Abstract
Background & Aims
Risk prediction models for Barrett’s esophagus (BE) have been developed using multiple demographic and clinical variables, but their predictive performance has been modest. Adding a multi-biomarker risk score may improve discriminatory ability.
Methods
We used data from 141 patients with definitive BE and 138 controls participating in a case–control study at the Michael E. DeBakey Veterans Affairs Medical Center in Houston, TX (97% male, 65% of controls white, 89% of cases white). We derived and compared 3 prediction models. Model 1 included only GERD frequency and duration; Model 2 included GERD frequency and duration, age, sex, race, waist-to-hip ratio, and Helicobacter pylori status; and Model 3 included the variables in Model 2 as well as a multi-biomarker risk score based on serum levels of interleukin (IL)12p70, IL6, IL8, IL10, and leptin. We assessed their predictive accuracy in terms of discrimination using area under the receiver operating characteristic curve (AUC) and calibration analyses.
Results
The multi-biomarker risk score was significantly associated with risk for BE. Compared to persons with a score of 0, persons with a score of 3 or more had greater than 10-fold increased risk for BE (biomarker risk score ≥3; OR=11.9; 95% confidence interval, 4.06–34.9; P-trend <.001). Risk prediction using the multi-biomarker score in conjunction with demographic and clinical features improved discrimination compared with using only GERD frequency and duration (AUC=0.85 vs 0.74, P=0.01).
Conclusions
Based on data from a case–control study of predominantly white male veterans, a risk prediction model including a multi-biomarker score, derived from serum levels of cytokines and leptin, as well as GERD frequency and duration, age, sex, race, waist-to-hip ratio, and H pylori infection, can more accurately identify persons in this population with BE than previous methods.
Keywords: Barrett’s esophagus, Biomarkers, Cytokines, Risk prediction
Introduction
The incidence of esophageal adenocarcinoma (EAC) and its precursor, Barrett’s esophagus (BE), continue to increase in Western populations.1, 2 Gastroesophageal reflux disease (GERD) is the primary causal factor for EAC and BE.3, 4 While obesity is associated with increased risk of GERD symptoms,5 epidemiological studies have implicated obesity as a risk factor for EAC and BE independently of GERD.6, 7 The mechanisms remain largely unknown, however we have demonstrated recently that visceral (as opposed to subcutaneous) abdominal fat is particularly important for promoting BE,8, 9 with high circulating pro-inflammatory cytokines and leptin, and low anti-inflammatory cytokines as possible mediators.10
Multiple BE predictive models have been developed using demographic and clinical variables, however none have shown high enough predictive ability to allow use in clinical practice.11–14 Given the complex interplay of mediators that may contribute to the development of BE, a multi-biomarker panel may provide additional information about BE risk above that provided by any single biomarker or by demographic and clinical features. The aim of this study was to evaluate the ability of a multi-biomarker risk score to predict the risk of BE. The risk score was based on levels of multiple circulating serum biomarkers that we have shown to be associated with BE, including serum levels of interleukin (IL)-12p70, IL-6, IL-8, IL-10 and leptin.10
Methods
We used data from a randomly selected subgroup of patients who participated in a case-control study of BE conducted at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas. The study was approved by the Institutional Review Boards for MEDVAMC and the Baylor College of Medicine.
Details of the overall9 and biomarker subgroup10 populations have been previously described. Briefly, participants were recruited between 1 September 2008 and 31 December 2011 from among consecutive patients attending one of seven selected MEDVAMC primary care clinics who were eligible for a routine screening colonoscopy. None of the patients attending primary care clinics were primarily referred for esophagogastroduodenoscopy (EGD) and the study EGD was performed during the same clinical visit as their colonoscopy. Our secondary recruitment source was consecutive eligible patients scheduled for an elective EGD at MEDVAMC for any indication. The eligibility criteria were: age between 50 and 80 years (and 40–80 years for the elective EGD group); no history of gastroesophageal surgery or diagnosis of cancer; not taking anticoagulants; no significant liver disease (indicated by platelet count below 70,000 or known gastroesophageal varices); and no history of major stroke or mental condition.
