Abstract
CCN proteins play crucial roles in cell motility, matrix turnover, and proliferation. In particular, CCN5 plays a role in cell motility and proliferation in several cell types; however, no functional binding proteins for CCN5 have been identified. In this study we report that CCN5 binds to the cell surface receptor integrin αvβ3 in vascular smooth muscle cells. Furthermore, this interaction takes place in podosomes, organelles known to degrade matrix and mediate motility. We show that CCN5 regulates the ability of podosomes to degrade matrix, but does not affect podosome formation. The level of CCN5 present in a podosome negatively correlates with its ability to degrade matrix. Conversely, knockdown of CCN5 greatly enhances the matrix-degrading ability of podosomes. These findings suggest that the antimotility effects of CCN5 may be mediated through the direct interaction of CCN5 and integrin αvβ3 in podosomes and the concomitant suppression of matrix degradation that is required for cell migration.
Keywords: CCN5, WISP-2, Integrin alpha v beta 3, Podosomes, Extracellular matrix, Matricellular proteins
Introduction
CCN5 is a member of the Cysteine-rich 61/Connective tissue growth factor/Nephroblastoma-overexpressed (CCN) family of genes (Perbal 2005). The six members of this family are matricellular proteins that have important functions in numerous cell and physiologic processes, including embryonic development, cell motility and proliferation, angiogenesis, and extracellular matrix remodeling (Perbal 2005; Leask & Abraham 2006; Perbal 2004; Rachfal & Brigstock 2005). CCN proteins consist of discrete domains that share identity with functional domains of other regulatory molecules. A prototypical CCN protein contains four functional domains: (i) an insulin- like growth factor binding protein-like module (IGFBP); (ii) a von Willebrand factor type C repeat module (VWC); (iii) a thrombospondin type-1 repeat module (TSP-1); and (iv) a carboxy terminal domain (CT). CCN5, also known as WISP-2 (Pennica et al. 1998), rCop-1(Zhang et al. 1998), COP-1(Delmolino et al. 2001), and CTGF-L (Kumar et al. 1999), is highly conserved among vertebrates and is the only CCN protein lacking the C-terminal domain (Gray MR 2005).
Originally discovered as a heparin-induced gene in vascular smooth muscle cells (VSMC), CCN5 behaves as a growth-arrest-specific gene in this cell type (Delmolino et al. 2001; Lake et al. 2003). CCN5 expression decreases in uterine fibroids and in the arterial wall after balloon angioplasty, and when over-expressed in in vivo models of fibroids and vascular injury, it significantly reduced SMC proliferation (Delmolino et al. 2001; Lake et al. 2003; Mason et al. 2004a). Cell culture studies demonstrate that CCN5 over-expression inhibits SMC proliferation and motility in vitro, whereas CCN5 knock-down via siRNA inhibits proliferation and increases basal locomotion (Lake et al. 2003; Mason et al. 2004a; Lake & Castellot 2003).
CCN proteins have been shown to mediate many of their activities through interactions with the integrin class of cell adhesion receptors (Lau & Lam 2005). The first documented interaction of integrins with CCN proteins is the direct binding of CCN1 to integrin αvβ3 to mediate endothelial cell adhesion (Kireeva et al. 1998). Since then, many studies have demonstrated the potential interaction of at least seven other integrins as signaling receptors for a diverse array of CCN-mediated functions including adhesion, migration, cell survival, and DNA synthesis (Chen & Lau 2009). Functional binding proteins and receptors for CCN5, however, have remained elusive. CCN5 has been shown to compete with fibrinogen for binding to integrin αvβ3 (Kumar et al. 1999), but no direct evidence of binding to integrin αvβ3 has been demonstrated. Like all CCN proteins, CCN5 lacks the prototypical integrin recognition sequence Arg-Gly-Asp (RGD), but it does contain a stretch of amino acids that is very similar to the integrin αvβ3 binding site in CCN1 (Chen et al. 2004) (Fig. 1).
Fig. 1.
