Abstract
Summary
Bisphosphonates reduce skeletal loss and fracture risk, but their use has been limited in patients with chronic kidney disease. This study shows skeletal benefits of zoledronic acid in an animal model of chronic kidney disease.
Introduction
Bisphosphonates are routinely used to reduce fractures but limited data exists concerning their efficacy in non-dialysis chronic kidney disease. The goal of this study was to test the hypothesis that zoledronic acid produces similar skeletal effects in normal animals and those with kidney disease.
Methods
At 25 weeks of age, normal rats were treated with a single dose of saline vehicle or 100 µg/kg of zoledronic acid while animals with kidney disease (approximately 30 % of normal kidney function) were treated with vehicle, low dose (20 µg/kg), or high dose (100 µg/kg) zoledronic acid, or calcium gluconate (3 % in the drinking water). Skeletal properties were assessed 5 weeks later using micro-computed tomography, dynamic histomorphometry, and mechanical testing.
Results
Animals with kidney disease had significantly higher trabecular bone remodeling compared to normal animals. Zoledronic acid significantly suppressed remodeling in both normal and diseased animals yet the remodeling response to zoledronic acid was no different in normal and animals with kidney disease. Animals with kidney disease had significantly lower cortical bone biomechanical properties; these were partially normalized by treatment.
Conclusions
Based on these results, we conclude that zoledronic acid produces similar amounts of remodeling suppression in animals with high turnover kidney disease as it does in normal animals, and has positive effects on select biomechanical properties that are similar in normal animals and those with chronic kidney disease.
Keywords: Anti-remodeling agents, Bisphosphonate, Bone mechanics, Remodeling suppression
Introduction
Disturbances in mineral metabolism are common in patients with chronic kidney disease (CKD) and have been classified as their own clinical entity known as CKD-Mineral and Bone Disorder (CKD-MBD) [1]. CKD-MBD begins early in the course of progressive disease, driven by secondary hyperparathyroidism, and results in altered bone remodeling, loss of bone mass, and increased risk of fracture in many patients with late stage (3–5) CKD compared to age-matched non-CKD individuals [2–5]. Unfortunately, there is little data on the prevention or treatment of fractures in this population, as abnormal kidney function and hyperparathyroidism is an exclusion criterion for nearly all clinical trials of osteoporotic therapies used in the general population.
Bisphosphonates have clear efficacy in reducing fracture risk in non-CKD patients [6–8]. There are no trials designed specifically to evaluate the skeletal benefits of bisphosphonates in CKD patients although secondary analyses of trials using risedronate and a large trial of alendronate [9, 10] demonstrated that patients with reduced kidney function still experience improvements in bone mineral density and fracture reduction with bisphosphonates. However, the presence of elevated creatinine or evidence of hyperparathryoidism, common features of CKD-MBD, were exclusion criteria in these studies [9, 10]. As a result, many of these participants had age-related alterations in kidney function as older individuals with low muscle mass may have altered kidney function despite creatinine values in the normal range. Estimating equations for glomerular filtration rates (eGFR) more readily identify such individuals and were used in these published post hoc analyses. These limitations resulted in Kidney Disease Improving Global Outcomes (KDIGO) international clinical practice guidelines recommending bisphosphonate use in patients with CKD stages 1–3 and normal PTH levels, but recommended not using bisphosphonates in patients with CKD stages 3–5 with biochemical evidence hyperparathyroidism unless a bone biopsy was done prior to administration [1].
A variety of animal models are use to study CKD, including surgically and diet induced CKD and/or genetic manipulation. While a number of skeletally targeted interventions have been tested in these models, such as phosphate binders [11, 12] and bone morphogenetic protein [13, 14], there are limited data regarding bisphosphonates [15, 16]. Thus, there is a relative paucity of both animal and clinical data that address the efficacy of bisphosphonates on bone properties in CKD stages 3–4 with hyperparathyroidism. The goal of this study was to determine the skeletal effects of zoledronic acid treatment, including bone mass, remodeling, and mechanical properties, in a model of slowly progressive kidney disease. Specifically, this study aimed to test the hypothesis that bisphosphonates would produce skeletal biomechanical benefits in animals with CKD and that effects on remodeling suppression would be similar in normal and CKD animals.
Methods
Animal model and experimental design
Male Cy/+ rats, a Han:SPRD rat with autosomal dominant polycystic kidney disease, and their non-affected (normal) littermates were used for this study. As shown previously, male heterozygous rats (Cy/+) develop characteristics of CKD (azotemia) around 10 weeks of age and the condition progresses with age [12, 17–19]. We have found that at 20 weeks of age, the urinary creatinine clearance in the Cy/+ rats is 50 % of normal animals, and at that stage the PTH and FGF23 are twice normal, and the 1,25(OH)2D levels are half normal with no difference in blood calcium or phosphate levels. Furthermore, the kidney klotho expression is less than half of normal supporting that CKD-MBD is quite apparent at 20 weeks [12, 17–19]. The secondary hyperparathyroidism progresses with slowly progressive kidney deterioration such that at 34 weeks of age, the animals show evidence of arterial and cardiac calcification, severe osteitis fibrosa cystica, and hyperphosphatemia consistent with all manifestations of CKD-MBD [18].
At 25 weeks of age, animals were assigned to treatment groups. In the CKD (Cy/+) animals, this age represents approximately 30 % of the kidney function of the normal littermates, and was chosen to simulate late stage 3, early stage 4 CKD, a stage at which there is elevated PTH yet normal calcium and phosphorus levels. In humans, bisphosphonates are not approved for clinical use at this level of GFR (and below) due to a lack of clinical studies. Normal animals (n=15) were given a single intraperitoneal dose of saline vehicle (VEH, 0.3 ml) or zoledronic acid (ZOL, 100 µg/kg body weight). CKD animals (n=25) were given a single intraperitoneal dose of VEH or ZOL (either 20 or 100 µg/kg body weight) or administered 3 % calcium gluconate in the drinking water. Both ZOL doses have been shown to be effective in preserving bone volume in ovariectomized rats when given as a single dose [20, 21]. The calcium gluconate group was used as a positive control to simulate calcium administration as a phosphate binder, which in CKD has been shown to reduce bone remodeling [22]. All animals were fed a casein diet (Purina AIN-76A; 0.53 % Ca and 0.56 % P) during the experiment to increase phosphorus availability which has been shown to produce a more consistent kidney disease in this model [18]. Two weeks prior to the end of the study, all animals were given an intraperitoneal injection of calcein (10 mg/kg body weight); a second injection was given 10 days later. At 30 weeks of age all animals were euthanized by an overdose of sodium pentobartibal. The 5-week study duration was chosen as it approximates one remodeling cycle in a rat [23] and is prior to the time when Cy/+ animals begin to become excessively sick from kidney disease complications.
At necropsy, blood and urine were collected by cardiac and bladder puncture, respectively. The paired kidneys were dissected, wet weights obtained, and then embedded in paraffin for qualitative evaluation. The right femur was wrapped in saline-soaked gauze and frozen for imaging and mechanical testing. Left tibiae were placed in 10 % neutral buffered formalin for 48 h and then changed to 70 % ethanol for histological processing. All procedures were reviewed and approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
Computed tomography
Morphological parameters of the proximal tibia were assessed using high-resolution micro-CT (Skyscan 1172). Bones were wrapped in parafilm to prevent drying during the scanning. Scans were obtained using an X-ray source, set at 60 kV and 167 µA over an angular range of 180° (rotational steps of 0.40°) with a 12-µm pixel size. Projection images were reconstructed using standard Skyscan software (NRecon). One millimeter of the proximal tibia (located ~0.5 mm from the growth plate) was analyzed by segmenting the trabecular bone from the cortical shell and calculating Trabecular bone volume per total volume (BV/TV), trabecular thickness (Tb.Th) and trabecular number (Tb.N) in accordance with recommended guidelines [24].
