Abstract
We examine the basis of Darwin’s corollary to Haldane’s rule, which describes viability and fertility differences between F1 produced from reciprocal crosses. We analyzed asymmetries in hybrid viability from >100 reciprocal crosses involving 36 toad species to test whether relatively high rates of mitochondrial vs. nuclear evolution produce dams with systematically less viable F1 hybrid progeny. We find no such effect, suggesting a predominant role for stochastic accumulation of asymmetric epistatic incompatibilities.
Keywords: Dobzhansky–Muller incompatibilities (DMIs), intrinsic postzygotic isolation, hybrid inviability, Darwin’s corollary to Haldane’s rule
AS Darwin (1859, Chap. 8) noted, interspecific reciprocal crosses often differ in whether fertilization occurs and in the viability and fecundity of F1 hybrids. Even under optimal laboratory conditions, reciprocal F1s often show viability or fecundity differences. This pattern of asymmetric intrinsic postzygotic isolation has been dubbed “Darwin’s corollary to Haldane’s rule” (Turelli and Moyle 2007). While asymmetric fertilization success can be produced under autosomal genetic control, Darwin’s corollary requires deleterious epistatic interactions (i.e., Dobzhansky–Muller incompatibilities, DMIs) involving uniparentally inherited factors such as mitochondria, sex chromosomes, epigenetic programming, or maternal effects (Turelli and Moyle 2007), because only uniparentally inherited factors will differentially affect F1 produced from reciprocal crosses.
Turelli and Moyle (2007) contrasted deterministic vs. stochastic explanations for Darwin’s corollary, depending on whether the cross that produces less fit hybrids is predictable from relative rates of evolution for uniparentally vs. biparentally inherited factors. Differences in the relative rates of evolution can lead to different expected fitnesses from reciprocal crosses. Consider the species pair A–B. If the proportion of mitochondrial to nuclear substitutions in lineage A exceeds that in lineage B, we expect more mitonuclear DMIs in AB F1 hybrids with A mothers vs. BA hybrids with B mothers, simply because there are more potential incompatibilities in the AB cross than in the BA cross. Thus, if mitonuclear DMIs contribute significantly to lowered interpopulation (Burton et al. 2006; Ellison and Burton 2008; Montooth et al. 2010; Meiklejohn et al. 2013) or interspecific (e.g., Fishman and Willis 2006; Lee et al. 2008; Chou et al. 2010; Rieseberg and Blackman 2010) fitness, as often argued (e.g., Rand et al. 2004; Gershoni et al. 2009; Chou and Leu 2010; Lane 2011; Burton and Barreto 2012), directional asymmetry may be predictable from relative rates of mitochondrial vs. nuclear evolution.
Turelli and Moyle (2007) conjectured that the deterministic signal associated with directional effects produced by a particular class of DMIs (e.g., mitonuclear incompatibilities) was likely to be overwhelmed by both stochastic effects and other classes of asymmetric incompatibilities. The theoretical analyses pioneered by Orr (1993) describe the accumulation of DMIs as rare––independent and inherently stochastic––events associated with molecular differences between diverging taxa. Different realizations of these stochastic processes will produce different outcomes. Thus, even when the expected number of DMIs between reciprocal crosses is equal (corresponding to equal numbers of nuclear and mitochondrial substitutions), the actual number and/or effects of DMIs will typically differ because of the stochasticity inherent in the emergence of DMIs from interspecific differences (Orr 1993; Turelli and Moyle 2007).
Despite these caveats, in the first investigation of deterministic asymmetry effects, Bolnick et al. (2008) found a weak but statistically significant signal associated with mitochondrial vs. nuclear evolution in centrarchid fishes. For 13 of 18 reciprocal crosses in their study (72%), viability was lower when the species with the relatively greater ratio of mtDNA to nuclear substitutions was used as the maternal parent. This observation is consistent with the deterministic asymmetry hypothesis, but was somewhat surprising in light of the relatively small effect expected with plausible parameters for mitonuclear contributions to hybrid inviability. Here we examine whether this deterministic pattern holds using more extensive data from another clade, toads of the genus Bufo (taxonomy following Pauly et al. 2009, which maintains consistency with the names used in Malone and Fontenot 2008). We describe patterns of asymmetry produced at different stages of F1 formation and development and at different levels of phylogenetic divergence.
