Abstract
There is convincing evidence from both human and animal studies suggesting that the infant intestinal microbiota plays an important role in regulating immune responses associated with the development of allergic diseases. To date there are, however, still no definite bacterial taxa or particular subsets of the microbiota that have been consistently associated with allergic diseases, which is mainly attributable to the methodological dissimilarities between studies. As such there is a need to apply different methodological concepts to enhance a deeper and more refined understanding of the relationship between the gut microbiota and allergies. Within our recent studies we reported that colonization by clostridia in early infancy increased the risk of atopic dermatitis. Using subsequent mediation analysis, we demonstrated that birth mode and having older siblings strongly impacted the infant microbiota which in turn affected the risk of atopic dermatitis. The results of these mediation analyses contributed stronger evidence for a causal link of birth mode and birth order on allergy risk through modulation of the microbiota composition.
Keywords: allergy, microbiota, hygiene hypothesis, epidemiology, infant
The Microbiota Hypothesis in Allergic Diseases
Allergic (or atopic) diseases, such as atopic dermatitis (eczema), food allergies, hay fever, and asthma, are chronic inflammatory disorders caused by aberrant immune responses against common “innocuous” environmental antigens (allergens) in susceptible individuals.1 An enhanced T helper (Th)2 immune response and the elaboration of cytokines such as interleukin (IL)-4, IL-13, and IL-5 contribute to the induction and maintenance of these diseases.2 Often, atopic dermatitis is the first manifestation of atopy in infants who will develop hay fever or asthma in later childhood.
The prevalence of allergic diseases has been on the rise for several decades, predominantly in industrialized nations and particularly among children.3,4 The pace of this increase has been much faster than the genetic constitution of any population can possibly shift;5 environmental changes associated with “Western” lifestyles are therefore generally believed to be responsible for the allergic epidemic.
In 1989, David Strachan introduced the “hygiene hypothesis,” postulating that reduced exposure to infections during childhood results in aberrant immune responses to innocuous antigens later in life.6,7 This hypothesis was based upon Strachan’s observations that infants with higher numbers of siblings were at decreased risk for developing allergies. Sibship size7,8 and other indirect markers of microbial exposure, such as farm living with especially close contact with livestock,9 were consistently shown to be associated with a decreased risk of developing allergic diseases; studies on the association between bacterial and viral infections and allergies were less consistent.10,11
An alternative interpretation of this hypothesis suggests that an altered normal intestinal colonization pattern in infancy, which fails to induce immunological tolerance, could be responsible for the increase in allergies rather than a decrease in viral or bacterial infections.12
The gut microbiota is indeed a key source of microbial-driven immune regulation and tolerance induction in early life. Animals bred in a germ-free environment show low densities of lymphoid cells in the gut mucosa, the specialized follicle structures are small, and circulating immunoglobulin levels are low. Immediately after exposure to microbes, the number of mucosal lymphocytes expands, germinal centers are formed, and immunoglobulin producing cells appear rapidly in follicles and in the lamina propria, accompanied by a significant increase in serum immunoglobulin levels.13,14 Furthermore, animal studies have shown that it is difficult to achieve oral tolerance in germ-free animals15 and that administration of lipopolysaccharides—constituents of the outer membrane of gram-negative bacteria—together with food antigens increases the tolerizing effect of feeding.16 Additionally, oral administration of bacterial lysates (BL, Enterococcus faecalis, and E. coli) to neonatal rats has been shown to attenuate experimental food allergies. BL-treated animals showed reduced allergen-specific IgE and IgG serum levels upon sensitization as compared with sham treated rats. Concomitantly, allergen-stimulated lymphocytes from spleen and mesenteric lymph nodes of BL-treated animals showed a significantly elevated IL-10 production in vitro.17
Moreover, recent studies have reported marked geographic differences in the composition of the gut microbiota in both children and adults, especially between Westernized and non-Westernized countries.18,19
It seems therefore plausible that perturbations in the gut microbiota composition (due to, e.g., increased hygiene, antibiotic use, and changes in diet, lifestyle, and family size) are involved in the pathogenesis of allergic diseases.