Femurs were scanned using peripheral quantitative computed tomography (pQCT , Norland Stratec XCT Research SA+; Stratec). Prior to scanning, the mid-diaphysis was marked and a single bone slice was imaged at that site using a scanning resolution of 0.07×0.07×0.50 mm. Bone mineral content (BMC, mg/mm), density (BMD, mg/cm3), area (B.Ar, mm2), medial-lateral diameter (MLdia, mm), cortical thickness (Ct.Th, mm) and cross-sectional moment of inertia (CSMI, mm4) were obtained using standard scanner software.
Bone mechanics
Femora were tested via three-point bending using standard methods [25] as previously described for the rat [26]. Femora were thawed to room temperature, presoaked in 0.9 % saline, and placed posterior side down on the bottom support of a servohydraulic test system (Test Resources). All bones were loaded to failure using a displacement rate of 2 mm/min with force versus displacement data collected at 10 Hz. Structural mechanical properties were determined from the load–deformation curves using standard definitions. Material properties at both sites were calculated using standard equations, as previously described [25, 26].
Bone histology
Tibia were embedded in methylmethacrylate as previously described [27]. The proximal tibia metaphysis was thin sectioned (4 µm) and tibia–fibula junction thick sectioned (100 µm; hand ground to ~50 µm) and mounted unstained using non-fluorescent medium. Additional sections of the proximal tibia metaphysis were stained with McNeal’s tetrachrome for osteoid assessment and TRAP for osteoclast assessment.
Sections were analyzed using a microscope interfaced with a semiautomatic analysis system (Bioquant OSTEO 7.20.10, Bioquant Image Analysis Co.). Each animal was assessed for presence of double label at both the proximal tibia and tibia–fibula junction. Presence of double label at either site, inside or outside the histomorphometry region of interest (ROI), suggests the two injections were properly administered. Lack of double label anywhere is interpreted to indicate either mis-injection (due to injection directly into bladder) or sufficiently low turnover rates that result in only one label to be present. All animals that could be confirmed to have been properly administered both calcein labels had single calcein-labeled surface within the cancellous ROI; in these animals we have measured and reported percent single labeled surface. Of the animals that two labels could be observed, all treated with VEH (both NL and CKD) had double label surface within the cancellous ROI, while only a subset of animals treated with ZOL had double label within the ROI. Thus MAR and BFR were considered missing data in several animals from the latter groups in accordance with recent recommendations [28].
For trabecular bone, ROI of approximately 8 mm2 within the secondary spongiosa (~ 0.5 mm from the growth plate) was defined and then measures of single- and double-label perimeter (sL.Pm, dL.Pm), total bone perimeter (B.Pm) and interlabel width (Ir.L.Wi) were conducted. Cortical bone primary measures included periosteal sL.Pm, dL.Pm, B.Pm, as well as Ir.L.Wi. Due to the paucity of label on the endocortical surfaces among all animals, parameters were not assessed on that surface. From these primary measurements, derived parameters were calculated for each surface as: mineralizing surface (MS/BS = [1/2sL.Pm + dL.Pm]/B.Pm; %), mineral apposition rate (MAR = Ir.l.W/ days between labels; µm/day), and bone formation rate (BFR/BS = MAR×MS/BS×3.65; µm3/µm2/year). Osteoid thickness was assessed on the endocortical surface at a location approximately 2 mm below the distal edge of the growth plate on longitudinal sections. This location was chosen at it consistently showed osteoid in all animals. Traditional measures of osteoid surface and volume were not undertaken as such data would not be expected to provide additional information beyond that from the dynamic measurements. Osteoclast surface per bone surface was assessed in a similar ROI as described above for dynamic properties. All parameters were measured and calculated in accordance with recommended standards [29].
Biochemistry
Serum calcium, phosphorus and blood urea nitrogen, and urine microalbumin, and creatinine were determined using colorimetric assay kits (Pointe Scientific, Canton, MI, or Sigma, St. Louis, MO). Serum intact PTH was determined by ELISA (Alpco, SalemNH). Serum FGF23 was measured by ELISA kits (Immunotopics, San Clemente, CA) according to the manufacturer's instruction.
Statistics
All analyses were run using SAS software. All data were compared using a one-way ANOVA with Fisher’s LSD post-hoc tests when appropriate. A p value of ≤0.05 was used to determine statistical significance. Data are presented as mean and standard error.
Results
Animal characteristics and biochemistry
There was no significant difference among groups for body weight at the end of the experiment (Table 1). Kidney weight was significantly higher in the four CKD groups compared to normal VEH-treated animals due to the cystic kidney growth with no effect of ZOL. Serum calcium and phosphorus were not different among the groups. PTH and FGF23 were significantly higher in VEH-treated CKD compared to those in VEH-treated normal rats. The low dose ZOL had no significant effect on the PTH or FGF23, whereas there was a modest decline in PTH but not FGF23 in the high dose ZOL group. There was a marked suppression of PTH and rise in FGF23 in the calcium treatment group compared to VEH-treated CKD. Similar to humans with CKD, there is known variability of PTH in this animal model as evident by the wide range in PTH values. BUN was significantly higher in all CKD groups compared to normal VEH-treated groups again with no effect of ZOL treatment in either genotype or Ca treatment in the CKD genotype. Microalbumin was significantly elevated in the CKD animals compared to normals. It was also higher in the high dose ZOL group compared to CKD-VEH. This latter effect was influenced by two animals which had urinary albumin/creatinine ratios of ug/mg. Qualitative histological analyses of the kidneys (blinded review by V.G.) from these two animals did not reveal any differences from the other high dose ZOL animals nor any vehicle-treated CKD animals.
Table 1.
