Introduction
Gastrin is the primary hormone that induces gastric acid secretion (Edkins 1906). In humans, the gene encoding for gastrin is located on chromosome 17q21 (Lund et al. 1986). This hormone is produced by the G-cells of the antrum of stomach as preprogastrin, which comprises of 101 amino acids and is cleaved between Ala-21 and Ser-22 to yield progastrin (Reeve et al. 1984). Progastrin is then sequentially cleaved by prohormone convertase and carboxypeptidase E to yield glycine-extended gastrins- a 35-amino acid gastrin-34-Gly (G34-Gly) or an 18-amino acid gastrin-17-Gly (G17-Gly) in endocrine cells (Varro et al. 1995; Lacourse et al. 1997). G34-Gly and G17-Gly are amidated at their carboxyl terminal groups by the enzyme peptidyl alpha-amidating mono-oxygenase to form amidated gastrin.
Gastrin interacts with the membrane-bound G-protein coupled cholecystokinin receptor group consisting of Cholecystokinin A receptor (CCKAR) and Cholecystokinin B receptor (CCKBR). CCKAR possesses high affinity for cholecystokinin and has a negligible affinity for gastrin (Ferrand and Wang 2006; Dufresne et al. 2006). However, CCKBR has a high affinity for gastrin and their carboxyl amidated analogues. CCKBR is expressed in the brain, smooth muscle cells, and parietal cells (Kopin et al. 1992; Berna et al. 2007). In addition to this, gastrin has also been reported to interact with annexin 2, and thereby exerts proliferation effects in gastrointestinal cancers (Singh et al. 2007; Singh 2007; Sarkar et al. 2012).
The release of gastrin is induced by gastrin-releasing peptide, a neurotransmitter which acts on its basolateral receptor in the G-cells. Binding of gastrin to CCKBR present in parietal and enterochromaffin-like (ECL) cells (Schmitz et al. 2001; Kulaksiz et al. 2000)induces gastric acid secretion by parietal cells as well as histamine release by ECL cells (Dockray et al. 2005). The released histamine reaches parietal cells by paracrine diffusion where it binds H2 receptors and induces gastric acid secretion. The secretion of gastric acid inhibits the release of gastrin hormone rendering a negative feedback control, further preventing excess acid secretion. In addition, low pH value in the stomach inhibits gastrin release through stimulating somatostatin secretion by antral D-cells (Bloom et al. 1974).
Gastrin is a well-known growth factor for the gastrointestinal tract. Gastrin stimulates proliferation of gastric mucosal cells (Hansen et al. 1976), maturation of parietal cells and enterochromaffin-like cells (Jain and Samuelson 2006), and promotes islet differentiation in the pancreas (Wang et al. 1993). It has also been shown to stimulate proliferation of gastric (Ishizuka et al. 1992) and colon cancer cells (Watson et al. 1989). Gastrin modulates invasion (Wroblewski et al. 2002), apoptosis (Przemeck et al. 2008; Todisco et al. 2001) and migration (Noble et al. 2003) in epithelial cells. Both glycine-extended gastrin and amidated gastrin have been reported to induce angiogenesis (Clarke et al. 2006; Lefranc et al. 2004). Moreover, the hypergastrinemic state has been associated with diverse physiological disorders in humans and other mammals. These include atrophic gastritis (Lehy et al. 2000), pernicious anemia (Orlando et al. 2007), excess acid secretion leading to duodenal ulcer disease in Helicobacter pylori infection (Berna et al. 2006; Jensen 2002; Scarpignato et al. 1996) and gastrinoma (Kloppel and Anlauf 2007).