Participants underwent a study EGD with systematic recording of suspected BE according to the Prague C & M classification and at least one targeted biopsy was taken from suspected BE areas using Jumbo biopsy forceps. The control group included patients from the primary care group with no endoscopically suspected BE on their study EGD. BE cases included patients from either the primary care group or the elective EGD group with both endoscopically-suspected and histologically confirmed BE. We excluded patients who had endoscopically suspected BE but without specialized intestinal metaplasia on biopsy from the analysis.
Data Collection
All participants completed a computer assisted survey prior to their study EGD. The survey elicited information about race and ethnicity, social background, cigarette smoking, alcohol use, medical history, and use of proton pump inhibitors (PPIs), H2-receptor antagonists, aspirin and nonsteroidal anti-inflammatory drugs. We ascertained a history of heartburn and regurgitation symptoms using a slightly modified version of the validated Gastroesophageal Reflux Questionnaire (GERQ).15 We defined duration of GERD as the sum of the duration of at least weekly heartburn or regurgitation symptoms. Frequency of current GERD symptoms (<weekly, ≥weekly) were further classified on the basis of current PPI use (yes, no) to identify those without frequent symptoms but who were using PPIs. Height and weight were measured prior to the study EGD and were used to calculate body mass index (BMI). A flexible tape measure was used to measure waist and hip circumference. Participants were defined as H pylori positive if organisms were seen on histopathology of any of the study gastric biopsies. If biopsy results were not available, participants were defined as positive for H pylori if review of the medical record showed a previous positive biopsy, presence of serum antibodies, or treatment received.
The measurements of circulating serum levels of inflammatory cytokines and leptin were performed at the MEDVAMC Division of Endocrinology laboratory using commercially available electro-chemiluminescence assay kits from Mesoscale (Gaithersburg, MD) and according to the manufacturers’ instructions. The intraassay coefficient of variance (CV) was ≤10% for all assays; and the sensitivities were 0.77 pg/mL, 0.18 pg/mL, 0.1 pg/mL, 7.5 pg/mL, and 43 pg/mL for IL-12p70, IL-6, IL-8, IL-10 and leptin, respectively.
Statistical analysis
To examine the joint predictive value of several serum biomarkers, we derived a biomarker risk score by assigning one point for each biomarker value (for IL-12p70, IL-6, IL-8 and leptin) that was in the highest quartile of the control group distribution. Since serum IL-10 is inversely associated with BE, we assigned one point to those in the lowest quartile. Odds ratios (OR) and 95% confidence intervals (95%CI) for the association between the biomarker score and BE risk were estimated using unconditional multivariable logistic regression. We developed three risk prediction models for BE. The first model included terms for frequency and duration of GERD symptoms. The second model included GERD frequency and duration, age, sex, race, waist-to-hip ratio (WHR), and H pylori status. Smoking was not included as it is not associated with BE in our study.16 The third model additionally included the biomarker risk score. We assessed the ability of the models to discriminate between cases and controls by calculating the area under the receiver operating characteristic curve (AUC), and used 10-fold cross validation and a bootstrap re-sampling method to evaluate each model’s internal validity and to correct for over-fitting. Model calibration was assessed using the Hosmer-Lemeshow goodness-of-fit test, where P>0.05 indicates a well-calibrated model. Analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC) and R software. Statistical significance was determined at α = 0.05 and all tests for statistical significance were two-sided.
Results
We had demographic, clinical and serological data available for 138 controls and 141 BE cases for analysis. Characteristics of the study participants are shown in Table 1. The majority of participants were white (77%) and male (97%). As reported previously, BE cases were more likely to be white, have a larger WHR, and have experienced GERD symptoms and have a history of PPI use compared with controls. In contrast, BE cases were less likely to be H pylori positive than controls. After adjusting for potential confounders, we found statistically significant linear trends between serum levels of leptin (P-trend=0.001), IL-12p70 (P-trend=0.02), IL-8 (P-trend<0.001), and IL-10 (P-trend<0.001) and the risk of BE; there was a borderline significant association between serum IL-6 and BE (P-trend=0.06).