Establishment of the Gut Microbiota in Newborns
The fetal intestine is bathed in swallowed amniotic fluid and thought to be sterile. However, this dogma of a sterile womb has been challenged by several recent studies reporting on the presence of bacteria in umbilical cord blood, amniotic fluid, fetal membranes, and meconium of healthy, term infants.20 This suggests that colonization of the fetal intestine might already occur before delivery by bacterial transmission through the placental barrier.
During delivery, the intestines become colonized by a variety of microorganisms.21 Intestinal colonization involves a succession of bacterial populations waxing and waning as the diet changes and the host develops.22 According to studies of cultured microbes, facultative anaerobes initially colonize the neonatal intestinal canal, reducing the oxygen content and thereby paving the way for strict anaerobes to follow.21 The latter organisms soon outnumber the facultative anaerobes as the microbiota starts to diversify. Diversification continues until around the age of one up to three years when the microbiota turns into an adult-type microbiota containing hundreds to thousands of different species.19,23
Factors influencing the intestinal microbiota composition can be divided into host factors (such as pH, bile acids, pancreatic enzymes, mucus composition, and transit time), non-host factors (such as diet, medication, and environmental factors), and bacterial factors (such as adhesion capacity, enzymes, and metabolic capacities).24
Of particular interest to explain the rise of allergic diseases are the changes in non-host factors due to modern life style. Antibiotic use, diet, smaller family sizes, increased hygiene, and high cesarean section rates may all result in perturbations in the gastrointestinal (GI) microbiota composition thereby interfering with the mechanisms involved in the development of immunological tolerance.25
One of the most extensively studied determinants of the gut microbiota during infancy is mode of delivery. The first inoculum in vaginally born infants is composed of maternal fecal and vaginal microbes, whereas infants born by cesarean section are initially exposed to bacteria originating from healthcare workers and the hospital’s environment.26,27
In our recent study, we examined the influence of non-host factors, including birth mode, on the establishment of the gut microbiota throughout the first seven months of life for 571 newborns.28 In agreement with previous studies,27,29,30 we demonstrated that delivery by cesarean section strongly impacts the infant microbiota, especially by decreasing colonization rates of bacteroides and increasing the prevalence of clostridia, an effect that was still notable at the age of seven months postpartum. Another recent prospective study on the influence of birth mode on microbiota development in early life not only confirmed a delayed Bacteroidetes colonization but also showed reduced microbial diversity and reduced Th1 responses in children born by cesarean section.31
Other factors that can influence the neonatal intestinal microbiota are: prematurity, medication, hygiene measures, the type of infant feeding (breastmilk or formula), and as recently shown, family size and household composition.28,29,32-34,35
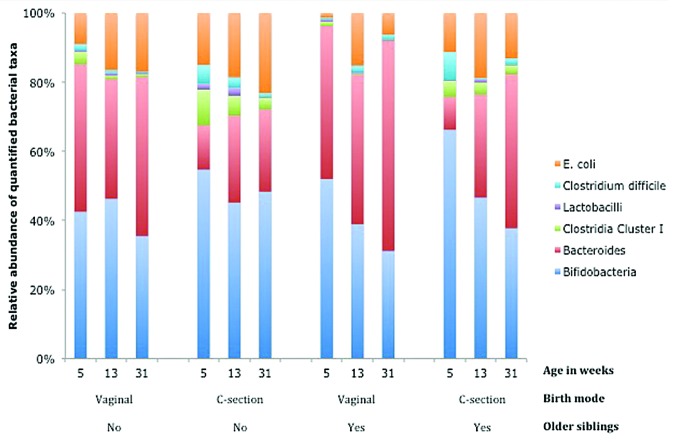
We found that the colonization pattern of firstborns, like the colonization pattern of infants born by cesarean section, was characterized by a higher colonization rate of C. difficile and other clostridia and lower rates of lactobacilli, bifidobacteria, bacteroides, and E. coli.28 Moreover, the effects of birth mode and birth order (having older siblings) appear to be independent. Figure 1 shows a low relative abundance (partly explained by low colonization rates) of bacteroides in infants born by cesarean section, which largely disappears by the age of seven months in children with siblings; however, this is only partly true for children without siblings. Additionally, the relative abundance of clostridia (cluster I) and E. coli are highest in firstborn children delivered by cesarean section and lowest in vaginally delivered children with older siblings.