Recently, a number of research groups have explored the feasibility of using gastrin to treat various diseases. Gastrin has been used in combination therapy with epidermal growth factor to increase beta-cell mass which reversed hyperglycemia in diabetic mice (Suarez-Pinzon et al. 2005). Gastrin-stimulated beta cell neogenesis when used in combination with glucagon-like peptide 1 in human pancreatic duct cell transplanted in immunodeficient diabetic mice (Suarez-Pinzon et al. 2008). Gastrins and their receptors have been suggested as potential targets to treat gastrointestinal and pancreatic cancers (Rengifo-Cam and Singh 2004). Gastrin has also been shown to have therapeutic promise as it inhibits the growth of cholangiocarcinoma cells by promoting apoptosis (Kanno et al. 2001).
On account of the functional significance of different natural forms of gastrin, we have assembled signaling pathway reactions induced by them upon binding to gastrin receptor(s) in different human cell types. These manually curated signaling events are made available through NetPath (http://www.netpath.org/) (Kandasamy et al. 2010) in different formats for analysis by the scientific community.
Material and methods
Annotation of gastrin signaling events
A literature survey was performed using PubMed to retrieve articles related to gastrin signaling, using the search term ‘Gastrin’. Signaling events observed under the stimulation of various gastrins including progastrin, glycine-extended gastrins and amidated gastrins were identified from literature. These events were categorized into protein-protein interactions (PPIs), enzyme-catalyzed events, site-specific post-translational modifications (PTMs), changes in protein localization across subcellular compartments and plasma membrane, activation/inhibition events with respect to their activity and gene regulation events. For manual curation of these events, we considered the NetPath annotation criteria as previously described for the series of NetPath pathways (Raju et al. 2011a; Soman et al. 2013). We used the software PathBuilder, developed by our group, to manually document the signaling events (Kandasamy et al. 2009). Information pertaining to protein–protein interactions, enzyme-catalyzed reactions, translocation events, and the activation/inhibition reactions of protein was captured from articles studying human and other mammalian cell/cell lines. The genes that were reported to be regulated by gastrins in different human cell types were also documented. Transcription factors/co-activators or repressors of gastrin regulated genes identified specific to gastrin signaling were also included. Each reaction annotated in gastrin signaling pathway has been linked to the respective research article from which the data was extracted or curated. We have also provided a textual description for each reaction specifying the cell lines and the form(s) of gastrin used for the simulation.
Visualization of gastrin signaling pathway
A pictorial representation of the pathway reactions was drawn using PathVisio (van Iersel et al. 2008). The reactions induced by gastrins were topologically arranged from the receptors to the specific transcriptionally regulated genes and their functional role using the information obtained by inhibition, activation, silencing and/or mutation studies. The molecules regulated by multiple signaling modules such as ERK1/2, PI3K/AKT or p38MAPK were also represented. Considering the complexity in visualization of the gastrin signaling map, we also represented a simplified version of the map as described previously (Raju et al. 2011a, b).
Results and discussion
We screened over 20,000 research articles from PubMed related to gastrin/gastrin signaling and identified molecules and signaling events reported to be induced by different forms of gastrin from 550 research articles. From these articles, we documented 97 unique proteins that were experimentally identified to be participant in one or more of the 18 PPI’s, 60 catalysis events or PTM’s, 17 protein translocation events, 24 activation, and 1 inhibition reactions. We have also provided 46 genes (37 upregulated and 9 down regulated genes) identified to be transcriptionally regulated by gastrin(s) in different human cell types.
