Abstract
A fundamental question in hematopoietic development is how multipotent progenitors achieve precise identities, while the progenitors themselves maintain quiescence. In Drosophila melanogaster larvae, multipotent hematopoietic progenitors support the production of three lineages, exhibit quiescence in response to cues from a niche, and from their differentiated progeny. Infection by parasitic wasps alters the course of hematopoiesis. Here we address the role of Notch (N) signaling in lamellocyte differentiation in response to wasp infection. We show that Notch activity is moderately high and ubiquitous in all cells of the lymph gland lobes, with crystal cells exhibiting the highest levels. Wasp infection reduces Notch activity, which results in fewer crystal cells and more lamellocytes. Robust lamellocyte differentiation is induced even in N mutants. Using RNA interference knockdown of N, Serrate, and neuralized (neur), and twin clone analysis of a N null allele, we show that all three genes inhibit lamellocyte differentiation. However, unlike its cell-autonomous function in crystal cell development, Notch’s inhibitory influence on lamellocyte differentiation is not cell autonomous. High levels of reactive oxygen species in the lymph gland lobes, but not in the niche, accompany NRNAi-induced lamellocyte differentiation and lobe dispersal. Our results define a novel dual role for Notch signaling in maintaining competence for basal hematopoiesis: while crystal cell development is encouraged, lamellocytic fate remains repressed. Repression of Notch signaling in fly hematopoiesis is important for host defense against natural parasitic wasp infections. These findings can serve as a model to understand how reactive oxygen species and Notch signals are integrated and interpreted in vivo.
Keywords: Drosophila, innate immunity, Notch, parasite, reactive oxygen species, complex genetics, tolerance, complex immunity, infection, resistance
IN mammalian hematopoiesis, multipotent stem cells follow a tightly regulated developmental program to specify the correct proportions of at least eight mature lineages. A complex set of hierarchical decisions are executed to supply the body with fully functional cells, while also renewing the original stem/progenitor population. Various lines of experimental evidence suggest that signaling pathways and molecular networks of transcription factors constitute key aspects of stem cell regulation (Orkin and Zon 2008). Functional homologs of many of these signaling pathways and transcription factors are present in Drosophila and mutations in their respective fly genes give rise to hematopoietic defects (Crozatier and Meister 2007; Krzemien et al. 2010a; Fossett 2013; Honti et al. 2013).
The future larval hematopoietic organ in Drosophila, called the lymph gland, differentiates from a cluster of embryonic mesodermal cells. Notch limits cardioblast (vascular) development in favor of embryonic lymph gland tissue (Mandal et al. 2004). Lymph gland lobe development continues through the first and second larval instars. By the mid-third instar, the organ has a pair of anterior lobes, and two sets of smaller posterior lobes, that flank the dorsal vessel. Genetic experiments suggest that progenitors of the three major blood cell types quiesce in the medullary zone or medulla (Jung et al. 2005; Krzemien et al. 2010b; Minakhina and Steward 2010; Kalamarz et al. 2012). The cortex harbors a mixture of maturing and fully differentiated blood cells. Lineage development in the cortex is tightly regulated. The medulla/cortex boundary is not sharp; cortical cells in an intermediate state of differentiation are closer to the medulla; the terminally differentiated cells are most peripheral in location (Jung et al. 2005; Krzemien et al. 2010b; Kalamarz et al. 2012). Plasmatocytes (90–95%) appear as single cells or in clusters and crystal cells (5–10%) are distributed singly. Crystal cells produce enzymes for melanization. Like mammalian macrophages, plasmatocytes engulf bacteria and apoptotic cells and make up the majority of circulating cells. Plasmatocytes and crystal cells circulating in the hemolymph have an embryonic origin and represent a developmental compartment that is distinct from the lymph gland (reviewed in Crozatier and Meister 2007; Fossett 2013). A non-hematopoietic niche, also referred to as the posterior signaling center, located at the base of the anterior lobes maintains progenitor quiescence in the anterior lobes (Lebestky et al. 2003; Krzemien et al. 2007; Mandal et al. 2007). It also mediates lamellocyte differentiation but mainly in response to wasp infection via the transcription factor Knot/Collier (Crozatier et al. 2004).
The lymph gland lobes are immune responsive (Lanot et al. 2001; Sorrentino et al. 2002). Parasitic wasps of the Leptopilina genus attack second and early third instar larvae. Wasp infection alters the course of basal hematopoiesis and triggers cell division, lamellocyte differentiation, aggregation, and melanization (Lee et al. 2009b). Lamellocytes are dedicated to encapsulating wasp eggs. Wasp attack reduces the abundance of crystal cells in the anterior lobes (Krzemien et al. 2010b). It also activates NF-κB signaling (Gueguen et al. 2013) and oxidative stress (Sinenko et al. 2012) in the niche. Activated NF-κB signaling in the lobes favors lamellocyte differentiation and inhibits crystal cell development (Gueguen et al. 2013). The effects of wasp-induced increase in reactive oxygen species is mediated by EGF signaling (Sinenko et al. 2012), although its effects on crystal cell development are not known. While it is clear that the lymph gland disperses after wasp infection and some plasmatocytes and lamellocytes arising in the lymph gland transfer into the hemolymph (Lanot et al. 2001; Sorrentino et al. 2002, 2004), how wasp attack alters hematopoiesis to promote lamellocyte differentiation in the first place is not well understood.
In this study, we analyze the role of Notch signaling in lamellocyte and crystal cell development. Notch signaling is evolutionarily conserved and regulates the diversification of cell fates in animals ranging from flies to mammals (Bray 2006; Koch et al. 2013). It also plays a role in the maintenance of hematopoietic stem cells (Bigas et al. 2012). In Drosophila, Notch’s role in crystal cell development is well documented. It regulates embryonic crystal cell development (Bataille et al. 2005). In the late embryo, N restricts the developmental potential of multipotent progenitors: at restrictive temperature, late stage-16 embryos, Nts1 embryos are unable to specify crystal cells (Krzemien et al. 2010b). In larval stages, Notch signaling regulates commitment to crystal cell lineage (Duvic et al. 2002; Lebestky et al. 2003); in procrystal cells, the Notch receptor is activated by Serrate (Ser), expressed in the niche cells. Procrystal cells express high levels of Acute Myeloid Leukemia-like transcription factor, Lozenge, and Suppressor of Hairless (Su(H)), a transcriptional activator mediating Notch signaling (Lebestky et al. 2003; Terriente-Felix et al. 2013). Notch and Lozenge select specific target genes to mediate crystal cell development (Terriente-Felix et al. 2013). Ligand-independent noncanonical Notch signaling also promotes crystal cell survival during basal hematopoiesis and hypoxic stress (Mukherjee et al. 2011).
Notch was reported to be essential for wasp-induced differentiation of lamellocytes (Duvic et al. 2002), although the mechanism behind its requirement is not known. We now report an unexpected non-cell-autonomous role for Notch signaling in restricting and not promoting differentiation of hematopoietic progenitors into lamellocytes. This inhibition appears to be independent of oxidative stress levels in the niche, although surprisingly, knockdown of N leads to increased reactive oxygen species (ROS) production in the lobes. Thus, Notch signaling plays an essential role in host defense against parasites in Drosophila. These results highlight an unexpected pleiotropy for Notch and ROS signaling in regulating the production of alternative lineages during larval hematopoiesis.
Materials and Methods
Stocks, crosses, and wasp infections
HmlΔ-GAL4 (Sinenko et al. 2004) was received from S. Bhattacharya. Dome-GAL4 (Ghiglione et al. 2002) was a gift from M. Crozatier, and Antp-GAL4 was received from S. Minakhina (Emerald and Cohen 2004). y w;UAS-mCD8-GFP (Sinenko et al. 2004) was incorporated into appropriate GAL4 backgrounds via standard crosses. UAS-RNAi lines for Notch (valium 1 and 10), Serrate (valium 10) neuralized (valium 10), and Su(H) [Vienna Drosophila RNAi Center (VDRC) line KK] were obtained from the Transgenic RNAi Project (TRiP) collection (Ni et al. 2008, 2009). msnf9-cherry (Tokusumi et al. 2009) was a gift from R. Schulz. arm-lacZ 19A/FM7, hs-FLP (Vincent et al. 1994) was obtained from N. Baker. A chromosome bearing null allele (N55e11) (Heitzler and Simpson 1991) N55e11 FRT19A/FM7 (Ohlstein and Spradling 2007) was obtained from B. Ohlstein. UAS-NICD encoding the Notch intracellular domain, NICD (Struhl and Adachi 1998) was obtained from K. Irvine. 12X Su(H)-lacZ (Go et al. 1998) was obtained from S. Artavanis-Tsakonas. The Dome-Meso-GFP (medulla is GFP positive) stock was a gift of M. Crozatier. y1 Nts1 and wa Nts2 rb flies were obtained from the Bloomington Stock Center.