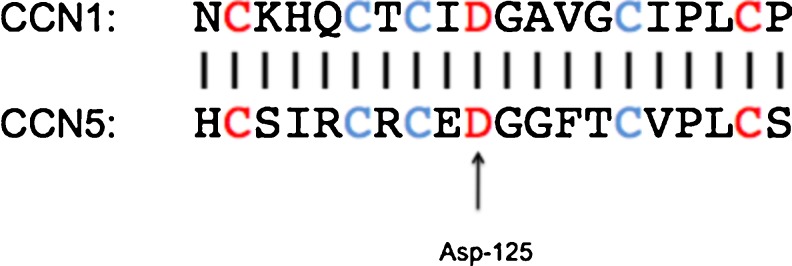
CCN5 contains region analogous to CCN1 Integrin αvβ3 binding domain. The 20 amino acid sequence (corresponding to amino acids 116–135 of the mouse CCN1) in the Von Willebrand factor type C domain of CCN1, as shown by (Chen et al. 2004) is critical for CCN1 binding to integrin αvβ3. CCN5 has an analogous region in amino acids 116–135 that contains Asp-125 as well flanking Cys residues (shown in red), which are necessary for binding of CCN1 to integrin αvβ3 as well as other conserved Cys residues (shown in blue) enhance binding affinity
Based on these considerations, we hypothesized that CCN5 interacts with integrin αvβ3, and that this interaction mediates, at least in part, the ability of CCN5 to inhibit cell proliferation and migration. In the present study, we show that CCN5 and integrin αvβ3 co-immunoprecipitate and co-localize in podosomes, cellular structures that degrade matrix and regulate cell motility and adhesion. Furthermore, we show that CCN5 strongly down-regulates the matrix-degrading activity of podosomes.
Materials and methods
Cell culture
Vascular smooth muscle cells (VSMC) were obtained from aortae of Sprague–Dawley rats (Charles River Breeding Laboratories, Inc. Wilmington MA). They were isolated, cultured, and characterized as previously described (Castellot et al. 1982). Briefly, the abdominal segment of the aorta was removed and the fascia cleaned away under a dissecting microscope. The aorta was cut longitudinally, and small pieces of the media were carefully stripped from the vessel wall. Two or three such strips were placed in 60 mm dishes. Within 1–2 weeks, VSMC migrated from the explants; they were capable of being passaged approximately 1 week after the first appearance of cells. They were identified as VSMC by their characteristic hill-and-valley growth pattern and indirect immunofluorescence staining for VSMC specific α-actin. All cultures were maintained in RPMI-1640 medium containing 10 % bovine growth serum (BGS) at 37 °C in a humidified, 5 % CO2/95 % air atmosphere.
Adenovirus production and infection
An adenovirus expressing both Green Fluorescent Protein (GFP) or CCN5, each tagged by a nine amino acid HA epitope on the C-terminus, was produced using the Ad-Easy system (He et al. 1998), as previously described (Lake et al. 2003). Briefly, cells were washed and exposed to various amounts of virus for 2 h in serum free RPMI-1640 with occasional gentle agitation. RPMI-1640 medium containing 10 % BGS was then added back to the cells. After 1–2 days, infected cells can be trypsinized and used for experiments. Infection can be monitored quickly by observing the fraction of cells expressing GFP in the population. Using this method, we achieve >90 % infection efficiency (Lake et al. 2003).
Design, construction, and Use of lentiviral shRNA against Rat CCN5
Rat CCN5 (RefSeq NM_031590) genomic sequence and splicing sites were obtained from University of California Santa Cruz (UCSC) Genome Bioinformatics database. shRNAs against CCN5 were designed by siDESIGN in Dharmacon website:
(http://www.dharmacon.com/designcenter/designcenterpage.aspx)
One shRNA used the following oligos:
Forward:CCGGTCCCATCTCTTAAGCACTCGCAAATTCAAGA- GATTTGCGAGTGCTTAAGAGATTTTTG; Reverse: AATTCAAAAATCTCTTA-AGCACTCGCAAATCTCTTGAATTTGCGAGTGCTTAAGAGATGGGA.
The second shRNA used the following oligos:
Forward: CCGGTCCCAGATGATGAA- TGAACTCGTATTCAAGAGATACGAGTTCATTCATCATCTTTTTG;
Reverse:AATTCAAAAAGATGATGAATGAACTCGTATCTCTTGAATACGAGTT-CATTCATCATCTGGGA. All oligos were synthesized by Invitrogen, then cloned into vector pLK0.1puro (Addgene, Cambridge MA) at AgeI/EcoRI sites. Identified clones were confirmed by NdeI/EcorI digestion and sequencing.
Constructs of pLK0.1puro-sh-CCN5 variants were mixed with two packing vectors, pCMV-dR8.2-dvpr and pCMV-VSV-G, at a ratio of 3:2:1. Mixed plasmids were co-transfected with Lipofectamine 2000 (Invitrogen, Carlsbad CA) into 293 T cells, following the protocol previously described (Wei, 2009). 48 h after transfection, culture media containing lentivirus was filtered with 0.45 μm Millex syringe driven filter unit (Millipore, Billerica MA). Target cells were grown in 6-well plates for lentiviral infection and seeded onto multi-well slides (Erie Scientific, Portsmouth NH) or grown and infected directly on slides for immunofluorescence microscopy. When cells reached approximately 50 % confluence, we replaced the growth media with the filtered virus containing media, and then added polybrene to a final concentration of 10 μg/ml. After overnight infection, cells were switched back to normal media. Media containing 2.5ug/ml puromycin was used for selection of infected cells.