Table 1.
Characteristics of controls and patients with Barrett’s esophagus
| Variables | Categories | Controls (n=138) | BE Cases (n=141) | P-value |
|---|---|---|---|---|
|
| ||||
| n (%) | n (%) | |||
| Age, years | Mean (s.d.) | 61.6 (6.9) | 62.8 (6.7) | 0.16 |
| Sex | 0.72 | |||
| Male | 134 (97.1) | 138 (97.9) | ||
| Female | 4 (2.9) | 3 (2.1) | ||
| Race | <0.001 | |||
| White | 89 (64.5) | 126 (89.4) | ||
| African American | 44 (31.9) | 12 (8.5) | ||
| Other | 5 (3.6) | 3 (2.1) | ||
| WHR ratio* | 0.03 | |||
| High | 118 (85.5) | 132 (93.6) | ||
| Low | 20 (14.5) | 9 (6.4) | ||
| Smoking status | 0.92 | |||
| Non-smoker | 33 (25.2) | 30 (23.1) | ||
| Ex-smoker | 61 (46.6) | 62 (47.7) | ||
| Current smoker | 37 (28.2) | 38 (29.2) | ||
| Missing | 7 | 11 | ||
| Combined frequency of current GERD symptoms and current PPIs | <0.001 | |||
| < weekly GERD, no PPI | 93 (70.6) | 41 (31.3) | ||
| < weekly GERD, yes PPI | 29 (22.0) | 62 (47.3) | ||
| ≥ weekly GERD, no PPI | 4 (3.0) | 3 (2.3) | ||
| ≥ weekly GERD, yes PPI | 66 (4.5) | 25 (19.1) | ||
| Missing | 6 | 10 | ||
| NSAID use | 0.84 | |||
| Ever | 82 (62.1) | 83 (63.4) | ||
| Never | 50 (37.9) | 48 (36.6) | ||
| Missing | 6 | 10 | ||
| H pylori status | 0.002 | |||
| Never | 96 (71.1) | 120 (86.3) | ||
| Ever | 39 (28.9) | 19 (13.7) | ||
| Missing | 3 | 2 | ||
BE, Barrett’s esophagus; GERD, gastroesophageal reflux disease; NSAID, non-steroidal anti-inflammatory drug; PPIs, proton pump inhibitors; WHR, waist-to-hip ratio.
High WHR cutoff was considered ≥0.90 for males and ≥0.85 for females.
In multivariable analysis, the biomarker risk score was significantly associated with risk of BE (Table 2). Compared to persons with a score of 0, persons with a score of 3 or more had approximately 12-fold increased risk of BE (biomarker risk score ≥3, OR=11.9, 95%CI 4.06–34.9; P-trend<0.001). We found an even stronger effect for the score when we restricted the analyses to 209 (n=86 controls, n=123 cases) white males (biomarker risk score ≥3, OR=15.9, 95%CI 4.29–59.2; P-trend<0.001).
Table 2.