Females from respective driver stocks containing y w; UAS-mCD8-GFP; Antp-GAL4/TM6 Tb Hu, y w Dome-GAL4/FM7; UAS-mCD8-GFP and y w; HmlΔGFP/HmlΔGFP were crossed to males from respective RNAi stocks y v/Y; NRNAi/NRNAi and w1118/Y; UAS-NICD/TM3. Third instar animals, selected from 6- to 8-hr egg lays and reared at 27° were genotyped by GFP expression. Lymph gland basement membrane dispersal was scored according to Sorrentino et al. (2002, 2004).
For loss-of-function Notch clones (Xu and Rubin 1993), N55e11FRT19A/FM7 females were crossed to arm lacZ19A; hs-FLP/TM6 Tb Hu males to create N55e11/N55e11 clones marked by the absence of β-galactosidase. Homozygous wild-type N+/N+ clones were detected by higher than background β-galactosidase expression. N55e11 mutant clones were generated by 37° heat shock of second instar larvae for 30–40 min. To induce flp-out clones (Struhl and Basler 1993), developmentally synchronized 4-day-old larvae with the hybrid flip out and GAL4 activation system [hsp70-flp; Actin>CD2>GAL4] and UAS-NLS-GFP transgenes or those with an additional UAS-NICD transgene, were heat shocked at 37° in a water bath for 15 min. NRNAi (valium 10) flp-out clones were similarly induced with a 1-hr heat shock of developmentally synchronized mid-to-late second instar animals. After recovery at 25°, lymph glands were dissected on day 6 from mid-third instar larvae, 20 hr after heat shock. Nts1 or Nts2 stocks or progeny from crosses were maintained at 18°. At the early second larval instar stage, animals were shifted to 29° through the third instar. Animals were matched for biological age to compare phenotypes. Most mutant males did not emerge; escapers showed notched wings.
Wasp infections were performed as follows: from a 6-hr egg lay, second instar wild-type or mutant larvae were subjected to infection by Leptopilina boulardi G486 (Sorrentino et al. 2002) or L. boulardi Lb17 (Schlenke et al. 2007) for 6–12 hours, after which wasps were removed and host larvae were allowed to develop to third instar stage. Uninfected controls followed the same growth regimen.
Immunohistochemistry and ROS detection
Unless otherwise specified, mid-third instar larvae from 6-hr or 12-hr egg lays were used in antibody staining experiments. Staining was performed according to Paddibhatla et al. (2010). Primary antibodies were as follows: mouse anti-Notch (C17.9C6, detects the intracellular product; Developmental Studies Hybridoma Bank) (Fehon et al. 1990) 1:10, mouse anti-Nimrod C/P1 and mouse anti-L1/Atilla (1:10) (Kurucz et al. 2007), mouse anti-prophenol oxidase (1:10, T. Trenczek, University of Giessen), 1:10, anti-integrin βPS (CF.6G11; Developmental Studies Hybridoma Bank) (Brower et al. 1984) 1:10, anti-proPO (Christophides et al. 2002), anti-β-galactosidase from Capell (1:2000 clone analysis), or from Immunology Consultants (1:200 for Notch activity). Secondary antibodies were as follows: Cy3 AffiniPure donkey anti-chicken (1:500 Jackson Immuno Research) or anti-mouse (1:200 Jackson Immuno Research or 1:500 Invitrogen). Rhodamine-, Alexa Fluor 488-, or Alexa Fluor 647-tagged phalloidin and nuclear dye Hoechst 33258 (all Invitrogen) were used to examine cell morphology. ROS detection (Invitrogen D11347) was carried out according to Owusu-Ansah and Banerjee (2009).
Lamellocytes were visualized by (a) their cell shape (filamentous actin), or (b) expression of integrin βPS (Stofanko et al. 2008), or (c) Atilla expression (L1) (Kurucz et al. 2007), or (d) the misshapen transcriptional enhancer msnf9 linked to cherry expression (Tokusumi et al. 2009). msnf9-cherry-positive lamellocytes are also strongly positive for F-actin with Alexa Fluor 488-labeled phalloidin (data not shown).
Data collection and statistical analysis
Samples were imaged with a Zeiss LSM 510 confocal microscope; 0.8 µm–1.5 µm thick optical images were analyzed according to Gueguen et al. (2013). Select images were imported into Adobe Photoshop CS5. The eyedropper tool was used to define and extract a signal intensity value from a 5 × 5 square of 25 pixels in random regions of the lobes. Intensity data were collected from 20 cells per lobe in at least 12 animals. Images showing signal saturation were not used for quantification. Niche areas (in square micrometers, μm2) were measured on confocal images using Path Tool of AxioVision 4.8 software. The average number of niche cells per lobe was scored by manually counting the mCD8-GFP- and Hoechst-positive cells in merged z-stacked images of the niche. For niche size and cell number, data were tested for normality before performing statistics using R software (R Development Core Team 2010). All other data were analyzed using Microsoft Excel 2010.
Results
Notch expression in third instar lymph glands
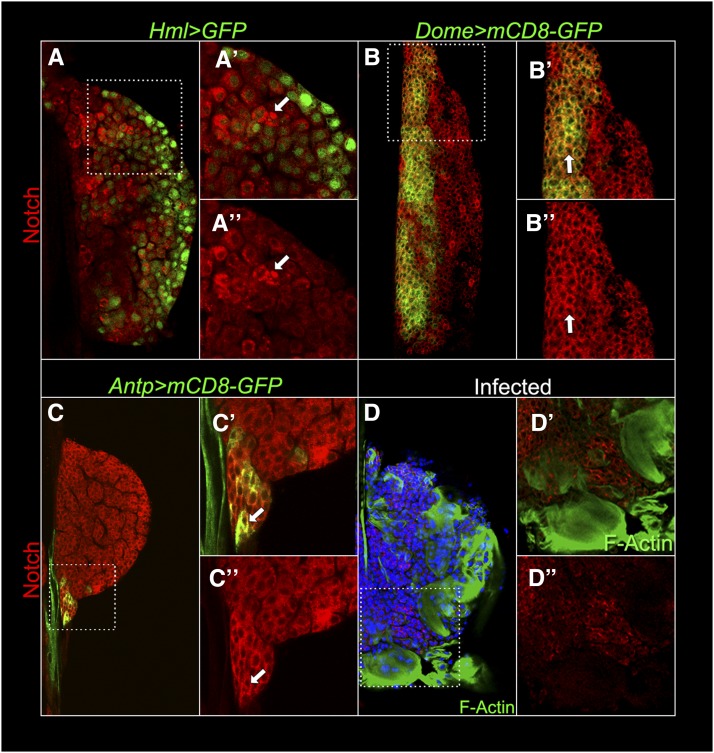
To examine the expression of the intracellular domain of Notch, we stained lymph glands with anti-Notch antibody. Notch signal is highest in some GFP-negative cells (arrow) while the staining signal is low in cortical Hml > GFP cells (Figure 1, A–A′′). Notch staining is high in Dome > mCD8-GFP-positive cells of the medulla of anterior (Figure 1, B–B′′) and posterior lobes (data not shown), as well as Antp > mCD8-GFP-positive niche cells (Figure 1, C–C′′). In none of these populations, is the Notch signal nuclear. We were surprised to find that Notch levels are low or undetectable in lamellocytes induced by wasp attack (Figure 1, D–D′′) or those in the gain-of-function hopscotchTumorous-lethal (hopTum-l) (Luo et al. 1995) background, where ectopic JAK-STAT signaling promotes lamellocyte differentiation in the absence of parasite attack (data not shown). Specificity of Notch staining and successful knockdown of Notch protein was confirmed by staining Antp > NRNAi glands, where the Notch signal was either reduced or not detected in GFP positive niche cells (Supporting Information, Figure S1). Differences in Notch levels and its subcellular localization in the lymph gland suggest different states of Notch activity in different cell populations and likely distinct functions of Notch in hematopoietic development.
Figure 1.
Notch intracellular domain expression in third instar lymph glands. (A) Variable Notch expression (red) in the cortical cells of a mid-third instar lymph gland. (A′ and A′′) Magnification shows weak Notch signal in Hml > GFP-positive cells, whereas some Hml > GFP-negative cells have high punctate cytoplasmic staining (arrow). (B–B′′) In the medulla, high anti-Notch staining colocalizes with the Dome > mCD8-GFP membrane signal (arrow). (C–C′′) Notch also colocalizes with Antp > mCD8-GFP signal in an early third instar lymph gland niche (arrow). (D–D′′) Notch staining is lower or is not detected in large lamellocytes (Alexa 488-phalloidin positive, green) in wild-type mid-third instar lymph glands from wasp-infected animals. Lamellocytes are rarely found in uninfected wild-type lymph glands (see Figure 6H). Hoechst (blue) stains DNA.