Body and tissue weights and biochemistry
| Normal |
CKD |
One-way ANOVA |
|||||
|---|---|---|---|---|---|---|---|
| Vehicle | ZOL 100 µg | Vehicle | ZOL 20 µg | ZOL 100 µg | Calcium | ||
| N | 8 | 7 | 6 | 7 | 5 | 6 | - |
| Final body weight, g | 495±13 | 496±13 | 502±8 | 505±8 | 491±5 | 495±10 | 0.94 |
| Kidney weight, g | 3.73±0.08 | 3.61±0.10* | 5.47±0.4# | 5.36±0.21# | 5.51 ±0.46# | 5.23±0.41# | <0.0001 |
| Calcium, mg/dl (range) | 11.1±0.18 (10.5–12.0) | 10.7±0.26 (9.6–11.5) | 10.7±0.33 (9.5–11.9) | 11.4±0.10 (10.9–11.7) | 11.2±0.33 (10.6–11.9) | 11.2±0.4 (9.6–12.5) | 0.34 |
| Phosphorus, mg/dl (range) | 5.1 ±0.21 (4.8–6.4) | 5.1 ±0.48 (4.5–5.8) | 5.4±0.20 (4.6–5.7) | 5.8±0.34 (4.8–7.0) | 6.5±0.70 (5.0–9.0) | 5.8±0.4 (4.5–6.9) | 0.07 |
| PTH, pg/ml (range) | 251±34 (104–382) | 233 ± 16 (174–303) | 853±227# (445–1,870) | 593±95# (238–950) | 558±203* (217–4,662) | 162±69* (22–420) | 0.0009 |
| FGF23, pg/ml (range) | 1,220±238 (866–1,596) | 1,288±276 (1,084–1,821) | 3,594±1,565# (2,235–6,075) | 3,835±1,542# (2,490–6,951) | 3,643± 1,925# (1,960–6,729) | 5,902± 1,382#*‡ (3,193–7,122) | <0.0001 |
| BUN, mg/dl (range) | 23.0±1.7* (12.5–28.0) | 23.6±0.7* (20.9–26.4) | 49.6±4.5# (39.1–65.7) | 51.2±3.4# (32.7–65.4) | 57.5±5.7# (40.6–72.8) | 50.4±4.6# (36.1–68.8) | <0.0001 |
| Microalbumin/creatinine, µg/mg (range) | 7.2±1.7 (0.6–16.6) | 7.91 ±2.0 (0.3–13.2) | 28.9±7.5# (10.6–55.3) | 12.7±2.9 (5.5–26.2) | 62.1 ±22.4#* (6.0–117.2) | 18.0±1.9 (9.7–23.4) | 0.0003 |
Data as mean ± SE
PTH parathyroid hormone, BUN blood urea nitrogen
p<0.05 versus normal vehicle group
p<0.05 versus CKD vehicle
p<0.0001 versus CKD with ZOL
Proximal tibia trabecular bone morphology and remodeling
Trabecular bone volume and trabecular number was significantly higher in CKD animals treated with ZOL or Ca compared to both normal-VEH and CKD-VEH animals (Table 2, Fig. 1). There was no significant difference between the two ZOL doses or between ZOL and Ca groups.
Table 2.
Proximal tibia trabecular bone morphology and remodeling
| Normal |
CKD |
One-way ANOVA |
|||||
|---|---|---|---|---|---|---|---|
| Vehicle | ZOL 100 µg | ANOVA | ZOL 20 µg | ZOL 100 µg | Calcium | ||
| BV/TV (%) | 14.5±1.4 | 18.6±1.2 | 13.9±1.8 | 24.1±1.6#* | 27.5±5.4#* | 24.9±1.0#* | 0.0002 |
| Tb.N (#/mm) | 1.54±0.12 | 2.08±0.09 * | 1.41±0.18 | 2.52±0.16 #* | 2.77±0.44 #* | 2.49±0.08 #* | <0.0001 |
| Tb.Th (µm) | 93 ±3 | 89±3 | 99±2 | 96±1 | 97±6 | 100±2 | 0.14 |
| Single label in animal, no. of animals | 8/8 | 7/7 | 6/6 | 7/7 | 5/5 | 6/6 | |
| Single label surface/bone surface (%) | 23.5±1.5 | 5.3±1.2# | 21.2±2.2 | 13.8±3.0#* | 8.4±2.1#* | 16.4±1.5# | <0.0001 |
| Double label in animal, # of animalsa | 7/8 | 3/7 | 5/6 | 4/7 | 5/5 | 4/6 | |
| Single label in ROI, # of animals | 7/7 | 3/3 | 5/5 | 4/4 | 5/5 | 4/4 | |
| Double label in ROI, # of animals | 7/7 | 0/3 | 5/5 | 0/4 | 2/6 | 4/4 | |
| MAR (µm/day) | 1.47±0.07 | – | 2.28±0.25# | – | 0.66±0.13#* | 1.19±0.12* | 0.0002 |
| BFR/BS (µm3/µm2/year) | 38.3±4.5 | – | 88±12# | – | 4.29±1.1#* | 14.1±2.4#* | <0.0001 |
| Osteoid thickness (µm) | 8.9±0.5 | 7.8±0.6 * | 9.5±0.4 | 7.0±0.5 #* | 7.3±0.5#* | 6.6±0.4 #* | 0.0010 |
| Osteoclast surface/bone surface (%) | 0.57±0.23 | 2.00±0.72 | 1.42±0.54 | 3.83±0.87 #* | 3.30±1.22# | 0.54±0.32 | 0.007 |
Data presented as mean ± SE
BV/TV bone volume per tissue volume, Tb.N trabecular number, Tb.Th trabecular thickness, MAR mineral apposition rate, MS/BS mineralizing surface per bone surface, BFR/BS bone formation rate per bone surface
Double labels were sought throughout three sections of the proximal tibia, both within and outside the measurement region of interest to confirm true administration of both labels, as well as in three sections of the cortical diaphysis on both periosteal and endocortical surfaces
p<0.05 versus normal vehicle group
p<0.05 versus CKD vehicle
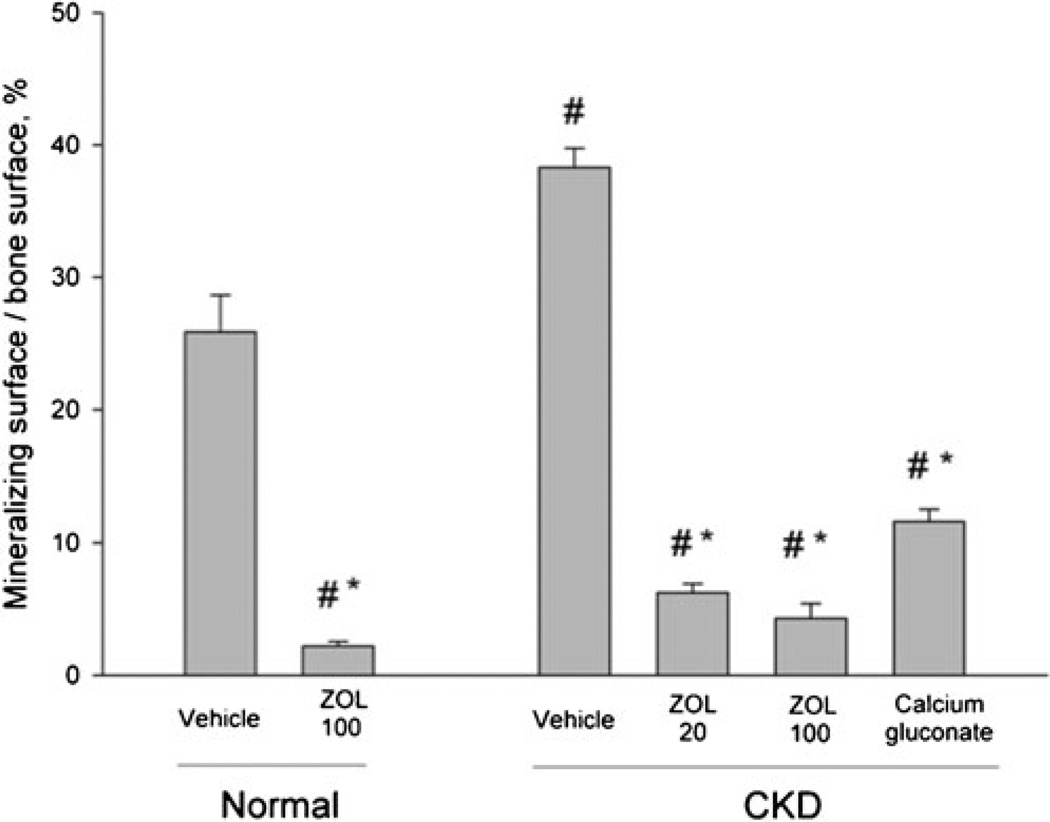
Fig. 1.
