Abstract
Objective
Deep brain stimulation (DBS) for Parkinson’s disease (PD) has been associated with psychiatric adverse effects (PAEs) including anxiety, depression, mania, psychosis and suicide. The purpose of this study was to evaluate safety of DBS in a large PD clinical practice.
Methods
Patients approved for surgery by Mayo Clinic DBS clinical committee participated in a 6 month prospective naturalistic follow-up study. In addition to the Unified Parkinson’s Disease Rating Scale (UPDRS), stability and psychiatric safety was measured using: Beck Depression Inventory (BDI-II), Hamilton Depression Rating Scale (HAMD-17), and Young Mania Rating scale (YMRS). Outcomes were compared in PD patients with past psychiatric history to PD patients with no comorbid psychiatric history.
Results
Forty-nine of 54 patients completed the study. Statistically significant 6-month baseline to endpoint improvement was found in motor and mood scales. No significant differences were found in psychiatric outcomes based on presence or absence of psychiatric comorbidity.
Conclusions
Our study suggests PD patients with a history of psychiatric comorbidity can safely respond to DBS with no greater risk of PAE occurrence. A multi-disciplinary team approach including careful psychiatric screening ensuring mood stabilization and psychiatric follow-up should be viewed as standard of clinical care to optimize psychiatric outcome in the course of DBS treatment.
INTRODUCTION
Deep brain stimulation (DBS) is an FDA-approved surgical treatment for management of advanced Parkinson’s disease (PD). In comparison to medication management, DBS has shown to significantly improve motor symptoms and quality of life in PD (1, 2).
The most common lead placement for PD DBS is the subthalamic nucleus (STN) which has been associated with new onset psychiatric adverse events (PAEs) including anxiety (2%), depression (8%), hypomania/mania (4%) and suicide (0.4%) (3). As the suicide rate in PD has been reported to be 10 times lower than in the general population (4), studies that report completed suicide rates in PD patients after STN DBS, between 1-4% (i.e. higher than general population (5),(6)), underscore the need to better understand risk and neurobiological underpinnings of psychiatric adverse events in DBS treated Parkinson’s patients.
Parkinson’s disease itself is associated with substantial comorbidity with estimate ranges of approximately 13% for psychotic disorders, 20% for anxiety disorders, 25% for depression, and up to 50% for sleep disturbances (5), (6), (7). The additional risk of DBS associated PAEs warrants prospective studies to ascertain the safety of DBS from a psychiatric perspective in PD patients(8). Our study aims were to evaluate: 1) safety of DBS surgery in PD patients with psychiatric comorbidity by evaluating baseline symptoms of depression and change (i.e. mania or hypomania induction) over the postoperative course during DBS parameter optimization and 2) whether psychiatric comorbidity influenced DBS PD motor outcomes.
METHODS
This study was approved by the Mayo Clinic Institutional Review Board (IRB #07-004602 PI: Frye). During the period of study enrollment (July 2008-December 2010), 63 PD patients were clinically evaluated by the Mayo Clinic DBS Clinical Committee. Inclusion criteria for this naturalistic followup research study were: diagnosis of Parkinson’s disease established by Mayo Clinic, age 18-85 years, and Mayo Clinic DBS Clinical Committee approval of surgery. The DBS Clinical Committee generally required 6 months of psychiatric stability, assessed by a board certified Mayo Clinic psychiatrist, for consideration of surgery. Medication adjustments and psychosocial interventions were made to stabilize psychiatric symptoms in PD patients. Those patients with unstable psychiatric condition, despite optimal psychiatric management, were not approved for surgery by the DBS clinical committee. Exclusion criteria included: inability to attend clinical and study visits, inability to speak English, and inability to provide informed consent. Five patients were not approved by DBS clinical committee for surgery (cognitive decline=1, inadequate dopaminergic medication response=2, inadequate dopaminergic medication trials=2). All approved patients (n=58) were asked to participate in our study; four patients declined. Following detailed discussion 54 signed an IRB approved informed consent enrolling them into our study.