N activities in larval lymph gland lobes
To test whether N’s influence on progenitor differentiation is more or less ubiquitous, or if Notch activity is localized to crystal cells, we examined Notch activity using specific reporters. We found that a reporter of Notch and Su(H) transcription, Su(H)-lacZ (Go et al. 1998), is expressed at moderate levels in wild-type anterior lobe cells in all three larval stages, although the reporter activity levels are distinctly higher in the third instar lobes (Figure 2, A–D). Additionally, only at the third instar stage, high N target gene activity is also detected in scattered cells (Figure 2D, arrows). A significant fraction of these cells (37.3%; Figure 6, C and C′, stars) are also pro-PO positive, suggesting an ontogenetic relationship among the Su(H)-lacZ- and pro-PO-positive cell populations. Developmentally, the appearance of mature crystal cells in the lymph gland, positive for Lozenge and Su(H) proteins, coincides with the second-to-third instar molt (Lebestky et al. 2000).
Figure 2.
Ubiquitous and localized N activity in the larval lymph glands. (A–D) Anti-β-galactosidase staining detects moderate ubiquitous expression of Su(H)-lacZ reporter transgene in lobes of first, second, or third instar larvae. Higher expression is detected in a few scattered cells, presumed crystal cells, in lobes from the third instar animals. (Also see Figure 6).
Figure 6.
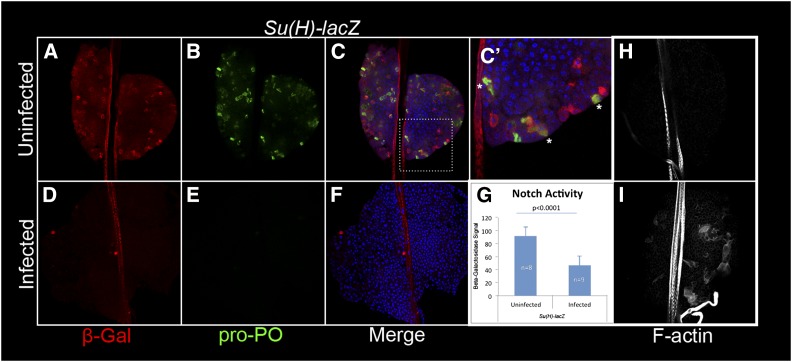
Notch activity is reduced after wasp infection. Anti-β-galactosidase antibody (red)-stained lobes from Su(H)-lacZ animals costained with anti-pro-PO (green) to detect coincidence of high Notch activity in crystal cells (stars). Cells expressing Su(H)-lacZ only make up 25.3% of all labeled cells; cells positive for pro-PO only constitute 37.3% of all labeled cells. The remainder labeled cell population (37.3%) are double positive (n = 406 cells from 20 lobes). Control animals did not experience wasp attack (A–C′), or animals were exposed to wasps (D–F) before dissection. Upon infection, Notch activity decreases (D–G). Standard deviation is shown in G. (H and I) Lamellocyte differentiation in Su(H)-lacZ lobes is normal. Anterior lobes are from uninfected Su(H)-lacZ (H) or infected (I) animal. Lamellocytes are labeled with phalloidin tagged with Alexa Fluor-647 (white), which also labels the dorsal vessel.
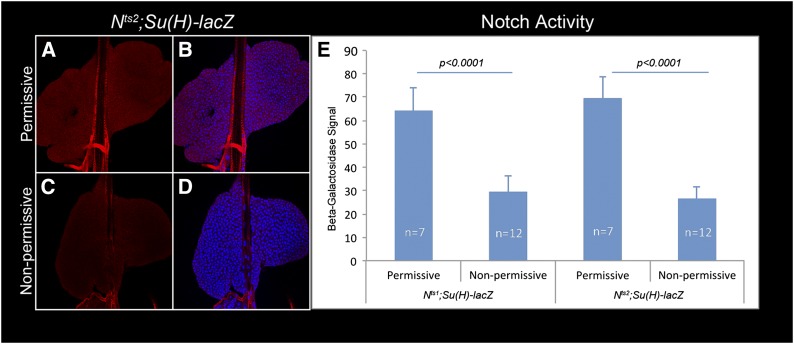
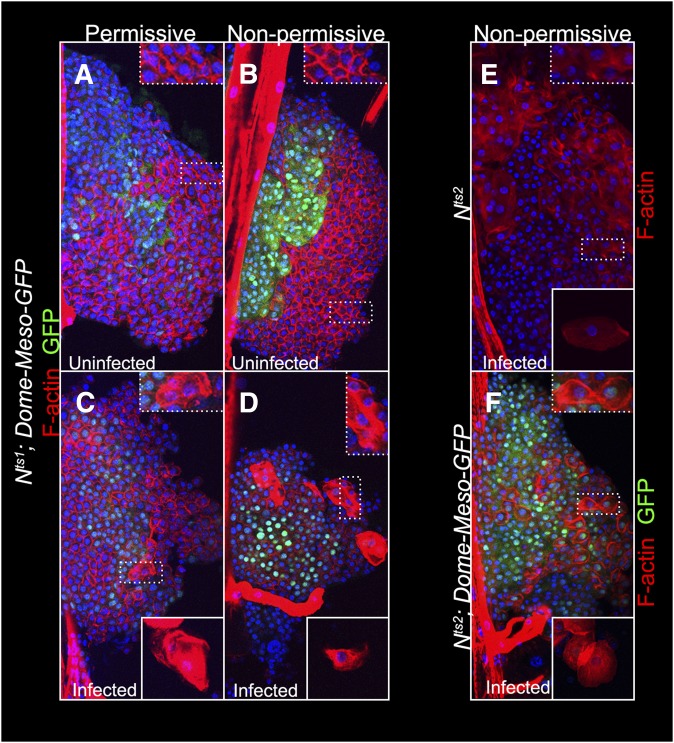
Ubiquitous moderate activity from the Su(H)-lacZ reporter is detected in the Nts1 or Nts2 lobes reared at a permissive 18° temperature [one Su(H)-lacZ copy in Nts/Y males; Figure 3, A, B, and E], although high signal in crystal cells is rarely observed in the Nts backgrounds presumably due to the inhibitory effect of the mutation. At nonpermissive temperature, however, both the moderate ubiquitous and high local Su(H)-lacZ signals are reduced in the Nts1 and Nts2 backgrounds (Figure 3, C–E). Unlike crystal cell differentiation, in Nts/Y lobes, neither the medulla/cortex sizes (assessed by Dome-Meso-GFP expression (Figure 7, A and B) nor plasmatocyte development (anti-Nimrod C staining; data not shown) appear to be affected at 29°. Thus, it appears that, consistent with Notch expression, Notch signaling is widespread in third instar anterior lobes. We hypothesized that the moderate ubiquitous Notch activity is needed for selection and patterning, i.e., the distributed appearance of crystal cells in the cortex, in a step prior to their maturation, which requires robust cell-autonomous Notch activity.
Figure 3.
Ubiquitous Notch reporter activity depends on functional N product. Lymph glands from Nts2/Y; Su(H)-lacZ larvae at permissive (A and B) or nonpermissive (C and D) temperature, stained with anti-β-galactosidase antibody (red). Moderate Notch activity at permissive temperature (A and B) is significantly reduced at nonpermissive temperature (C–E). Standard deviation is shown.
Figure 7.
Lymph glands from Nts animals are competent for lamellocyte differentiation. Lymph gland lobes (A–F), or circulating cells in the hemolymph (C–F, bottom insets), stained with rhodamine-phalloidin to detect the presence of lamellocytes in Nts1 (A–D) or Nts2 (E and F) mutants reared at permissive (A and C) or nonpermissive (B and D–F) temperatures are shown.
NICD expression promotes development of crystal cell clusters
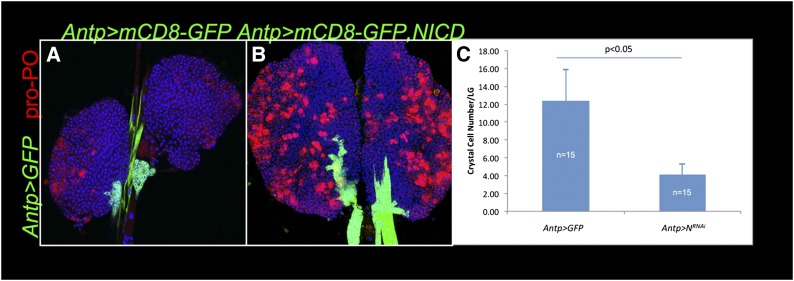
We first examined Notch’s role in crystal cell development. We were surprised to find that the expression of the Notch intracellular domain (NICD) in the niche (Antp > NICD) results in a significant increase in crystal cell number (average 67 crystal cells/lobe in Antp > NICD glands, compared to 18 crystal cells/lobe in control glands, n = 6 lobes for each genotype; P < 0.05) (Figure 4, A and B). Many of these crystal cells develop in clusters, a pattern rarely observed in wild-type glands. Conversely, RNAi knockdown of N via Antp led to a significant reduction in crystal cell number (Figure 4C).
Figure 4.
A non-cell-autonomous role for N in crystal cell development. (A) Anterior lobes of Antp > mCD8-GFP lymph gland stained with anti-pro-PO antibody (red) marks crystal cells in control lobes. (B) Antp > NICD lobes stained with anti-pro-PO antibody (red) have more crystal cells compared to control lobes. (C) A significant reduction in crystal cell number is observed in Antp > NRNAi lobes. Samples were taken from mid-third instar animals. LG, lymph gland. Standard error and P-value are shown.