Mineralizing surface per bone surface of trabecular bone in the proximal tibia metaphysis. Vehicle-treated animals with chronic kidney disease (CKD) had a significantly higher percent of bone surface undergoing active bone remodeling compared to normal vehicle-treated animals (+48 %). Treatment of normal animals with zoledronic acid (ZOL, 100 µm/kg) has significantly lower mineralizing surface (−92 %) compared to normal vehicle. Both doses of ZOL reduced mineralizing surface in CKD animals (−84 and −88 %) compared to CKD-VEH; Ca treatment reduced values 70 % relative to CKD-vehicle. There was no significant difference between high dose ZOL in normal and CKD animals nor between any treatment in the CKD animals. p<0.05 versus normal-vehicle (#) and CKD-vehicle (*)
All animals that confirmed to have been properly administered both calcein labels had single calcein-labeled surface within the cancellous ROI. All VEH-treated animals (both NL and CKD) had double label surface within the cancellous ROI while only a subset of animals treated with ZOL had double label within the ROI. Compared to normal VEH animals, CKD-VEH animals had significantly higher MS/BS (+48 %; Fig. 1), MAR, and BFR. The low dose ZOL resulted in significantly lower MS/BS compared to both VEH-treated CKD (−84 %) and VEH-treated normal (−76 %); the high dose ZOL also had lower MS/BS (−88 % versus CKD and −83 % versus normal). Calcium gluconate treatment resulted in lower MS/BS (−70 % versus CKD and −57 % versus normal). There was no significant difference in MS/BS between the two ZOL doses, or between either dose of ZOL and Ca treatments. High dose ZOL resulted in lower MS/BS (−92 %) in normal animals compared to their VEH-treated controls. Percent single labeled surfaced revealed effects of ZOL consistent with MS/BS. Osteoid thickness was not significantly different between vehicle treated groups; ZOL and Ca each significantly reduced thickness in CKD animals compared to the VEH controls. Osteoclast surface was non-significantly higher (+150 %) in CKD-VEH compared to normal.
There were no significant differences among groups for dynamic bone formation properties on the periosteal surface of the tibia–fibula junction.
Cortical density, geometry, mechanical properties, and bone formation
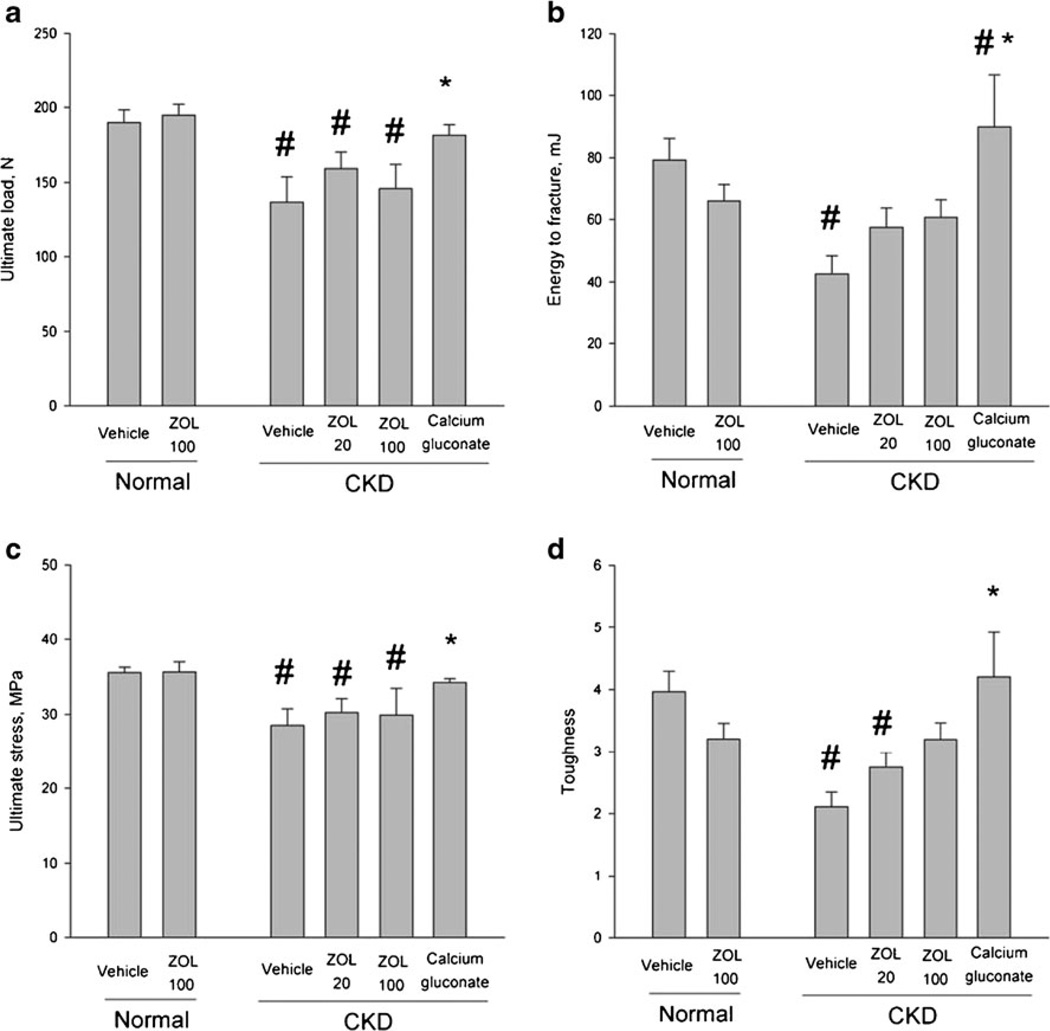
Femoral diaphysis structural mechanical properties were significantly altered by CKD (Table 3, Fig. 2). Vehicle-treated CKD animals had significantly lower ultimate load (−28 %), stiffness (−17 %) and energy to fracture (−46 %) compared to normal VEH-treated animals. Low and high dose ZOL in CKD animals each produced intermediate structural mechanical properties, with ultimate load and energy to fracture values between normal- and CKD-VEH groups. Ca treatment of CKD animals resulted in ultimate load and energy absorption values significantly higher than CKD-VEH and similar to normal vehicle.
Table 3.