Antibodies
CCN5 protein was detected using a highly specific, peptide affinity-purified rabbit polyclonal antibody to a polypeptide fragment from amino acids 103–117 of the von Willebrand Factor-C (VWC) domain of CCN5 (Gray MR 2005; Lake et al. 2003; Lake & Castellot 2003; Mason et al. 2004b). This recognizes full-length 27 kDa CCN5 protein on Western blots (Gray MR 2005; Lake et al. 2003; Lake & Castellot 2003; Mason et al. 2004b). Alternatively, CCN5 protein was detected using a highly specific mouse monoclonal antibody to the von Willebrand Factor-C domain of CCN5 (Wei et al. 2009). Antibodies against the following proteins were also used: cortactin (Abcam, Cambridge MA); integrin αvβ3, integrin αv, integrin β3, and integrin αIIB (Millipore, Billerica MA); and HA (Roche, Indianapolis IN).
Western blot analysis
Proteins were denatured at 95 °C for 5 min with 2X loading buffer (100 mM Tris-Cl, pH6.8, 4 % SDS, 20 % glycerol, 200 mM dithiothreitol, 0.2 % Bromophenol Blue), and then separated by 12 % SDS-PAGE and transferred onto 0.2-μm pore size Immuno-Blot polyvinylidene fluoride membranes (PVDF) (Bio-Rad, Hercules CA). Dried membranes were re-wetted and blocked with 5 % non-fat milk in TBST (137 mM NaCl, 20 mM Tris, pH7.6, 0.2 % Tween 20). Primary antibodies were diluted in milk-TBST; horseradish peroxidase (HRP)-conjugated secondary antibody anti-mouse or anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz CA) was diluted 1:5000. All washing steps were done three times with PBST for 10 min each. Membranes were developed with ECL reagent (GE-Amersham, Amersham UK), and exposed with X-Omat Blue XB-1 Film (Kodak, Rochester NY).
Immunocytochemistry
In experiments in which podosomes were induced, cells were treated with 2 μM phorbol 12,13-dibutyrate (PDBU) (Fisher, Hampton NH) for 1 h at 37°C prior to processing for immunostaining. Cells were fixed with 3 % formaldehyde in PBS for 7–10 min. In some cases cells were permeabilized by washing three times with 1x PBST (PBS containing 0.1 % Triton X − 100) for 3 min each at room temperature. Wells were washed with PBS three times before adding primary antibody. Unless otherwise noted, primary antibodies were normally diluted 1:100 in PBS, and the secondary antibody was diluted 1:300 in PBS. Cells were incubated with antibodies for 1 h at room temperature. All washing steps between incubations were done 3 times with PBS for 5 min each. Coverslips were mounted using VECTASHIELD (Vector Laboratories, Burlingame CA), which contains DAPI to visualize nuclei. Pictures were taken at room temperature through a Carl Zeiss Axiomat fluorescence microscope with a digital camera system (SPOT; Diagnostic Instruments) and analyzed by NIS-Elements software by Nikon (Tokyo, Japan).
Co-immunoprecipitation
Cultured cells were washed twice with cold PBS for 5 min each, then lysed with buffer (1 % NP-40, 0.15 M NaCl, 0.05 M Tris–HCl, pH8.0, 0.001 M MgCl2, 0.001 M CaCl2) plus 1:50 protease inhibitor (Sigma, P8340). Immunoprecipitations were carried out with Protein-G-conjugated Dynabeads (Invitrogen, Carlsbad CA), according to the manufacturer’s protocol. Briefly, protein-G coated magnetic beads were bound to antibody in buffer (0.1 M sodium phosphate, 0.01 % Tween 20, pH 7.4) for 10 min at RT. Dynabeads bound to antibody were then incubated with lysates for 30 min at RT. Beads were then washed in buffer (PBS, 0.01 % Tween 20) three times at RT. The bound protein complexes were then eluted from the beads at 70°C for 10 min in loading buffer (100 mM Tris-Cl, pH6.8, 4 % SDS, 20 % glycerol, 200 mM DTT, 0.2 % Bromophenol Blue). Bound proteins in the eluate were resolved by SDS-PAGE and analyzed by Western Blot using an antibody against the desired protein.