Association between the biomarker risk score and risk of Barrett’s esophagus
| Biomarker risk score | Controls n (%) | BE Cases n (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI)* |
|---|---|---|---|---|
| 0 | 30 (21.7) | 11 (7.8) | 1.00 (Ref) | 1.00 (Ref) |
| 1 | 59 (42.8) | 34 (24.1) | 1.57 (0.70–3.53) | 1.30 (0.51–3.27) |
| 2 | 35 (25.4) | 45 (31.9) | 3.51 (1.54–7.96) | 3.75 (1.44–9.78) |
| ≥3 | 14 (10.1) | 51 (36.2) | 9.94 (4.00–24.7) | 11.9 (4.06–34.9) |
| P-trend | <0.001 | |||
| White males only | ||||
| 0 | 19 (22.1) | 10 (8.1) | 1.00 (Ref) | 1.00 (Ref) |
| 1 | 40 (46.5) | 30 (24.4) | 1.43 (0.58–3.51) | 0.96 (0.34–2.69) |
| 2 | 22 (25.6) | 39 (31.7) | 3.37 (1.33–8.51) | 2.82 (0.99–8.00) |
| ≥3 | 5 (5.8) | 44 (35.8) | 16.7 (5.03–55.6) | 15.9 (4.29–59.2) |
| P-trend | <0.001 |
BE, Barrett’s esophagus; CI, confidence interval; OR, odds ratio.
Adjusted for age, sex, race, waist-to-hip ratio, PPI use, use of NSAIDs, and H pylori status.
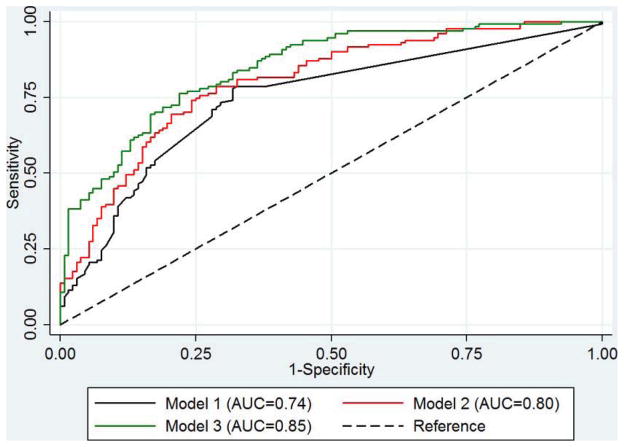
From the first to the third prediction models for BE, the range of predicted risk increased in cases and controls. Importantly, however, the difference in predicted risks between cases and controls increased and the discriminatory performance of the models improved as more risk factors were added (Figure 1). The AUC for Model 1 (GERD symptoms only) was 0.74 (95%CI 0.69–0.80). The addition of risk factors significantly associated with BE in our study (Model 2) improved the AUC to 0.80 (95%CI 0.75–0.85). However, this did not reach statistical significance (P=0.15). Further addition of the biomarker risk score (Model 3) significantly improved the AUC above that for Model 1 (AUC=0.85, 95%CI 0.80–0.89; P=0.01), but not above that for Model 2 (P=0.21). Each model showed good internal validity and we found little evidence of over-fitting (10-fold cross validation AUCs: Model 1, AUC=0.73, 95%CI 0.63–0.83; Model 2, AUC=0.75, 95%CI 0.67–0.84; Model 3, AUC=0.82, 95%CI 0.75–0.88). The three risk prediction models were well-calibrated according to the Hosmer-Lemeshow test (P=0.12, P=0.67, P=0.59, respectively). The models performed equally well when we restricted our analyses to white males (Model 1: AUC=0.76, 95%CI 0.69–0.82; Model 2: AUC=0.78, 95%CI 0.71–0.84; Model 3: AUC=0.84, 95%CI 0.78–0.89).
Figure 1.
Comparison of ROC curves for: Model 1, GERD frequency and duration only; Model 2, GERD frequency and duration, age, sex, race, WHR and H pylori status; Model 3, Model 2 + biomarker risk score.
Discussion
We have previously shown that high circulating serum levels of IL-12p70, IL-6, IL-8 and leptin, and low circulating serum levels of IL-10 are separately statistically significantly associated with increased risk of BE.10 Here, we assessed their combined effect on BE risk and investigated the potential clinical utility of a multi-biomarker score for predicting risk of BE. BE risk increased linearly with increasing biomarker score. When ≥3 biomarkers were elevated, risk was especially high (>10-fold). Further, the multi-biomarker score had strong discriminatory power for predicting risk of BE.
