Abstract
Intussusceptive angiogenesis is a dynamic intravascular process capable of dramatically modifying the structure of the microcirculation. The distinctive structural feature of intussusceptive angiogenesis is the intussusceptive pillar—a cylindrical microstructure that spans the lumen of small vessels and capillaries. The extension of the intussusceptive pillar appears to be a mechanism for pruning redundant or inefficient vessels, modifying the branch angle of bifurcating vessels and duplicating existing vessels. Despite the biological importance and therapeutic potential, intussusceptive angiogenesis remains a mystery, in part, because it is an intravascular process that is unseen by conventional light microscopy. Here, we review several fundamental questions in the context of our current understanding of both intussusceptive and sprouting angiogenesis. 1) What are the physiologic signals that trigger pillar formation? 2) What endothelial and blood flow conditions specify pillar location? 3) How do pillars respond to the mechanical influence of blood flow? 4) What biological influences contribute to pillar extension? The answers to these questions are likely to provide important insights into the structure and function of microvascular networks.
The growth of new blood vessels from existing vessels—a process known as angiogenesis---occurs in normal development as well as in pathologic conditions involving tissue repair (1), organ regeneration (2) and tumorigenesis (3). In adult animals, early intravital microscopy observations in living tissue demonstrated that new vessels formed by “the sending out of sprouts from the vessel already present” as in early growth in an embryo (4,5). In other cases, “numerous new branches and short connections” rapidly formed without obvious sprouts (6). These intravital observations are now considered to represent the two fundamental processes of new vessel growth: sprouting and nonsprouting angiogenesis.
The process of nonsprouting or “intussusceptive” angiogenesis was formally identified in 1986 (7), although earlier reports described a similar process (8,9). To visualize blood vessel structure, Caduff and colleagues studied the developing rat lung using corrosion casting and scanning electron microscopy (SEM). During the phase of rapid alveolarization and capillary growth (7-13 days), they observed no capillary sprouts, but small holes in the sheet-like alveolar microvasculature (7). These regular and nonrandom holes were temporally and spatially associated with rapid expansion of the microcirculation. Importantly, the diameter of the alveolar capillaries was smaller after, rather than prior to, expansion suggesting that the holes were involved in not only capillary replication, but also capillary remodeling (7). The authors concluded that the small holes reflected a mechanism of “in-itself or intussusceptional growth”— a process that made sprouting of individual capillary segments unnecessary (7).
Because the holes were seen in casts of the vessel lumen, the holes reflected a “pillar” or “post” spanning the lumen of the blood vessel (Figure 1). Pillar-like microstructures spanning a conduit are unique in mammalian anatomy; however, a similar structure exists in the gills of fish, molluscs and crustaceans (10,11). In these organisms, blood flows between two thin epithelial plates separated by a series of pillars or trabeculae composed of characteristic “pillar cells” (12). In both mammalian blood vessels and fish gills, pillars are a highly adaptive design feature for optimizing bulk fluid transport. In mammalian vessels, the selective growth or extension of intravascular pillars can be used to efficiently modify vessel structure. Depending upon several influences, including the intravascular flow field, pillar extension can 1) modify the branching angle of a bifurcating vessel, 2) duplicate an existing vessel, or 3) prune a redundant or energetically inefficient vessel (Figure 2). In addition, the presence of an intraluminal tissue bridge provides an opportunity for local exposure to a variety of blood-borne elements including soluble factors and progenitor cells.
Figure 1.
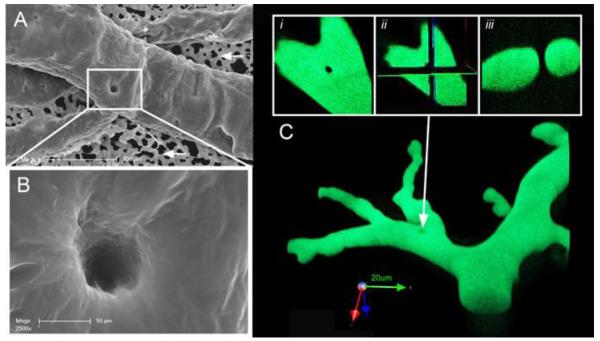
Intussusceptive pillars in the chick chorioallantoic membrane (CAM). A) Corriosion casting of the CAM microcirculation was imaged with scanning electron microscopy. B) Because the casting media fills the intraluminal space, the intussusceptive pillar is seen as a hole in the vessel. C) Confocal microscopy of fluorescent casts demonstrates the transluminal orientation of the pillar. An en face view of the vessel (i) was analyzed in orthogonal planes (ii) demonstrating a typical appearance of an intussusceptive pillar in cross-section (iii). Unpublished figures courtesy of Drs. Maximilian’ Ackermann and Grace Lee.
Figure 2.
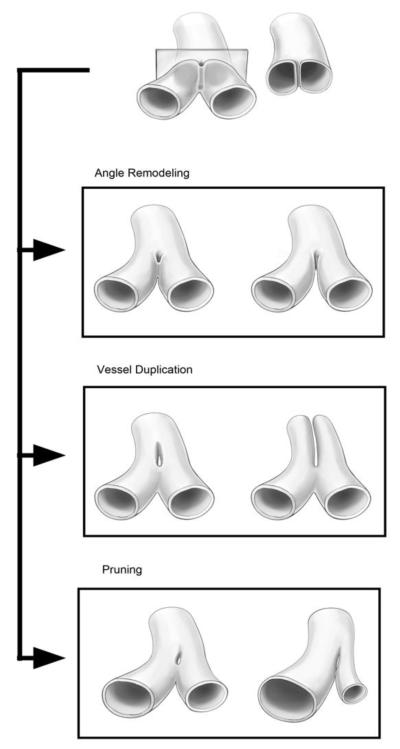
Schematic representation of pillar extension with three different results. Pillar growth toward the vessel angle results in the remodeling of vessel bifurcation. Pillar extension down the axis of the vessel results in vessel duplication. Asymmetric pillar growth can result in pruning of a redundant or energetically inefficient vessel.
The process of sprouting capillaries can be quantitatively studied because individual sprouts can be counted and the rate of growth assessed by light microscopy. In contrast, nonsprouting angiogenesis is an intravascular process. Unseen by standard light microscopy, nonsprouting or intussusceptive angiogenesis is an underappreciated process of multiplicative blood vessel growth and network remodeling.