In situ zymography
In situ zymography was performed essentially as described by Bowden et al (Bowden et al. 2001). Glass coverslips were coated with 50 μg/mL fluorescein-conjugated gelatin (Invitrogen, Carlsbad CA), cross-linked for 15 min with 0.25 % glutaraldehyde in PBS at 37°C, and incubated for 3 min with 5 mg/ml NaBH4 in PBS at 37°C. After quenching with RPMI at 37°C, cells were plated on coated coverslips in RPMI containing 10 % BGS and incubated overnight at 37°C. Cells were then treated with 2 μM Phorbol 12,13-Dibutyrate (PDBU) (Fisher, Hampton NH) for 1 h at 37°C to induce podosome formation before processing for immunostaining. Pictures were taken at room temperature through a Carl Zeiss Axiomat fluorescence microscope with a digital camera system (SPOT; Diagnostic Instruments) and analyzed by NIS-Elements software by Nikon (Tokyo, Japan). Uniform magnification and exposure times for each channel were used for every photograph. Pixel intensities were quantified with Adobe Photoshop CS by recording the mean pixel intensity/area for the CCN5 signal and gelatin remaining under each podosome.
Results
CCN5 Interacts with integrin αvβ3
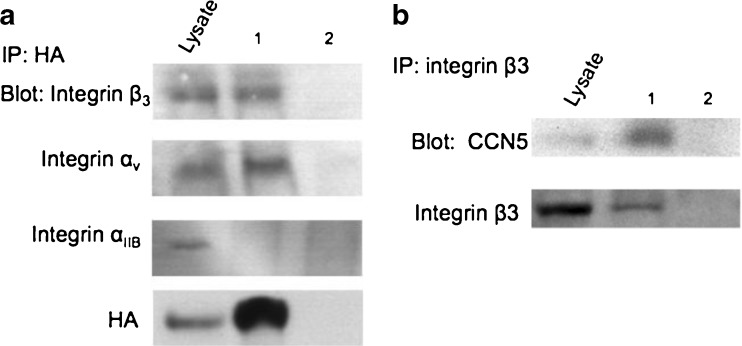
To determine if CCN5 and integrin αvβ3 interact, VSMC were infected with either adenovirus expressing HA-tagged CCN5 or adenovirus expressing GFP prior to making whole cell lysates. We then carried out immunoprecipitation with anti-HA antibody on these lysates, followed by immunoblotting with both anti-integrin αv and anti-integrin β3 antibodies. A Western blot using integrin αIIB was performed as a control for nonspecific binding as it is the only other integrin subunit known to form a heterodimer with integrin β3. Binding between HA-CCN5 and integrin αvβ3 was demonstrated based on the ability to detect integrin αv and integrin β3 on the Western blot (Fig. 2a).
Fig. 2.
CCN5 Binds Integrin αvβ3. a Exponentially growing VSMC were infected with either adenovirus expressing an HA tagged CCN5 (lane 1) or adenovirus expressing GFP (lane 2), after which cell lysates were prepared in an NP-40 based lysis buffer. Equal amounts of lysate protein were immunoprecipitated using anti-HA bound magnetic beads. Bound proteins were resolved by SDS-PAGE and analyzed by Western blotting using an antibody against Integrin β3, Integrin αv, Integrin αIIB (as a control for nonspecific integrin binding), or HA (to demonstrate HA-CCN5 binding to beads). b VSMC were growth arrested by serum starvation, after which, cell lysates were prepared in an NP-40 based lysis buffer. Equal amounts of lysate protein were immunoprecipitated using magnetic beads bound to either antibody against Integrin β3 (lane 1) or mixed IgG (lane 2). Bound proteins were resolved by SDS-PAGE and analyzed by Western blotting using an antibody against CCN5, or Integrin β3 (to demonstrate binding of Integrin β3 to beads). Both experiments replicated three times
Additional immunoprecipitation studies were performed using growth-arrested VSMCs to determine if this interaction was observed with endogenous CCN5. Whole cell lysates were made and immunoprecipitation was carried out using either anti-integrin β3 antibody or mixed IgG, followed by immunoblotting with mouse monoclonal anti-CCN5 antibody. Binding of integrin β3 and endogenous CCN5 was demonstrated based on the ability to detect CCN5 on the Western blot of the anti-integrin β3 immunoprecipitation, but not on the Western blot of the mixed IgG immunoprecipitation (Fig. 2b). These observations strongly suggest that CCN5 has a specific interaction with integrin αvβ3.
