Abstract
BACKGROUND
Electrical impedance myography (EIM) and quantitative ultrasound (QUS) are two non-invasive, painless, and effort-independent approaches for assessing neuromuscular disease. Both techniques have potential to serve as useful biomarkers in clinical trials in Duchenne muscular dystrophy (DMD). However, their comparative sensitivity to disease status and how they relate to one another is unknown.
METHODS
We performed a cross-sectional analysis of EIM and QUS in 24 healthy boys and 24 with DMD, aged 2-14 years with trained research assistants performing all measurements. Three upper and three lower extremity muscles were studied unilaterally in each child and the data averaged for each individual.
RESULTS
Both EIM and QUS differentiated healthy boys from those with DMD (p<0.001 for both). QUS values correlated with age in DMD boys (rho=0.45, p=0.029), whereas EIM did not (rho=−0.31, p=0.14). However, EIM phase correlated with age in healthy boys (rho=0.51, p= 0.012) whereas QUS did not (rho =−0.021, p =0.92). In DMD boys, EIM phase correlated with the North Star Ambulatory Assessment (EIM: rho=0.65, p=0.022); QUS showed a near-significant association (rho=−0.56, p=0.060). The two technologies trended toward a moderate correlation with one another in the DMD cohort but not in the healthy group (rho=−0.40, p=0.054, rho=−0.32, p=0.13, respectively).
CONCLUSIONS
EIM and QUS are complementary modalities for the assessment of boys with DMD; further study and application of these two modalities alone or in combination in a longitudinal fashion is warranted.
Keywords: electrical impedance myography, quantitative ultrasound, Duchenne muscular dystrophy, biomarker, outcome measure
INTRODUCTION
Outcome measures for DMD clinical trials remain limited to functional measures such as the six-minute walk test (6MWT) and North Star Ambulatory Assessment (NSAA).1,2 While valuable, such functional measures are constrained by subjective elements including effort and mood, as well by variability in younger boys and inapplicability to non-ambulatory older boys. In addition, given recent failures in two clinical trials in DMD to demonstrate definite improvements using the 6MWT,3 there is interest in developing more sensitive and reliable measures of disease status than those currently in use.
Two non-invasive and easily applied technologies in children with potential use for this purpose are quantitative ultrasound (QUS)4,5 and electrical impedance myography (EIM).6 Ultrasound shows increased echointensity in dystrophic muscle due to adipose and connective tissue deposition;7 these changes can be quantified by measuring grayscale luminosity (GSL) values using image analysis software such as Photoshop® or Matlab®. In EIM, changes in localized impedance data reflect alterations in the underlying structure and composition of the muscle.6 EIM has been shown to be sensitive to the severity of other neuromuscular diseases, including spinal muscular atrophy,8 and quantifies disease progression in amyotrophic lateral sclerosis (ALS).9 Recently, it has also been shown to be sensitive to disease status in mdx mice.10 In short, both QUS and EIM measure the compositional and structural status of muscle, QUS relying on backscattered acoustic energy and EIM on transmitted electrical energy.
QUS and EIM are both attractive candidates for assessing neuromuscular pathology in children since both techniques are painless, require no patient effort, and can be rapidly assessed from multiple muscles in a clinical trial-type setting. QUS has already been studied to some extent in boys with DMD,11,12 and our preliminary assessments with EIM have recently been presented.13 The objective of this cross-sectional analysis is to more completely evaluate how well EIM and QUS identify disease status with trained non-physician evaluators performing measurements, how each correlates with both age and NSAA as surrogates of disease severity, and how the two technologies correlate with one another.