Cortical bone density, geometry, mechanical properties
| Normal |
CKD |
1way ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Vehicle | ZOL 100 µg | Vehicle | ZOL 20 µg | ZOL 100 µg | Calcium | ||
| BMC (mg/mm) | 11.9±0.3 | 12.4±0.18 | 10.9±0.6# | 12.0±0.20 * | 11.7±0.12 | 12.2±0.3 * | 0.04 |
| BMD (mg/cm3) | 1469±1.4 | 1472±2 | 1477±3 | 1474±3 | 1459±12 | 1474±5 | 0.18 |
| Bone area (mm2) | 8.13±0.19 | 8.39±0.12 | 7.36±0.4 # | 8.16±0.13 * | 8.05±0.09 * | 8.26±0.20 * | 0.03 |
| Bone diameter (mm) | 3.6±0.05 | 3.58±0.04 | 3.25±0.15# | 3.43±0.05 | 3.50±0.05 * | 3.39±0.05 # | 0.01 |
| Cortical thickness (mm) | 0.82±0.01 | 0.85±0.01 | 0.79±0.01 | 0.86±0.01 #* | 0.86±0.01 #* | 0.88±0.01 #* | <0.0001 |
| CSMIp (mm4) | 21.7±1.0 | 22.1 ±0.7 | 18.1 ±2.2# | 20.5±1.1 | 19.3±0.55 | 20.2±1.0 | 0.23 |
| Stiffness (N/mm) | 442±14 | 442±22 | 366±40 # | 393 ±20 | 351±31# | 424±11 | 0.04 |
| Modulus (MPa) | 312±8 | 304±10 | 322±29 | 294±13 | 278±30 | 321±8 | 0.50 |
| Single label on periosteal surface (n) | 7/7 | 4/4 | 6/6 | 5/5 | 5/5 | 4/4 | – |
| Double label on periosteal surface (n) | 7/7 | 4/4 | 5/6 | 4/5 | 5/5 | 4/4 | – |
| Periosteal MAR (µm/day) | 1.28±0.11 | 1.35±0.11 | 1.49±0.18 | 1.39±0.09 | 1.57±0.09 | 1.54±0.09 | 0.43 |
| Periosteal MS/BS (%) | 58±6 | 64±3 | 65±12 | 59±6 | 70±8 | 56±12 | 0.89 |
| Periosteal BFR/BS (µm3/µm2/year) | 74±11 | 86±8 | 118±24 | 89±13 | 110±16 | 90±22 | 0.38 |
Data presented as mean ± SE
BMC bone mineral content, BMD bone mineral density, CSMIp polar cross-sectional moment of inertia, MAR mineral apposition rate, MS/BS mineralizing surface per bone surface, BFR bone formation rate
p<0.05 versus normal vehicle group
p<0.05 versus CKD vehicle
Fig. 2.
Biomechanical and geometric properties of mid-femoral diaphysis cortical bone. Whole bone structural biomechanical properties (a, b), material-level biomechanical properties (c, d). Material-level properties are calculated by adjusting structural mechanical properties for differences in bone size and reflect an estimate of the properties of the bone tissue itself regardless of how much is present or how it is distributed. Overall ANOVA p values were significant for all variables (a, p=0.0035; b, p=0.0096; c, p=0.019; d, p=0.0032). CKD chronic kidney disease, ZOL zoledronate. p<0.05 versus normal-vehicle (#) and CKD-vehicle (*)
Mid-femoral cortical BMC (−8 %), bone area (−9 %), and bone diameter (−10 %) were all significantly lower in CKD-VEH animals compared to normal VEH animals. These properties were normalized by all three treatments in CKD animals.
Material-level biomechanical properties were adversely affected by CKD. Vehicle-treated CKD animals had significantly lower ultimate stress (−20 %) and toughness (−47 %) compared to normal VEH-treated animals. Modulus, the material stiffness, was not significantly different among the groups. Low dose ZOL and high dose ZOL treatment in CKD animals resulted in intermediate toughness values between normal- and CKD-VEH groups. Ca treatment resulted in ultimate stress values that were significantly higher than CKD-VEH and not different from normal animals.
Discussion
The current study demonstrates two important finding regarding bisphosphonates in the setting of CKD. First, CKD animals treated with zoledronic acid experience levels of remodeling suppression similar to those of non-CKD animals and similar to calcium ingestion. Second, we show a significant mechanical benefit of zoledronic acid in CKD animals.
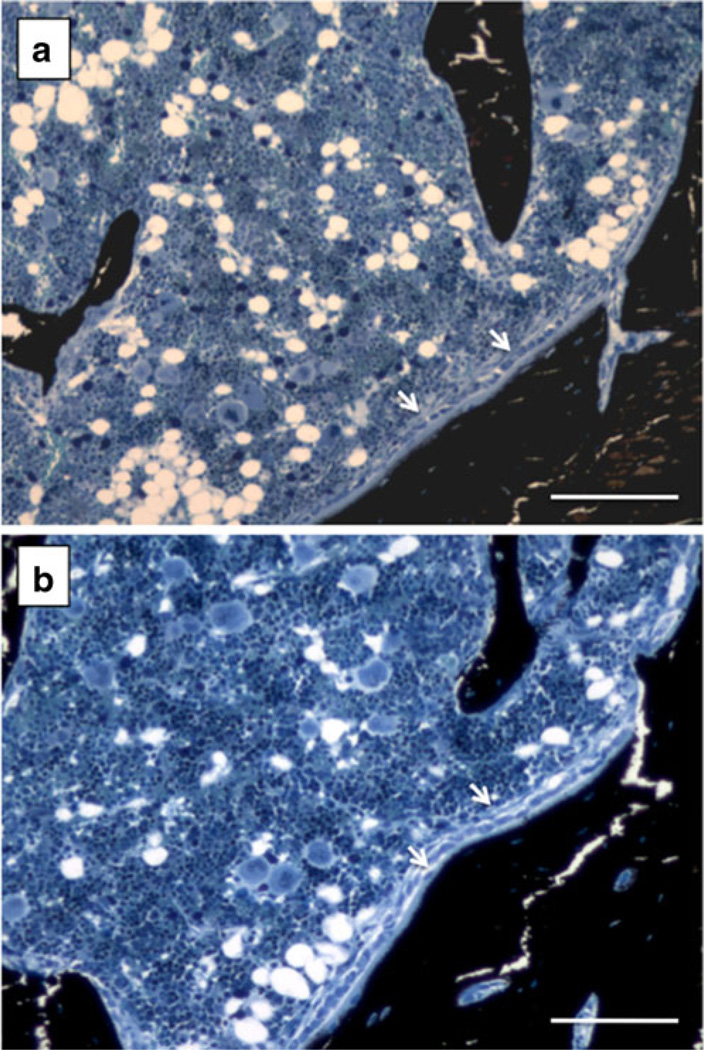
Using dynamic histomorphometry, we show that animals with progressive CKD and an elevated trabecular bone remodeling rate experience zoledronic acid-induced remodeling suppression that is comparable to animals with normal kidney function (−88 % and 92 %, respectively). Despite these significant levels of suppression, some remodeling still exists in the trabecular bone of the proximal tibia. These data are consistent with previous work using pamidronate in a model of adenine-induced CKD [16] and suggests that in situations of high turnover CKD disease bisphosphonates are not associated with adynamic bone disease. In this context it is important to differentiate low bone remodeling from adynamic bone disease. Historically the term adynamic bone disease, along with aplastic bone disease (defined as the inability to form bone), was used to describe the histological phenotype of patients with aluminum toxicity in which there was no formation (and few to no cells) on trabecular surfaces [30–33]. More recent work has shown patients with various conditions, including CKD, which meet this definition of adynamic/aplastic bone disease, as they have no cellular activity. A number of papers in the nephrology literature define and use the term adynamic bone disease to mean low (not absent) bone remodeling [34, 35]. This can cause confusion, as most individuals on anti-remodeling treatment such as bisphosphonates, in patients without CKD, would thus be classified as having adynamic bone disease given that bisphosphonates suppress remodeling by 70–90 % compared to controls. The remodeling suppression by bisphosphonates in post-menopausal women is not generally considered to be adynamic bone disease [36]. Thus our data, as well as others [16], show that bisphosphonate treatment results in low remodeling, but not adynamic bone, in the setting of high-turnover CKD given that bone cells are present and functional (Fig. 3). Given the number of animals in which double labels were not observed (even in VEH animals) the sample size of these analyses were low and thus future work is warranted to confirm the lack of adynamic bone in this animal model. Certainly, it is necessary to determine if these levels of remodeling suppression are stable with longer duration treatment (using multiple doses of ZOL) and if these results are applicable to situations where bone remodeling is low (such as occurs in some CKD patients) prior to bisphosphonates treatment. It is also important to note that recent associations between potent remodeling suppression and rare side effects such as osteonecrosis of the jaw and atypical femoral fractures raise questions about the long-term safety of very low remodeling rates, even if adynamic bone is not present.