Participants consisted of 39 males and 15 females with a mean age of 62 years. Study visits (Baseline, 2 weeks, 4 weeks, and 2-6 months post DBS) corresponded to the clinical appointments.
Unified Parkinson’s disease rating scale (UPDRS) (9) was used for objective assessment of motor symptoms of PD at baseline. Levodopa equivalent daily dose (LEDD) to compare the amount of antiparkinsonian medications was computed based on the following formula: total L-dopa = (regular L-dopa dose × 1) + (L-dopa controlled release × 0.75) + (pramipexole dose × 67) + (ropinirole dose × 16.67) + (pergolide dose × 100) + (bromocriptine dose × 10), + [(L-dopa + L-dopa controlled release × 0.75) × 0.25 if taking tolcapone (regardless of tolcapone dose)](10).
Mood rating scales included the Mood Disorder Questionnaire (MDQ) (11) for screening bipolar disorder (at baseline only), Beck’s Depression Inventory (BDI-II) (12), Hamilton Depression score (HAMD-17) (13) to measure depressive symptoms, and Young Mania Rating scale (YMRS) (14) to assess hypomanic/manic symptoms, and the Quality of Life Enjoyment and Satisfaction Questionnaire scale (Q-LES-Q-SF) (15). Baseline visit ratings were completed by study P.I. psychiatrist (M.A.F) and clinical research coordinator discussing any discrepancies in scores. All subsequent research visits were completed by the clinical research coordinator at time of the prescheduled clinical appointment with a neurologist and DBS nurse.
A majority of the PD patients (51/54) underwent bilateral subthalamic nucleus (STN) stimulation with the rest (3/54) receiving bilateral globus pallidus (GPi) stimulation. DBS stimulation parameters including electrode contacts, voltage, pulse width and frequency were recorded during each clinical visit.
Presence or absence of comorbidity was quantified by semi-structured clinical assessment based on DSM criteria completed or supervised by senior psychiatrist (M.A.F) during pre DBS clinical evaluation. Patients were divided into three broad groups, including: depression (current or history of major depressive disorder or depression secondary to general medical condition), mania/impulse dyscontrol (current or history of bipolar disorder or mania/impulse symptoms secondary to dopaminergic medications) and no comorbidity.
Demographics among the 3 groups were compared using a chi-squared test for gender, and analysis of variance (ANOVA) for continuous measures of age and the rating scales. Change in rating scores from baseline to 6 months follow-up amongst motor, mood and quality of life variables were assessed for significance using paired t-tests. Cohen’s D effect size was calculated for baseline to end point change in mood rating scales. The 6 month changes in scores were also compared among the 3 groups using ANOVA. A p-value less than 0.05 were considered statistically significant. All statistical analyses were conducted in SAS (version 9.3, Cary, NC).
RESULTS
Baseline demographics are presented in Table 1. Other than a greater percentage of women in the depression comorbid group, as well as increased depression and reduced quality of life scores, there was no statistically significant difference between groups.
Table 1.