Figure 1. Relative proportion of bacterial taxa in the fecal microbiota at the ages of 5, 13, and 31 wk postpartum in vaginally delivered and C(esarean)-section delivered infants with or without older siblings.28
Two consecutive papers studying the intestinal microbiota in a subset of infants from the Canadian Healthy Infant Longitudinal Development (CHILD) birth cohort recently confirmed the impact of both mode of delivery32 and older siblings33 and additionally identified pet exposure as a determinant of the infant gut microbiota composition.
Gut Microbiota and Allergic Diseases
During the past 15 y, numerous human epidemiological studies have been conducted on the association between the gut microbiota composition and allergy risk. The vast majority of these studies suggest that altered microbial exposure during childhood is associated with allergic sensitization and/or allergic manifestations, especially atopic dermatitis.
However, to date there are still no definite bacterial taxa or particular subsets of the microbiota that have been consistently associated with allergic diseases. Several studies have given rise to the hypothesis that a reduced intestinal microbial diversity, rather than the presence or abundance of particular taxa, increases the risk of allergic manifestations in childhood. At least four studies have linked a lower bacterial diversity to an increased risk of atopic dermatitis,36-39 whereas a recent birth cohort study found a lower bacterial diversity to be associated with allergic rhinitis and sensitization but not with atopic dermatitis. In contrast, Nylund and colleagues reported a higher bacterial diversity in the fecal microbiota of infants having eczema as compared with healthy controls.40
Publication bias and false positive findings due to multiple comparisons likely contribute to, but cannot completely explain, the high percentages of studies that report an actual association between the gut microbiota and allergic diseases. But why are results far from being consistent? In large part, it is due to methodological dissimilarities; differences in study populations (high risk vs. general populations), in study designs (prospective vs. cross-sectional), in the bacteria under study and the techniques used to identify them, and probably most importantly in the individuals’ age at which the intestinal microbiota is being studied; these are sufficient explanations of results being inconsistent in present studies. There is substantial evidence in both human and animal studies indicating that the most important “window of opportunity” for immune education is early infancy, when the maturation of the immune system is not yet completed and is still building up immune tolerance against food and microbial antigens.7,41 Thus, it seems unlikely that perturbations in the intestinal microbiota beyond infancy may still have an effect on the etiology of allergic diseases. It is more plausible that these differences reflect disturbances in the gut microbiota already present in early life or reflect perturbations caused by the allergic disease that had manifested already (reverse causation). Birth cohort studies quantifying the intestinal microbiota in early life and relating this to allergic manifestations later on in childhood are therefore the most powerful studies to establish (the direction of) causality.
The methodological heterogeneity between studies to date still hampers conclusive evidence for the role of the microbiota in allergic diseases. As such there is a need to apply different methodological concepts to enhance a deeper and more refined understanding of the relationship between the gut microbiota and allergies. One such concept is to move beyond the simple cause-and-effect relationships and attempt to understand what bridges the causal relationships and what alters the magnitude or direction of the causal relationship.42 More specifically, using integrated research designs, it is possible to test whether the reported associations between birth mode and birth order and the risk of allergic diseases is indeed mediated by their impact on the indigenous microbiota (Fig. 2).
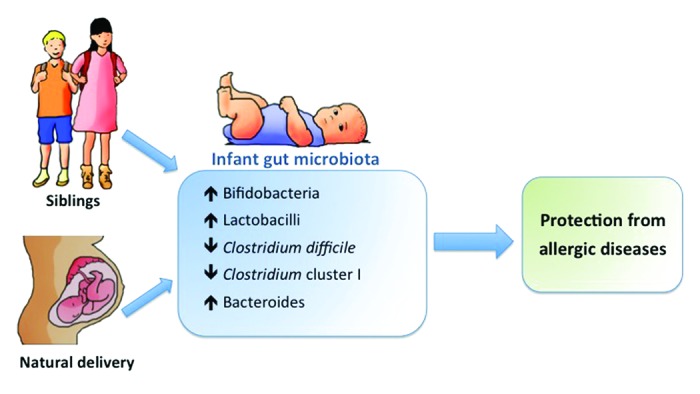
Figure 2. Indirect effects of birth mode and older siblings on the risk of developing allergic diseases through their influence on the establishment of the gut microbiota composition in early life.