METHODS
Subjects and Recruitment
Boston Children's Hospital Institutional Review Board approved the protocol, and parents and children provided written consent and verbal assent respectively. Both healthy boys and those with DMD aged 2 to 14 years were recruited into the study through the neuromuscular clinic at Boston Children's Hospital, with the goal of following them for a 2-year period. Subjects were not permitted to have a pacemaker or other electrical device for inclusion in the study. All subjects with DMD had genetic confirmation of disease or were a brother of a family member with previously diagnosed disease. DMD boys were excluded if they were involved in an ongoing therapeutic clinical trial or if they had a concomitant neuromuscular or other medical condition that substantially impacted health. Healthy boys had no history of neuromuscular disease or other disorder that would substantially affect muscle health and were recruited via advertisement and word-of-mouth.
Evaluators
The NSAA was performed by a trained and experienced pediatric physical therapist (AP). The individuals performing EIM were all research assistants with no previous experience with radiological or electrophysiological testing, and were taught how to perform basic ultrasound by the senior authors; their data showed good reproducibility for US measures.14 For EIM, they were shown where to place the probes and what data was technically poor based on a display of the multifrequency data; such an approach has previously shown good reproducibility.15 No ongoing oversight was provided outside of this basic training, similar to what might be performed in a clinical trial in which these modalities would be utilized.
EIM Measurements
EIM measurements were obtained with the Imp SFB7 (Impedimed, Inc, Sydney, Australia), using a custom hand-held array,15 with three different probe sizes being used depending on the child's size. The array dimensions are: Small: 4 × 1.5cm; Medium 5 × 2cm; Large: 7 × 2.5 cm. Unilateral measurements were performed on the dominant extremities on deltoid, biceps, wrist flexors, quadriceps, tibialis anterior, and medial gastrocnemius muscles. Measurements were performed transversely across the muscle in the same direction as the ultrasound measurements, with the 50 kHz phase as the standard outcome parameter. The probe was placed over the center of the bulk of the muscle using simple measurement paradigms based on the location of standard boney prominences.
Ultrasound Measurements
US images were obtained using the Terason t3000 system (Teracorp, Inc, Burlington, MA) with a 10 MHz probe. Measurements were performed on the same muscles and locations as the EIM measurements, with the probe placed transversely and identical US settings for all image acquisition in all participants.14 All images were converted to .jpg files and analyzed using Matlab® (MathWorks, Inc, Natick, MA) to obtain the brightness of the region of interest, measured as median grayscale level (GSL). The region of interest (ROI) was defined as a region of fixed dimensions (130 pixels × 64 pixels) and placed directly below the subcutaneous fat layer. From this position, additional displacement of the ROI left or right and minor changes in the dimensions were allowed to avoid inter- or intra-muscular fascial planes.
Data Analysis
Prior to final data analysis, all ultrasound and EIM data were reviewed for quality and any spurious data were excluded. Mann-Whitney tests were performed to distinguish between DMD and healthy boys, and the median and ranges were reported as (median, range). Spearman correlations were performed to determine the relationships between EIM 50 kHz phase, age, and NSAA. A p-value of <0.05 was considered significant. For simplicity of presentation, the data from all 6 muscles was averaged for all analyses included here and defined here as the “average” value for each subject.
RESULTS
Subject Demographics
Twenty-four boys with DMD and 24 healthy boys were recruited. Boys with DMD had a median age of 8.2±3.2 and healthy boys had a median age of 8.0±3.2 years. At the time of the study, 9 of the 24 boys with DMD were on corticosteroids. Only 12 DMD boys were in the 4-10 year age range and could participate in NSAA testing. In 6 of the healthy and 13 of DMD boys the smaller probe was used; the medium sized probe was used in 18 of the healthy versus 11 DMD; and the large probe was used in only one boy (with DMD).
Data quality review
All ultrasound images appeared to be of sufficient quality and were used in the analyses that follow. However, we identified 5 individual muscle EIM measurements out of the total 138 that were deemed spurious based on erratic spectral appearance (e.g. negative values). These were identified in 3 muscles in one DMD patient, and in 1 muscle each in two other DMD patients. These data points were excluded from the analysis that follows.