We examine the success of Blair’s (1972) experimental crosses as summarized by Malone and Fontenot (2008) across three stages of toad development: fertilization, hatching, and metamorphosis. These stages differ in whether mitonuclear incompatibilities can plausibly explain observed asymmetries:
Fertilization (measured as the proportion of eggs fertilized): Fertilization can depend on sperm–egg incompatibilities (e.g., Vacquier and Swanson 2011), but is very unlikely to reflect F1 mitonuclear DMIs as envisioned in the deterministic theory. Hence, fertilization informs the tempo of asymmetric reproductive isolation, but is a negative control for the importance of mitonuclear DMIs.
Hatching (measured as the proportion of fertilized eggs that hatched): Hatching of fertilized eggs involves interactions between alleles in the F1 as well as interactions between the F1 embryo and maternal contributions to the egg; it therefore compounds offspring genotype with another uniparental factor (maternal effects) that may swamp the signal of deterministic effects associated with mitonuclear incompatibilities.
Metamorphosis (measured as the proportion of larvae metamorphosed): The period from hatching to metamorphosis completes the maternal–zygotic transition (Wang and Dey 2006). It provides the best case for testing the deterministic effects of mitonuclear incompatibilities.
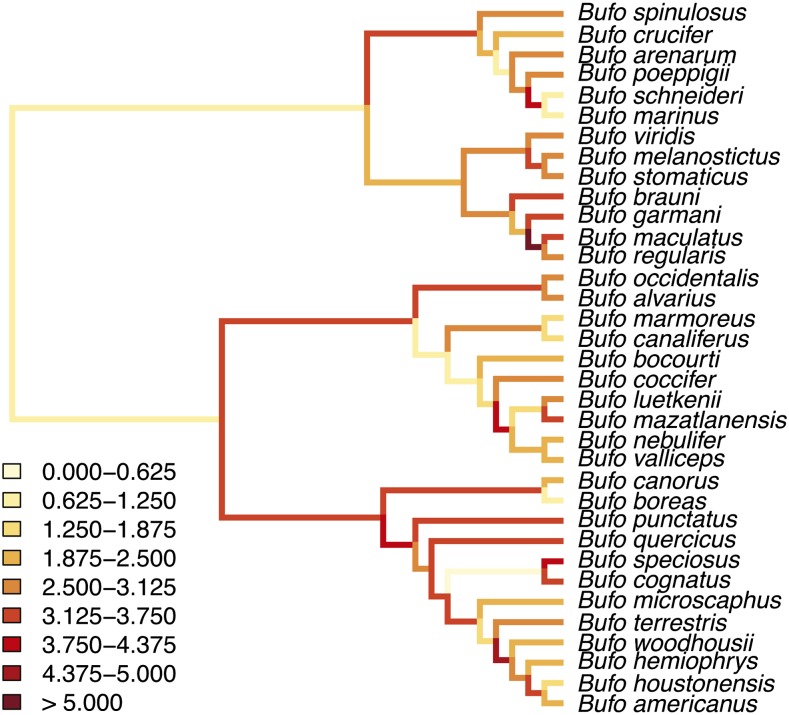
Testing the deterministic theory requires estimates of mitochondrial and nuclear branch lengths on a shared topology. We performed phylogenetic analyses for each gene region using MrBayes 3.2 (Ronquist et al. 2012) to identify substitution models for each locus (one mitochondrial and five nuclear loci; see Methods in Supporting Information File S1, Table S1, Table S2, Figure S1, Figure S2, Figure S3, Figure S4, Figure S5, Figure S6, Figure S7, Figure S8, and Figure S9). To generate a shared mitonuclear topology for our analysis (i.e., a species tree), we constrained the topology of our combined nuclear–mitochondrial analysis on the deeper clade relationships evident in the nuclear trees (Figure 1), allowing us to accommodate an apparent mitochondrial introgression event that caused a deep discordance between nuclear and mitochondrial gene trees (see Methods and Results in Supporting Information File S1 and Figure S10). The resulting species tree is highly supported (i.e., high clade posterior probabilities; see Figure S11) and provides estimates of mitonuclear substitution rate asymmetry (see Figure S12 and Figure S13). Excluding crosses spanning the mitochondrial capture event does not alter our qualitative results (not shown).