The Gut Microbiota as a Mediator
Biostatisticians and epidemiologists use the method of “causal models” to explain a possible causal relationship between a risk factor (the independent variable) and an outcome (the dependent variable). In such causal models, a mediator is an intermediary process that leads from the risk factor to the outcome, and therefore explains, “why” or “how” a risk factor results in an effect. In other words, the independent variable is presumed to cause the mediator, and in turn, the mediator causes the dependent variable.42 Demonstrating such mediation strengthens the evidence for causality of reported associations.
During the past decades birth by cesarean section has increased rapidly in many Western countries,43 whereas family sizes have been decreasing.44 Moreover, both birth order (number of older siblings) and birth mode have been identified as risk factors for allergic diseases8,45 and, as discussed above, strongly influence the establishment of the neonatal microbiota. As such, it has often been postulated that the gut microbiota composition acts as a mediator in the association between birth mode and order and the risk of allergic diseases. Within the Dutch KOALA Birth Cohort Study, mediation-analysis was applied for the first time to test potential mediation by gut microbes in the association between place and mode of delivery and the risk of allergic manifestations. Within this population-based study we demonstrated that Clostridium difficile mediated the relation between birth mode and birthplace (home vs. hospital delivery), as well as both eczema and asthma.46 As home birth is still very common in the Netherlands, in strong contrast to most other developed countries, we were able to demonstrate that the environment during delivery also strongly influences the intestinal colonization process of newborns.
In our recent study among German high-risk neonates (with a positive family history of atopy), mediation analyses confirmed a statistically significant indirect effect of cesarean section delivery, via microbiota pertubations (increase in Clostridium Cluster I), on atopic dermatitis risk. Moreover, this study demonstrated a “beneficial” influence of older siblings on the microbiota composition subsequently linked to a lower risk of atopic dermatitis. This suggests that the microbiota may be one of the biological mechanisms underlying the sibling effect.
Altogether, the results of the mediation analyses in these two populations contributed stronger evidence for a causal link of birth mode and birth order on allergy risk through modulation of the microbiota composition.
It has to be noted that both studies used a targeted approach to quantify several of the most abundant bacterial taxa in neonates instead of applying more comprehensive methods such as next-generation sequencing. As a consequence, it cannot be ruled out that reported associations merely reflect other unmeasured shifts in the microbiota composition.
Therefore, a next logical step would therefore be to integrate the power of extensive microbial profiling methods and (molecular) epidemiological concepts within the context of well-defined prospective (birth) cohort studies. Further, there is a clear need for studies carefully monitoring the day-to-day succession of microbial species during early life in order to guide large-scale cohorts in choosing the appropriate time-points for fecal sampling. Together, such studies may elucidate additional mediating or moderation mechanisms by other microorganisms and may provide new leads for the primary prevention of allergies.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- 1.Romagnani S. Regulatory T cells: which role in the pathogenesis and treatment of allergic disorders? Allergy. 2006;61:3–14. doi: 10.1111/j.1398-9995.2006.01005.x. [DOI] [PubMed] [Google Scholar]
- 2.Ngoc PL, Gold DR, Tzianabos AO, Weiss ST, Celedón JC. Cytokines, allergy, and asthma. Curr Opin Allergy Clin Immunol. 2005;5:161–6. doi: 10.1097/01.all.0000162309.97480.45. [DOI] [PubMed] [Google Scholar]