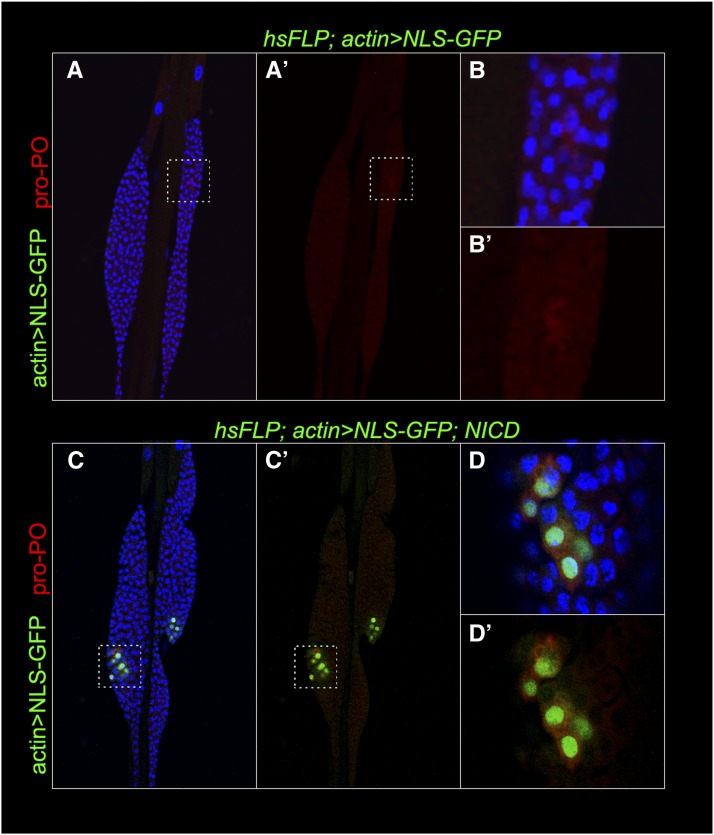
“Flp-out” clones of constitutively active NICD strongly induced crystal cell fates in third instar lymph glands: All (99%, n = 89 cells) GFP-positive clonal cells expressing NICD are also pro-PO positive (Figure 5, C–D′′), compared to 0.5%, (n = 236 cells) of pro-PO-positive cells in control clones (Figure 5, A–B′′). We also observed rare pro-PO-expressing cell clusters in NICD-expressing posterior lobes (Figure S2, C–D′). Crystal cells normally do not develop in posterior lobes (Figure S2, A–B′). Thus, ectopic NICD expression makes all daughter cells unresponsive to their normal internal and external developmental cues and, instead, programs them into crystal cells.
Figure 5.
A cell-autonomous role for the Notch intracellular domain in crystal cell development. (A–B′′) hsFLP; actin > NLS-GFP control clones marked with GFP are negative for pro-PO (red). High magnification of the defined areas A′ and A′′ from panel A and defined areas B′ and B′′ in panel B are shown. (C–D′′) Most cells in hsFLP; actin > NLS-GFP, NICD clones express pro-PO (red), but not Nimrod C (P1). High magnification of the defined areas C′ and C′′ in panel C, and D′ and D′′ in panel D are shown. Hoechst channel is omitted in B and D for clarity.
Consistent with the Antp > NICD result above (Figure 4, A–C), numerous crystal cells were also found outside the actin > NICD flp-out clones (Figure 5, D and D′′). This observation further supports an indirect positive influence of Notch activity on crystal cell differentiation. In addition, actin > NICD flp-out lobes contain mature anti-Nimrod C/P1-positive plasmatocytes at the lobe periphery. These plasmatocytes do not express NLS-GFP and are therefore NICD-negative (Figure 5, C and C′′). Together, these results not only confirm that high Notch activity is sufficient for crystal cell development as described in previous studies (Duvic et al. 2002; Lebestky et al. 2003) but also that Notch-mediated non-cell-autonomous mechanism patterns the cortex with dispersed crystal cells.
Notch activity is quenched after wasp infection
To examine whether wasp infection might decouple the ubiquitous and localized N activities, we infected second instar Su(H)-lacZ transgenic animals (wild type for N) and stained lymph glands from mid-third instar animals with anti-β-galactosidase antibody. We found a significant, twofold reduction in the ubiquitous signal in lobes of wasp-infected Su(H)-lacZ animals compared to lobes from uninfected animals (Figure 6G). In addition, the number of cells with high Notch activity (some of which also express the pro-PO enzyme) (green, Figure 6 C′, stars), is significantly reduced (Figure 6, A–F), supporting the idea that moderate ubiquitous Notch activity may be needed to specify crystal cell development, while acquisition of their final fates requires higher Notch activity in the maturing crystal cells, consistent with Notch’s cell-autonomous role (Lebestky et al. 2003). The anterior lobes of wasp-infected Su(H)-lacZ glands revealed strong lamellocyte differentiation (Figure 6, H and I), raising the possibility that reduction in Notch activity may not compromise lamellocyte differentiation as previously reported (Duvic et al. 2002).
NotchRNAi promotes lamellocyte differentiation
To examine whether Nts/Y; Dome-Meso-GFP/+ mutant lymph glands support differentiation of lamellocytes, we exposed second instar animals to L. boulardi. Unexpectedly, wasp infection of temperature-restricted Nts1 or Nts2 animals results in robust lamellocyte differentiation and lobe dispersal in mid-third instar animals (Figure 7, A–F).
The expression of Serrate, the Notch ligand, is restricted to few cells of the niche (Lebestky et al. 2003). To examine whether reduction in Notch signaling in this cell population induces lamellocyte differentiation, we conducted cell-specific knockdown using UAS-NRNAi in either valium 1 or valium 10 vector (Ni et al. 2008, 2009) in the niche or cortex, utilizing the Antp and Hml GAL4 drivers, respectively. The driver stock contains the UAS-GFP reporter transgene, marking the location of GAL4-positive cells. In each case, lamellocytes were detected in NRNAi lobes, but not in control lobes. Lamellocytes themselves were GFP negative and were located in the vicinity of the GFP-positive cells (Figure 8, A–D′′), suggesting an inductive relationship. The strongest effects were observed upon knockdown with either valium 1 or valium 10 in the niche (Table S1; Figure 8). As is the case with wasp-infected animals, mature lamellocytes were most often in the cortical zone, rarely at the medulla/cortex junction, and never in the niche or posterior lobes.
Figure 8.
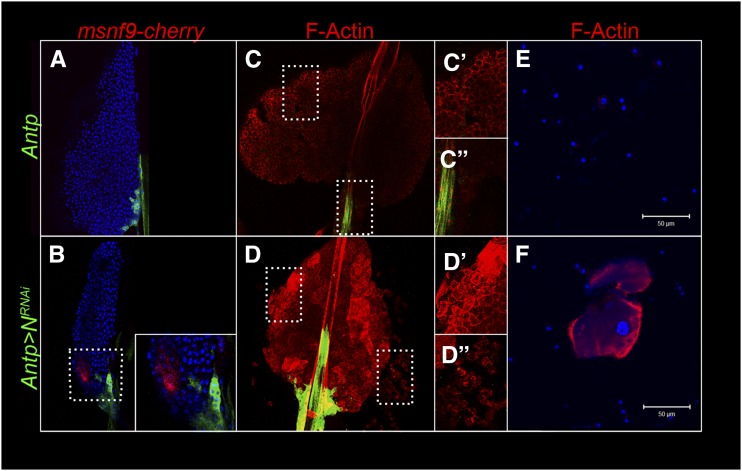
NotchRNAi correlates with lamellocyte differentiation and lobe dispersal. Supernumerary lamellocytes are detected with msnf9-cherry reporter (B) or with rhodamine phalloidin (D) in Antp > NRNAi lobes (B and D–D′′) and not in control lobes (A and C–C′′). Lamellocyte differentiation accompanies lobe dispersal (D–D′′). Hemolymph smears from control (E) and Antp > NRNAi (F) animals stained with rhodamine-phalloidin show circulating lamellocytes in experimental but not control samples.
Since basement membrane disruption correlates with the presence of lamellocytes (Sorrentino et al. 2002, 2004), we inspected the integrity of the basement membrane in both control and NRNAi lobes (Table S1). Basement membrane in the control lobes were largely intact (Figure 8, A and C), but NRNAi lobes were frequently dispersed (Figure 8, B, D, D′, and D′′; Table S1). Lamellocytes were also observed in smear preparations of circulating hemolymph of NRNAi animals (Figure 8F) but not in control smears (Figure 8E).With both drivers, knockdown with valium 10 UAS-NRNAi resulted in stronger dispersal relative to valium 1 UAS-NRNAi knockdown. These results imply that lamellocyte precursors are sensitive to Notch activity in neighboring cells.