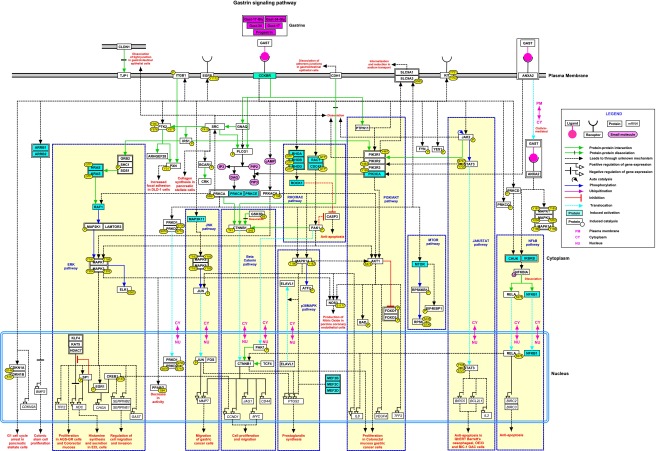
The gastrin pathway data can be accessed at NetPath (http://www.netpath.org/pathways?path_id=NetPath_154). A pictorial representation of the complete set of curated reactions in gastrin signaling pathway is shown in Fig. 1. The NetSlim version of the map and a detailed description of the gastrin induced signaling events are available at http://www.netpath.org/netslim/Gastrin_pathway.html. This version of the gastrin signaling pathway represents 76 molecules involved in 97 reactions based on the NetSlim criteria (Raju et al. 2011b). An interactive version of this map can be obtained by clicking on “map with citations”. Each node (protein) is linked to their corresponding NetPath molecule page; and the edges (relationship among proteins) and the PTMs are linked to their respective PubMed identifiers.
Fig. 1.
Schematic representation of the gastrin signaling pathway: this map represents the reactions of the gastrin signaling pathway present in NetPath. The different types of reactions are color coded as described in the legend. The signaling pathways are also distinguished by colored boxes
The Gastrin pathway page in NetPath provides information about the pathway, the molecules participating in the pathway and the statistics for the number of molecules and reactions annotated for this pathway. Each molecule has been linked to a molecule page in NetPath which provides a brief description about the molecule. Through the molecule page, every protein captured in NetPath has also been linked to other protein-centric resources including Human Protein Reference Database (HPRD) (Prasad et al. 2009a, b), Entrez gene (Maglott et al. 2011), Swiss-Prot (Boeckmann et al. 2003) and OMIM (Hamosh et al. 2005). The gastrin signaling pathway data can be downloaded from both NetPath and NetSlim in various standard data exchange formats such as BioPAX level 3.0 (Demir et al. 2010), PSI-MI version 2.5 (Hermjakob et al. 2004) and SBML level 2.1 (Hucka et al. 2003). The gene regulation data is available in Microsoft Excel and tab-delimited formats. The pathway maps can be downloaded for customization and analysis in .gpml and .GenMAPP formats from NetSlim.
Conclusions
Gastrin is known to play an important role in gastrointestinal disorders and carcinogenesis. Channeling research on gastrin signaling could provide novel insights into diagnostic, prognostic and therapeutic strategies in gastrointestinal cancers and other disorders. Information on gastrin signaling and the pathway map designed in this study are available in different standard formats compatible with multiple pathway analysis software. Thus, the gastrin pathway data organized in this study will serve as a template for gene set enrichment and pathway analysis of data from multi-omics platforms related to these disorders.
Acknowledgements
We thank the Department of Biotechnology, Government of India for research support to the Institute of Bioinformatics, Bangalore. YS is a recipient of Senior Research Fellowship from University Grants Commission (UGC), Government of India.
Conflict of interest
No potential conflict of interest declared.
Abbreviations
- CCKAR
Cholecystokinin A receptor
- CCKBR
Cholecystokinin B receptor
- ECL
Enterochromaffin-like
- PPI
Protein-protein interaction
- PTM
Post-translational modification
- HPRD
Human protein reference database
- SBML
Systems biology markup language
- PSI-MI
Proteomics standards initiative for molecular interaction
- BioPax
Biological pathway exchange
Footnotes
Yashwanth Subbannayya and Kumari Anuja contributed equally.
Contributor Information
Yashwanth Subbannayya, Email: yashwanth@ibioinformatics.org.
Kumari Anuja, Email: anuja.sharma192@gmail.com.
Jayshree Advani, Email: jayshree@ibioinformatics.org.
Urmesh Kumar Ojha, Email: urmesh@ibioinformatics.org.
Vishalakshi Nanjappa, Email: vishalakshi@ibioinformatics.org.