- 3.Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, Williams H, ISAAC Phase Three Study Group Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–43. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 4.Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR, International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947–54, e15. doi: 10.1016/j.jaci.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Nowak D, Suppli Ulrik C, von Mutius E. Asthma and atopy: has peak prevalence been reached? Eur Respir J. 2004;23:359–60. doi: 10.1183/09031936.04.00134004. [DOI] [PubMed] [Google Scholar]
- 6.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strachan DP. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax. 2000;55(Suppl 1):S2–10. doi: 10.1136/thorax.55.suppl_1.S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karmaus W, Botezan C. Does a higher number of siblings protect against the development of allergy and asthma? A review. J Epidemiol Community Health. 2002;56:209–17. doi: 10.1136/jech.56.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Mutius E. Environmental factors influencing the development and progression of pediatric asthma. J Allergy Clin Immunol. 2002;109(Suppl):S525–32. doi: 10.1067/mai.2002.124565. [DOI] [PubMed] [Google Scholar]
- 10.Björkstén B. Effects of intestinal microflora and the environment on the development of asthma and allergy. Springer Semin Immunopathol. 2004;25:257–70. doi: 10.1007/s00281-003-0142-2. [DOI] [PubMed] [Google Scholar]
- 11.Flohr C, Pascoe D, Williams HC. Atopic dermatitis and the ‘hygiene hypothesis’: too clean to be true? Br J Dermatol. 2005;152:202–16. doi: 10.1111/j.1365-2133.2004.06436.x. [DOI] [PubMed] [Google Scholar]
- 12.Wold AE. The hygiene hypothesis revised: is the rising frequency of allergy due to changes in the intestinal flora? Allergy. 1998;53(Suppl):20–5. doi: 10.1111/j.1398-9995.1998.tb04953.x. [DOI] [PubMed] [Google Scholar]
- 13.Butler JE, Sun J, Weber P, Navarro P, Francis D. Antibody repertoire development in fetal and newborn piglets, III. Colonization of the gastrointestinal tract selectively diversifies the preimmune repertoire in mucosal lymphoid tissues. Immunology. 2000;100:119–30. doi: 10.1046/j.1365-2567.2000.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falk PG, Hooper LV, Midtvedt T, Gordon JI. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol Rev. 1998;62:1157–70. doi: 10.1128/mmbr.62.4.1157-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997;159:1739–45. [PubMed] [Google Scholar]
- 16.Kim JH, Ohsawa M. Oral tolerance to ovalbumin in mice as a model for detecting modulators of the immunologic tolerance to a specific antigen. Biol Pharm Bull. 1995;18:854–8. doi: 10.1248/bpb.18.854. [DOI] [PubMed] [Google Scholar]
- 17.Ahrens B, Quarcoo D, Buhner S, Matricardi PM, Hamelmann E. Oral administration of bacterial lysates attenuates experimental food allergy. Int Arch Allergy Immunol. 2011;156:196–204. doi: 10.1159/000322352. [DOI] [PubMed] [Google Scholar]
- 18.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–6. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funkhouser LJ, Bordenstein SR. Mom knows best: the universality of maternal microbial transmission. PLoS Biol. 2013;11:e1001631. doi: 10.1371/journal.pbio.1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl. 2003;91:48–55. doi: 10.1111/j.1651-2227.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 22.Noverr MC, Huffnagle GB. The ‘microflora hypothesis’ of allergic diseases. Clin Exp Allergy. 2005;35:1511–20. doi: 10.1111/j.1365-2222.2005.02379.x. [DOI] [PubMed] [Google Scholar]
- 23.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goossens D, Jonkers D, Stobberingh E, van den Bogaard A, Russel M, Stockbrügger R. Probiotics in gastroenterology: indications and future perspectives. Scand J Gastroenterol Suppl. 2003;(239):15–23. doi: 10.1080/00855920310002645. [DOI] [PubMed] [Google Scholar]
- 25.Noverr MC, Huffnagle GB. Does the microbiota regulate immune responses outside the gut? Trends Microbiol. 2004;12:562–8. doi: 10.1016/j.tim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–21. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 28.Penders J, Gerhold K, Stobberingh EE, Thijs C, Zimmermann K, Lau S, Hamelmann E. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J Allergy Clin Immunol. 2013;132:601–7, e8. doi: 10.1016/j.jaci.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 29.Adlerberth I, Strachan DP, Matricardi PM, Ahrné S, Orfei L, Aberg N, Perkin MR, Tripodi S, Hesselmar B, Saalman R, et al. Gut microbiota and development of atopic eczema in 3 European birth cohorts. J Allergy Clin Immunol. 