CCN5 Interacts with integrin αvβ3 in podosomes
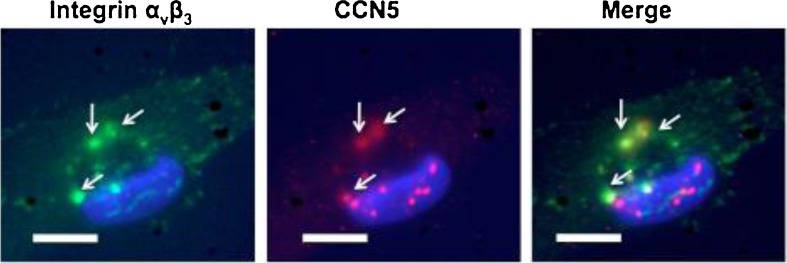
The two most prominent subcellular localizations of integrin αvβ3 are found at focal adhesions and podosomes. To ascertain if CCN5 was interacting with integrin αvβ3 at either of these cell structures, we performed immunofluorescence studies to look for colocalization of CCN5 and integrin αvβ3. CCN5 could be seen in relatively large and densely staining structures (Fig. 3). These structures appeared morphologically distinct from focal adhesion-like structures and strongly resemble podosomes.
Fig. 3.
Integrin αvβ3 and CCN5 colocalize at structures which resemble podosomes. Exponentially growing VSMCs were fixed and stained with antibodies for Integrin αvβ3 (green), CCN5 (red), and DAPI (blue). Bars = 5 μm
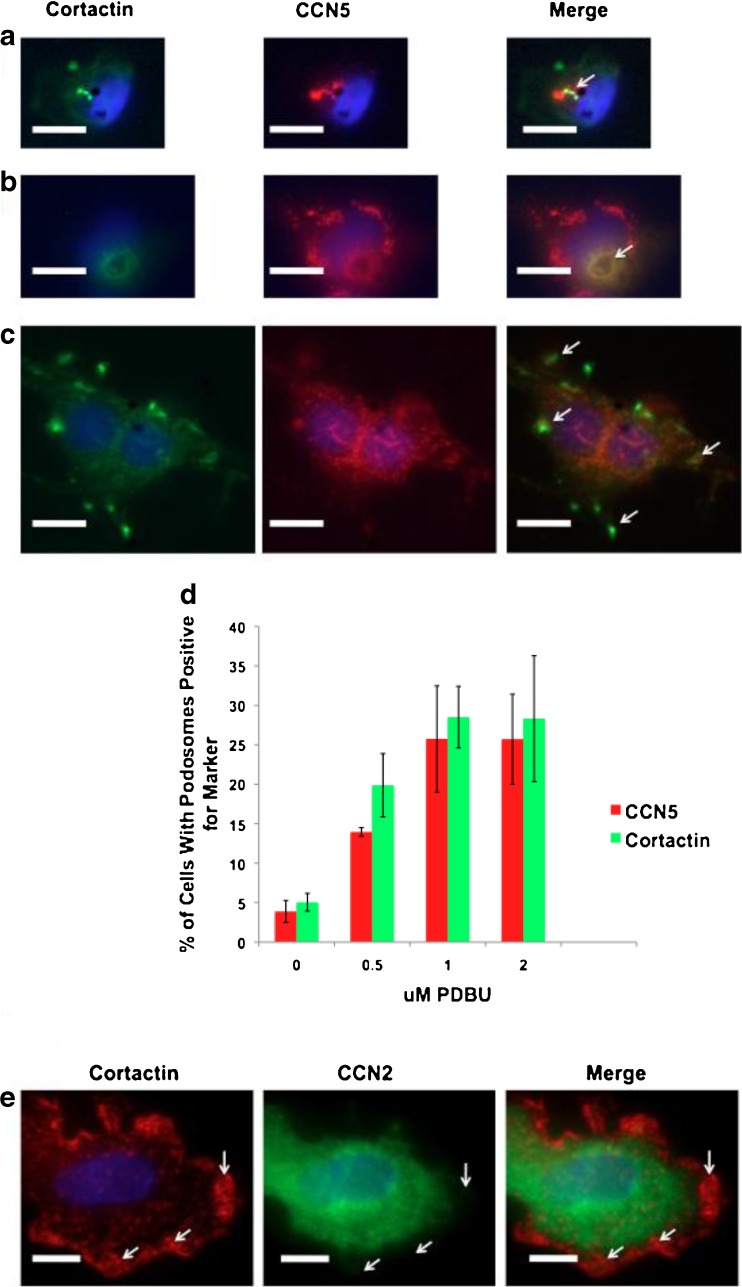
To confirm that the observed CCN5-containing structures were podosomes, we co-labeled VSMCs with CCN5 and the classic podosomal marker, cortactin. The CCN5-positive structures also displayed cortactin labeling >95 % of the time (Fig. 4a). Individual podosomes assemble and form higher order conglomerates of podosomes known as rosettes, due to their flower-like appearance (Tarone et al. 1985). We also observed a colocalization of CCN5 and cortactin in rosette structures in close proximity to the nucleus (Fig. 4b), as well as on the periphery of the cell in what appear to be filopodia (Fig. 4c). Because it has been well established that the phorbol ester PDBU induces podosome formation in VSMC (Hai et al. 2002), we treated VSMC with PDBU and observed that the percentage of cells with podosomes containing CCN5 increased in a strongly dose- dependent fashion (Fig. 4d). CCN2 and CCN5 have opposite expression patterns in VSMC. We therefore examined CCN2 expression in podosomes (Fig. 4e). Unlike CCN5, CCN2 was completely excluded from podosomes. These observations indicate that CCN5 is present in virtually all podosomes and is likely to interact with integrin αvβ3 in these structures.