Bijesh George, Email: bijesh@ibioinformatics.org.
Avinash Sonawane, Email: asonawane@kiitbiotech.ac.in.
Rekha V. Kumar, Email: rekha_v_kumar@yahoo.co.in
Girija Ramaswamy, Email: girijaramaswamy@yahoo.co.in.
Akhilesh Pandey, Email: pandey@jhmi.edu.
B. L. Somani, Email: somani@ibioinformatics.org
Rajesh Raju, Phone: +91-080-28416140, Email: rajesh@ibioinformatics.org.
References
- Berna MJ, Hoffmann KM, Serrano J, Gibril F, Jensen RT. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore) 2006;85:295–330. doi: 10.1097/01.md.0000236956.74128.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berna MJ, Tapia JA, Sancho V, Jensen RT. Progress in developing cholecystokinin (CCK)/gastrin receptor ligands that have therapeutic potential. Curr Opin Pharmacol. 2007;7:583–592. doi: 10.1016/j.coph.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom SR, Mortimer CH, Thorner MO, Besser GM, Hall R, Gomez-Pan A, Roy VM, Russell RC, Coy DH, Kastin AJ, Schally AV. Inhibition of gastrin and gastric-acid secretion by growth-hormone release-inhibiting hormone. Lancet. 1974;2:1106–1109. doi: 10.1016/S0140-6736(74)90869-1. [DOI] [PubMed] [Google Scholar]
- Boeckmann B, Bairoch A, Apweiler R, Blatter MC, Estreicher A, Gasteiger E, Martin MJ, Michoud K, O’Donovan C, Phan I, Pilbout S, Schneider M. The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Res. 2003;31:365–370. doi: 10.1093/nar/gkg095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PA, Dickson JH, Harris JC, Grabowska A, Watson SA. Gastrin enhances the angiogenic potential of endothelial cells via modulation of heparin-binding epidermal-like growth factor. Cancer Res. 2006;66:3504–3512. doi: 10.1158/0008-5472.CAN-05-0280. [DOI] [PubMed] [Google Scholar]
- Demir E, Cary MP, Paley S, Fukuda K, Lemer C, Vastrik I, Wu G, D’Eustachio P, Schaefer C, Luciano J, Schacherer F, Martinez-Flores I, Hu Z, Jimenez-Jacinto V, Joshi-Tope G, Kandasamy K, Lopez-Fuentes AC, Mi H, Pichler E, Rodchenkov I, Splendiani A, Tkachev S, Zucker J, Gopinath G, Rajasimha H, Ramakrishnan R, Shah I, Syed M, Anwar N, Babur O, Blinov M, Brauner E, Corwin D, Donaldson S, Gibbons F, Goldberg R, Hornbeck P, Luna A, Murray-Rust P, Neumann E, Ruebenacker O, Samwald M, van Iersel M, Wimalaratne S, Allen K, Braun B, Whirl-Carrillo M, Cheung KH, Dahlquist K, Finney A, Gillespie M, Glass E, Gong L, Haw R, Honig M, Hubaut O, Kane D, Krupa S, Kutmon M, Leonard J, Marks D, Merberg D, Petri V, Pico A, Ravenscroft D, Ren L, Shah N, Sunshine M, Tang R, Whaley R, Letovksy S, Buetow KH, Rzhetsky A, Schachter V, Sobral BS, Dogrusoz U, McWeeney S, Aladjem M, Birney E, Collado-Vides J, Goto S, Hucka M, Le Novere N, Maltsev N, Pandey A, Thomas P, Wingender E, Karp PD, Sander C, Bader GD. The BioPAX community standard for pathway data sharing. Nat Biotechnol. 2010;28:935–942. doi: 10.1038/nbt.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray G, Dimaline R, Varro A. Gastrin: old hormone, new functions. Pflugers Arch. 2005;449:344–355. doi: 10.1007/s00424-004-1347-5. [DOI] [PubMed] [Google Scholar]
- Dufresne M, Seva C, Fourmy D. Cholecystokinin and gastrin receptors. Physiol Rev. 2006;86:805–847. doi: 10.1152/physrev.00014.2005. [DOI] [PubMed] [Google Scholar]