Discrimination of healthy boys from those with DMD
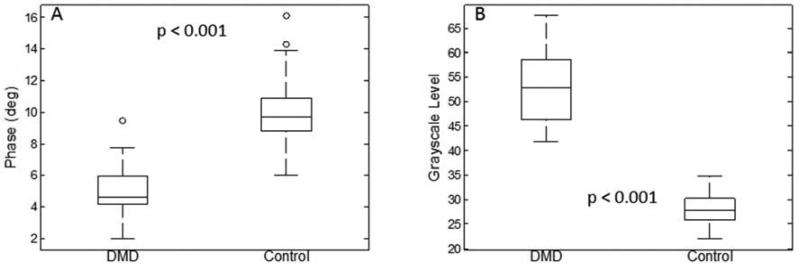
The average 50 kHz phase was lower in boys with DMD than healthy boys (Figure 1A). GSL measurements were higher in DMD boys than the healthy boys (Figure 1B) consistent with previous literature.4
Figure 1.
Box plots comparing both EIM and QUS data (6-muscle average) for healthy boys and those with DMD. Both technologies readily discriminate between groups.
Correlations of EIM and GSL with Age in boys with DMD and controls
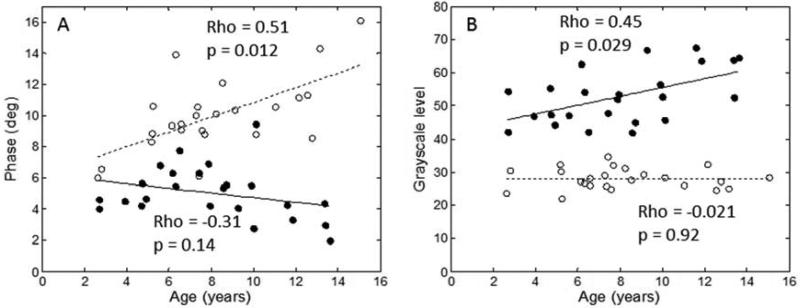
The average EIM 50 kHz phase did not correlate significantly with age in boys with DMD (rho = −0.31, p = 0.14) but did increase with age in healthy boys (rho =0.51, p =0.012, Figure 2A), consistent with previous findings.8 (Of interest, the one 10-year-old DMD boy who was a marked outlier in terms of his EIM data with a phase value of over 9 degrees (see Figure 2A) was also high functioning with an NSAA score of 26.) Unlike EIM, results of QUS did correlate with age in DMD boys. Average GSL increased, on average, with increasing age in boys with DMD (rho=0.45, p, =0.029, Figure 2B) but not in the healthy boys (rho =−0.021, p =0.92). Thus, both technologies revealed an increasing difference between healthy children and those with DMD as age increased; however, in the case of EIM it was due mainly to increasing phase values in normal children whereas with QUS it was due mainly to increasing GSL values in boys with DMD.
Figure 2.
Correlations between EIM data and age for both healthy and diseased boys (open circles, healthy boys; closed circles, boys with DMD). GSL correlates across all ages in the boys with DMD whereas EIM shows a relatively poor correlation. In contrast, in healthy boys, GSL shows no age-related changes whereas EIM does.
EIM and GSL compared with NSAA in DMD
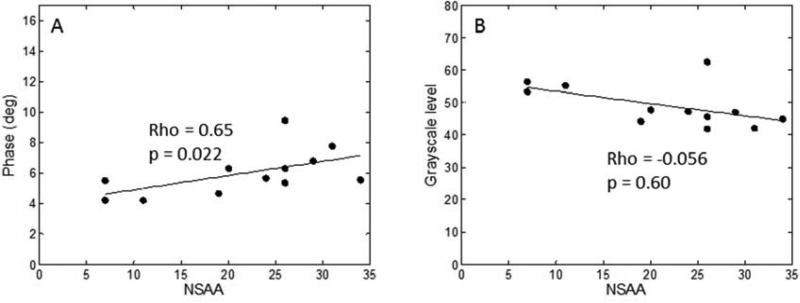
Average 50 kHz phase showed a significant correlation with NSAA (rho=0.65, p=0.022, Figure 3); QUS showed a near-significant correlation (rho=−0.56, p=0.060, respectively).