Intussusceptive pillars
The pillars identified by Caduff et al. during rat lung development were intravascular microstructures characterized by their intraluminal location. In a variety of experimental models, intravascular pillars have been identified in capillaries and small vessels. For example, intravascular pillars have been identified in the developing chick chorioallantoic membrane (13), murine chemically-induced colitis (14), a variety of tumors (15) and the physiologic angiogenesis associated with skeletal muscle training (16). Despite the many different model systems, there are currently no reliable histologic markers or convincing in vitro models of intussusceptive pillars. Because of their intraluminal location, intussusceptive pillars have been largely defined by corrosion casting and SEM.
Corrosion casting is an investigative method that perfuses the vascular system with a low viscosity resin that polymerizes within the microvasculature. To visualize the intraluminal polymer, the surrounding tissue is digested and the remaining cast of the lumen is examined by SEM (17,18). SEM is a high resolution imaging technique that is sufficiently scalable to characterize the structure of vascular networks as well as individual vessels. Using SEM, the corrosion casts can provide useful data about vascular density, the presence of intussusceptive pillars and the orientation of vascular lining cells. High-quality casts can reveal endothelial nuclear imprints. Because endothelial nuclei align with flow (19-22), these corrosion casts can provide a measure of cell density and the direction of blood flow. In several model systems, including the chick chorioallantoic membrane (23), nuclear imprints suggest that multiple endothelial cells contribute to the initial pillar structure.
An interesting observation is that corrosion casts of the vessel lining cells neighboring the intussusceptive pillars are typically unremarkable. Membrane irregularities, micro-spikes or distinctive mural contours are not associated with the presence of intraluminal pillars. There are no intraluminal features that would indicate, or specify, the location of the pillar. Whether this observation reflects the positive pressure instillation of the resin, or is an accurate reflection of luminal morphology, is unknown. Of note, technical artifacts such as extravasation, resin shrinkage and residual intravascular particulates would not account for the observations attributable to tissue pillars (17,24).
Pillar ultrastructure
A disadvantage of corrosion casting is that the surrounding tissue is digested away—casts provide little information regarding the cellular or extracellular composition of the pillar. To image the ultrastructural features of the pillar, the most commonly used approach is serial transmission electron microscopy (TEM) (25). TEM has the advantage of ultrastructural detail of the endothelial cells and tissue comprising the pillar. Identifying a tissue pillar in a 2-dimensional analysis, however, requires hundreds of serial TEM sections. In most cases, reliable TEM analysis requires a tissue with a high density of intussusceptive pillars, such as the developing rat lung during alveologenesis (7).
Without a sufficiently high frequency of intussusceptive pillars with a predictable orientation, pillars can be difficult to reliably identify in 2 dimensions. For example, when viewed in tangential TEM sections, a looping vessel may give the impression of a pillar. Because of the potential for misattribution, some investigators have limited the diameter range of a potential pillar from 1-2.5 μm (26). In practice, TEM is time-consuming and economically infeasible for most laboratories. Nonetheless, TEM has identified the composition of the pillars in several tissues including the developing rodent lung (26), skeletal muscle (16,27) and murine colitis (14). These studies suggest that a typical intussusceptive pillar is composed of endothelial membranes, occasional myofibroblasts and rare pericytes.
The endothelial cell origin and the tubular configuration of the intussusceptive pillar is reminiscent of capillary sprouts. An appealing idea is that the intussusceptive pillar reflects a mirror image of abluminal sprouting (Figure 3) (14,28). In sprouting angiogenesis, the basement membrane is degraded and endothelial “tip cells” project into the extracellular matrix (29). At least in the murine colitis (14) and rat skeletal muscle (8,30) models of intussusceptive angiogenesis, the basement membrane is not degraded. In addition, the endothelial cell projections are not oriented into the extracellular matrix, but across the vessel lumen. In both sprouting and intussusceptive angiogenesis, the endothelial cell projections are believed to contain contractile elements and a shape consistent with filopodia.
Figure 3.
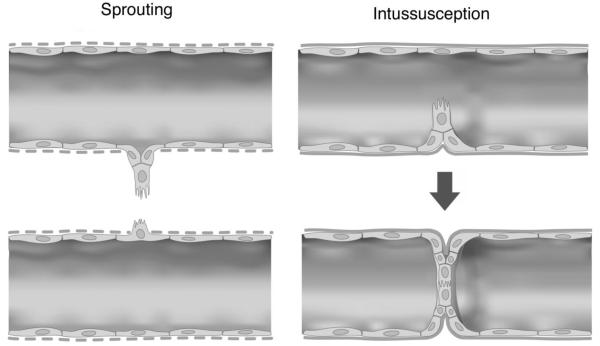
Speculative comparison of the early processes of sprouting and intussusceptive angiogenesis. In sprouting angiogenesis, tip cells project into the extracellular matrix through a disrupted basement membrane (dotted lines). In contrast, intussusceptive pillars may project across the vessel lumen with an intact basement membrane.
Endothelial cell filopodia are finger-like projections filled with tight parallel bundles of filamentous (F)-actin. In sprouting angiogenesis, the elongation of these filaments projects the leading edge of the endothelial cell into the extracellular matrix. Growth factor receptors, such as VEGF receptors (VEGFR2) on the filopodia likely provide guidance cues for endothelial cell migration (31). Although the model of inward sprouting provides an appealing symmetry for intussusceptive angiogenesis, most endothelial cell membranous projections visualized by TEM do not contain contractile elements (9,14) and have been characterized by the more general term “pseudopodial” processes (32). Furthermore, it is unclear whether the actin network in the endothelial cell cytoskeleton is sufficiently rigid to resist blood flow in a distended capillary or have sufficient protrusive force to project across a lumen filled with blood.
In sprouting angiogenesis, sprout fusion appears to occur as a result of filopodial interactions between two approaching tip cells. In intussusceptive angiogenesis, there is no evidence that the lumen-spanning endothelial cells reflect tip cell characteristics; that is, dynamic filopodia, migratory behavior, and growth factor responsiveness (31). Also, there is inconclusive data to support “stalk cell” function as there is little endothelial cell proliferation in the early stages of intussusceptive angiogenesis despite the formation of a nascent pillar lumen (33,34). Another important distinction with sprouting angiogenesis is that the projections extend into the lumen of a functioning capillary; that is, pillar formation occurs without an obvious growth factor diffusion gradient to guide endothelial cell projections. Since pillars develop with an intact basement membrane and without obvious extraluminal growth factors, the potential role for morphogen gradients in regulating endothelial cell protrusion and endothelial cell-cell fusion is unclear.
