: 10.1515/reveh.2008.23.4.243. [DOI] [PubMed] [Google Scholar]
- 7.Vincent R, Bjarnason SG, Adamson IY, Hedgecock C, Kumarathasan P, Guénette J, Potvin M, Goegan P, Bouthillier L. Acute pulmonary toxicity of urban particulate matter and ozone. Am J Pathol. 1997;151:1563–70. [PMC free article] [PubMed] [Google Scholar]
- 8.Bhalla DK. Ozone-induced lung inflammation and mucosal barrier disruption: toxicology, mechanisms, and implications. J Toxicol Environ Health B Crit Rev. 1999;2:31–86. doi: 10.1080/109374099281232. [DOI] [PubMed] [Google Scholar]
- 9.Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, et al. American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 10.Möller W, Häussinger K, Winkler-Heil R, Stahlhofen W, Meyer T, Hofmann W, Heyder J. Mucociliary and long-term particle clearance in the airways of healthy nonsmoker subjects. J Appl Physiol (1985) 2004;97:2200–6. doi: 10.1152/japplphysiol.00970.2003. [DOI] [PubMed] [Google Scholar]
- 11.Beamish LA, Osornio-Vargas AR, Wine E. Air pollution: An environmental factor contributing to intestinal disease. J Crohns Colitis. 2011;5:279–86. doi: 10.1016/j.crohns.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 12.De Brouwere K, Buekers J, Cornelis C, Schlekat CE, Oller AR. Assessment of indirect human exposure to environmental sources of nickel: oral exposure and risk characterization for systemic effects. Sci Total Environ. 2012;419:25–36. doi: 10.1016/j.scitotenv.2011.12.049. [DOI] [PubMed] [Google Scholar]
- 13.Lomer MC, Thompson RP, Powell JJ. Fine and ultrafine particles of the diet: influence on the mucosal immune response and association with Crohn’s disease. Proc Nutr Soc. 2002;61:123–30. doi: 10.1079/PNS2001134. [DOI] [PubMed] [Google Scholar]
- 14.Lomer MC, Hutchinson C, Volkert S, Greenfield SM, Catterall A, Thompson RP, Powell JJ. Dietary sources of inorganic microparticles and their intake in healthy subjects and patients with Crohn’s disease. Br J Nutr. 2004;92:947–55. doi: 10.1079/BJN20041276. [DOI] [PubMed] [Google Scholar]
- 15.Calkins BM. A meta-analysis of the role of smoking in inflammatory bowel disease. Dig Dis Sci. 1989;34:1841–54. doi: 10.1007/BF01536701. [DOI] [PubMed] [Google Scholar]
- 16.Cosnes J, Carbonnel F, Beaugerie L, Le Quintrec Y, Gendre JP. Effects of cigarette smoking on the long-term course of Crohn’s disease. Gastroenterology. 1996;110:424–31. doi: 10.1053/gast.1996.v110.pm8566589. [DOI] [PubMed] [Google Scholar]
- 17.Cosnes J, Beaugerie L, Carbonnel F, Gendre JP. Smoking cessation and the course of Crohn’s disease: an intervention study. Gastroenterology. 2001;120:1093–9. doi: 10.1053/gast.2001.23231. [DOI] [PubMed] [Google Scholar]
- 18.Sands BE, Arsenault JE, Rosen MJ, Alsahli M, Bailen L, Banks P, Bensen S, Bousvaros A, Cave D, Cooley JS, et al. Risk of early surgery for Crohn’s disease: implications for early treatment strategies. Am J Gastroenterol. 2003;98:2712–8. doi: 10.1111/j.1572-0241.2003.08674.x. [DOI] [PubMed] [Google Scholar]
- 19.Benjamin JL, Hedin CR, Koutsoumpas A, Ng SC, McCarthy NE, Prescott NJ, Pessoa-Lopes P, Mathew CG, Sanderson J, Hart AL, et al. Smokers with active Crohn’s disease have a clinically relevant dysbiosis of the gastrointestinal microbiota. Inflamm Bowel Dis. 2012;18:1092–100. doi: 10.1002/ibd.21864. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan GG, Dixon E, Panaccione R, Fong A, Chen L, Szyszkowicz M, Wheeler A, MacLean A, Buie WD, Leung T, et al. Effect of ambient air pollution on the incidence of appendicitis. CMAJ. 2009;181:591–7. doi: 10.1503/cmaj.082068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–48. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Kinross JM, Darzi AW, Nicholson JK. Gut microbiome-host interactions in health and disease. Genome Med. 2011;3:14. doi: 10.1186/gm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van de Wiele T, Vanhaecke L, Boeckaert C, Peru K, Headley J, Verstraete W, Siciliano S. Human colon microbiota transform polycyclic aromatic hydrocarbons to estrogenic metabolites. Environ Health Perspect. 2005;113:6–10. doi: 10.1289/ehp.7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kish L, Hotte N, Kaplan GG, Vincent R, Tso R, Gänzle M, Rioux KP, Thiesen A, Barkema HW, Wine E, et al. Environmental particulate matter induces murine intestinal inflammatory responses and alters the gut microbiome. PLoS One. 2013;8:e62220. doi: 10.1371/journal.pone.0062220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zarling EJ, Ruchim MA. Protein origin of the volatile fatty acids isobutyrate and isovalerate in human stool. J Lab Clin Med. 1987;109:566–70. [PubMed] [Google Scholar]
- 26.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–7. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 27.Choi JJ, Eum SY, Rampersaud E, Daunert S, Abreu MT, Toborek M. Exercise attenuates PCB-induced changes in the mouse gut microbiome. Environ Health Perspect. 2013;121:725–30. doi: 10.1289/ehp.1306534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salim SY, Söderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:362–81. doi: 10.1002/ibd.21403. [DOI] [PubMed] [Google Scholar]
- 29.Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55:1512–20. doi: 10.1136/gut.2005.085373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mutlu EA, Engen PA, Soberanes S, Urich D, Forsyth CB, Nigdelioglu R, Chiarella SE, Radigan KA, Gonzalez A, Jakate S, et al. Particulate matter air pollution causes oxidant-mediated increase in gut permeability in mice. Part Fibre Toxicol. 2011;8:19. doi: 10.1186/1743-8977-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osornio-Vargas AR, Serrano J, Rojas-Bracho L, Miranda J, García-Cuellar C, Reyna MA, Flores G, Zuk M, Quintero M, Vázquez I, et al. In vitro biological effects of airborne PM₂.₅ and PM₁₀ from a semi-desert city on the Mexico-US border. Chemosphere. 2011;83:618–26. doi: 10.1016/j.chemosphere.2010.11.073. [DOI] [PubMed] [Google Scholar]
- 32.van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, Qui D, Vincent R, Hogg JC. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)) Am J Respir Crit Care Med. 2001;164:826–30. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- 33.Sawyer K, Mundandhara S, Ghio AJ, Madden MC. The effects of ambient particulate matter on human alveolar macrophage oxidative and inflammatory responses. J Toxicol Environ Health A. 2010;73:41–57. doi: 10.1080/15287390903248901. [DOI] [PubMed] [Google Scholar]
- 34.Park CY, Hill KM, Elble AE, Martin BR, DiMeglio LA, Peacock M, McCabe GP, Weaver CM. Daily supplementation with 25 μg cholecalciferol does not increase calcium absorption or skeletal retention in adolescent girls with low serum 25-hydroxyvitamin D. J Nutr. 2010;140:2139–44. doi: 10.3945/jn.110.124891. [DOI] [PubMed] [Google Scholar]
- 35.Jalava PI, Hirvonen MR, Sillanpää M, Pennanen AS, Happo MS, Hillamo R, Cassee FR, Gerlofs-Nijland M, Borm PJ, Schins RP, et al. Associations of urban air particulate composition with inflammatory and cytotoxic responses in RAW 246.7 cell line. Inhal Toxicol. 2009;21:994–1006. doi: 10.1080/08958370802695710. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonora E. The metabolic syndrome and cardiovascular disease. Ann Med. 2006;38:64–80. doi: 10.1080/07853890500401234. [DOI] [PubMed] [Google Scholar]
- 38.Badman MK, Flier JS. The adipocyte as an active participant in energy balance and metabolism. Gastroenterology. 2007;132:2103–15. doi: 10.1053/j.gastro.2007.03.058. [DOI] [PubMed] [Google Scholar]