Baseline demographics of Depression, Mania/Impulse and No Comorbidity
| Total | Depression | Mania/Impulse | No Comorbidity | ||
|---|---|---|---|---|---|
| Mean (STD) | Mean (STD) | Mean (STD) | Mean (STD) | p- value |
|
| M/F | N=54 (39/M,15/F) | N=16 (6/M,10/F) | N=11 (9/M,2/F) | N=27 (24/M,3/F) | 0.001 |
| Age | 62 (+/− 9) | 64 (+/− 6.8) | 58 (+/−9.8) | 62 (+/− 8.9) | 0.255 |
| UPDRS off medication |
35.20 (+/− 10.06) | 33.8 (+/− 9.4) | 36.8 (+/− 12.9) | 35.4 (+/− 9.4) | 0.749 |
| UPDRS on medication |
16.40 (+/− 7.91) | 15.6 (+/− 8.9) | 13.3 (+/− 7.9) | 18.1 (+/− 7.1) | 0.216 |
| LEDD | 1636.2 (+/− 1020.6) |
1442.9 (+/− 560.1) |
2284.6 (+/− 1498.2) |
1486.5 (+/− 924.6) |
0.058 |
| BDI | 9.0 (+/− 7.7) | 12.7 (+/− 8.1) | 9.2 (+/− 8.9) | 6.8 (+/− 6.2) | 0.047 |
| Ham-D | 8.7 (+/− 4.2) | 10.7 (+/− 4.9) | 8.6 (+/− 4.3) | 7.5 (+/− 3.4) | 0.055 |
| YMRS | 2.0 (+/− 2.5) | 2.6 (+/− 3.4) | 2.2 (+/− 3.0) | 1.6 (+/− 1.6) | 0.433 |
| Q-LES-SF-Q | 3.4 (+/− 0.9) | 3.1 (+/− 1.0) | 3.3 (+/− 0.8) | 3.7 (+/− 0.7) | 0.046 |
| AD meds | N=13 (24%) | N=11 (68.7%) | N=0 | N=2 (7.4%) | |
| Mood Stabilizer Med |
N=9 (16.6%) | N=2* (12.5%) | N=4* (36.4%) | N=2* (7.4%) | |
| Anti-Psychotic Med |
N=0 | N=0 | N=0 | N=0 |
UPDRS: Unified Parkinson’s disease rating scale; LEDD: levodopa equivalent daily dosage; BDI: Beck’s Depression Inventory; Ham-D: Hamilton Depression Scale; YMRS: Young Mania Rating Scale; Q-LES- Q-SF: Quality of Life Rating Scores-Short Form
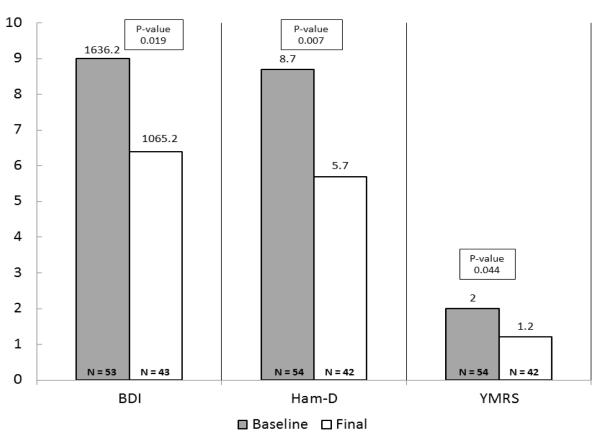
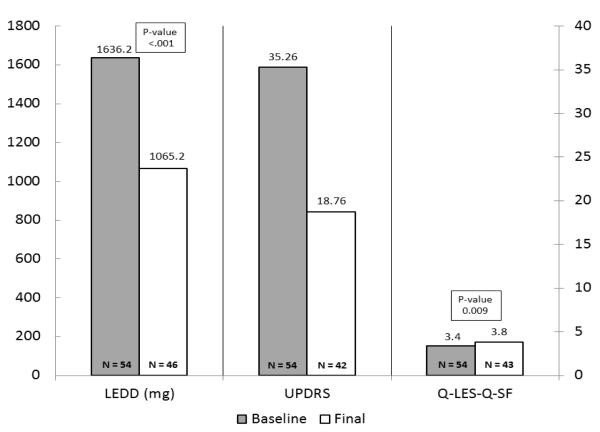
Forty-nine of 54 patients completed 6 months follow-up. Five participants voluntarily withdrew from the study citing: time constraints (n=1), mood assessments causing emotional discomfort (n=1), and patient decision citing no reason (n=3). There was statistically significant reduction in LEDD, UPDRS, all mood ratings, and commensurate improvement in quality of life (Figures 1a, 1b). At study endpoint, significant improvements in UPDRS scores (on/off dopaminergic medications) accompanied marked reduction in LEDD (p=.001) in all PD patients. There was no significant difference in motor, mood outcomes by presence of comorbidity.