Figure 3.
Correlations between EIM and GSL with NSAA in boys with DMD.
EIM correlation with GSL in both healthy and diseased boys
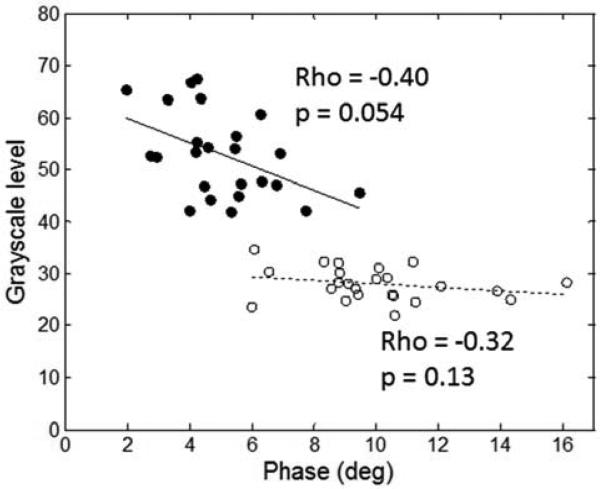
The average 50 kHz EIM phase trended toward a moderate correlation with the QUS data in the DMD subject group (rho=−0.40, p=0.054) but the correlation was not significant for the healthy subject group (rho=−0.32, p=0.13, Figure 4).
Figure 4.
Correlation between EIM and GSL for boys with DMD and for healthy subjects. A near-significant relationship was found for the boys with DMD but not for the healthy ones. Open circles, healthy boys; closed circles, boys with DMD.
DISCUSSION
These results indicate that both EIM and GSL parameters can readily discriminate healthy from DMD muscle and that this difference increases in older children. In the case of EIM, this is consistent with recently published work that identified major differences in both in vivo and ex vivo EIM values in mdx mice as compared to control animals.10 This pattern also follows closely that observed previously in children with spinal muscular atrophy.16 In the case of QUS, this ability to readily discriminate healthy boys from those with DMD has been described previously.5,12
Despite this consistent finding for both techniques, the correlations of 50 kHz phase and GSL with age in DMD and in healthy boys showed substantial differences. We hypothesized that a correlation with age would be present for both modalities since the disease progresses as a child grows. In the case of QUS, a moderate correlation between GSL values and age was identified, consistent with previously reported work.11,12 In normal subjects, however, no change was observed in GSL. In contrast, for EIM no such correlation was identified in boys with DMD, whereas a major positive trend was present for the healthy boys.
The reason underlying this difference is that the two techniques are sensitive to distinct aspects of muscle pathology. In the case of QUS, the presence of fat and connective tissue in DMD muscle creates heterogeneities in the tissue that produce increased echo-intensity and higher GSL values as a child ages. However, in healthy muscle there are no such abnormal depositions, and thus the GSL values remain similar across all ages. With EIM, in contrast, other structural effects are at play. In normal subjects, muscle fiber size increases with age throughout the childhood years.17 This has the effect of increasing muscle capacitance and thus increasing muscle phase values as a healthy child grows; indeed, a strong relationship between EIM and muscle fiber diameter has been previously described.18,19 However, in DMD boys, fat and connective tissue reduce EIM phase values since these tissues also have low capacitance and, in the case of fat, high resistivity. Thus, these effects, in combination with reduced muscle fiber size, lower EIM phase values even in young DMD children (see Figure 2). However, as a child grows, muscle fiber size will increase to some extent too, thus counteracting these negative effects, resulting in static EIM values across childhood in DMD boys.