- Edkins JS. The chemical mechanism of gastric secretion. J Physiol. 1906;34:133–144. doi: 10.1113/jphysiol.1906.sp001146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrand A, Wang TC. Gastrin and cancer: a review. Cancer Lett. 2006;238:15–29. doi: 10.1016/j.canlet.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Hamosh A, Scott AF, Amberger JS, Bocchini CA, McKusick VA. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005;33:D514–D517. doi: 10.1093/nar/gki033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen OH, Pedersen T, Larsen JK, Rehfeld JF. Effect of gastrin on gastric mucosal cell proliferation in man. Gut. 1976;17:536–541. doi: 10.1136/gut.17.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermjakob H, Montecchi-Palazzi L, Bader G, Wojcik J, Salwinski L, Ceol A, Moore S, Orchard S, Sarkans U, von Mering C, Roechert B, Poux S, Jung E, Mersch H, Kersey P, Lappe M, Li Y, Zeng R, Rana D, Nikolski M, Husi H, Brun C, Shanker K, Grant SG, Sander C, Bork P, Zhu W, Pandey A, Brazma A, Jacq B, Vidal M, Sherman D, Legrain P, Cesareni G, Xenarios I, Eisenberg D, Steipe B, Hogue C, Apweiler R. The HUPO PSI’s molecular interaction format–a community standard for the representation of protein interaction data. Nat Biotechnol. 2004;22:177–183. doi: 10.1038/nbt926. [DOI] [PubMed] [Google Scholar]
- Hucka M, Finney A, Sauro HM, Bolouri H, Doyle JC, Kitano H, Arkin AP, Bornstein BJ, Bray D, Cornish-Bowden A, Cuellar AA, Dronov S, Gilles ED, Ginkel M, Gor V, Goryanin II, Hedley WJ, Hodgman TC, Hofmeyr JH, Hunter PJ, Juty NS, Kasberger JL, Kremling A, Kummer U, Le Novere N, Loew LM, Lucio D, Mendes P, Minch E, Mjolsness ED, Nakayama Y, Nelson MR, Nielsen PF, Sakurada T, Schaff JC, Shapiro BE, Shimizu TS, Spence HD, Stelling J, Takahashi K, Tomita M, Wagner J, Wang J. The systems biology markup language (SBML): a medium for representation and exchange of biochemical network models. Bioinformatics. 2003;19:524–531. doi: 10.1093/bioinformatics/btg015. [DOI] [PubMed] [Google Scholar]
- Ishizuka J, Martinez J, Townsend CM, Jr, Thompson JC. The effect of gastrin on growth of human stomach cancer cells. Ann Surg. 1992;215:528–534. doi: 10.1097/00000658-199205000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RN, Samuelson LC. Differentiation of the gastric mucosa. II. Role of gastrin in gastric epithelial cell proliferation and maturation. Am J Physiol Gastrointest Liver Physiol. 2006;291:G762–G765. doi: 10.1152/ajpgi.00172.2006. [DOI] [PubMed] [Google Scholar]
- Jensen RT. Involvement of cholecystokinin/gastrin-related peptides and their receptors in clinical gastrointestinal disorders. Pharmacol Toxicol. 2002;91:333–350. doi: 10.1034/j.1600-0773.2002.910611.x. [DOI] [PubMed] [Google Scholar]