Regardless of the mechanism of transluminal fusion, the capillary endothelial cells involved in both sprouting and intussusceptive angiogenesis necessarily remodel their local endothelial cell-cell attachments (35). Perhaps a reflection of this flexible polarity, endothelial cells are recognized to have less apical and basal lateral sorting of proteins than epithelial cells (36,37). Another potential distinction is the interaction of endothelial cells with the extracellular matrix. In sprouting angiogenesis, the endothelial cell-extracellular matrix interaction appears to play a functional role during lumenogenesis (38); however, it is unclear how endothelial interactions with the extracellular matrix are potentially involved in pillar formation.
Pillars and blood flow
The development of intravascular pillars produces a curious “symmetry” with fused sprouts (Figure 4). An important distinction is that intussusceptive pillars remain within the original flow stream and are potentially responsive to intravascular flow fields. Although the early pillars identified by TEM and SEM are typically cylindrical structures less than 5 μm in diameter, later imaging indicates that intussusceptive pillars can grow and extend to create duplicated and remodeled blood vessels. Pillar extension down the axis of the vessel appears to be the mechanism responsible for vessel duplication without the need for endothelial cell proliferation (16). Similarly, pillar extension in non-axial directions appears to be the mechanism responsible for remodeling the branch angles of bifurcating vessels (39) or pruning redundant or energetically inefficient vessel branches (23)
Figure 4.
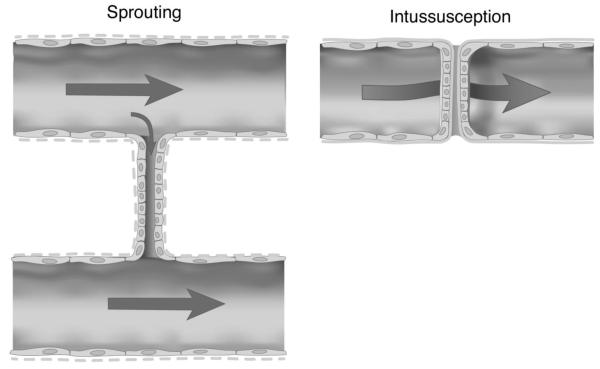
Schematic comparison of the later stages of sprouting and intussusceptive angiogenesis. The fusion of abluminal sprouts may or may not be influenced by dominant flow fields within the microcirculation. In contrast, intraluminal pillars are necessarily exposed to blood flow within the vessel undergoing intussceptive remodeling (arrow denotes blood flowing around the intraluminal pillar).
To identify intussusceptive pillars and provide a simultaneous assessment of blood flow, we have adapted an intravital video microscopy system to the planar networks in the mouse colon and the chick chorioallantoic membrane (CAM) (23,40). Using fluorescent plasma markers, intravascular pillars can be identified by persistent flow voids and confirmed by time-lapse digital recombination. This analysis focused on the larger conducting vessels, rather than the capillary meshwork (Figure 1A, white arrows) in the chick CAM because of the complex network flow interactions in the meshwork as well as the difficulty in distinguishing tissue islands incidentally entrapped by sprout fusion from active intussusceptive pillars.
Analysis of the CAM conducting vessels has demonstrated that approximately 1% of the non-mesh vascular network is comprised of flow voids consistent with intussusceptive pillars (40). Most of intussusceptive pillars were identified at vessel bifurcations; 80% of those bifurcations reflected convergent flow. In most cases, the single pillar located at the site of convergent flow was oriented orthogonal to the bifurcation plane. Confirmed by corrosion casting and SEM as well as confocal microscopy, the orientation of the pillar relative to the bifurcation plane--as well as its typical location through the central axis of the vessel (e.g. Figure 1C)--suggests that pillar orientation is determined, in part, by blood flow from both converging vessels (40).
To investigate the influence of blood flow on pillar extension, we have developed 3-D finite element models of intussusceptive angiogenesis in murine colitis (41) and the chick CAM (23). Based on measurable flow dynamics and essential structural features of the blood vessels identified by intravital microscopy, these computational models have allowed us to map the distribution of mechanical forces within the vessels containing an intussusceptive pillar. An assumption of this work is that the blood is treated as a Newtonian fluid—effectively discounting mechanical effects of blood cells. Justifying this assumption, the chick and murine computational models have strikingly similar results despite a wide variation in blood cell concentrations. Based on the comparisons of these two models (23,41), we suspect that our computational models closely approximate the distribution of forces in vivo.
Using finite element flow models, we can perform computational “pillar deletion” experiments; that is, we can calculate the distribution of forces in the same vessel with and without intraluminal pillars (Figure 5). These studies suggest that individual pillars, or the first pillar in a series of pillars, form in regions of the vessel with minimal shear stress. Pillars are primarily located in micro-hemodynamic “dead zones” within the vessel (23,41); that is, pillars form in flow regions with shear stress below 1 dyn/cm2 (23)(Figure 5B, white arrow). Because regions of low wall shear stress are not always associated with pillars, we have postulated a permissive role of wall shear stress in pillar development. We suspect that regions of low wall shear stress are necessary, but not sufficient, for the development of intussusceptive pillars. In addition to low shear stress, endothelial cell activation from extravascular or intraluminal signals may be required for pillar formation (23).
Figure 5.
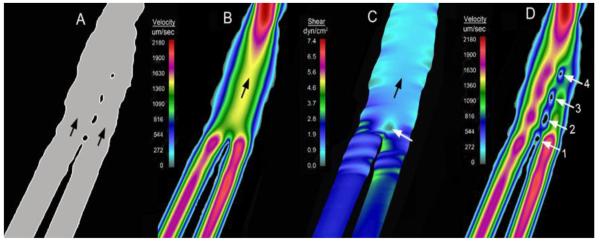
Finite element models of bifurcating vessels in the chick CAM (after (23)). A) An example of a vessel with multiple or “serial” pillars (blood flow indicated by black arrows). B) A finite element model of the vessel without the pillars demonstrating the flow velocity streamlines. C) Wall shear stress calculations of the vessel without pillars demonstrate low shear stress in the region of the first pillar (white arrow). D) Similar flow conditions with the sequential addition of the pillars (1 through 4) suggest that pillars in series form “downstream” from the initial pillar.