Fig. 4.
CCN5 colocalizes with cortactin in podosomes of several classic morphologies, and CCN2 does not a-c Podosomes induced by stimulation of VSMCs with 2 μM PDBU phorbol ester phorbol 12,13-dibutyrate (PDBu) for 1 h. Cells were then fixed and stained with antibodies for cortactin (green), CCN5 (red), and DAPI (blue). Bars = 10 μm d Podosomes induced by stimulation of VSMCs with 0-2 μM PDBU phorbol ester phorbol 12,13-dibutyrate (PDBU) for 1 h. Percent of cells containing podosomes positive for CCN5 and/or cortactin were counted and graphed against increasing PDBU concentration. Error Bars = standard deviation, n = 3. e Podosomes induced by stimulation of VSMCs with 2 μM PDBU phorbol ester phorbol 12,13-dibutyrate (PDBu) for 1 h. Cells were then fixed and stained with antibodies for cortactin (red), CCN5 (green), and DAPI (blue). Bars = 10 μm
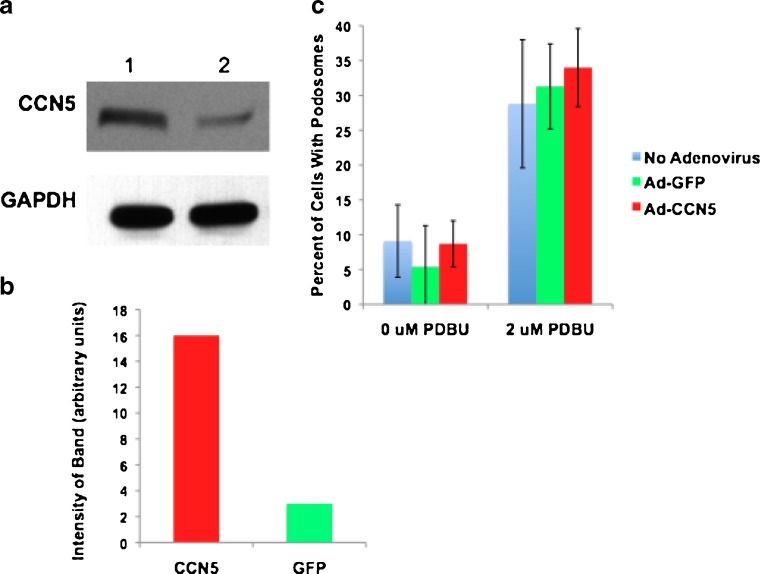
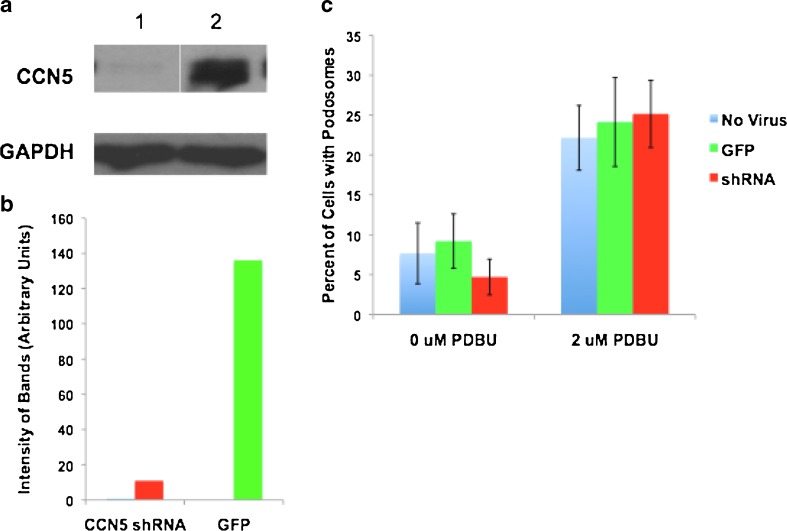
CCN5 Over-expression does Not induce podosome formation
After establishing that CCN5 was present in podosomes, we investigated the possibility that CCN5 was involved in podosome formation. The formation of podosomes is an extremely complex process that is still not completely understood, though the basic players involved are well characterized (Osiak et al. 2005; Linder et al. 2000a; Linder et al. 1999; Burns et al. 2001). CCN5 knockdown results in structural changes in the actin cytoskeleton (Lake & Castellot 2003) and therefore may be playing a role in regulating assembly of actin-based structures such as podosomes. To address this possibility, we overexpressed CCN5 in VSMC using an adenovirus (Fig. 5a-b), after which cells were treated with PDBU to induce podosome formation. The fraction of cells with podosomes was then calculated for each experimental condition (Fig. 5c). Cells overexpressing CCN5 did not show a significant change in podosome formation with respect to GFP or uninfected controls. As a complementary experiment, we knocked down CCN5 in VSMCs using a lentivirally delivered shRNA (Fig. 6a-b), after which cells were treated with PDBU to induce podosome formation. The fraction of cells with podosomes was then calculated for each condition (Fig. 6c). Cells with CCN5 knockdown did not show a significant change in podosome formation with respect to GFP or uninfected controls. These data indicate that altered expression of CCN5 does not result in a change in the number of podosomes.