- Kandasamy K, Keerthikumar S, Raju R, Keshava Prasad TS, Ramachandra YL, Mohan S, Pandey A. PathBuilder–open source software for annotating and developing pathway resources. Bioinformatics. 2009;25:2860–2862. doi: 10.1093/bioinformatics/btp453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy K, Mohan SS, Raju R, Keerthikumar S, Kumar GS, Venugopal AK, Telikicherla D, Navarro JD, Mathivanan S, Pecquet C, Gollapudi SK, Tattikota SG, Mohan S, Padhukasahasram H, Subbannayya Y, Goel R, Jacob HK, Zhong J, Sekhar R, Nanjappa V, Balakrishnan L, Subbaiah R, Ramachandra YL, Rahiman BA, Prasad TS, Lin JX, Houtman JC, Desiderio S, Renauld JC, Constantinescu SN, Ohara O, Hirano T, Kubo M, Singh S, Khatri P, Draghici S, Bader GD, Sander C, Leonard WJ, Pandey A. NetPath: a public resource of curated signal transduction pathways. Genome Biol. 2010;11:R3. doi: 10.1186/gb-2010-11-1-r3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno N, Glaser S, Chowdhury U, Phinizy JL, Baiocchi L, Francis H, LeSage G, Alpini G. Gastrin inhibits cholangiocarcinoma growth through increased apoptosis by activation of Ca2 + -dependent protein kinase C-alpha. J Hepatol. 2001;34:284–291. doi: 10.1016/S0168-8278(00)00025-8. [DOI] [PubMed] [Google Scholar]
- Kloppel G, Anlauf M. Gastrinoma–morphological aspects. Wien Klin Wochenschr. 2007;119:579–584. doi: 10.1007/s00508-007-0885-1. [DOI] [PubMed] [Google Scholar]
- Kopin AS, Lee YM, McBride EW, Miller LJ, Lu M, Lin HY, Kolakowski LF, Jr, Beinborn M. Expression cloning and characterization of the canine parietal cell gastrin receptor. Proc Natl Acad Sci U S A. 1992;89:3605–3609. doi: 10.1073/pnas.89.8.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulaksiz H, Arnold R, Goke B, Maronde E, Meyer M, Fahrenholz F, Forssmann WG, Eissele R. Expression and cell-specific localization of the cholecystokinin B/gastrin receptor in the human stomach. Cell Tissue Res. 2000;299:289–298. doi: 10.1007/s004410050027. [DOI] [PubMed] [Google Scholar]
- Lacourse KA, Friis-Hansen L, Rehfeld JF, Samuelson LC. Disturbed progastrin processing in carboxypeptidase E-deficient fat mice. FEBS Lett. 1997;416:45–50. doi: 10.1016/S0014-5793(97)01164-2. [DOI] [PubMed] [Google Scholar]
- Lefranc F, Mijatovic T, Mathieu V, Rorive S, Decaestecker C, Debeir O, Brotchi J, Van Ham P, Salmon I, Kiss R. Characterization of gastrin-induced proangiogenic effects in vivo in orthotopic U373 experimental human glioblastomas and in vitro in human umbilical vein endothelial cells. Clin Cancer Res. 2004;10:8250–8265. doi: 10.1158/1078-0432.CCR-04-0343. [DOI] [PubMed] [Google Scholar]
- Lehy T, Roucayrol AM, Mignon M. Histomorphological characteristics of gastric mucosa in patients with Zollinger-Ellison syndrome or autoimmune gastric atrophy: role of gastrin and atrophying gastritis. Microsc Res Tech. 2000;48:327–338. doi: 10.1002/(SICI)1097-0029(20000315)48:6<327::AID-JEMT3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Lund T, Geurts van Kessel AH, Haun S, Dixon JE. The genes for human gastrin and cholecystokinin are located on different chromosomes. Hum Genet. 1986;73:77–80. doi: 10.1007/BF00292669. [DOI] [PubMed] [Google Scholar]
- Maglott D, Ostell J, Pruitt KD, Tatusova T. Entrez Gene: gene-centered information at NCBI. Nucleic Acids Res. 2011;39:D52–D57. doi: 10.1093/nar/gkq1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble PJ, Wilde G, White MR, Pennington SR, Dockray GJ, Varro A. Stimulation of gastrin-CCKB receptor promotes migration of gastric AGS cells via multiple paracrine pathways. Am J Physiol Gastrointest Liver Physiol. 2003;284:G75–G84. doi: 10.1152/ajpgi.00300.2002. [DOI] [PubMed] [Google Scholar]