Figure 1a.
Baseline-6 months Mood outcomes
Figure 1b.
Baseline-6 months LEDD, Motor symptoms, Quality of Life outcomes
Both depressive and manic/hypomanic symptoms improved significantly at endpoint as evident by reduction in BDI-II (p=0.019, effect size 0.38), HAM-D (p=0.007, effect size 0.65), and YMRS (p=0.044, effect size = 0.37) scores. The three groups did not differ significantly in terms of change in BDI-II (p=0.696), HAM-D (p=0.801), YMRS (p=0.891), quality of life (p=0.626) rating scores and LEDD (p=0.768) dose at six month follow-up when compared to the baseline shown in Table 2. In terms of DBS associated PAEs, only one patient in the no comorbidity group developed mania with psychotic features requiring psychiatric hospitalization. No attempted or completed suicides were observed.
Table 2.
Outcomes by Depression, Mania/Impulse, and No Comorbidity
| Depression | Mania/Impulse | No Comorbidity | |||||
|---|---|---|---|---|---|---|---|
| N | Mean±SD | N | Mean±SD | N | Mean±SD | p-value* | |
| Change in BDI | 13 | −1.6 ±6.1 | 9 | −4.0 ±5.8 | 20 | −2.1 ±7.4 | 0.696 |
| Change in YMRS | 13 | −0.7 ±3.0 | 10 | 0.6 ±2.2 | 19 | −1.0 ±1.9 | 0.891 |
| Change in Ham-D | 13 | −1.7 ±5.3 | 10 | −3.3 ±5.6 | 19 | −2.6 ±6.3 | 0.801 |
| Change in QLES | 13 | 0.2 ±1.1 | 10 | 0.6 ±0.7 | 20 | 0.4 ±0.9 | 0.626 |
| Change in LEDD | 14 | −618.1 ±542.2 | 11 | −626.4 ±709.5 | 21 | −478.1 ±716.1 | 0.768 |
| Change in UPDRS | 13 | −14.69 ±5.8 | 8 | −17.25 ±15.2 | 21 | −17.33 ±9.5 | 0.73 |
comparison between 3 groups using one-way ANOVA
BDI: Beck’s Depression Inventory; Ham-D: Hamilton Depression Scale; YMRS: Young Mania Rating Scale; Q-LES- Q-SF: Quality of Life Rating Scores-Short Form; LEDD: levodopa equivalent daily dosage; UPDRS: Unified Parkinson’s disease rating scale
DISCUSSION
The safety of DBS from a psychiatric perspective has been a clinical concern when judging therapeutic benefit for Parkinson’s disease (16), (17) (18), (19), (20). Exacerbation of pre-existing psychiatric illness, familial diathesis, medication effects (mood changes secondary to dopaminergic medications), and neurobiological changes induced by DBS have been implicated etiologic factors contributing to DBS associated PAEs. There is a lack of large-scale prospective studies focusing on DBS associated PAEs and the existing studies are not without methodological limitations including very small sample sizes and lack of control groups.