Fig. 3.
Photomicrographs depicting active osteoid seams in both untreated normal (a) and high dose zoledronate-treated CKD animal (b). Images were chosen from an animal in each of these two groups that lacked double label. The presence of osteoid and active osteoblasts (arrows), based on the cuboidal nature of the cells atop the osteoid, in animals that lack double label supports the conclusions that these animals do not have adynamic bone disease. Scale bar = 100 µm
There are limited histomorphometry data from humans with compromised kidney function describing bisphosphonate effects. In one study, patients having undergone renal transplantation were treated with pamidronate, calcitriol and calcium carbonate or calcitriol and calcium carbonate alone [37]. Pre-transplantation and 6 month post-biopsies of the iliac crest were taken. The authors concluded that all patients treated with pamidronate developed adynamic disease although three of the six patients had measurable activation frequency, all had measurable osteoid, and five of the six patients had measurable osteoclast surface. Of the eight patients not treated with pamidronate, five of seven patients had measurable activation frequency, all had measurable osteoid, and only 50 % had measurable osteoclasts. Thus, with the presence of cellular activity, others would not have concluded that adynamic bone was induced but rather than bone remodeling was lowered. These results highlight the need for consensus as to the definition of adynamic bone disease and an understanding of the clinical consequences of suppression of remodeling (as seen in our study and in humans without CKD treated with bisphosphonates) compared to the consequences of low remodeling and acellular bone. More importantly, they highlight that the effects of bisphosphonates on dynamic properties necessitate much more research to understand the cellular changes to bone surfaces.
Our model of CKD represents a high-turnover disease, illustrated by both high dynamic bone turnover rate and the trend toward higher osteoclast surface (+150 %) in untreated CKD animals compared to normals. Higher osteoclast surface is consistent with higher TRAP gene expression in femora of CKD animals compared to normals [12]. Also consistent with previous literature, although counterintuitive, is that treatment with ZOL was associated with higher osteoclast surface compared to untreated animals. As bisphosphonate treatment renders osteoclasts inactive, it is not uncommon to find equal or even higher amounts of osteoclast surface compared to untreated controls [38, 39]. Bisphosphonate-induced increases in osteoclast surface have also been shown in other CKD models[16].
Bisphosphonates are excreted unmetabolized by the kidney and have been shown to have adverse effects on the kidney tissue including acute tubular necrosis and glomerular changes (summarized in [40]). This has led to concern that patients with decreased renal function could experience renal toxicity, as the drugs would remain in the system for longer periods of time and therefore circulate through the kidney for longer and at higher concentrations. Two animals treated with high dose ZOL had microalbumin/creatine ratios >100 µg/mg although the review of kidney pathology did not reveal any evidence of acute tubular necrosis or changes in glomerular pathology compared to the other animals in this same group. There was no difference in kidney function (BUN) or kidney size in those CKD or normal animals treated with ZOL versus VEH. We cannot exclude the possibility that repeat dosing over longer duration treatment could produce more dramatic kidney effects, although the doses used in the current study were significantly higher than what would be used clinically and nearly all reports of bisphosphonate renal toxicity were acute changes. Nonetheless, future studies in humans should clearly evaluate this potential adverse effect.
CKD patients have an increased risk of fracture compared to an age-matched population [3–5]. Our animal model of progressive CKD is consistent with this clinical observation as we show a clear and significant mechanical phenotype. Vehicle-treated CKD animals had significantly lower bone strength (ultimate load), stiffness, and energy absorption (energy to fracture) of the femoral diaphysis compared to normal animals. In general, reductions in both ultimate load and energy absorption are consistent with more easily fractured bone [25]. Whole bone mechanical properties are determined by several factors including the amount of bone, its distribution, and its material properties (properties of the tissue independent of amount) [25, 41]. pQCT analyses of the cortical diaphysis revealed that CKD animals have smaller bones (lower BMC, bone area, diameter, thickness, CSMI) compared to normal animals. These differences, which could be due to a number of factors including alterations in normal growth, do not completely explain the structural phenotype as calculation of the material-level biomechanical properties show that both ultimate stress and toughness are significantly lower in CKD animals compared to normal. This points to a deficit in the material properties within the bone as the predominant mechanism of the reduced mechanical properties in CKD. Deficits in material-level properties shown here are in line with recent work in a separate animal model of CKD [42] which quantified differences in collagen cross linking of CKD animals compared to normal. Interestingly, collagen cross-linking changes have the most dramatic effects on bone toughness [43, 44], a parameter that was significantly affected in our model.
Treatment with zoledronic acid in our CKD model resulted in a mechanical phenotype intermediate to normal and CKD vehicle animals. At the lower dose of zoledronic acid, energy to fracture, a structural mechanical property, was not significantly higher than CKD-vehicle animals, but it was also not significantly lower than normal vehicle animals. Previous work with pamidronate presented limited mechanical data yet showed nonsignificantly higher ultimate load compared to non-treated animals [16]. Material-level toughness remained significantly lower than normal vehicle animals. The pattern of mechanical properties was similar with the higher dose of zoledronic acid with the exception of toughness, which was also not significantly higher than CKD-vehicle animals nor significantly lower than normal vehicle. The benefits to whole bone mechanical properties with zoledronic acid appear to be driven by both improvements in the bone structure and material properties. The mechanisms underlying improvements in material properties are not entirely clear as the majority of data on bisphosphonates treatment and collagen cross-linking would suggest an increase in cross-links [45, 46], which would tend to reduce toughness, not improve toughness as is noted in this study. Despite additional work being needed to understand the mechanism underlying the finding, our results clearly depict a positive trajectory for the mechanical profile of CKD-affected bone with zoledronic acid treatment.
In contrast to the acute hypocalcemic effects of bisphosphonates in patients with normal kidney function, no hypocalcemia was noted in the current study. There was a modest but significant decrease in the PTH with the high dose zoledronic acid treatment that is not readily explained, as there was no difference in calcium, phosphorus, or FGF23 in this treatment groups. In contrast, the animals treated with calcium gluconate had a more marked suppression in PTH and an increase in FGF23. The latter is presumably due to a decrease in 1,25 (OH)2D levels, but we were unable to measure levels in the current study to confirm/refute this hypothesis. However, 1,25 (OH)2D levels were very low in this model at 20 weeks [9], and thus would be expected to be very low in the current study as well. We cannot rule out a direct effect of calcium on FGF23 secretion. Taken together, zoledronic acid had very little effect on biochemical parameters of CKD-MBD whereas calcium gluconate decreased PTH and increased FGF23.
CKD animals treated with calcium gluconate had both structural and material biomechanical properties that were comparable to vehicle-treated normal animals. As the amount of bone remodeling suppression was similar in both ZOL- and Ca-treated CKD groups, this suggests that the benefits to biomechanical properties were independent of bone remodeling. Of the data we collected the one factor that potentially explains this result is serum PTH, as values in Ca-treated animals were 80 % lower than those in ZOL-treated animals and comparable to those of normal animals. This leads us to speculate that levels of serum PTH affect bone mechanical properties independent of effects on remodeling. Alternatively, there may be specific effects of calcium gluconate on bone mechanical properties that we are not able to directly tease out without a normal group treated with calcium. Previous studies in this animal model have demonstrated that the administration of calcium gluconate leads to cardiac and aorta calcification [47], limiting the safety of calcium as a therapeutic treatment. In contrast, in animal models [15], and in a small study in dialysis patients [48], bisphosphonates reduce vascular calcification.