Figure 1.
The majority-rule consensus tree for the genus Bufo. Branch lengths are relative to time on an ultrametric tree. Branch colors represent the relative amount of mitochondrial to nuclear evolution [log(mitochondrial branch length/nuclear branch length)] as indicated by the legend (estimated using MrBayes 3.2; Ronquist et al. 2012). We estimated the expected amount of evolution for each branch under the topological constraint described in the text with MrBayes 3.2 by unlinking nuclear and mitochondrial branch lengths to estimate mitonuclear asymmetry on a shared topology. We estimated relative divergence times using BEAST (Drummond et al. 2012). Data were gathered from GenBank with additional sequencing to complete the data matrix (accession numbers, primers, alignments, additional descriptions of our methods, and code for tree generation and computing relative branch lengths are presented in File S1, Table S1 and Table S2; File S2, alignments; and File S3, code). Statistical analyses were performed in R (R Development Core Team 2008).
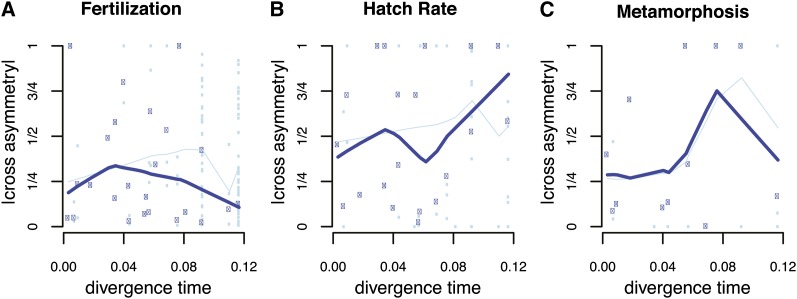
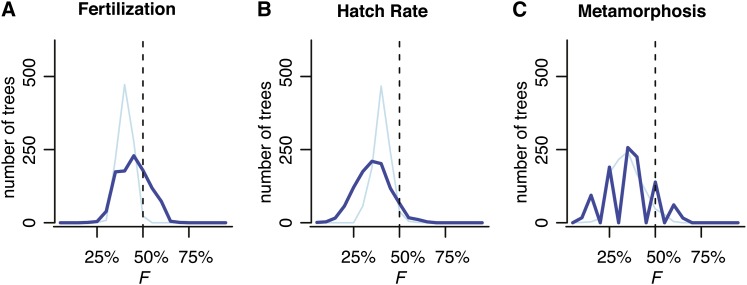
Figure 2 displays the tempo of evolution of asymmetric reproductive isolation, for both the raw data small light blue dots and thin light blue lines in Figure 2) and the node-weighted averages (Fitzpatrick 2002; dark blue squares and thick lines in Figure 2). Not surprisingly, asymmetries are found in fertilization success (Figure 2A) and through development (Figure 2, B and C) across all levels of divergence. We test the deterministic theory by asking whether taxa with higher relative mitochondrial-to-nuclear substitution rates (see the definition of δA in Figure 3) are more likely to be worse dams in a reciprocal cross (Figure 3). We find no support for this prediction. Rather, we observe a trend in the other direction across all developmental stages. This result is robust to uncertainty in tree topology and branch lengths; for a large majority of trees sampled from the posterior, across all developmental stages, the relationship between relative mitochondrial to nuclear substitution rates and reciprocal cross asymmetry trended against the prediction of the deterministic theory (Figure 3). In fact, aside from one chance observation in our negative control (fertilization), none of the 2000 (nonindependent) tests (1000 trees × 2 measures [hatching and metamorphosis]) provided statistically significant support for the deterministic theory (Figure 3).