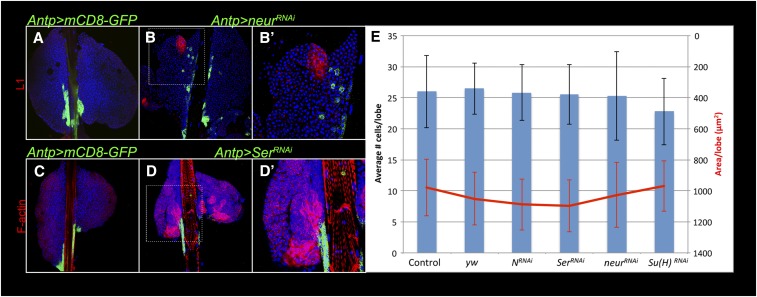
To further assess the role of Notch signaling in lamellocyte differentiation, we created RNAi knockdown lymph glands where levels of other N pathway components, Ser, neur, or Su(H) were similarly reduced in the niche. In all cases, supernumerary lamellocytes were induced (Figure 9 and data not shown). In some Antp > neurRNAi glands, ectopic lamellocytes were observed close to the GFP-positive cells (Figure 9, A–B′), but away from the niche. Knockdown of Ser (Figure 9, C–D′) or Su(H) (data not shown) had similar effects.
Figure 9.
Lamellocyte differentiation is induced by neurRNAi and SerRNAi. (A) Control Antp > GFP and (B and B′) Antp > neurRNAi lymph glands stained with anti-L1 antibody (red). L1-positive lamellocytes are found in the cortex adjacent to satellite niche cells (green). (C) Control Antp > GFP and Antp > SerRNAi (D and D′) lymph glands stained with rhodamine-phalloidin. F-actin-rich lamellocytes (red) are adjacent to the GFP-positive niche cells. Hoechst stains DNA (blue). (E) Niche size is unaffected by knockdown of N pathway components. Left: Average number of GFP-positive cells per niche (n ≥ 20) are not significantly different in control (Antp > mCD8-GFP and Antp > y w) lobes and experimental (RNAi of N, Ser, neur or Su(H)) lobes. Right: The average area of GFP-positive cells per niche (n ≥ 20) is not significantly different between control and experimental lymph glands. Standard deviations for both parameters are shown.
Thus it appears that N pathway components alter cellular properties of the niche, which in turn affects progenitor differentiation. Knockdown of these proteins, however, affects neither the number of Antp > mCD8-GFP-positive niche cells nor the size of the niche (Figure 9E). In none of these six backgrounds, did we detect actin-rich processes arising from the niche into the progenitor population as reported by others (Crozatier et al. 2007; Mandal et al. 2007; Tokusumi et al. 2012). Thus, Notch signaling does not affect specification or growth of the niche itself, but instead Notch signaling contributes to the production of a signal needed to maintain lamellocyte progenitor quiescence. RNAi results above (Figure 8, Figure 9, and Table S1) suggest that this inhibitory influence on lamellocyte differentiation, while stronger in the niche, is not limited to this compartment, and can also be exerted in other parts of the lobe.
Notch’s inhibitory influence on lamellocyte differentiation is non-cell-autonomous
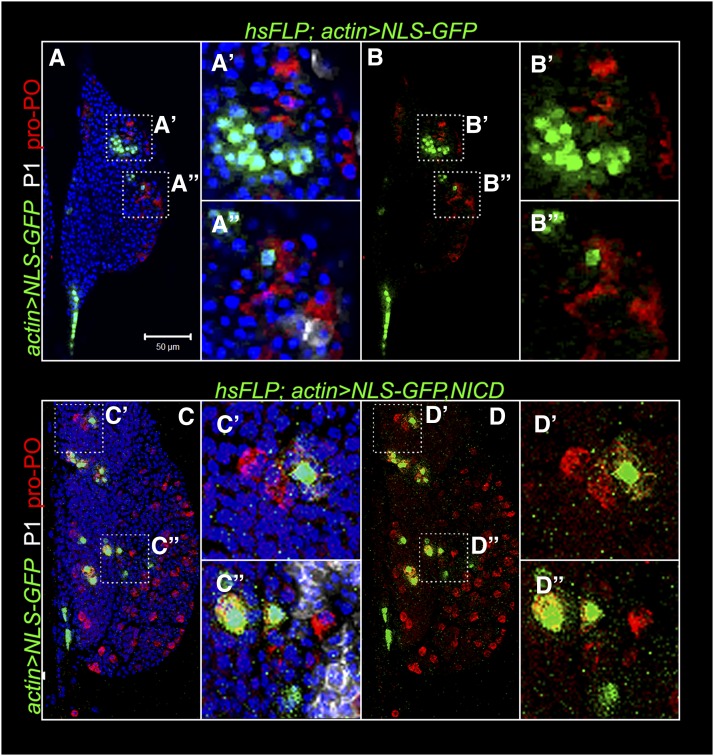
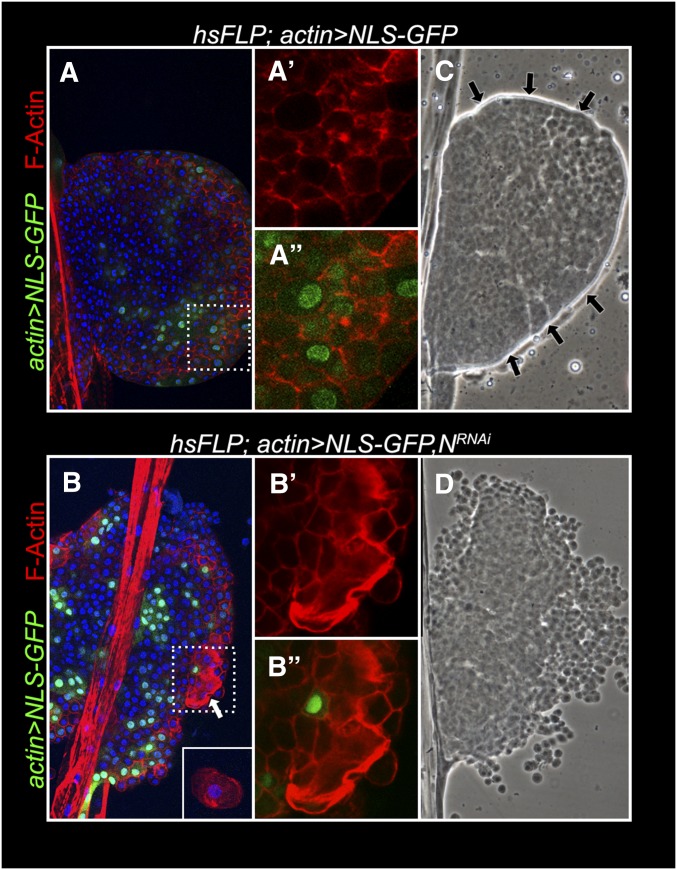
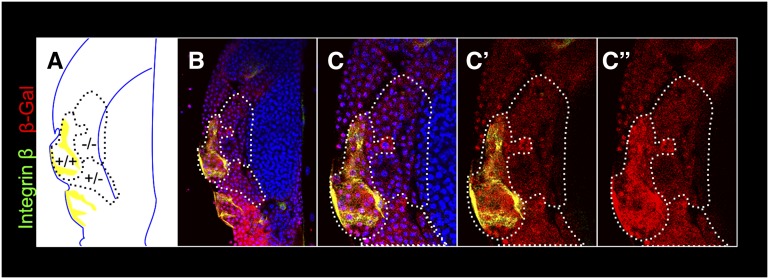
Non-cell-autonomy of N was examined in two independent experiments. Flp-out clones, marked with GFP and expressing NRNAi produce descendants that fail to differentiate into lamellocytes. However cells in the vicinity of GFP-positive NRNAi cells (but not neighboring GFP-expressing cells without the NRNAi transgene) show lamellocyte morphology (Figure 10, A and B, arrow) and strong lobe dispersal (Figure 10, C and D). We also induced twin clones (Xu and Rubin 1993) in heterozygous N55e11/N+ glands. N55e11 is a strong loss-of-function allele (Diaz-Benjumea and Garcia-Bellido 1990). In this technique, somatic recombination in heterozygous cells produces daughter cells that are either homozygous mutant (N55e11/N55e11) or wild type (N+/N+). Wild-type clones are identified by two copies of the marker protein, β-galactosidase, encoded by the lacZ transgene present in cis to N+. Figure 11 shows that integrin β-positive lamellocytes also express high levels of β-galactosidase. These double-positive cells are not found within the mutant N clone that is negative for the β-galactosidase signal (Figure 11, A–C′′), and a majority of newly differentiated cells are observed in the cortex (Figure 11). Thus, consistent with the NRNAi experiments, lamellocyte differentiation is non-cell-autonomous and a majority of newly differentiated cells are observed in the cortex (Figure 11).
Figure 10.