A functional measure, the NSAA, correlated with EIM values and showed a nearly significant correlation with GSL. This finding supports that both technologies are associated with function capability and have clinical meaning. Finally, we also evaluated the relationship between EIM and QUS. Interestingly, the correlation in normal subjects was highly non-significant and the correlation in boys with DMD only trended toward significance with an R value of 0.40. These data confirm that both technologies are mostly assessing complementary aspects of DMD pathology. We note that, in our previously published study,13 we included the 6-minute walk test; here we chose to focus on the NSAA because we had twice the number of data points since the many more children could participate in the NSAA than the 6-minute walk test.
There are several limitations of this work. First, the sample size included here (N = 24 for DMD, N=24 for healthy) is not large and even fewer (n=12) were able to perform the NSAA; hence, there is considerable opportunity for type 2 error in all of these analyses. In addition, this analysis was cross-sectional in nature and only a longitudinal study can truly determine either technique's capability for identifying a treatment effect in a clinical therapeutic trial. Moreover, both technologies were applied in limited fashion here. In the case of ultrasound, we only evaluated grayscale data and not quantified raw backscatter signals; in the case of EIM, only a single frequency of data was used in this analysis. Another concern is that we used 3 different probe sizes to collect EIM data given the considerable variation in limb size encountered during the study, and the smaller probe was used more frequently in the DMD boys. However, we suspect that any contribution from probe size to our results to be minor since phase values should not be readily affected by electrode size (although the raw impedance values—reactance and resistance—would be significantly altered); moreover, the probe size relative to a child's individual muscle size is similar. Finally, we have not pursued an individual muscle-by-muscle analysis in this work; such an analysis may also provide additional useful information.
In summary, both EIM and QUS technologies effectively discriminate healthy boys from those with DMD. However, the relationship between alterations in QUS GSL and in EIM phase with disease status is complex. While both techniques are similar in that they quantify muscle pathology by evaluating how applied energy is transformed by muscle tissue, they appear to provide complementary information. Each has the potential for being used alone, but this difference offers the prospect of employing both techniques in combination to provide a single more potent index of muscle health. We have recently demonstrated such an approach in spinal muscular atrophy.20 The application of such a strategy in DMD may ultimately assist in speeding clinical therapeutic trials.
Acknowledgments
This work was funded by the NIH (R01AR060850-02). Irina Shklyar's participation was funded by the Howard Hughes Medical Institute (HHMI) and Duchenne Research Fund (DRF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.McDonald CM, Henricson EK, Han JJ, et al. The 6-minute walk test as a new outcome measure in Duchenne muscular dystrophy. Muscle Nerve. 2010 Apr;41(4):500–510. doi: 10.1002/mus.21544. [DOI] [PubMed] [Google Scholar]
- 2.Mazzone ES, Messina S, Vasco G, et al. Reliability of the North Star Ambulatory Assessment in a multicentric setting. Neuromuscul Disord. 2009 Jul;19(7):458–461. doi: 10.1016/j.nmd.2009.06.368. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman EP, Connor EM. Orphan drug development in muscular dystrophy: update on two large clinical trials of dystrophin rescue therapies. Discovery medicine. 2013 Nov;16(89):233–239. [PubMed] [Google Scholar]
- 4.Pillen S, Arts IM, Zwarts MJ. Muscle ultrasound in neuromuscular disorders. Muscle Nerve. 2008 Jun;37(6):679–693. doi: 10.1002/mus.21015. [DOI] [PubMed] [Google Scholar]