Figure 2.
Reciprocal-cross asymmetry across fertilization and through development. The x-axis represents ultrametric distance between two nodes (Figure 1). The relative difference in reciprocal cross success is ρ = (A × B – B × A)/(A × B + B × A). The y-axis represents the absolute value of ρ. The entire data set is presented with light blue points, and the thin light blue line displays local smoothing by lowess (R Development Core Team 2008). Dark blue boxes and thick lines present node-weighted average values that correct for phylogenetic nonindependence (Fitzpatrick 2002). Developmental stages are: (A) Fertilization, (B) hatching, and (C) metamorphosis.
Figure 3.
Asymmetries in mitochondrial vs. nuclear rates of evolution do not explain asymmetries in mating or hybrid viability. We examined asymmetries across a distribution of 1000 plausible phylogenetic trees (of varying topologies and branch lengths). Let C denote the most recent common ancestor of species A and B, and let L(m)iC (L(n)iC) denote the estimated number of mitochondrial (nuclear) substitutions (branch length) separating species C and i. Following Bolnick et al. (2008, Equation 1 and Figure 1), we calculate the relative rate of mtDNA evolution for lineage A vs. lineage B as δA = [L(m)AC/(L(m)AC + L(m)BC)] – [L(n)AC/(L(n)AC + L(n)BC)]. For each tree, we calculated F, the fraction of crosses in which the lineage with the elevated relative rate of mtDNA to nuclear evolution is the worse dam (i.e., produces lower rates of fertilization, hatching, or metamorphosis). This corresponds to δAρ < 0. In each panel, the x-axis is F and the y-axis is the number of trees (from our posterior distribution of 1000) that produce each F. The y-axis accounts for phylogenetic uncertainty. Light blue lines include all data and dark blue lines are node-weighted averages (i.e., the data after phylogenetic correction). If the deterministic theory was supported, we expect F > 0.5 for a significant majority of trees, at least for B and C, which report phenotypes likely to be affected by mitonuclear incompatibilities. (A) Lower fertilization occurs when the maternal lineage has an elevated relative rate of mtDNA evolution (δA > 0) for only 43.4 of 107 crosses when averaged over our 1000 trees, i.e., F = 0.41 (10.90 of 23.98 after phylogenetic correction, P = 0.45). After phylogenetic correction, only one-fifth of trees (200 of 1000) produce F > 0.5, and only 1 of our 1000 trees produces F significantly larger than 0.5. (B) For hatching, on average 29.68 of 74 crosses support the deterministic theory, F = 0.40 (7.10 of 20.00 after phylogenetic correction, P = 0.36). Only one-thirtieth of trees (33 of 1000) produce F > 0.5, and none of our 1000 trees produces F significantly larger than 0.5. (C) In metamorphosis, on average 7.97 of 22 crosses support the deterministic theory, F = 0.36 (4.32 of 12 after phylogenetic correction, F = 0.36). After phylogenetic correction, only one-thirteenth (33 of 1000) of trees produce F > 0.5, and none of our thousand trees does so significantly.
Thus, reciprocal–cross asymmetry, while pervasive across toad development, is not driven by the deterministic effects of mitonuclear incompatibilities, at least as estimated from relative rates of mitochondrial vs. nuclear evolution. Although theoretical analysis predicted a very weak effect (Turelli and Moyle 2007), Bolnick et al. (2008) found tentative support for the deterministic prediction using molecular data comparable to ours. Specifically, 13 of 18 reciprocal crosses reported by Bolnick et al. (2008) went in the direction expected under the deterministic theory—a significant departure from the null (one-tailed binomial P = 0.048). Given our results, we could dismiss the results of Bolnick et al. (2008) as a chance observation that fell slightly below nominal significance. Although our results are statistically inconsistent with theirs, the discrepancy is relatively slight. For instance, in Figure 3B, only 30 of 74 crosses support the deterministic theory. If we use a χ2 test to compare that with 13 of 18 from Bolnick et al. (2008), we reject homogeneity with P = 0.03. Yet, each data set is consistent with a very small positive signal associated with mitonuclear DMIs. (For instance, if the binomial probability (p) that the dam with δA > 0 produces the less fit F1 is raised to 0.507 instead of 0.5, the probability of seeing as many “concordant” results as Bolnick et al. (2008) becomes P > 0.05. But seeing as few concordant results as we did also has P > 0.05.) Thus, both results could be somewhat unlikely outcomes of a subtle influence of relative mitochondrial-to-nuclear substitution rates on viability in reciprocal crosses. Our negative result cannot be simply attributed to misidentifying the sign of relative mitonuclear rates in cases with subtle differences—even the lineage with the highest relative rate of mitochondrial substitution does not produce particularly poor dams (see Table S3, Table S4, and File S1).