Results of our prospective longitudinal study underscore overall safety of DBS in carefully screened PD patients with and without pre-existing psychiatric disorders. In our study, despite previous history of psychiatric disorders in 27/54 (50%) of the patients, depressive symptoms improved in all three groups as evident by modest reduction in BDI-II and HAM-D scores during 6 month follow-up. Lower incidence of new onset PAEs in our study is likely related to both small sample size and study duration, particularly for suicidality, and a comprehensive psychiatric screening and followup ensuring mood stabilization in PD patients with psychiatric comorbidity prior to the DBS surgery. This carefully screened population does limit ability to generalize to larger clinical trial populations as it relates to safety. Our results are consistent with improvement in depression after DBS as noted in a previous study during the immediate postoperative period (21). The authors have reported similar results as DBS was noted to be safe in terms of PAEs when comparing PD patients receiving best medical treatment (n=63) with patients who received STN DBS (n=60) in a 6 month randomized controlled study (21). Although, selective decrease in frontal cognitive functions (not impacting quality of life) in STN DBS group was noted in this study. Patients were carefully screened by psychiatrists preoperatively and those with current or history of severe depression and psychosis were excluded from the study (21).
In another prospective controlled study, authors reported decrease in positive affect, increase in emotional lability and psychiatric complications in STN DBS group (n=103) as compared to control group including patients with idiopathic PD (n=39) during 6 month follow-up (22). STN group showed significantly more negative and significantly less quality of life compared to the control group in this study (22). Additionally, a recent study has reported worsening of cognitive-emotional symptoms of depression in STN DBS group (n=17) as compared to matched non-surgical PD patients (n=22) over 6 months follow-up. STN DBS group had reported higher levels of depression at baseline in this study (23).
Long term follow-up studies on mood-depressive symptoms have noted to be improved at one year follow-up with return to baseline depression scores at three years follow-up in another study (24) suggesting that mood may vary during long term follow-up after STN DBS surgery. Although, the authors reported that PD patients with mild psychiatric disturbances should not be excluded from having DBS surgery (24). It may be difficult to predict long-term mood outcomes in our study participants as follow-up duration was only six months and more studies are needed to ascertain long term mood stability and psychiatric safety in carefully screened PD patients.
In a multicentre case control study (n=5311), STN-DBS has been associated with completed suicide rate of 0.45% and attempted suicide rate of 0.90% (25). Postoperative depression, being single, previous history of impulse control disorders or compulsive medication use, being younger, younger PD onset and a previous suicide attempt were considered risk factors associated with attempted/completed suicides (25). In our study, DBS was found to be safe as none of the patients attempted or completed suicide. This could be attributed to careful psychiatric screening which led to exclusion of patients with unstable psychiatric disorders and those at high risk of self-harm. Also, depressive symptoms improved at endpoint in PD patients with and without psychiatric disorders which is seemingly protective as postoperative depression has been strongly correlated with attempted or completed suicide in PD patients (25).
Our study has significant clinical implications in provision of care of PD patients considered for DBS surgery. Despite significant motoric improvements in PD patients, PAEs associated with DBS can have devastating outcomes in terms of worsening depression, mania and attempted or completed suicides. Therefore, clinical strategies focussing on prevention and treatment of PAEs deserve utmost attention in ensuring safety of DBS in PD patients. Our study highlights the need for multidisciplinary teams, such as Mayo Clinic DBS committee, in medical centers across the nation to provide highest standards of care to PD patients undergoing DBS surgery. The multidisciplinary team ideally comprises of neurosurgeons, neurologists, psychiatrists, neuropsychologists, radiologists and nurses. Comprehensive psychiatric evaluation should be considered to both screen PD patients for undiagnosed and or unstable psychiatric disorders and optimize their psychiatric treatment prior to DBS surgery. Adequate psychiatric follow-up should be provided after DBS surgery ensuring psychiatric stability of patients and manage PAEs associated with DBS.
Our study has a number of limitations that include small sample size, lack of structured diagnostic interview confirming diagnosis, and lack of randomization in study design. The sample size and short study duration make it difficult to generalize about safety issues which have been a focused area of interest for DBS. We cannot make any meaningful conclusions about suicidality or suicide completion in this small study. Secondly, the psychiatric diagnosis was made by clinical evaluation, not structured interview where additional comorbidities (both lifetime and current) identified may have influenced outcome. Finally, the study was not randomized based on presence or absence of comorbidity. However, when compared to randomized controlled study by Witt et al, comprised of patients with careful psychiatric screening prior to enrollment, our results are similar in terms of psychiatric outcomes after DBS surgery. We acknowledge a possibility of patient selection bias as this study was conducted at single tertiary care teaching hospital and these patients were followed up for a duration of only six months. Therefore, we recommend multicenter and longer term follow-up studies to assess mood and psychiatric safety in PD patients undergoing DBS surgery.