- 5.Pillen S, Verrips A, van Alfen N, Arts IM, Sie LT, Zwarts MJ. Quantitative skeletal muscle ultrasound: diagnostic value in childhood neuromuscular disease. Neuromuscul Disord. 2007 Jul;17(7):509–516. doi: 10.1016/j.nmd.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Rutkove SB. Electrical Impedance Myography: Background, Current State, and Future Directions. Muscle Nerve. 2009;40:936–946. doi: 10.1002/mus.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pillen S, Tak RO, Zwarts MJ, et al. Skeletal muscle ultrasound: correlation between fibrous tissue and echo intensity. Ultrasound Med Biol. 2009 Mar;35(3):443–446. doi: 10.1016/j.ultrasmedbio.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Rutkove SB, Gregas MC, Darras BT. Electrical impedance myography in spinal muscular atrophy: A longitudinal study. Muscle Nerve. 2012 May;45(5):642–647. doi: 10.1002/mus.23233. [DOI] [PubMed] [Google Scholar]
- 9.Rutkove SB, Caress JB, Cartwright MS, et al. Electrical impedance myography as a biomarker to assess ALS progression. Amyotrophic Lateral Sclerosis. 2012 Jun 7; doi: 10.3109/17482968.2012.688837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Geisbush TR, Rosen GD, Lachey J, Mulivor A, Rutkove SB. Electrical impedance myography for the in and ex vivo assessment of muscular dystrophy (mdx) mouse muscle. Muscle Nerve. 2013 doi: 10.1002/mus.24086. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansen M, van Alfen N, Nijhuis van der Sanden MW, van Dijk JP, Pillen S, de Groot IJ. Quantitative muscle ultrasound is a promising longitudinal follow-up tool in Duchenne muscular dystrophy. Neuromuscul Disord. 2012 Apr;22(4):306–317. doi: 10.1016/j.nmd.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Zaidman CM, Connolly AM, Malkus EC, Florence JM, Pestronk A. Quantitative ultrasound using backscatter analysis in Duchenne and Becker muscular dystrophy. Neuromuscul Disord. 2010 Dec;20(12):805–809. doi: 10.1016/j.nmd.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutkove SB, Darras BT. Electrical impedance myography for the assessment of children with muscular dystrophy: a preliminary study. Journal of physics. Conference series. 2013;434(1) doi: 10.1088/1742-6596/434/1/012069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaidman CM, Wu J, Wilder S, Darras BT, Rutkove SB. Minimal training is required to reliably perform quantitative ultrasound of muscle. Muscle Nerve. 2013 doi: 10.1002/mus.24117. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narayanaswami P, Spieker AJ, Mongiovi P, Keel JC, Muzin SC, Rutkove SB. Utilizing a handheld electrode array for localized muscle impedance measurements. Muscle Nerve. 2012 Aug;46(2):257–263. doi: 10.1002/mus.23307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutkove SB, Shefner JM, Gregas M, et al. Characterizing spinal muscular atrophy with electrical impedance myography. Muscle Nerve. 2010 Dec;42(6):915–921. doi: 10.1002/mus.21784. [DOI] [PubMed] [Google Scholar]
- 17.Brooke MH, Engel WK. The histographic analysis of human muscle biopsies with regard to fiber types. 4. Children's biopsies. Neurology. 1969 Jun;19(6):591–605. doi: 10.1212/wnl.19.6.591. [DOI] [PubMed] [Google Scholar]
- 18.Ahad MA, Fogerson PM, Rosen GD, Narayanswami P, Rutkove SB. Electrical characteristics of rat skeletal muscle in immaturity, adulthood, and after sciatic nerve injury and their relation to muscle fiber size. Physiol Meas. 2009;30:1415–1427. doi: 10.1088/0967-3334/30/12/009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakhilin S, Turner G, Katz M, et al. Electrical impedance as a novel biomarker of myotube atrophy and hypertrophy. J Biomol Screen. 2011 Jul;16(6):565–574. doi: 10.1177/1087057111401392. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava T, Darras BT, Wu JS, Rutkove SB. Machine learning algorithms to classify spinal muscular atrophy subtypes. Neurology. 2012 Jul 24;79(4):358–364. doi: 10.1212/WNL.0b013e3182604395. [DOI] [PMC free article] [PubMed] [Google Scholar]