Once the pillar is formed, pillar extension appears to be limited by wall shear stress. Multiple, or serial, pillars in the chick CAM provide a useful model for examining both pillar formation and pillar extension. In most cases, new pillars in a pillar sequence form sequentially; in other words, the next pillar in a sequence forms “downstream” from the pre-existing pillars (e.g. Figure 5). Although pillar extension occurs within hours, it is generally too slow for real-time video microscopy. Nonetheless, the shape and extension of existing pillars suggests these intravascular structures are limited by wall shear stress (40).
Network structure
The performance of vascular networks is highly dependent on their architecture. Tissues undergoing regeneration or repair often demonstrate heterogeneous vascular microarchitecture because angiogenesis is driven by stochastic processes. Initiated by metabolic demands and guided by morphogen gradients, the mechanisms of capillary sprouting and sprout fusion create heterogeneity that has implications for both blood flow distribution and the energy cost of blood transport.
An underappreciated role of intussusceptive angiogenesis is that it provides a mechanism for modifying the structure of microvascular networks. The responsiveness of intravascular pillars to blood flow features suggest that intravascular pillars are a fundamental mechanism for optimizing the structure of the microcirculation.
In addition to modifying and optimizing the structure of microcirculations, an intriguing therapeutic possibility is the potential for Intussusceptive angiogenesis to expand vascular networks. For example, pillars in a high flow setting could serially divide the vessel and rapidly expand the existing microcirculatory network. Multiplicative expansion of the network would be particularly useful in tissues with ischemia or high metabolic demands. The existing data suggest two intriguing mechanisms for initiating intussusceptive angiogenesis: one mechanism is triggered by intraluminal events and the second is triggered by extravascular events.
Intraluminal stimulus
Vessel growth in skeletal muscle occurs under physiologic circumstances. Skeletal muscles demonstrate an adaptive response to changing conditions including physiologic stress in the form of exercise training. By increasing the blood flow to muscles 3-6 fold, endurance training can result in a 15-20% increase in both capillary density as well as capillary:fiber ratio (42-44). In perhaps the earliest electron microscopic description of intussusceptive angiogenesis, Ogawa studied rats subjected to one hour of daily exercise training (8). After 60 days, he demonstrated a significant increase in vascularity characterized by intussusceptive luminal division (8).
Although these results suggested that the capillary growth was triggered by increased blood flow associated with endurance training-stimulated capillary growth, the interpretation of these experiments was complicated by the many systemic changes associated with exercise training (45). Activation of specific muscle groups with electrical stimulation provides a more controlled experimental alternative. When compared to endurance training, electrical stimulation produced a similar pattern of capillary growth, but without the confounding systemic effects of exhaustive training (46). The results of both exercise training and electrical stimulation suggested that increased blood flow alone could induce intussusceptive angiogenesis (30,44,47).
To examine the influence of enhanced muscle flow without training-associated muscular contraction, several studies have used chronic peripheral arteriolar dilatation (48,49). Using alpha-1 receptor antagonist prazosin, capillary shear stress was 3-fold (4 to 15 dyn/cm2) greater than in control animals or animals treated by chronic electrical stimulation alone (27,50). Although prazosin was associated with little demonstrable endothelial cell proliferation by PCNA staining (16,51), there were elevated levels of VEGF in both the prazosin and chronic electrical stimulation conditions (50). Most importantly, histologic assessment of the prazosin-treated muscle (2 weeks) demonstrated a 40% increase in capillary:fiber ratio (50). TEM of the muscle in similar treatment conditions has shown intussusceptive pillars and an intact capillary basement membrane (50).
These results suggest that shear stress alone, in the absence of obvious extraluminal growth factor or metabolic stimulation, can trigger intussusceptive angiogenesis. The preservation of intact basement membranes (8,14,30) suggests that the process of intussusceptive angiogenesis has important distinctions from typical sprouting angiogenesis; namely, endothelial cell proliferation, basement membrane degradation and abluminal endothelial cell migration appear to be unnecessary for intussusceptive capillary replication. These observations, however, raise several intriguing questions. First, how are the divided capillaries redistributed within the muscle fibers? After 2 weeks, the capillaries appear to be uniformly surrounding the skeletal muscle (52). The process of capillary redistribution is likely associated with gradual basement membrane remodeling and perhaps distraction forces unique to skeletal muscle.
Second, how does this process occur with minimal endothelial cell proliferation? Capillary luminal division alone—without a concomitant increase in endothelial cell circumference--results in a 50% decrease in cross-sectional area. Although post-intussusceptive endothelial cell enlargement has been observed (9), it is likely that the growth phase of intussusceptive angiogenesis in skeletal muscle is also associated with endothelial cell division; however, we speculate that the proliferation is temporally more protracted and spatially more distributed than is observed in models of sprouting angiogenesis.
Third, how does the increased wall shear stress trigger the development of intussusceptive pillars? To date, in vitro studies of endothelial cell responses to variable levels of shear stress have not identified pillar-like endothelial cell structures (22). The absence of shear stress-induced in vitro pillars may reflect insufficiently sensitive assessments of endothelial morphology. Alternatively, shear stress-induced endothelial cell changes may require in vivo elements—such as an intact capillary basement membrane—that are necessary for the initiation of intussusceptive angiogenesis. Of note, measures of endothelial cell transcriptional responses to shear stress have demonstrated the potential involvement of many genes and signaling pathways (53,54).
Finally, the elevated shear stress associated with muscle training in intussusceptive angiogenesis appears to be in conflict with our blood flow simulations in the chick CAM and mouse colitis models; namely, the computational observations that pillars appear to form in regions of low shear stress and are limited by high shear stress. A potential explanation for this apparent paradox is the temporal pattern of blood flow in skeletal muscle. It is possible that it is the sequential exposure of the vessel lining cells to high and low shear stress leads to pillar formation. The capillary leak associated with muscle training (8) may also facilitate endothelial contact reminiscent of the Baxter-Jain model of blood flow heterogeneity in tumors (55).
Extraluminal stimulus
A complementary model of adult intussusceptive angiogenesis is murine colitis. In chemically-induced murine colitis, the administration of chemicals such as dextran sodium sulfate (oral) or trinitrobenzene sulfonic acid (rectal instillation) results in both acute and chronic colitis. In acute colitis, murine video endoscopy shows inflammation of the colonic mucosa (56). Histology of the colon demonstrates an infiltration of the superficial mucosa with an inflammatory mononuclear infiltrate (57). In chronic colitis, the mucosa demonstrates increased vascularity. Corrosion casting and SEM of the chronically inflamed mucosal microcirculation has demonstrated duplicated and triplicated mucosal vessels (14).