CONCLUSIONS
Our study suggests PD patients with a history of psychiatric comorbidity show significant reduction of symptoms of depression and can safely respond to DBS with no greater risk of PAE occurrence.
Acknowledgements
This publication [or project] was supported by CTSA Grant Number UL1 TR000135 from National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent official views of NIH.
Disclosure: Mark A. Frye, M.D.
Grant Support: Pfizer, Myriad, National Institute of Mental Health (NIMH), National Institute of Alcohol Abuse and Alcoholism (NIAAA), Mayo Foundation
Consultant (Unpaid): Allergan, Myriad, Sunovion, Teva Pharmaceuticals
CME Activities: CME Outfitters Inc.
Kendall H. Lee, MD, PhD
Patents pending for DBS technology and industry support from St. Jude Neuromodulation.
Work supported by NIH (K08 NS 52232 award) and Mayo Foundation (2008-2010 Research Early Career Development Award for Clinician Scientists) to KHL.
Julie A. Fields, PhD
Consultant: Medtronic, Inc.
Footnotes
The remaining authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the Annual Meeting of the American Psychiatric Association, Philadelphia, May 2012
REFERENCES
- 1.Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. New England Journal of Medicine. 2003;349:1925–34. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- 2.Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Jr., et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Temel Y, Kessels A, Tan S, Topdag A, Boon P, Visser-Vandewalle V. Behavioural changes after bilateral subthalamic stimulation in advanced Parkinson disease: a systematic review. Parkinsonism & Related Disorders. 2006;12:265–72. doi: 10.1016/j.parkreldis.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Myslobodsky M, Lalonde FM, Hicks L. Are Patients With Parkinson’s Disease Suicidal? J Geriatr Psychiatry Neurol. 2001 Fall;:120–4. doi: 10.1177/089198870101400304. [DOI] [PubMed] [Google Scholar]
- 5.Burkhard PR, Vingerhoets FJ, Berney A, Bogousslavsky J, Villemure JG, Ghika J. Suicide after successful deep brain stimulation for movement disorders. Neurology. 2004;63:2170–2. doi: 10.1212/01.wnl.0000145603.48221.b5. [DOI] [PubMed] [Google Scholar]
- 6.Soulas T, Gurruchaga JM, Palfi S, Cesaro P, Nguyen JP, Fenelon G. Attempted and completed suicides after subthalamic nucleus stimulation for Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2008;79:952–4. doi: 10.1136/jnnp.2007.130583. [DOI] [PubMed] [Google Scholar]
- 7.Riedel O, Klotsche J, Spottke A, Deuschl G, Forstl H, Henn F, et al. Frequency of dementia, depression, and other neuropsychiatric symptoms in 1,449 outpatients with Parkinson’s disease. Journal of Neurology. 2010;257:1073–82. doi: 10.1007/s00415-010-5465-z. [DOI] [PubMed] [Google Scholar]
- 8.Chopra A, Tye SJ, Lee KH, Matsumoto J, Klassen B, Adams AC, Stead M, Sampson S, Kall BA, Frye MA. Voltage-dependent mania after subthalamic nucleus deep brain stimulation in Parkinson’s disease: a case report. Biological Psychiatry. 2011;70:e5–7. doi: 10.1016/j.biopsych.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 9.Fahn S, Elton RL, Committee UD. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent Developments in Parkinson’s Disease. McMillan Healthcare Information; Florham Park, NJ: 1987. pp. 153–63. [Google Scholar]