NRNAi–flp-out clones encourage lamellocyte differentiation and lobe dispersal. (A and C) Control lobes after induction of somatic recombination with NLS–GFP-positive cells but without NRNAi, do not induce lamellocyte differentiation (A) or lobe dispersal (C). The edge of the lobe in C is continuous (arrows), suggesting the presence of an intact basement membrane. (B and D) Clones with NLS–GFP and NRNAi induce lamellocytes (B, arrow) and lobe shows a discontinuous edge, indicating disrupted basement membrane (D).
Figure 11.
Notch clones reveal non-cell-autonomous function in lamellocyte differentiation. (A) Schematic of twin clones (genotypes labeled) and lamellocytes in the cortex of an N55e11/N+ anterior lobe. The schematic corresponds to an analysis of 14 z-stacked confocal images, each of 0.8-µm thickness; merged z-stacks are shown in B–C′′. (B) A mutant (N55e11/N55e11) clone, marked by the absence of β-galactosidase and a wild-type (N+/N+) clone detected by high levels of β-galactosidase (red). Lamellocytes positive for integrin β are also β-galactosidase-positive (yellow), and these cells are found outside the clone boundary (B–C′′). The region of interest in B is shown at a higher magnification in C–C′′, where double-positive N+/N+ lamellocytes with large, thin morphologies are more clearly evident. Hoechst stains DNA (blue).
Antp > NRNAi leads to changes in ROS levels in lymph gland lobes
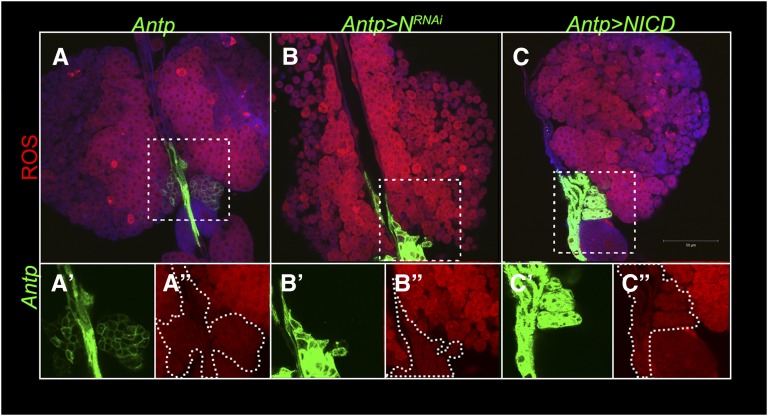
Recent work has linked increased ROS levels in the niche to lamellocyte differentiation after wasp infection (Sinenko et al. 2012 and Figure S3). To examine whether reduction in Notch activity has a similar effect on ROS levels, we stained control and Antp > NRNAi lymph glands with dihydroethidium. This cell-permeable probe reacts with cellular superoxide ions to generate oxyethidium (Zhao et al. 2003). The interaction of oxyethidium with nucleic acids is detected as red fluorescence (Tarpey et al. 2004). Figure 12 shows strong red fluorescence in the medulla in control glands, indicating high ROS levels. However, ROS are not detected in the niche and they are low in the cortex, where cells retain the unreacted probe (Figure 12, A and A′′, blue). In Antp > NRNAi lymph glands, the “high ROS” population (presumed medulla) is depleted (Figure 12B). However, Antp > mCD8-GFP-positive cells with NRNAi show no change in ROS levels and exhibit significantly low or no ROS (Figure 12B′′), much like the niche of control lobes (Figure 12A′′). Even NICD expression in the niche did not alter ROS levels in the niche (Figure 12C′′). However, ROS levels are significantly higher in the NRNAi cortex (Figure 12B) but not in NICD cortex (Figure 12C). These observations suggest that a ubiquitous effect of Notch signaling is to maintain low ROS in the anterior lobes, which may in turn maintain lamellocyte progenitors in an undifferentiated state.
Figure 12.
NRNAi induces high levels of ROS in blood cells, but not in the niche. ROS staining of freshly dissected lymph glands of control (Antp > mCD8-GFP, A–A′′), experimental (Antp > mCD8-GFP, NRNAi) (B–B′′), and (Antp > mCD8-GFP, NICD) (C–C′′) animals. High ROS is detected in the medulla and cortex of Antp > mCD8-GFP, NRNAi lymph glands. ROS levels are low or undetectable in the niche of all three genotypes (A′′, B′′, and C′′).
Discussion
In this study, we report a dual role for Notch signaling in larval hematopoiesis. Notch appears to play a reciprocal role in the development of crystal cells and in lamellocyte development. Moderate levels of wild-type Notch signaling promote crystal cell selection, but, contrary to expectation, support quiescence and not differentiation of lamellocyte progenitors. The inhibitory influence on lamellocyte differentiation is exerted non-cell-autonomously and correlates with high oxidative stress in lobes undergoing lamellocyte differentiation and dispersal.
Dual and reciprocal non-autonomous effects of N on lineage decision
Division and development of a heterogeneous hematopoietic progenitor population in the third instar medulla is arrested. Cortical cells in proximity to the medulla are less fully developed than peripheral cortical cells. Signals from the niche, cortex, and the medulla maintain medullary quiescence, and some of these signals must be modified to release progenitors from their quiescent state (reviewed in Fossett 2013). Our experiments reveal that (1) Notch protein, while ubiquitously expressed, shows considerably variable subcellular localization, suggesting different states of N activities in the organ (Figure 1); (2) Notch activity is low and ubiquitous in first and second instar lobes, but increases in the third instar stage as the host becomes competent for defense against parasite attack (Sorrentino et al. 2002) (Figure 2); (3) although reduction in Notch activity does not affect Antp > GFP expression, niche size, or the development of the plasmatocyte lineage, it has divergent effects on the other two lineages. While moderate Notch activity encourages the selection and development of individual crystal cells in the lobe cortex, the same activity maintains lamellocyte progenitors in an undifferentiated state (Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, and Figure 10). Lamellocytes themselves do not express high levels of Notch (Figure 1) and it is quite possible that Notch protein levels are down-regulated during the differentiation process.
Notch’s autonomous effects on crystal cell development are understood in some detail (Lebestky et al. 2003; Mukherjee et al. 2011; Terriente-Felix et al. 2013). Our results suggest that ubiquitous Notch activity in third instar lobes (Figure 2) in basal hematopoiesis is required for the selection of the correct proportion of crystal cells in the third instar lobe resulting in their dispersed distribution pattern. Other cell signaling mechanisms including Notch signaling itself, build on this Notch activity “pre-pattern” for continued crystal cell development and maturation. Temperature-restricted Nts lobes exhibit normal medulla/cortex zonation (Figure 7) and produce Nimrod-C-positive plasmatocytes in their correct location (our unpublished results) but are unable to produce crystal cells (Lebestky et al. 2003 and Figure 3). However, forced expression of NICD (or NRNAi) in the niche alone is sufficient to encourage (or discourage) crystal cell development (Figure 4). More significantly, ectopic NICD expression in mitotic clones alters and overcomes the effects of the normal pre-pattern in basal hematopoiesis and programs all clonal cells into crystal cells (Figure 5).
We were surprised to discover that temperature-restricted Nts lobes are immune competent and can produce lamellocytes (Figure 7). Thus, reduced Notch does not compromise lamellocyte differentiation (i.e., Notch may not be needed for lamellocyte differentiation). Instead, under low Notch, lamellocyte progenitors are specified and are developmentally competent. The inhibitory effect of Notch activity on lamellocyte progenitors in the absence of infection is observed upon RNAi knockdown in the niche or cortex (Figure 8 and Figure 9) or removal of N function in somatic clones (Figure 10 and Figure 11). We propose that a second role for moderate ubiquitous Notch activity is to generate a signal that shelters lamellocyte progenitors in the absence of infection. Infection reduces this ubiquitous Notch activity allowing lamellocyte progenitors to mature (Figure 6).
Are the reciprocal effects of N on crystal cells and lamellocytes linked? Recent studies have uncovered unexpected plasticity in hematopoietic development (Avet-Rochex et al. 2010; Honti et al. 2010; Stofanko et al. 2010; Kroeger et al. 2012). Wasp infection induces proliferation and lamellocyte differentiation (Sorrentino et al. 2002; Krzemien et al. 2010b), but infection reduces the abundance of crystal cells (Krzemien et al. 2010b). Our results provide a mechanism whereby reduction in Notch activity would coordinate alternative fate choice in basal vs. activated hematopoiesis; such a switch would allow progenitor reprogramming to tailor host defense to promote wasp egg encapsulation. As such, lymph gland dispersal and release of plasmatocytes and lamellocytes into the hemolymph alters the circulating blood cell population, enhancing the host’s immune competence. Our data reveal that Notch plays a critical role in this process. Similar scenarios for Notch-mediated restriction of alternative cell fates has been observed at multiple stages of vertebrate hematopoiesis. Notch signaling in zebra fish promotes the production of hematopoietic stem cells at the expense of endothelial cells from a common bipotent hemangioblast (Lee et al. 2009a). Notch1 signaling is critical in the mouse thymus, where Notch manages the checkpoint for T-cell commitment at the expense of the B-lineage program; and Notch1 collaborates with GATA-3 to commit progenitors to the T-cell lineage (Garcia-Ojeda et al. 2013). Further downstream, Notch favors adoption of an αβ over γδ T-cell fate (Radtke et al. 2005). Our study expands the recurrent and burgeoning theme of selecting alternative cell fates by activating Notch signaling at crucial lineage branch points in multiple development contexts and in diverse organisms.