- Orlando LA, Lenard L, Orlando RC. Chronic hypergastrinemia: causes and consequences. Dig Dis Sci. 2007;52:2482–2489. doi: 10.1007/s10620-006-9419-3. [DOI] [PubMed] [Google Scholar]
- Prasad TSK, Goel R, Kandasamy K, Keerthikumar S, Kumar S, Mathivanan S, Telikicherla D, Raju R, Shafreen B, Venugopal A, Balakrishnan L, Marimuthu A, Banerjee S, Somanathan DS, Sebastian A, Rani S, Ray S, Harrys Kishore CJ, Kanth S, Ahmed M, Kashyap MK, Mohmood R, Ramachandra YL, Krishna V, Rahiman BA, Mohan S, Ranganathan P, Ramabadran S, Chaerkady R, Pandey A. Human protein reference database–2009 update. Nucleic Acids Res. 2009;37:D767–D772. doi: 10.1093/nar/gkn892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad TSK, Kandasamy K, Pandey A. Human protein reference database and human proteinpedia as discovery tools for systems biology. Methods Mol Biol. 2009;577:67–79. doi: 10.1007/978-1-60761-232-2_6. [DOI] [PubMed] [Google Scholar]
- Przemeck SM, Varro A, Berry D, Steele I, Wang TC, Dockray GJ, Pritchard DM. Hypergastrinemia increases gastric epithelial susceptibility to apoptosis. Regul Pept. 2008;146:147–156. doi: 10.1016/j.regpep.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Raju R, Balakrishnan L, Nanjappa V, Bhattacharjee M, Getnet D, Muthusamy B, Kurian Thomas J, Sharma J, Rahiman BA, Harsha HC, Shankar S, Prasad TS, Mohan SS, Bader GD, Wani MR, Pandey A (2011a) A comprehensive manually curated reaction map of RANKL/RANK-signaling pathway. Database (Oxford) 2011:bar021 [DOI] [PMC free article] [PubMed]
- Raju R, Nanjappa V, Balakrishnan L, Radhakrishnan A, Thomas JK, Sharma J, Tian M, Palapetta SM, Subbannayya T, Sekhar NR, Muthusamy B, Goel R, Subbannayya Y, Telikicherla D, Bhattacharjee M, Pinto SM, Syed N, Srikanth MS, Sathe GJ, Ahmad S, Chavan SN, Kumar GS, Marimuthu A, Prasad TS, Harsha HC, Rahiman BA, Ohara O, Bader GD, Sujatha Mohan S, Schiemann WP, Pandey A (2011b) NetSlim: high-confidence curated signaling maps. Database (Oxford) 2011:bar032 [DOI] [PMC free article] [PubMed]
- Reeve JR, Jr, Walsh JH, Tompkins RK, Hawke D, Shively JE. Amino terminal fragments of human progastrin from gastrinoma. Biochem Biophys Res Commun. 1984;123:404–409. doi: 10.1016/0006-291X(84)90428-5. [DOI] [PubMed] [Google Scholar]
- Rengifo-Cam W, Singh P. Role of progastrins and gastrins and their receptors in GI and pancreatic cancers: targets for treatment. Curr Pharm Des. 2004;10:2345–2358. doi: 10.2174/1381612043383999. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Kantara C, Singh P. Clathrin mediates endocytosis of progastrin and activates MAPKs: role of cell surface annexin A2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G712–G722. doi: 10.1152/ajpgi.00406.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpignato C, Kisfalvi I, D’Amato M, Varga G. Effect of dexloxiglumide and spiroglumide, two new CCK-receptor antagonists, on gastric emptying and secretion in the rat: evaluation of their receptor selectivity in vivo. Aliment Pharmacol Ther. 1996;10:411–419. doi: 10.1111/j.0953-0673.1996.00411.x. [DOI] [PubMed] [Google Scholar]