- 10.Hobson DE, Lang AE, Martin WRW, Razmy A, Rivest J, Fleming J. Excessive daytime sleepiness and sudden-onset sleep in Parkinson disease: a survey by the Canadian Movement Disorders Group. JAMA. 2002;287:455–63. doi: 10.1001/jama.287.4.455. [DOI] [PubMed] [Google Scholar]
- 11.Hirschfeld RM, Williams JB, Spitzer RL, Calabrese JR, Flynn L, Keck PE, Jr., et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. American Journal of Psychiatry. 2000;157:1873–5. doi: 10.1176/appi.ajp.157.11.1873. [DOI] [PubMed] [Google Scholar]
- 12.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 15.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacology Bulletin. 1993;29:321–6. [PubMed] [Google Scholar]
- 16.Okun MS, Fernandez HH, Wu SS, Kirsch-Darrow L, Bowers D, Bova F, et al. Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Annals of Neurology. 2009;65:586–95. doi: 10.1002/ana.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zibetti M, Torre E, Cinquepalmi A, Rosso M, Ducati A, Bergamasco B, et al. Motor and nonmotor symptom follow-up in parkinsonian patients after deep brain stimulation of the subthalamic nucleus. European Neurology. 2007;58:218–23. doi: 10.1159/000107943. [DOI] [PubMed] [Google Scholar]
- 18.Funkiewiez A, Ardouin C, Caputo E, Krack P, Fraix V, Klinger H, et al. Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 2004;75:834–9. doi: 10.1136/jnnp.2002.009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volkmann J, Allert N, Voges J, Weiss PH, Freund HJ, Sturm V. Safety and efficacy of pallidal or subthalamic nucleus stimulation in advanced PD. [Erratum appears in Neurology 2001 Oct 9;57(7):1354] Neurology. 2001;56:548–51. doi: 10.1212/wnl.56.4.548. [DOI] [PubMed] [Google Scholar]
- 20.Saint-Cyr JA, Trepanier LL, Kumar R, Lozano AM, Lang AE. Neuropsychological consequences of chronic bilateral stimulation of the subthalamic nucleus in Parkinson’s disease. Brain. 2000;123:2091–108. doi: 10.1093/brain/123.10.2091. [DOI] [PubMed] [Google Scholar]
- 21.Witt K, Daniels C, Reiff J, Krack P, Volkmann J, Pinsker MO, et al. Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson’s disease: a randomised, multicentre study. Lancet Neurology. 2008;7:605–14. doi: 10.1016/S1474-4422(08)70114-5. [DOI] [PubMed] [Google Scholar]
- 22.Smeding HMM, Speelman JD, Koning-Haanstra M, Schuurman PR, Nijssen P, van Laar T, et al. Neuropsychological effects of bilateral STN stimulation in Parkinson disease: a controlled study. Neurology. 2006;66:1830–6. doi: 10.1212/01.wnl.0000234881.77830.66. [DOI] [PubMed] [Google Scholar]
- 23.Strutt AM, Simpson R, Jankovic J, York MK. Changes in cognitive-emotional and physiological symptoms of depression following STN-DBS for the treatment of Parkinson’s disease. European Journal of Neurology. 2012;19:121–7. doi: 10.1111/j.1468-1331.2011.03447.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser I, Kryspin-Exner I, Brucke T, Volc D, Alesch F. Long-term effects of STN DBS on mood: psychosocial profiles remain stable in a 3-year follow-up. BMC Neurology. 2008;8:43. doi: 10.1186/1471-2377-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voon V, Krack P, Lang AE, Lozano AM, Dujardin K, Schupbach M, et al. A multicentre study on suicide outcomes following subthalamic stimulation for Parkinson’s disease. Brain. 2008;131:2720–8. doi: 10.1093/brain/awn214. [DOI] [PMC free article] [PubMed] [Google Scholar]