Notch signaling blocks the production of high ROS in anterior lobes
Reduction of Notch signaling appears to influence progenitor quiescence via increased levels of superoxide ions in most lobe cells, but not in the niche (Figure 12). Surprisingly, cells in the lobe where levels of N (or N pathway components were reduced), show high levels of oxyethidium in response to high superoxide. Thus, it appears that Notch signaling can have systemic effects on the oxidative stress level of the organ. Aberrant increase in cellular ROS is associated with loss of quiescence and precocious differentiation of medullary progenitors (Owusu-Ansah and Banerjee 2009). In addition, the hypoxia protein HIF-α interacts with NICD to promote transcription of target genes that support crystal cell survival (Mukherjee et al. 2011). Wasp infection induces high ROS in the niche; infection results in the secretion of the epidermal growth factor-like cytokine, Spitz, whose activation of rhomboid and the EGF receptor induces lamellocyte differentiation in the cortex (Sinenko et al. 2012). Thus, oxidative stress via ROS has multiple and complex effects on hematopoietic cell quiescence, differentiation, or survival. Our results point to the possibility that Notch signaling maintains low oxidative stress in cells of the cortex (but not the niche) to preserve lamellocyte progenitor quiescence and promote crystal cell development and survival. Reduced N, or an increase in ROS, signals wasp-induced lamellocyte differentiation. The mechanism underlying ROS changes in the cortex remains to be explored.
The developing lymph gland has emerged as a powerful system to study mechanisms governing hematopoietic stem/progenitor cell quiescence and differentiation. We show that Notch activity controls quiescence vs. development for divergent lineages. Moderate Notch activity correlates with low ROS levels and lamellocyte quiescence. Experimental evidence suggests that a low-oxygen milieu of the bone marrow protects mammalian hematopoietic stem cells and the supporting niche cells from oxidative stress, whereas high ROS correlates with their ability to self-renew (Piccoli et al. 2005; Ito et al. 2006; Parmar et al. 2007; Tothova et al. 2007; Wang and Wagers 2011). Primitive hematopoietic stem cells with low ROS exhibit properties of quiescent stem cells that include slow cycling and expression of Notch1 (Jang and Sharkis 2007). Thus, it is possible that intracellular ROS levels coordinate with signaling pathways to specify quiescence vs. proliferation and differentiation. ROS have traditionally been considered to be agents of cell damage and are associated with many human pathologies (Balaban et al. 2005). Recent data link ROS more directly to cellular function and homeostasis via signaling pathways (Hamanaka et al. 2013). Given the extensive conservation of N functions in fly and mammalian hematopoiesis (Krzemien et al. 2010a; Fossett 2013), our studies open the possibility of more thoroughly understanding how signal transduction and ROS are integrated and how this integration coordinates basal and activated hematopoietic development.
Acknowledgments
We thank our colleagues, Vienna Drosophila RNAi Center, TRiP, and the Bloomington Stock Center for fly stocks and antibodies. We are especially grateful for Dr. M. Crozatier for sharing the Dome-Meso-GFP line before publication. We are grateful to R. Rajwani and Z. Papadopol for help with experiments, D. Fimiarz and J. Morales for help with microscopy, S. Hoskins, and R. Jha for editorial feedback, and members of the Govind lab for their input. This publication was made possible by grants from National Science Foundation (1121817), National Institutes of Health (S06 GM08168, 8G12MD007603-27, RISE 41399-009), Howard and Vicki Palefsky Fellowship, and Professional Staff Congress-City University of New York (66657-00 44).
Footnotes
Communicating editor: B. Lazzaro
Literature Cited
- Avet-Rochex A., Boyer K., Polesello C., Gobert V., Osman D., et al. , 2010. An in vivo RNA interference screen identifies gene networks controlling Drosophila melanogaster blood cell homeostasis. BMC Dev. Biol. 10: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban R. S., Nemoto S., Finkel T., 2005. Mitochondria, oxidants, and aging. Cell 120: 483–495 [DOI] [PubMed] [Google Scholar]
- Bataille L., Auge B., Ferjoux G., Haenlin M., Waltzer L., 2005. Resolving embryonic blood cell fate choice in Drosophila: interplay of GCM and RUNX factors. Development 132: 4635–4644 [DOI] [PubMed] [Google Scholar]
- Bigas A., D’Altri T., Espinosa L., 2012. The Notch pathway in hematopoietic stem cells. Curr. Top. Microbiol. Immunol. 360: 1–18 [DOI] [PubMed] [Google Scholar]
- Bray S. J., 2006. Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 7: 678–689 [DOI] [PubMed] [Google Scholar]
- Brower D. L., Wilcox M., Piovant M., Smith R. J., Reger L. A., 1984. Related cell-surface antigens expressed with positional specificity in Drosophila imaginal discs. Proc. Natl. Acad. Sci. USA 81: 7485–7489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophides G. K., Zdobnov E., Barillas-Mury C., Birney E., Blandin S., et al. , 2002. Immunity-related genes and gene families in Anopheles gambiae. Science 298: 159–165 [DOI] [PubMed] [Google Scholar]
- Crozatier M., Meister M., 2007. Drosophila haematopoiesis. Cell. Microbiol. 9: 1117–1126 [DOI] [PubMed] [Google Scholar]
- Crozatier M., Ubeda J. M., Vincent A., Meister M., 2004. Cellular immune response to parasitization in Drosophila requires the EBF orthologue collier. PLoS Biol. 2: E196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozatier M., Krzemien J., Vincent A., 2007. The hematopoietic niche: a Drosophila model, at last. Cell Cycle 6: 1443–1444 [PubMed] [Google Scholar]
- Diaz-Benjumea F. J., Garcia-Bellido A., 1990. Behaviour of cells mutant for an EGF receptor homologue of Drosophila in genetic mosaics. Proc. Biol. Sci. 242: 36–44 [DOI] [PubMed] [Google Scholar]
- Duvic B., Hoffmann J. A., Meister M., Royet J., 2002. Notch signaling controls lineage specification during Drosophila larval hematopoiesis. Curr. Biol. 12: 1923–1927 [DOI] [PubMed] [Google Scholar]
- Emerald B. S., Cohen S. M., 2004. Spatial and temporal regulation of the homeotic selector gene Antennapedia is required for the establishment of leg identity in Drosophila. Dev. Biol. 267: 462–472 [DOI] [PubMed] [Google Scholar]
- Fehon R. G., Kooh P. J., Rebay I., Regan C. L., Xu T., et al. , 1990. Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell 61: 523–534 [DOI] [PubMed] [Google Scholar]
- Fossett N., 2013. Signal transduction pathways, intrinsic regulators, and the control of cell fate choice. Biochim. Biophys. Acta 1830: 2375–2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ojeda M. E., Klein Wolterink R. G., Lemaitre F., Richard-Le Goff O., Hasan M., et al. , 2013. GATA-3 promotes T-cell specification by repressing B-cell potential in pro-T cells in mice. Blood 121: 1749–1759 [DOI] [PubMed] [Google Scholar]
- Ghiglione C., Devergne O., Georgenthum E., Carballes F., Medioni C., et al. , 2002. The Drosophila cytokine receptor Domeless controls border cell migration and epithelial polarization during oogenesis. Development 129: 5437–5447 [DOI] [PubMed] [Google Scholar]
- Go M. J., Eastman D. S., Artavanis-Tsakonas S., 1998. Cell proliferation control by Notch signaling in Drosophila development. Development 125: 2031–2040 [DOI] [PubMed] [Google Scholar]
- Gueguen G., Kalamarz M. E., Ramroop J., Uribe J., Govind S., 2013. Polydnaviral ankyrin proteins aid parasitic wasp survival by coordinate and selective inhibition of hematopoietic and immune NF-kappa B signaling in insect hosts. PLoS Pathog. 9: e1003580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka R. B., Glasauer A., Hoover P., Yang S., Blatt H., et al. , 2013. Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Sci. Signal. 6: ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzler P., Simpson P., 1991. The choice of cell fate in the epidermis of Drosophila. Cell 64: 1083–1092 [DOI] [PubMed] [Google Scholar]
- Honti V., Csordas G., Markus R., Kurucz E., Jankovics F., et al. , 2010. Cell lineage tracing reveals the plasticity of the hemocyte lineages and of the hematopoietic compartments in Drosophila melanogaster. Mol. Immunol. 47: 1997–2004 [DOI] [PubMed] [Google Scholar]
- Honti V., Csordas G., Kurucz E., Markus R., Ando I., 2013. The cell-mediated immunity of Drosophila melanogaster: hemocyte lineages, immune compartments, microanatomy and regulation. Dev. Comp. Immunol. 42: 47–56. [DOI] [PubMed] [Google Scholar]
- Ito K., Hirao A., Arai F., Takubo K., Matsuoka S., et al. , 2006. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 12: 446–451 [DOI] [PubMed] [Google Scholar]
- Jang Y. Y., Sharkis S. J., 2007. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 110: 3056–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S. H., Evans C. J., Uemura C., Banerjee U., 2005. The Drosophila lymph gland as a developmental model of hematopoiesis. Development 132: 2521–2533 [DOI] [PubMed] [Google Scholar]