- Schmitz F, Goke MN, Otte JM, Schrader H, Reimann B, Kruse ML, Siegel EG, Peters J, Herzig KH, Folsch UR, Schmidt WE. Cellular expression of CCK-A and CCK-B/gastrin receptors in human gastric mucosa. Regul Pept. 2001;102:101–110. doi: 10.1016/S0167-0115(01)00307-X. [DOI] [PubMed] [Google Scholar]
- Singh P. Role of Annexin-II in GI cancers: interaction with gastrins/progastrins. Cancer Lett. 2007;252:19–35. doi: 10.1016/j.canlet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Wu H, Clark C, Owlia A. Annexin II binds progastrin and gastrin-like peptides, and mediates growth factor effects of autocrine and exogenous gastrins on colon cancer and intestinal epithelial cells. Oncogene. 2007;26:425–440. doi: 10.1038/sj.onc.1209798. [DOI] [PubMed] [Google Scholar]
- Soman S, Raju R, Sandhya VK, Advani J, Khan AA, Harsha HC, Prasad TS, Sudhakaran PR, Pandey A, Adishesha PK. A multicellular signal transduction network of AGE/RAGE signaling. J Cell Commun Signal. 2013;7:19–23. doi: 10.1007/s12079-012-0181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Pinzon WL, Lakey JR, Rabinovitch A. Combination therapy with glucagon-like peptide-1 and gastrin induces beta-cell neogenesis from pancreatic duct cells in human islets transplanted in immunodeficient diabetic mice. Cell Transplant. 2008;17:631–640. doi: 10.3727/096368908786092775. [DOI] [PubMed] [Google Scholar]
- Suarez-Pinzon WL, Yan Y, Power R, Brand SJ, Rabinovitch A. Combination therapy with epidermal growth factor and gastrin increases beta-cell mass and reverses hyperglycemia in diabetic NOD mice. Diabetes. 2005;54:2596–2601. doi: 10.2337/diabetes.54.9.2596. [DOI] [PubMed] [Google Scholar]
- Todisco A, Ramamoorthy S, Witham T, Pausawasdi N, Srinivasan S, Dickinson CJ, Askari FK, Krametter D. Molecular mechanisms for the antiapoptotic action of gastrin. Am J Physiol Gastrointest Liver Physiol. 2001;280:G298–G307. doi: 10.1152/ajpgi.2001.280.2.G298. [DOI] [PubMed] [Google Scholar]
- van Iersel MP, Kelder T, Pico AR, Hanspers K, Coort S, Conklin BR, Evelo C. Presenting and exploring biological pathways with PathVisio. BMC Bioinforma. 2008;9:399. doi: 10.1186/1471-2105-9-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varro A, Voronina S, Dockray GJ. Pathways of processing of the gastrin precursor in rat antral mucosa. J Clin Invest. 1995;95:1642–1649. doi: 10.1172/JCI117839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TC, Bonner-Weir S, Oates PS, Chulak M, Simon B, Merlino GT, Schmidt EV, Brand SJ. Pancreatic gastrin stimulates islet differentiation of transforming growth factor alpha-induced ductular precursor cells. J Clin Invest. 1993;92:1349–1356. doi: 10.1172/JCI116708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S, Durrant L, Morris D. Gastrin: growth enhancing effects on human gastric and colonic tumour cells. Br J Cancer. 1989;59:554–558. doi: 10.1038/bjc.1989.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroblewski LE, Pritchard DM, Carter S, Varro A. Gastrin-stimulated gastric epithelial cell invasion: the role and mechanism of increased matrix metalloproteinase 9 expression. Biochem J. 2002;365:873–879. doi: 10.1042/BJ20020068. [DOI] [PMC free article] [PubMed] [Google Scholar]