GERD is increasingly common in Western populations,17 and is associated with increased risks of EAC and BE.3, 4 However, the yield associated with endoscopic screening for BE according to GERD symptoms is low because GERD symptoms have only modest ability to discriminate between people with and without BE.13, 14 In this study, we found that frequency and duration of GERD symptom had fair discriminatory power with an AUC of 0.73 after internal validation. The AUC for GERD frequency and duration was lower however in a recent study among Veterans in Michigan (0.61).12 Neither of these studies was externally validated.
Few tools have been developed by which clinicians can integrate multiple BE risk factors into a single score for BE risk stratification,11, 12 and none have shown high enough predictive ability to warrant clinical application. In the only externally validated model, the AUC for predicting BE among patients with GERD (based on their age, sex, BMI, education and PPI use) was 0.61.11 While age, sex, race, WHR and H pylori status are associated with BE risk in our study, adding these to the GERD only model did not significantly improve discrimination. However, risk prediction using the multi-biomarker score in conjunction with demographic and clinical features was superior to GERD alone (P=0.01). The modest relative gain in AUC between Models 2 and 3 suggests that the biomarker score adds some important information above WHR and other factors, but it is the combination of factors (e.g., WHR and the biomarker score) that is essential for improved discriminatory accuracy above GERD history alone.
While it is not our intention to define a threshold for “high-risk BE”, we found that at all potential risk thresholds more cases and fewer controls had risks above the risk threshold when using Model 3 compared with Models 1 and 2. Consequently, the risk distributions among cases and controls were more separated by Model 3 than by Models 1 and 2. Furthermore, the balance between sensitivity and specificity was also improved from Model 1 to 3 (e.g., for a specificity of 75%, sensitivity was 33% and 70% for Models 1 and 3, respectively).
We believe that the first priority is to validate the use of these biomarkers in large external cohorts, followed if needed by modifications of the predictive model. The current price of these biomarkers is approximately $890 as an out of pocket expense at commercial labs. However this high price partly reflects individual pricing for each biomarker, and is expected to drop significantly if a set of biomarkers are combined in one kit. Additionally, healthcare organizations are likely to obtain significant discounts; for example the VA has approximately 50% price discount on most of these biomarkers (personal communication with HES). It is possible that application of a predictive model that combines serum biomarkers and clinical factors might result in significant savings related to a reduced number of endoscopic procedures. However, formal analyses may be required to establish cost-effectiveness of the serum biomarkers once all the steps described above are accomplished.
Strengths of the present study include the prospective enrolment of study participants, the standardized histologic and endoscopic definition used to make formal diagnosis of BE and the use of a comprehensive questionnaire to capture detailed information on potential confounders. The study has a few limitations. Because we studied predominantly white male veterans, the findings from our study may not be generalizable to women and nonveterans. Furthermore, as we may have overestimated the performance of our models using internal validation methods, independent validation on an external cohort of patients is required.
In summary, this study demonstrated that a multi-biomarker risk score may provide useful information for stratifying risk of BE; however, this needs to be validated in other VA populations and there is a need for further research to determine whether such a score would help predict BE risk in the general population. Identifying additional serum biomarkers associated with risk of BE and adding these to the multi-biomarker score may further improve predictive ability and is the focus of our ongoing research.
Acknowledgments
Grant support: This study was funded by a grant from the National Institutes of Health (NIH) (CA116845) to HES. APT is supported by a Fellowship from the National Health and Medical Research Council of Australia (APP1052219). JMG receives financial support from the Department of Veterans Affairs (CX000174, BX000507) and the NIH (AG040583). This material is also based upon work supported with resources and the use of facilities at the Houston VA Health Services Research and Development Center of Excellence (HFP90-020). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Abbreviations
- AUC
area under the receiver operating characteristic curve
- BE
Barrett’s esophagus
- CI
confidence interval
- EAC
esophageal adenocarcinoma
- EGD
esophagogastroduodenoscopy
- GERD
Gastroesophageal reflux disease
- IL
interleukin
- OR
odds ratio
- PPI
proton pump inhibitor
- WHR
waist-to-hip ratio
Footnotes
Disclosures: None.