The blood flow to the colonic mucosa is supplied by a polygonal mucosal network surrounding the colonic crypts (58). Within the first 4 days of inflammation, corrosion casting and SEM demonstrate a significant increase in the diameter of mucosal plexus vessels. The diameter of the mucosal vessels nearly doubles (8-15 μm) in many regions of the mucosal plexus within 96 hours (57). Associated with this dilatation is a 50% reduction in flow velocity (1500 μm/s to 700 μm/s) (57). By 7 days after the onset of acute colitis, intravital microscopy of the mucosal plexus demonstrates complex flow patterns (58,59). In 15-25% of vessel segments, tracer flow is excluded. Flow variability appears to be spatially related to occlusive platelet aggregates within the mucosal plexus (60). Coincident with the decrease in blood flow and increase in excluded segments, corrosion casting and SEM demonstrates filling defects in the casts consistent with intussusceptive pillars (14). The pillars are consistently identified at vessel bifurcations and are oriented orthogonal to the bifurcation plane. TEM demonstrates that the pillar is composed of endothelial cells dividing the original mucosal plexus capillary lumen into 2 vessels (14).
Corrosion casting and SEM 28-31 days after the onset of inflammation demonstrates regions of the mucosa with significant vascular replication (14). Some regions of the mucosal plexus demonstrated multiple new layers of the vascular plexus. In both models of chemically-induced colitis, replication of the vessels in the mucosal plexus is heterogeneous with “patchy” areas demonstrating little or no angiogenesis.
In a time course similar to vessel duplication, a subset of vessel bifurcations in murine colitis demonstrate significant remodeling of the vessel branch angle. The morphologic features of the remodeled vessel angles are reminiscent of the intussusceptive process resulting in luminal duplication. In this case, however, pillar extension appears to extend toward the apex of the vessel angle. Also similar to other intussusceptive processes, the spatial orientation and the periodicity of the remodeled angles has suggested that remodeling was a response to regional flow patterns (39).
These results indicate that that pillar formation in two models of chemically-induced colitis leads to both intussusceptive angiogenesis and branch angle remodeling. Similar to skeletal muscle models, these models of extraluminal stimulation of intussusceptive angiogenesis suggest several outstanding questions. First, what is the influence of blood flow heterogeneities in intussusceptive angiogenesis? Platelet aggregates are likely a common source of heterogeneity in many models of tissue inflammation. In tumor biology, blood flow heterogeneities have been associated with leaky capillaries and interstitial hypertension (55). It is possible that there are features common to inflammation and tumorigenesis that contribute to intussusceptive angiogenesis.
Second, how are the duplicated capillaries redistributed in the tissue? The colitis model provides an example of vascular duplication without the contractions associated with skeletal muscle angiogenesis. In chronic colitis, the vessels were often distributed close together. The absence of significant distraction suggests that the arrangement of duplicated capillaries depends upon the anatomical constraints (crypts) and mechanical forces (skeletal muscle) in the surrounding tissue.
Third, what are the inflammatory signals that trigger intussusceptive angiogenesis? An interesting observation in murine colitis is the relative imbalance of blood vessel growth toward intussusceptive angiogenesis; little sprouting angiogenesis was observed after chemical stimulation. Whether the differential angiogenic response was a reflection of the inflammatory cell composition, growth factor milieu or tissue response is unknown.
Pillar extension
Although observations in the chick chorioallantoic membrane indicates that pillar extension often occurs “downstream” to the initial pillar, the mechanism of pillar extension is unknown. The morphologic evidence, based on corrosion casting and SEM, suggests endothelial nuclear impressions in the expanding pillar are indistinguishable from other mural lining cells. The endothelial cell nuclear impressions reflect comparable size and orientation relative to the presumed flow stream (23). There are no morphologic clues suggesting an explanation for the selective downstream proliferation of pillar endothelial cells. We have also been unable to identify focal areas of endothelial proliferation.
Although undetected endothelial proliferation is a possible explanation, it is also possible that the intussusceptive pillar functions as a “trap” for blood borne endothelial progenitor cells (EPCs). The presence of endothelial progenitor cells (EPC) in the blood has been suspected since DeBakey and colleagues suspended a small piece of crumpled Dacron— called a “hub”—with monofilament sutures in the aorta of small pigs (61). Within a month, the piece of Dacron was coated with endothelial cells as documented by electron microscopy (62). Although the report was underappreciated at the time, it is now clear that Stump, O’Neal, DeBakey and colleagues introduced the field of endothelial progenitor cell (EPC) biology. These studies also identified an essential feature of the EPC “trap”; that is, the efficient delivery of EPCs by placing the “hub” or “trap” within the flow stream.
Our group has developed a parabiotic cross-circulation model to investigate the possibility of an EPC trap (63). Parabiosis establishes a common blood circulation by surgical pairing; that is, creating a surgical union of twin animals or parabionts (64). The development of genetically engineered mice expressing green fluorescent protein (GFP) have provided an opportunity to track blood borne cells with a persistent cytoplasmic marker (65). Most importantly, parabiosis provides a fate map of cell migration without bone marrow transplantation or ex vivo labeling. Using the parabiosis model we have demonstrated dramatic numbers of EPCs migrating to, and integrating into, the vascular endothelium of the growing mouse lung (66).
We have also used the parabiosis model to investigate EPC migration in inflammatory colitis (14). In the colon, we tracked GFP+ cells into the colonic vasculature (integration was confirmed by confocal co-localization). An intriguing finding was the pattern of GFP+ migration. Readily analyzed because of the planar vascular network in the colon, concentrations of GFP+ cells were identified in regions of known intussusceptive angiogenesis; that is, at vessel bifurcations within the mucosal plexus (14). Further, the frequency of these aggregated GFP+cells was statistically identical to the frequency of intussusceptive pillars (14). Immunostaining demonstrated that these cells at the vessel bifurcations expressed the EPC marker CD34. Also, vessels already having undergone intussusceptive duplication demonstrate diffusely positive, but lower levels of GFP fluorescence—a characteristic of EPC that have transitioned into mature vascular lining (endothelial) cells (67). Together, these findings suggest that blood-borne EPC may co-localize at the site of intussusceptive angiogenesis.