As noted throughout, there are limitations to our study design that need to be kept in mind. Our study assessed properties after 5 weeks, equivalent to one remodeling cycle in a rat. This would translate to roughly 4–6 months in the human and therefore the conclusions of the current work cannot speak to long-term safety for either bone or kidney. Our animal model is also one of high turnover disease. Whether or not the results shown here would be similar in a low turnover CKD setting are unclear. Sample sizes for some analyses (histomorphometry, mechanical testing) were low and thus, despite statistical significance for many of them, future work will be necessary to confirm these outcomes in a larger sample set. Finally, although rats are the accepted FDA rodent model for anti-osteoporotic drug development and have shown results with bisphosphonates that have translated to humans, rats have continual longitudinal bone growth that could influence the results of this study.
Bisphosphonates are highly effective in reducing bone skeletal deterioration in non-CKD patients yet their use in CKD patients has been limited. In secondary analyses of post-menopausal osteoporosis patients, there was an improvement in bone mineral density and a reduction of fractures in patients with CKD stages 3 and 4 treated with bisphosphonates. As the patients in these studies had age-related decline in kidney disease (with normal PTH levels) [9, 10] the results may not be generalizable to those with CKD-MBD. Extrapolating the results from our current study would suggest a potential for efficacy of bisphosphonates in those with intrinsic disease (stage 3–4 CKD with elevated PTH). However, this must be tested in prospective human clinical fracture prevention trials so that long term risk to bone (such as atypical fractures) and kidney function can be assessed. In addition, bisphosphonates must be tested in CKD patients with low PTH and presumed low turnover disease at baseline. Such trials with bisphosphonates are not beyond possibility, as demonstrated by a recent study in which stage 3–4 CKD patents were treated for 18 months with alendronate in a clinical trial focused on vascular calcification [49].
In conclusion, we have documented that zoledronic acid produces similar amounts of remodeling suppression in the setting of high bone turnover chronic kidney disease as it does in conditions of normal kidney function. Furthermore, we show a clear mechanical phenotype in untreated animals with CKD that can be partially attenuated with a single dose of zoledronic acid or with calcium gluconate treatment, although the mechanisms appear to differ.
Acknowledgements
This work was supported by National Institutes of Health grant (AR058005) to SMM.
Footnotes
There are no disclosures to report related to this work.
Conflicts of interest None.
Contributor Information
M. R. Allen, Department of Anatomy and Cell Biology, MS 5035, Indiana, University School of Medicine, 635 Barnhill Drive, Indianapolis, IN 46202, USA, matallen@iupui.edu
N. X. Chen, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA
V. H. Gattone, II, Department of Anatomy and Cell Biology, MS 5035, Indiana, University School of Medicine, 635 Barnhill Drive, Indianapolis, IN 46202, USA.
X. Chen, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA
A. J. Carr, Department of Anatomy and Cell Biology, MS 5035, Indiana, University School of Medicine, 635 Barnhill Drive, Indianapolis, IN 46202, USA
P. LeBlanc, Department of Anatomy and Cell Biology, MS 5035, Indiana, University School of Medicine, 635 Barnhill Drive, Indianapolis, IN 46202, USA
D. Brown, Department of Anatomy and Cell Biology, MS 5035, Indiana, University School of Medicine, 635 Barnhill Drive, Indianapolis, IN 46202, USA
S. M. Moe, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA Roudebush VA Medical Center, Indianapolis, IN, USA.
References
- 1.Moe S, Dreke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 2.Sprague SM. The role of the bone biopsy in the diagnosis of renal osteodystrophy. Semi Dial. 2000;13:152–155. doi: 10.1046/j.1525-139x.2000.00042.x. [DOI] [PubMed] [Google Scholar]
- 3.Ball AM, Gillen DL, Sherrard D, Weiss NS, Emerson SS, Seliger SL, Kestenbaum BR, Stehman-Breen C. Risk of hip fracture among dialysis and renal transplant recipients. JAMA. 2002;288:3014. doi: 10.1001/jama.288.23.3014. [DOI] [PubMed] [Google Scholar]
- 4.Dooley AC, Weiss NS, Kestenbaum B. Increased risk of hip fracture among men with CKD. Am J Kidney Dis. 2008;51:38–44. doi: 10.1053/j.ajkd.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Jamal SA, West SL, Miller PD. Fracture risk assessment in patients with chronic kidney disease. Osteoporos Int. 2011;23:1191–1198. doi: 10.1007/s00198-011-1781-0. [DOI] [PubMed] [Google Scholar]
- 6.Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures — results from the fracture intervention trial. JAMA. 1998;280:2077–2082. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 7.Black DM, Cummings SR, Karpf DB, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 8.Reginster JY, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int. 2000;11:83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- 9.Miller PD, Roux C, Boonen S, Barton IP, Dunlap LE, Burgio DE. Safety and efficacy of risedronate in patients with age related reduced renal function as estimated by the cockcroft and gault method: a pooled analysis of nine clinical trials. J Bone Miner Res. 2005;20:2105–2115. doi: 10.1359/JBMR.050817. [DOI] [PubMed] [Google Scholar]
- 10.Jamal SA, Bauer DC, Ensrud KE, Cauley JA, Hochberg M, Ishani A, Cummings SR. Alendronate treatment in women with normal to severely impaired renal function: an analysis of the fracture intervention trial. J Bone Miner Res. 2007;22:503–508. doi: 10.1359/jbmr.070112. [DOI] [PubMed] [Google Scholar]
- 11.Matthew S, Lund RJ, Strebeck F, Tustison K, Gueurs T, Hruska K. Reversal of the adynamic bone bisorder and decreased vascular calcification in chronic kidney disease by sevelamer carbonate therapy. J Am Soc Nephrol. 2007;18:122–130. doi: 10.1681/ASN.2006050490. [DOI] [PubMed] [Google Scholar]
- 12.Moe S, Radcliffe J, White K, Gattone V, II, Seifert M, Chen X, Aldridge B, Chen N. The pathophysiology of early stage chronic kidney disease-mineral bone disorder (CKD-MBD) and response to phosphate binders in the rat. J Bone Miner Res. 2011;26:2672–2681. doi: 10.1002/jbmr.485. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez E, Lund R, Martin K, Mccartney J, Tondravi M, Sampath T, Hruska K. Treatment of a murine model of high-turnover renal osteodystrophy by exogenous BMP-7. Kidney Int. 2002;61:1322–1331. doi: 10.1046/j.1523-1755.2002.00258.x. [DOI] [PubMed] [Google Scholar]
- 14.Lund RJ, Davies M, Brown A, Hruska K. Successful treatment of an adynamic bone disorder with bone morphogenetic protein-7 in a renal ablation model. J Am Soc Nephrol. 2004;15:359–369. doi: 10.1097/01.asn.0000109671.99498.08. [DOI] [PubMed] [Google Scholar]