2007;120:343–50. doi: 10.1016/j.jaci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 30.Grönlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr. 1999;28:19–25. doi: 10.1097/00005176-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, Björkstén B, Engstrand L, Andersson AF. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by Caesarean section. Gut. 2013 doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 32.Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, Sears MR, Becker AB, Scott JA, Kozyrskyj AL, CHILD Study Investigators Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013;185:385–94. doi: 10.1503/cmaj.121189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Sears MR, Becker AB, Scott JA, Kozyrskyj AL. Infant gut microbiota and the hygiene hypothesis of allergic disease: impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin Immunol. 2013;9:15. doi: 10.1186/1710-1492-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penders J, Stobberingh EE, Thijs C, Adams H, Vink C, van Ree R, van den Brandt PA. Molecular fingerprinting of the intestinal microbiota of infants in whom atopic eczema was or was not developing. Clin Exp Allergy. 2006;36:1602–8. doi: 10.1111/j.1365-2222.2006.02599.x. [DOI] [PubMed] [Google Scholar]
- 35.Yap GC, Chee KK, Hong PY, Lay C, Satria CD, Sumadiono, Soenarto Y, Haksari EL, Aw M, Shek LP, et al. Evaluation of stool microbiota signatures in two cohorts of Asian (Singapore and Indonesia) newborns at risk of atopy. BMC Microbiol. 2011;11:193. doi: 10.1186/1471-2180-11-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012;129:434–40, e1-2. doi: 10.1016/j.jaci.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 37.Forno E, Onderdonk AB, McCracken J, Litonjua AA, Laskey D, Delaney ML, Dubois AM, Gold DR, Ryan LM, Weiss ST, et al. Diversity of the gut microbiota and eczema in early life. Clin Mol Allergy. 2008;6:11. doi: 10.1186/1476-7961-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ismail IH, Oppedisano F, Joseph SJ, Boyle RJ, Licciardi PV, Robins-Browne RM, Tang ML. Reduced gut microbial diversity in early life is associated with later development of eczema but not atopy in high-risk infants. Pediatr Allergy Immunol. 2012;23:674–81. doi: 10.1111/j.1399-3038.2012.01328.x. [DOI] [PubMed] [Google Scholar]
- 39.Wang M, Karlsson C, Olsson C, Adlerberth I, Wold AE, Strachan DP, Martricardi PM, Aberg N, Perkin MR, Tripodi S, et al. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J Allergy Clin Immunol. 2008;121:129–34. doi: 10.1016/j.jaci.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Nylund L, Satokari R, Nikkilä J, Rajilić-Stojanović M, Kalliomäki M, Isolauri E, Salminen S, de Vos WM. Microarray analysis reveals marked intestinal microbiota aberrancy in infants having eczema compared to healthy children in at-risk for atopic disease. BMC Microbiol. 2013;13:12. doi: 10.1186/1471-2180-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rautava S, Ruuskanen O, Ouwehand A, Salminen S, Isolauri E. The hygiene hypothesis of atopic disease--an extended version. J Pediatr Gastroenterol Nutr. 2004;38:378–88. doi: 10.1097/00005176-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Wu AD, Zumbo BD. Understanding and Using Mediators and Moderators. Soc Indic Res. 2008;87:367–92. doi: 10.1007/s11205-007-9143-1. [DOI] [Google Scholar]
- 43.Declercq E, Young R, Cabral H, Ecker J. Is a rising cesarean delivery rate inevitable? Trends in industrialized countries, 1987 to 2007. Birth. 2011;38:99–104. doi: 10.1111/j.1523-536X.2010.00459.x. [DOI] [PubMed] [Google Scholar]
- 44.Group ECW, ESHRE Capri Workshop Group Europe the continent with the lowest fertility. Hum Reprod Update. 2010;16:590–602. doi: 10.1093/humupd/dmq023. [DOI] [PubMed] [Google Scholar]
- 45.Bager P, Wohlfahrt J, Westergaard T. Caesarean delivery and risk of atopy and allergic disease: meta-analyses. Clin Exp Allergy. 2008;38:634–42. doi: 10.1111/j.1365-2222.2008.02939.x. [DOI] [PubMed] [Google Scholar]
- 46.van Nimwegen FA, Penders J, Stobberingh EE, Postma DS, Koppelman GH, Kerkhof M, Reijmerink NE, Dompeling E, van den Brandt PA, Ferreira I, et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol. 2011;128:948–55, e1-3. doi: 10.1016/j.jaci.2011.07.027. [DOI] [PubMed] [Google Scholar]