Our analyses can be refined in several ways. Analyses of complete nuclear and mitochondrial genomes could replace approximations of their relative rates of evolution. Alternatively, a clearer signal may be found by considering only nuclear and mitochondrial loci known to interact. Nevertheless, our molecular data are comparable to those of Bolnick et al. (2008), and we analyze many more crosses. Data from additional taxa would be welcome. Rapid advances in sequencing technology will simplify the molecular analyses, but experimental crosses will, unfortunately, remain laborious.
A notable finding of our analysis is a deep discordance between mitochondrial and nuclear topologies. This discordance and numerous other potential cases of mitochondrial introgression (Toews and Brelsford 2012) demonstrate that mitonuclear incompatibilities need not bar interspecific gene flow. In contrast, data mapping hybrid incompatibilities to mitochondria (Fishman and Willis 2006; Rieseberg and Blackman 2010) and physiological arguments (Rand et al. 2004; Gershoni et al. 2009; Chou and Leu 2010; Lane 2011; Burton and Barreto 2012) suggest that mitochondria can play a major role in reproductive isolation. These apparently contradictory results may simply reflect the stochasticity of substitutions and the heterogeneity of effects of mitochondrial introgressions into foreign genetic backgrounds. These effects can range from largely innocuous (or even favorable) to profoundly disruptive.
Acknowledgments
We thank Dan Bolnick, Daniel Matute, Leonie Moyle, and David Rand for their constructive comments. We also thank David Cannatella for permission to use previously unpublished RAG1 sequence data. For the loan of tissue samples or permission to use DNA extractions generated for prior research, we thank David Blackburn and Jens Vindum (California Academy of Sciences), Rafe Brown and Linda Trueb (University of Kansas Biodiversity Institute), Jonathan Campbell (University of Texas, Arlington Collection of Vertebrates), David Cannatella and Travis LaDuc (Texas Natural History Collections, University of Texas, Austin), Jim McGuire (Museum of Vertebrate Zoology, University of California, Berkeley), Alan Resetar (Field Museum of Natural History), and Addison Wynn (United States National Museum). This research was supported by grants from the National Science Foundation: DEB 0815145 to M.T., a National Science Foundation Bioinformatics postdoctoral fellowship to Y.B., and a predoctoral fellowship to M.R.M.; and by National Institutes of Health R01 GM104325 to M.T.