Author Contributions: APT, JMG,HES designed the study. JMG performed hormonal assays. APT analyzed the data. APT, HES drafted the manuscript. APT, JMG, HES reviewed and approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol. 2012;23:3155–3162. doi: 10.1093/annonc/mds181. [DOI] [PubMed] [Google Scholar]
- 2.van Soest EM, Dieleman JP, Siersema PD, et al. Increasing incidence of Barrett's oesophagus in the general population. Gut. 2005;54:1062–1066. doi: 10.1136/gut.2004.063685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lagergren J, Bergstrom R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman DA, Oehlke M, Helfand M. Risk factors for Barrett's esophagus in community-based practice. Am J Gastroenterol. 1997;92:1293–1297. [PubMed] [Google Scholar]
- 5.Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143:199–211. doi: 10.7326/0003-4819-143-3-200508020-00006. [DOI] [PubMed] [Google Scholar]
- 6.Singh S, Sharma AN, Murad MH, et al. Central adiposity is associated with increased risk of esophageal inflammation, metaplasia, and adenocarcinoma: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1399–1412. doi: 10.1016/j.cgh.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duggan C, Onstad L, Hardikar S, et al. Association between markers of obesity and progression from Barrett's esophagus to esophageal adenocarcinoma. Clin Gastroenterol Hepatol. 2013;11:934–943. doi: 10.1016/j.cgh.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Serag HB, Hashmi A, Garcia J, et al. Visceral abdominal obesity measured by CT scan is associated with an increased risk of Barrett's oesophagus: a case-control study. Gut. 2013 doi: 10.1136/gutjnl-2012-304189. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer JR, Fischbach LA, Richardson P, et al. Waist-to-hip ratio, but not body mass index, is associated with an increased risk of Barrett's esophagus in white men. Clin Gastroenterol Hepatol. 2013;11:373–381. doi: 10.1016/j.cgh.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia JM, Splenser AE, Kramer J, et al. Circulating inflammatory cytokines and adipokines are associated with Barrett's esophagus: a case-control study. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.07.038. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thrift AP, Kendall BJ, Pandeya N, et al. A clinical risk prediction model for Barrett esophagus. Cancer Prev Res (Phila) 2012;5:1115–1123. doi: 10.1158/1940-6207.CAPR-12-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubenstein JH, Morgenstern H, Appelman H, et al. Prediction of Barrett's esophagus among men. Am J Gastroenterol. 2013;108:353–362. doi: 10.1038/ajg.2012.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerson LB, Edson R, Lavori PW, et al. Use of a simple symptom questionnaire to predict Barrett's esophagus in patients with symptoms of gastroesophageal reflux. Am J Gastroenterol. 2001;96:2005–2012. doi: 10.1111/j.1572-0241.2001.03933.x. [DOI] [PubMed] [Google Scholar]
- 14.Locke GR, Zinsmeister AR, Talley NJ. Can symptoms predict endoscopic findings in GERD? Gastrointest Endosc. 2003;58:661–670. doi: 10.1016/s0016-5107(03)02011-x. [DOI] [PubMed] [Google Scholar]
- 15.Locke GR, Talley NJ, Weaver AL, et al. A new questionnaire for gastroesophageal reflux disease. Mayo Clin Proc. 1994;69:539–547. doi: 10.1016/s0025-6196(12)62245-9. [DOI] [PubMed] [Google Scholar]
- 16.Thrift AP, Kramer JR, Richardson PA, et al. No significant effects of smoking or alcohol consumption on risk of Barrett’s esophagus. Dig Dis Sci. 2013 doi: 10.1007/s10620-013-2892-6. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2013 doi: 10.1136/gutjnl-2012-304269. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]