Fig. 5.
CCN5 overexpression does not alter podosome formation. a VSMCs were infected with either adenovirus expressing CCN5 (lane 1) or adenovirus expressing GFP (lane 2). Cell lysates were then prepared from a fraction of the infected cells in an NP-40 based lysis buffer. Proteins were resolved by SDS-PAGE and analyzed by Western blotting using an antibody against CCN5 or GAPDH (loading control) b The level of CCN5 from a was quantitated using densitometry. c Another fraction of the cells infected with adenovirus (both CCN5 and GFP), as well as a group of uninfected cells were trypsinized and plated on glass slides. Half of each of the aforementioned three groups of cells were then treated with 2 μM phorbol ester phorbol 12,13-dibutyrate (PDBU) for 1 h to induce podosome formation, while the other half was untreated. Cells were then fixed and stained for cortactin (podosomal marker). Percentage of cells containing podosomes were then calculated and graphed. Error Bars = standard deviation, n = 3
Fig. 6.
CCN5 shRNA knockdown does not alter podosome formation. a VSMCs were infected with either lentivirus expressing shRNA against CCN5 (lane 1) or lentivirus expressing GFP (lane 2). Cell lysates were then prepared from a fraction of the infected cells in an NP-40 based lysis buffer. Proteins were resolved by SDS-PAGE and analyzed by Western blotting using an antibody against CCN5 or GAPDH (loading control) b The level of CCN5 from a was quantitated using densitometry. c. Another fraction of the cells infected with lentivirus (both CCN5 shRNA and GFP), as well as a group of uninfected cells were trypsinized and plated on glass slides. Half of each of the aforementioned three groups of cells were then treated with 2 μM phorbol ester phorbol 12,13-dibutyrate (PDBU) for 1 h to induce podosome formation, while the other half was untreated Cells were then fixed and stained for cortactin (podosomal marker). Percentage of cells containing podosomes were then calculated and graphed. Error Bars = standard deviation, n = 3
CCN5 Regulates the matrix degrading capacity of podosomes