- 15.Lomashvili KA, Monier-Faugere M-C, Wang X, Malluche HH, O'Neill WC. Effect of bisphosphonates on vascular calcification and bone metabolism in experimental renal failure. Kidney Int. 2009;75:617–625. doi: 10.1038/ki.2008.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jokihaara J, Pörsti IH, Kööbi P, et al. Treatment of experimental renal osteodystrophy with pamidronate. Kidney Int. 2008;74:319–327. doi: 10.1038/ki.2008.180. [DOI] [PubMed] [Google Scholar]
- 17.Cowley B, Gudapaty S, Kraybill AL, Barash BD, Harding MA, Calvet JP, Gattone V. Autosomal-dominant polycystic kidney disease in the rat. Kidney Int. 1993;43:522–522. doi: 10.1038/ki.1993.79. [DOI] [PubMed] [Google Scholar]
- 18.Moe SM, Chen NX, Seifert MF, Sinders RM, Duan D, Chen X, Liang Y, Radcliff JS, White KE, Gattone VH. A rat model of chronic kidney disease-mineral bone disorder. Kidney Int. 2009;75:176–184. doi: 10.1038/ki.2008.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowley B. Modification of disease progression in rats with inherited polycystic kidney disease. Am J Kidney Dis. 1996;27:865–879. doi: 10.1016/s0272-6386(96)90525-9. [DOI] [PubMed] [Google Scholar]
- 20.Gasser JA, Ingold P, Venturiere A, Shen V, Green JR. Long term protective effects of zoledronic acid on cancellous and cortical bone in the ovariectomized rat. J Bone Miner Res. 2008;23:544–551. doi: 10.1359/jbmr.071207. [DOI] [PubMed] [Google Scholar]
- 21.Brouwers JEM, van Rietbergen B, Bouxsein ML. Influence of early and late zoledronic acid administration on vertebral structure and strength in ovariectomized rats. Calcif Tissue Int. 2008;83:186–191. doi: 10.1007/s00223-008-9160-3. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira A, Frazao JM, Monier-Faugere MC, Gil C, Galvao J, Oliveira C, Baldaia J, Rodrigues I, Santos C, Ribeiro S. Effects of sevelamer hydrochloride and calcium carbonate on renal osteodystrophy in hemodialysis patients. J Am Soc Nephrol. 2008;19:405. doi: 10.1681/ASN.2006101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Jee W, Ke H, Mori S. Age-related changes of cancellous and cortical bone histomorphometry in female Sprague-Dawley rats. Cells Mater. 1991;1:25–25. [Google Scholar]
- 24.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone micro-structure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 25.Turner CH, Burr DB. Basic biomechanical measurements of bone — a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs RK, Allen MR, Condon KW, Reinwald S, Miller LM, McClenathan D, Keck B, Phipps RJ, Burr DB. Strontium ranelate does not stimulate bone formation in ovariectomized rats. Osteoporos Int. 2008;19:1331–1341. doi: 10.1007/s00198-008-0602-6. [DOI] [PubMed] [Google Scholar]
- 27.Allen MR, Iwata K, Phipps R, Burr DB. Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone. 2006;39:872–879. doi: 10.1016/j.bone.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 28.Recker RR, Kimmel DB, Dempster D, Weinstein RS, Wronski TJ, Burr DB. Issues in modern bone histomorphometry. Bone. 2011;49:955–964. doi: 10.1016/j.bone.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry - standaridization of nomenclature, symbols, and units. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 30.Spasovski G. Bone biopsy as a diagnostic tool in the assessment of renal osteodystrophy. Int J Artif Organs. 2004;27:918. doi: 10.1177/039139880402701103. [DOI] [PubMed] [Google Scholar]
- 31.Malluche HH, Faugere MC. Atlas of mineralized bone histology. Basel, Switzerland: S. Karger Pub; 1986. [Google Scholar]
- 32.Andress DL, Maloney NA, Endres DB, Sherrard DJ. Aluminum associated bone disease in chronic renal failure: high prevalence in a long term dialysis population. J Bone Miner Res. 1986;1:391–398. doi: 10.1002/jbmr.5650010503. [DOI] [PubMed] [Google Scholar]
- 33.De Vernejoul M, Belenguer R, Halkidou H, Buisine A, Bielakoff J, Miravet L. Histomorphometric evidence of deleterious effect of aluminum on osteoblasts. Bone. 1985;6:15–20. doi: 10.1016/8756-3282(85)90401-6. [DOI] [PubMed] [Google Scholar]
- 34.Heaf J. Causes and consequences of adynamic bone disease. Nephron. 2000;88:97–106. doi: 10.1159/000045968. [DOI] [PubMed] [Google Scholar]
- 35.Brandenburg VM, Floege J. Adynamic bone disease: bone and beyond. NDT Plus. 2008;1:135–147. doi: 10.1093/ndtplus/sfn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Recker RR, Delmas PD, Halse J, et al. Effects of intravenous zoledronic acid once yearly on bone remodeling and bone structure. J Bone Miner Res. 2008;23:6–16. doi: 10.1359/jbmr.070906. [DOI] [PubMed] [Google Scholar]
- 37.Coco M. Prevention of bone loss in renal transplant recipients: a prospective, randomized trial of intravenous pamidronate. J Am Soc Nephrol. 2003;14:2669–2676. doi: 10.1097/01.asn.0000087092.53894.80. [DOI] [PubMed] [Google Scholar]
- 38.Smith SY, Recker RR, Hannan M, Muller R, Bauss F. Intermittent intravenous administration of the bisphosphonate ibandronate prevents bone loss and maintains bone strength and quality in ovariectomized cynomolgus monkeys. Bone. 2003;32:45–55. doi: 10.1016/s8756-3282(02)00923-7. [DOI] [PubMed] [Google Scholar]
- 39.Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med. 2009;360:53–62. doi: 10.1056/NEJMoa0802633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller PD. The kidney and bisphosphonates. Bone. 2011;49:77–81. doi: 10.1016/j.bone.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 41.Seeman E, Delmas PD. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 42.Iwasaki Y, Kazama JJ, Yamato H, Fukagawa M. Changes in chemical composition of cortical bone associated with bone fragility in rat model with chronic kidney disease. Bone. 2011;48:1260–1267. doi: 10.1016/j.bone.2011.03.672. [DOI] [PubMed] [Google Scholar]
- 43.Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001;28:195–201. doi: 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Shen X, Li X, Agrawal CM. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31:1–7. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- 45.Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int. 2009;20:887–894. doi: 10.1007/s00198-008-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allen MR, Gineyts E, Leeming DJ, Burr DB, Delmas PD. Bisphosphonates alter trabecular bone collagen cross-linking and isomerization in beagle dog vertebra. Osteoporos Int. 2008;19:329–337. doi: 10.1007/s00198-007-0533-7. [DOI] [PubMed] [Google Scholar]
- 47.Moe SM, Seifert MF, Chen NX, Sinders RM, Chen X, Duan D, Henley C, Martin D, Gattone VH. R-568 reduces ectopic calcification in a rat model of chronic kidney disease-mineral bone disorder (CKD-MBD) Nephrol Dial Transplant. 2009;24:2371. doi: 10.1093/ndt/gfp078. [DOI] [PubMed] [Google Scholar]
- 48.Nitta K, Akiba T, Suzuki K, Uchida K, Watanabe RI, Majima K, Aoki T, Nihei H. Effects of cyclic intermittent etidronate therapy on coronary artery calcification in patients receiving long-term hemodialysis. Am J Kidney Dis. 2004;44:680–688. [PubMed] [Google Scholar]
- 49.Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG. Effect of alendronate on vascular calcification in CKD Stages 3 and 4: a pilot randomized controlled trial. Am J Kidney Dis. 2010;56:57–68. doi: 10.1053/j.ajkd.2009.12.039. [DOI] [PubMed] [Google Scholar]