- Kalamarz M. E., Paddibhatla I., Nadar C., Govind S., 2012. Sumoylation is tumor-suppressive and confers proliferative quiescence to hematopoietic progenitors in Drosophila melanogaster larvae. Biol. Open 1: 161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch U., Lehal R., Radtke F., 2013. Stem cells living with a Notch. Development 140: 689–704 [DOI] [PubMed] [Google Scholar]
- Kroeger P. T., Jr, Tokusumi T., Schulz R. A., 2012. Transcriptional regulation of eater gene expression in Drosophila blood cells. Genesis 50: 41–49 [DOI] [PubMed] [Google Scholar]
- Krzemien J., Dubois L., Makki R., Meister M., Vincent A., et al. , 2007. Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature 446: 325–328 [DOI] [PubMed] [Google Scholar]
- Krzemien J., Crozatier M., Vincent A., 2010a Ontogeny of the Drosophila larval hematopoietic organ, hemocyte homeostasis and the dedicated cellular immune response to parasitism. Int. J. Dev. Biol. 54: 1117–1125 [DOI] [PubMed] [Google Scholar]
- Krzemien J., Oyallon J., Crozatier M., Vincent A., 2010b Hematopoietic progenitors and hemocyte lineages in the Drosophila lymph gland. Dev. Biol. 346: 310–319. [DOI] [PubMed] [Google Scholar]
- Kurucz E., Vaczi B., Markus R., Laurinyecz B., Vilmos P., et al. , 2007. Definition of Drosophila hemocyte subsets by cell-type specific antigens. Acta Biol. Hung. 58(Suppl): 95–111 [DOI] [PubMed] [Google Scholar]
- Lanot R., Zachary D., Holder F., Meister M., 2001. Postembryonic hematopoiesis in Drosophila. Dev. Biol. 230: 243–257 [DOI] [PubMed] [Google Scholar]
- Lebestky T., Chang T., Hartenstein V., Banerjee U., 2000. Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science 288: 146–149 [DOI] [PubMed] [Google Scholar]
- Lebestky T., Jung S. H., Banerjee U., 2003. A Serrate-expressing signaling center controls Drosophila hematopoiesis. Genes Dev. 17: 348–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Y., Vogeli K. M., Kim S. H., Chong S. W., Jiang Y. J., et al. , 2009a Notch signaling functions as a cell-fate switch between the endothelial and hematopoietic lineages. Curr. Biol. 19: 1616–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. J., Kalamarz M. E., Paddibhatla I., Small C., Rajwani R., et al. , 2009b Virulence factors and strategies of Leptopilina spp.: selective responses in Drosophila hosts. Adv. Parasitol. 70: 123–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Hanratty W. P., Dearolf C. R., 1995. An amino acid substitution in the Drosophila hopTum-l Jak kinase causes leukemia-like hematopoietic defects. EMBO J. 14: 1412–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal L., Banerjee U., Hartenstein V., 2004. Evidence for a fruit fly hemangioblast and similarities between lymph-gland hematopoiesis in fruit fly and mammal aorta-gonadal-mesonephros mesoderm. Nat. Genet. 36: 1019–1023 [DOI] [PubMed] [Google Scholar]
- Mandal L., Martinez-Agosto J. A., Evans C. J., Hartenstein V., Banerjee U., 2007. A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature 446: 320–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakhina S., Steward R., 2010. Hematopoietic stem cells in Drosophila. Development 137: 27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee T., Kim W. S., Mandal L., Banerjee U., 2011. Interaction between Notch and Hif-alpha in development and survival of Drosophila blood cells. Science 332: 1210–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J. Q., Markstein M., Binari R., Pfeiffer B., Liu L. P., et al. , 2008. Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat. Methods 5: 49–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J. Q., Liu L. P., Binari R., Hardy R., Shim H. S., et al. , 2009. A Drosophila resource of transgenic RNAi lines for neurogenetics. Genetics 182: 1089–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B., Spradling A., 2007. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science 315: 988–992 [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Zon L. I., 2008. Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132: 631–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Ansah E., Banerjee U., 2009. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature 461: 537–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddibhatla I., Lee M. J., Kalamarz M. E., Ferrarese R., Govind S., 2010. Role for sumoylation in systemic inflammation and immune homeostasis in Drosophila larvae. PLoS Pathog. 6: e1001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar K., Mauch P., Vergilio J. A., Sackstein R., Down J. D., 2007. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc. Natl. Acad. Sci. USA 104: 5431–5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli C., Ria R., Scrima R., Cela O., D’Aprile A., et al. , 2005. Characterization of mitochondrial and extra-mitochondrial oxygen consuming reactions in human hematopoietic stem cells. Novel evidence of the occurrence of NAD(P)H oxidase activity. J. Biol. Chem. 280: 26467–26476 [DOI] [PubMed] [Google Scholar]
- R Development Core Team, 2010 R: a language and environment for statistical computing http://www.R-project.org/ R Foundation for Statistical Computing, Vienna, Austria.
- Radtke F., Wilson A., MacDonald H. R., 2005. Notch signaling in hematopoiesis and lymphopoiesis: lessons from Drosophila. Bioessays 27: 1117–1128 [DOI] [PubMed] [Google Scholar]
- Schlenke T. A., Morales J., Govind S., Clark A. G., 2007. Contrasting infection strategies in generalist and specialist wasp parasitoids of Drosophila melanogaster. PLoS Pathog. 3: 1486–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinenko S. A., Kim E. K., Wynn R., Manfruelli P., Ando I., et al. , 2004. Yantar, a conserved arginine-rich protein is involved in Drosophila hemocyte development. Dev. Biol. 273: 48–62 [DOI] [PubMed] [Google Scholar]
- Sinenko S. A., Shim J., Banerjee U., 2012. Oxidative stress in the haematopoietic niche regulates the cellular immune response in Drosophila. EMBO Rep. 13: 83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino R. P., Carton Y., Govind S., 2002. Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev. Biol. 243: 65–80 [DOI] [PubMed] [Google Scholar]
- Sorrentino R. P., Melk J. P., Govind S., 2004. Genetic analysis of contributions of dorsal group and JAK-Stat92E pathway genes to larval hemocyte concentration and the egg encapsulation response in Drosophila. Genetics 166: 1343–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stofanko M., Kwon S. Y., Badenhorst P., 2008. A misexpression screen to identify regulators of Drosophila larval hemocyte development. Genetics 180: 253–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stofanko M., Kwon S. Y., Badenhorst P., 2010. Lineage tracing of lamellocytes demonstrates Drosophila macrophage plasticity. PLoS ONE 5: e14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G., Basler K., 1993. Organizing activity of wingless protein in Drosophila. Cell 72: 527–540 [DOI] [PubMed] [Google Scholar]
- Struhl G., Adachi A., 1998. Nuclear access and action of notch in vivo. Cell 93: 649–660 [DOI] [PubMed] [Google Scholar]
- Tarpey M. M., Wink D. A., Grisham M. B., 2004. Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286: R431–R444 [DOI] [PubMed] [Google Scholar]
- Terriente-Felix A., Li J., Collins S., Mulligan A., Reekie I., et al. , 2013. Notch cooperates with Lozenge/Runx to lock haemocytes into a differentiation programme. Development 140: 926–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokusumi T., Sorrentino R. P., Russell M., Ferrarese R., Govind S., et al. , 2009. Characterization of a lamellocyte transcriptional enhancer located within the misshapen gene of Drosophila melanogaster. PLoS ONE 4: e6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokusumi Y., Tokusumi T., Shoue D. A., Schulz R. A., 2012. Gene regulatory networks controlling hematopoietic progenitor niche cell production and differentiation in the Drosophila lymph gland. PLoS ONE 7: e41604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothova Z., Kollipara R., Huntly B. J., Lee B. H., Castrillon D. H., et al. , 2007. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128: 325–339 [DOI] [PubMed] [Google Scholar]
- Vincent J. P., Girdham C. H., O’Farrell P. H., 1994. A cell-autonomous, ubiquitous marker for the analysis of Drosophila genetic mosaics. Dev. Biol. 164: 328–331 [DOI] [PubMed] [Google Scholar]
- Wang L. D., Wagers A. J., 2011. Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nat. Rev. Mol. Cell Biol. 12: 643–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Rubin G. M., 1993. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117: 1223–1237 [DOI] [PubMed] [Google Scholar]
- Zhao H., Kalivendi S., Zhang H., Joseph J., Nithipatikom K., et al. , 2003. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic. Biol. Med. 34: 1359–1368 [DOI] [PubMed] [Google Scholar]