Summmary
In this review, we discussed our current understanding of the structure and function of the intussusceptive pillar in the process of intussusceptive angiogenesis. 1) Observations in the chick CAM suggest that pillars form in areas of low shear stress. An unresolved paradox is that skeletal muscle intussusceptive angiogenesis appears to be stimulated by high blood flow and elevated wall shear stress. 2) The nonrandom orientation of pillars orthogonal to the bifurcation plane of branching vessels in most models suggests that pillar location is dependent upon blood flow, but the requisite conditions for pillar formation remain unclear. 3) Finite element models of the chick CAM and murine colitis models suggest that shear stress has both a permissive and limiting influence on intussusceptive pillar; however, these observations are unresolved with the high blood flow conditions associated with skeletal muscle intussusceptive angiogenesis. 4) The intravascular location of the intussusceptive pillar suggests its potential role in localizing blood-borne elements, such as endothelial progenitor cells, to areas of the microcirculation undergoing active angiogenesis. We anticipate that further studies in these areas will provide fundamental insights into the remodeling and expansion of microvascular networks.
Abbreviations
- CAM
chick chorioallantoic membrane
- EPC
endothelial progenitor cell
- GFP
green fluorescent protein
- SEM
scanning electron microscopy
- TEM
transmission electron microscopy
Footnotes
Presented, in part, at the Trans-NIH Angiogenesis Conference, May 21, 2014
References
- 1.Eming SA, Brachvogel B, Odorisio T, Koch M. Regulation of angiogenesis: wound healing as a model. Prog. Histochem. Cytochem. 2007;42:115–70. doi: 10.1016/j.proghi.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends Mol. Med. 2011;17:424–432. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Zhao Y, Bao Q, Renner A, Camaj P, Eichhorn M, Ischenko I, Angele M, Kleespies A, Jauch KW, Bruns C. Cancer stem cells and angiogenesis. Int. J. Dev. Biol. 2011;55:477–482. doi: 10.1387/ijdb.103225yz. [DOI] [PubMed] [Google Scholar]
- 4.Clark ER. Studies on the growth of blood-vessels in the tail of the frog larva--by observation and experiment on the living animal. Am. J. Anat. 1918;23:37–88. [Google Scholar]
- 5.Schulte HW. Early stages of vasculogenesis in the cat (Felis Domestica) with especial reference to the mesenchymal origin of endothelium. The Wistar Institute of Anatomy and Biology. 1914 [Google Scholar]
- 6.Clark ER, Clark EL. Microscopic observations on the extra-endothelial cells of living mammalian blood vessels. Am. J. Anat. 1940;66:1–49. [Google Scholar]
- 7.Caduff JH, Fischer LC, Burri PH. Scanning electron microscope study of the developing microvasculature in the postnatal rat lung. Anat. Rec. 1986;216:154–64. doi: 10.1002/ar.1092160207. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa Y. On the fine structural changes of the microvascular beds in skeletal muscle. J. Yokohama City Univ. Ser. Sport Sci. Med. 1977;6:1–19. [Google Scholar]
- 9.Appell H-J. Morphological studies on skeletal muscle under conditions of high altitude training. Int. J. Sports Med. 1980;1:103–109. [Google Scholar]
- 10.Hughes GM, Morgan M. Structure of fish gills in relation to their respiratory function. Biol. Rev. Camb. Philos. Soc. 1973;48:419. &. [Google Scholar]
- 11.Olson KR, Dewar H, Graham JB, Brill RW. Vascular anatomy of the gills in a high energy demand teleost, the skipjack tuna (Katsuwonus pelamis) J. Exp. Zool. 2003;297A:17–31. doi: 10.1002/jez.a.10262. [DOI] [PubMed] [Google Scholar]
- 12.Mongera A, Singh AP, Levesque MP, Chen YY, Konstantinidis P, Nusslein-Volhard C. Genetic lineage labeling in zebrafish uncovers novel neural crest contributions to the head, including gill pillar cells. Development. 2013;140:916–925. doi: 10.1242/dev.091066. [DOI] [PubMed] [Google Scholar]
- 13.Makanya AN, Hlushchuk R, Djonov VG. Intussusceptive angiogenesis and its role in vascular morphogenesis, patterning, and remodeling. Angiogenesis. 2009;12:113–23. doi: 10.1007/s10456-009-9129-5. [DOI] [PubMed] [Google Scholar]
- 14.Konerding MA, Turhan A, Ravnic DJ, Lin M, Fuchs C, Secomb TW, Tsuda A, Mentzer SJ. Inflammation-induced intussusceptive angiogenesis in murine colitis. Anat. Rec. 2010;293:849–857. doi: 10.1002/ar.21110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribatti D, Djonov V. Intussusceptive microvascular growth in tumors. Cancer Lett. 2012;316:126–131. doi: 10.1016/j.canlet.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 16.Egginton S, Zhou AL, Brown MD, Hudlicka O. Unorthodox angiogenesis in skeletal muscle. Cardiovasc. Res. 2001;49:634–646. doi: 10.1016/s0008-6363(00)00282-0. [DOI] [PubMed] [Google Scholar]
- 17.Konerding MA. Scanning electron microscopy of corrosion casting in medicine. Scanning Microsc. 1991;5:851–65. [PubMed] [Google Scholar]
- 18.Lametschwandtner A, Aharinejad S. Recent Adv. Microsc. Cells Tis. Organs. Antonio Delfino; Rome: 1997. Scanning electron microscopy/corrosion casting technique in biological and medical research. State of the art and perspectives; pp. 51–58. [Google Scholar]
- 19.Gottlieb AI, Langille BL, Wong MK, Kim DW. Structure and function of the endothelial cytoskeleton. Lab Invest. 1991;65:123–37. [PubMed] [Google Scholar]
- 20.Malek AM, Izumo S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J. Cell Sci. 1996;109(Pt 4):713–26. doi: 10.1242/jcs.109.4.713. [DOI] [PubMed] [Google Scholar]