Footnotes
Communicating editor: S. I. Wright
Literature Cited
- Blair W. F., 1972. Evolution in the Genus Bufo. University of Texas Press, Austin, TX [Google Scholar]
- Bolnick D. I., Turelli M., López-Fernández H., Wainwright P. C., Near T. J., 2008. Accelerated mitochondrial evolution and ‘Darwin’s corollary’: asymmetric viability of reciprocal F1 hybrids in centrarchid fishes. Genetics 178: 1037–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton R. S., Barreto F. S., 2012. A disproportionate role for mtDNA in Dobzhansky–Muller incompatibilities? Mol. Ecol. 21: 4942–4957 [DOI] [PubMed] [Google Scholar]
- Burton R. S., Ellison C. K., Harrison J. S., 2006. The sorry state of F2 hybrids: consequences of rapid mitochondrial DNA evolution in allopatric populations. Am. Nat. 168: S14–S24 [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Leu L. Y., 2010. Speciation through cytonuclear incompatibility: insights from yeast and implications for higher eukaryotes. BioEssays 32: 401–411 [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Hung Y. S., Lin K. H., Lee H. Y., Leu J. Y., 2010. Multiple molecular mechanisms cause reproductive isolation between three yeast species. PLoS Biol. 8: e1000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C., 1859. On the Origin of Species. Murray, London [Google Scholar]
- Drummond A. J., Suchard M. A., Xie D., Rambaut A., 2012. Bayesian phylogenetics with BEAUTi and the BEAST 1.7. Mol. Biol. Evol. 29: 1969–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison C. K., Burton R. S., 2008. Interpopulation hybrid breakdown map to the mitochondrial genome. Evolution 62: 631–638 [DOI] [PubMed] [Google Scholar]
- Fishman L., Willis J. H., 2006. A cytonuclear incompatibility causes anther sterility in Mimulus hybrids. Evolution 60: 1372–1381 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick B. M., 2002. Molecular correlates of reproductive isolation. Evolution 56: 191–198 [DOI] [PubMed] [Google Scholar]
- Gershoni M., Templeton A. R., Mishmar D., 2009. Mitochondrial bioenergetics as a major motive force of speciation. BioEssays 31: 642–650 [DOI] [PubMed] [Google Scholar]
- Lane N., 2011. Mitonuclear match: optimizing fitness and fertility over generations drives ageing within generations. BioEssays 33: 860–869 [DOI] [PubMed] [Google Scholar]
- Lee H. Y., Chou J. Y., Cheong L., Chang N. H., Yang S. Y., et al. , 2008. Incompatibility of nuclear and mitochondrial genomes causes hybrid sterility between two yeast species. Cell 135: 1065–1073 [DOI] [PubMed] [Google Scholar]
- Malone J. H., Fontenot B. E., 2008. Patterns of reproductive isolation in toads. PLoS ONE 3: e3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiklejohn C. D., Holmbeck M. A., Siddiq M. A., Abt D. N., Rand D. M., et al. , 2013. An incompatibility between a mitochondrial tRNA and its nuclear-encoded tRNA synthetase compromises development and fitness in Drosophila. PLoS Genet. 9: e1003238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montooth K. L., Meiklejohn C. D., Abt D. N., Rand D. M., 2010. Mitochondrial-nuclear epistasis affects fitness within species but does not contribute to fixed incompatibilities between species of Drosophila. Evolution 64: 3364–3379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr H. A., 1993. A mathematical model of Haldane’s rule. Evolution 47: 1606–1611 [DOI] [PubMed] [Google Scholar]
- Pauly G. B., Hillis D. M., Cannatella D. C., 2009. Taxonomic freedom and the role of official lists of species names. Herpetologica 65: 115–128 [Google Scholar]
- R Development Core Team, 2008 R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
- Rand D. M., Haney R. A., Fry A. J., 2004. Cytonuclear cooperation: the genomics of cooperation. Trends Ecol. Evol. 19: 645–653 [DOI] [PubMed] [Google Scholar]
- Rieseberg L. H., Blackman B. K., 2010. Speciation genes in plants. Ann. Bot. 106: 439–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F., Teslenko M., van der Mark P., Ayres D., Darling A., et al. , 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 54: 401–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toews D. P. L., Brelsford A., 2012. The biogeography of mitochondrial and nuclear discordance in animals. Mol. Ecol. 21: 3907–3930 [DOI] [PubMed] [Google Scholar]
- Turelli M., Moyle L. C., 2007. Asymmetric postmating isolation: Darwin’s corollary to Haldane’s rule. Genetics 176: 1059–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacquier V. D., Swanson W. J., 2011. Selection in the rapid evolution of gamete recognition proteins in marine invertebrates. Cold Spring Harb. Perspect. Biol. 3: a002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Dey S. K., 2006. Roadmap to embryo implantation: clues from mouse models. Nat. Rev. Genet. 7: 185–199 [DOI] [PubMed] [Google Scholar]