- 21.Galbraith CG, Skalak R, Chien S. Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil. Cytoskelet. 1998;40:317–330. doi: 10.1002/(SICI)1097-0169(1998)40:4<317::AID-CM1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Barbee KA, Davies PF, Lal R. Shear stress-induced reorganization of the surface topography of living endothelial cells imaged by atomic force microscopy. Circ. Res. 1994;74:163–71. doi: 10.1161/01.res.74.1.163. [DOI] [PubMed] [Google Scholar]
- 23.Lee GS, Filipovic N, Lin M, Gibney BC, Simpson DC, Konerding MA, Tsuda A, Mentzer SJ. Intravascular pillars and pruning in the extraembryonic vessels of chick embryos. Dev. Dyn. 2011;240:1335–1343. doi: 10.1002/dvdy.22618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schraufnagel DE, Schmid A. Microvascular casting of the lung: effects of various fixation protocols. Journal of Electron Microscopy Technique. 1988;8:185–91. doi: 10.1002/jemt.1060080205. [DOI] [PubMed] [Google Scholar]
- 25.Nico B, Crivellato E, Ribatti D. The importance of electron microscopy in the study of capillary endothelial cells: An historical review. Endothelium. 2007;14:257–264. doi: 10.1080/10623320701746289. [DOI] [PubMed] [Google Scholar]
- 26.Burri PH, Tarek MR. A novel mechanism of capillary growth in the rat pulmonary microcirculation. Anat. Rec. 1990;228:35–45. doi: 10.1002/ar.1092280107. [DOI] [PubMed] [Google Scholar]
- 27.Williams JL, Cartland D, Rudge JS, Egginton S. VEGF trap abolishes shear stress- and overload-dependent angiogenesis in skeletal muscle. Microcirculation. 2006;13:499–509. doi: 10.1080/10739680600785717. [DOI] [PubMed] [Google Scholar]
- 28.Paku S, Dezso K, Bugyik E, Tovari J, Timar J, Nagy P, Laszlo V, Klepetko W, Dome B. A new mechanism for pillar formation during tumor-induced intussusceptive angiogenesis. Am. J. Pathol. 2011;179:1573–1585. doi: 10.1016/j.ajpath.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eming SA, Hubbell JA. Extracellular matrix in angiogenesis: dynamic structures with translational potential. Exp. Dermatol. 2011;20:605–613. doi: 10.1111/j.1600-0625.2011.01309.x. [DOI] [PubMed] [Google Scholar]
- 30.Brown MD, Hudlicka O. Modulation of physiological angiogenesis in skeletal muscle by mechanical forces: Involvement of VEGF and metalloproteinases. Angiogenesis. 2003;6:1–14. doi: 10.1023/a:1025809808697. [DOI] [PubMed] [Google Scholar]
- 31.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen-Smith FM, Hudlicka O, Egginton S. In vivo angiogenesis in adult rat skeletal muscle: Early changes in capillary network architecture and ultrastructure. Cell Tissue Res. 1996;286:123–136. doi: 10.1007/s004410050681. [DOI] [PubMed] [Google Scholar]
- 33.Djonov V, Baum O, Burri PH. Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 2003;314:107–17. doi: 10.1007/s00441-003-0784-3. [DOI] [PubMed] [Google Scholar]
- 34.Makanya AN, Stauffer D, Ribatti D, Burri PH, Djonov V. Microvascular growth, development, and remodeling in the embryonic avian kidney: The interplay between sprouting and intussusceptive angiogenic mechanisms. Journal of Electron Microscopy Technique. 2005;66:275–288. doi: 10.1002/jemt.20169. [DOI] [PubMed] [Google Scholar]
- 35.Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr. Opin. Cell Biol. 2010;22:617–625. doi: 10.1016/j.ceb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Wacker A, Gerhardt H. Endothelial development taking shape. Curr. Opin. Cell Biol. 2011;23:676–685. doi: 10.1016/j.ceb.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Lee CY, Bautch VL. Ups and Downs of Guided Vessel Sprouting: The Role of Polarity. Physiology. 2011;26:326–333. doi: 10.1152/physiol.00018.2011. [DOI] [PubMed] [Google Scholar]
- 38.Iruela-Arispe ML, Davis GE. Cellular and Molecular Mechanisms of Vascular Lumen Formation. Dev. Cell. 2009;16:222–231. doi: 10.1016/j.devcel.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ackermann M, Tsuda A, Secomb TW, Mentzer SJ, Konerding MA. Intussusceptive remodeling of vascular branch angles in chemically-induced murine colitis. Microvasc. Res. 2013;87:75–82. doi: 10.1016/j.mvr.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee GS, Filipovic N, Miele LF, Simpson DC, Lin M, Konerding MA, Tsuda A, Mentzer SJ. Blood flow shapes intravascular pillar geometry in the chick chorioallantoic membrane. J. Angiogenes. Res. 2010;2:11–20. doi: 10.1186/2040-2384-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filipovic N, Tsuda A, Lee GS, Miele L, Lin M, Konerding MA, Mentzer SJ. Computational flow dynamics in a geometric model of intussusceptive angiogenesis. Microvasc. Res. 2009;78:286–293. doi: 10.1016/j.mvr.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hudlicka O, Brown MD, Egginton S. Myology: Basic and Clinical. McGraw-Hill; London: 2004. Microcirculation in muscle; pp. 511–533. [Google Scholar]
- 43.Egginton S, Hudlicka O, Brown MD, Walter H, Weiss JB, Bate A. Capillary growth in relation to blood flow and performance in overloaded rat skeletal muscle. J. Appl. Physiol. 1998;85:2025–2032. doi: 10.1152/jappl.1998.85.6.2025. [DOI] [PubMed] [Google Scholar]
- 44.Hudlicka O, Brown M, Egginton S. Angiogenesis in skeletal and cardiac-muscle. Physiol. Rev. 1992;72:369–417. doi: 10.1152/physrev.1992.72.2.369. [DOI] [PubMed] [Google Scholar]
- 45.Green DJ, Maiorana AJ, Cable NT. Point : Counterpoint: Exercise training does/does not induce vascular adaptations beyond the active muscle beds. J. Appl. Physiol. 2008;105:1002–1004. doi: 10.1152/japplphysiol.90570.2008. [DOI] [PubMed] [Google Scholar]
- 46.Milkiewicz M, Hudlicka O, Brown MD, Silgram H. Nitric oxide, VEGF, and VEGFR-2: interactions in activity-induced angiogenesis in rat skeletal muscle. American Journal of Physiology-Heart and Circulatory Physiology. 2005;289:H336–H343. doi: 10.1152/ajpheart.01105.2004. [DOI] [PubMed] [Google Scholar]
- 47.Hudlicka O, Brown MD. Adaptation of Skeletal Muscle Microvasculature to Increased or Decreased Blood Flow: Role of Shear Stress, Nitric Oxide and Vascular Endothelial Growth Factor. J. Vasc. Res. 2009;46:504–512. doi: 10.1159/000226127. [DOI] [PubMed] [Google Scholar]
- 48.Tornling G, Adolfsson J, Unge G, Ljungqvist A. Capillary neoformation in skeletal-muscle of dipyridamole-treated rats. Arzneimittel-Forsch. 1980;30-1:791–792. [PubMed] [Google Scholar]
- 49.Hudlicka O, Komarek J, Wright AJA. The effect of a xanthine derivative, 1-(5′-oxohexyl)-3-methyl-7-propylxanthine (hwa-285), on heart performance and regional blood-flow in dogs and rabbits. Br. J. Pharmacol. 1981;72:723–730. doi: 10.1111/j.1476-5381.1981.tb09154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milkiewicz M, Brown MD, Egginton S, Hudlicka O. Association between shear stress, angiogenesis, and VEGF in skeletal muscles in vivo. Microcirculation. 2001;8:229–241. doi: 10.1038/sj/mn/7800074. [DOI] [PubMed] [Google Scholar]
- 51.Hudlicka O, Graciotti L, Fulgenzi G, Brown MD, Egginton S, Milkiewicz M, Granata AL. The effect of chronic skeletal muscle stimulation on capillary growth in the rat: are sensory nerve fibres involved? J. Physiol.-London. 2003;546:813–822. doi: 10.1113/jphysiol.2002.030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rivilis I, Milkiewicz M, Boyd P, Goldstein J, Brown MD, Egginton S, Hansen FM, Hudlicka O, Haas TL. Differential involvement of MMP-2 and VEGF during muscle stretch-versus shear stress-induced angiogenesis. American Journal of Physiology-Heart and Circulatory Physiology. 2002;283:H1430–H1438. doi: 10.1152/ajpheart.00082.2002. [DOI] [PubMed] [Google Scholar]
- 53.Resnick N, Yahav H, Schubert S, Wolfovitz E, Shay A. Signalling pathways in vascular endothelium activated by shear stress: relevance to atherosclerosis. Curr. Opin. Lipidol. 2000;11:167–177. doi: 10.1097/00041433-200004000-00010. [DOI] [PubMed] [Google Scholar]
- 54.Ando J, Yamamoto K. Effects of Shear Stress and Stretch on Endothelial Function. Antioxid. Redox Signal. 2011;15:1389–1403. doi: 10.1089/ars.2010.3361. [DOI] [PubMed] [Google Scholar]
- 55.Baxter LT, Jain RK. Vascular-permeability and interstitial diffusion in superfused tissues - a two-dimensional model. Microvasc. Res. 1988;36:108–115. doi: 10.1016/0026-2862(88)90043-x. [DOI] [PubMed] [Google Scholar]
- 56.Ravnic DJ, Konerding MA, Huss HT, Wolloscheck T, Pratt JP, Mentzer SJ. Murine microvideo endoscopy of the colonic microcirculation. J. Surg. Res. 2007;142:97–103. doi: 10.1016/j.jss.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ravnic DJ, Konerding MA, Tsuda A, Jiang X, Huss HT, Pratt JP, Mentzer SJ. Structural adaptations in the murine colon microcirculation associated with hapten-induced inflammation. Gut. 2007;56:518–523. doi: 10.1136/gut.2006.101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turhan A, Konerding MA, Tsuda A, Ravnic DJ, Hanidizar D, Lin MY, Mentzer SJ. Bridging mucosal vessels associated with rhythmically oscillating blood flow in murine colitis. Anat. Rec. 2007;291:74–92. doi: 10.1002/ar.20628. [DOI] [PubMed] [Google Scholar]
- 59.Tsuda A, Turhan A, Konerding MA, Ravnic DJ, Hanidziar D, Lin M, Mentzer SJ. Bimodal oscillation frequencies of blood flow in the inflammatory colon microcirculation. Anat. Rec. 2009;292:65–72. doi: 10.1002/ar.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miele LF, Turhan A, Lee GS, Lin M, Ravnic DJ, Tsuda A, Konerding MA, Mentzer SJ. Blood flow patterns spatially associated with platelet aggregates in murine colitis. Anat. Rec. 2009;292:1143–1153. doi: 10.1002/ar.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stump MM, Jordan GL, Jr., Debakey ME, Halpert B. Endothelium Grown from Circulating Blood on Isolated Intravascular Dacron Hub. Am. J. Pathol. 1963;43:361–7. [PMC free article] [PubMed] [Google Scholar]
- 62.O’Neal RM, Jordan GL, Jr., Rabin ER, Debakey ME, Halpert B. Cells Grown on Isolated Intravascular Dacron Hub; an Electron Microscopic Study. Exp. Mol. Pathol. 1964;90:403–12. doi: 10.1016/0014-4800(64)90022-x. [DOI] [PubMed] [Google Scholar]
- 63.Gibney B, Chamoto K, Lee GS, Simpson DC, Miele L, Tsuda A, Konerding MA, Wagers A, Mentzer SJ. Cross-circulation and cell distribution kinetics in parabiotic mice. J. Cell. Physiol. 2012;227:821–828. doi: 10.1002/jcp.22796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bunster E, Meyer RK. An improved method of parabiosis. Anat. Rec. 1933;57:339–343. [Google Scholar]
- 65.Swenson ES, Price JG, Brazelton T, Krause DS. Limitations of green fluorescent protein as a cell lineage marker. Stem Cells. 2007;25:2593–600. doi: 10.1634/stemcells.2007-0241. [DOI] [PubMed] [Google Scholar]
- 66.Chamoto K, Gibney BC, Lee GS, Lin M, Simpson DC, Voswinckel R, Konerding MA, Tsuda A, Mentzer SJ. CD34+ progenitor to endothelial cell transition in post-pneumonectomy angiogenesis. Am. J. Resp. Cell Mol. Biol. 2012;46:283–289. doi: 10.1165/rcmb.2011-0249OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chamoto K, Gibney BC, Lee GS, Ackermann M, Konerding MA, Tsuda A, Mentzer SJ. Migration of CD11b+ accessory cells during murine lung regeneration. Stem Cell Res. 2013;10:267–277. doi: 10.1016/j.scr.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]