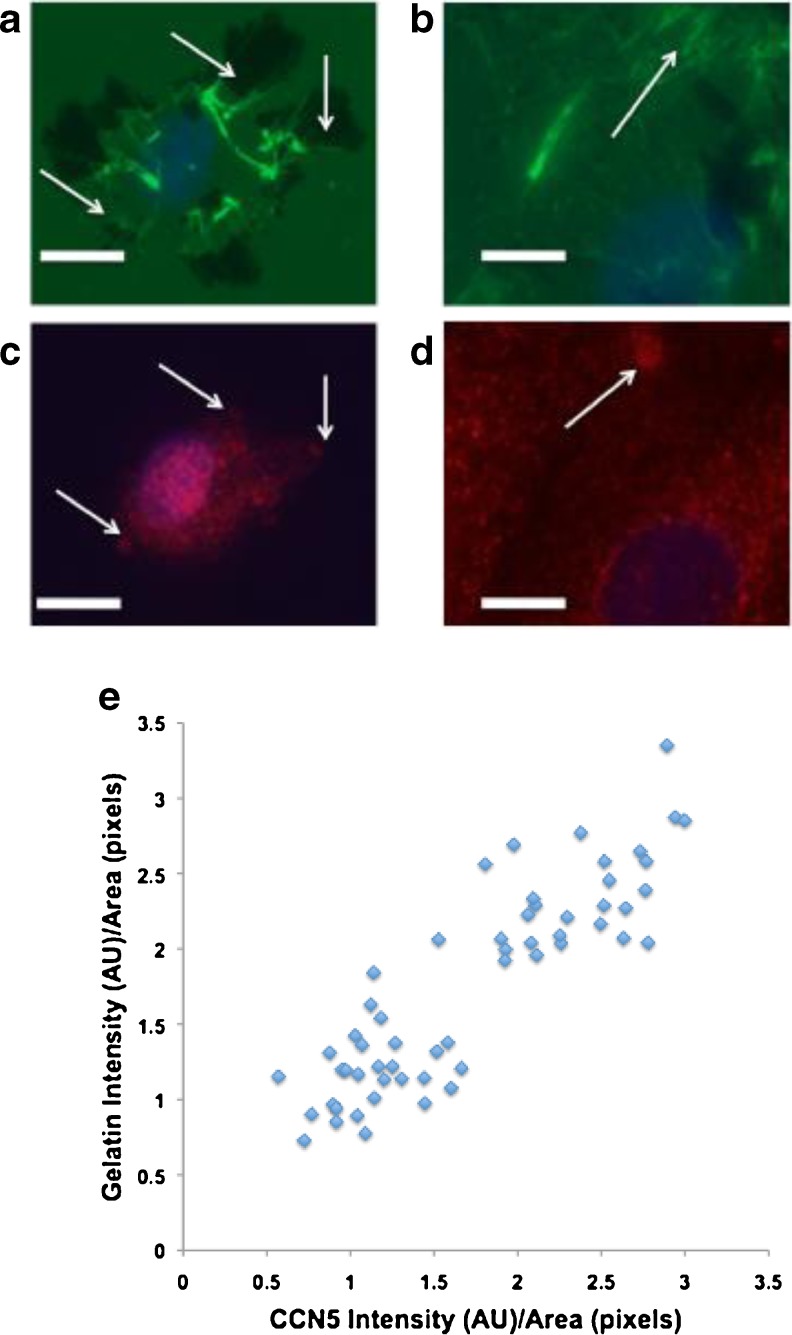
While examining CCN5 containing podosomes with immunofluorescence, we noticed that the intensity of CCN5 was widely variable between podosomes. To assess whether the CCN5 level in a podosome correlated with its ability to degrade the matrix we carried out an in situ zymography assay as described by Tatin et al. (Tatin et al. 2006). VSMC were seeded onto glass slides pre-coated with cross-linked, fluorsceinated gelatin and treated for 1 h with PDBU. Following PDBU treatment, gelatin degradation was visualized as dark areas in the matrix, and podosomes were visualized by CCN5 labeling. As essentially all CCN5-labeled structures that resemble podosomes also label with cortactin, CCN5 is a suitable marker for podosomes. Most of the non-fluorescent patches (i.e., degraded matrix) co-localized with podosomes. Podosomes with robust areas of matrix degradation below them always had a much lower intensity of CCN5 labeling (Fig. 7a,c), whereas podosomes with little or no matrix degradation below them were invariably higher in CCN5 intensity (Fig. 7b,d). This correlation was quantified by measuring pixel intensity (Fig. 7e). Due to the linear appearance of the data as well as its apparent non-parametric distribution we calculated the Spearman correlation coefficient (rs =0.828) on the rank order of all the data points from three separate experiments. This analysis indicates a very high likelihood that CCN5 levels and matrix degradation are negatively correlated.
Fig. 7.
CCN5 level in a podosome correlates with decreased the ability of podosomes to degrade matrix. VSMCs were plated on glass coverslips coated with cross-linked fluorescein–conjugated gelatin. After 24 h, cells were treated with 2 μM phorbol ester phorbol 12,13-dibutyrate (PDBU) for 1 h to induce podosome formation. Cells were then fixed and stained for CCN5 and DAPI. a–b Images of gelatin (green) and DAPI (blue). c-d Superimposed images of the same fields in a-b corresponding to CCN5 (red) and DAPI (blue). Bars = 10 μm e CCN5 signal intesity/area of podosome and gelatin signal intesity/area of below corresponding podosome was quantitated using Adobe Photoshop CS. This data was then graphed in a scatterplot. n = 57. Spearman rank-order correlation coefficient (r s) = 0.828
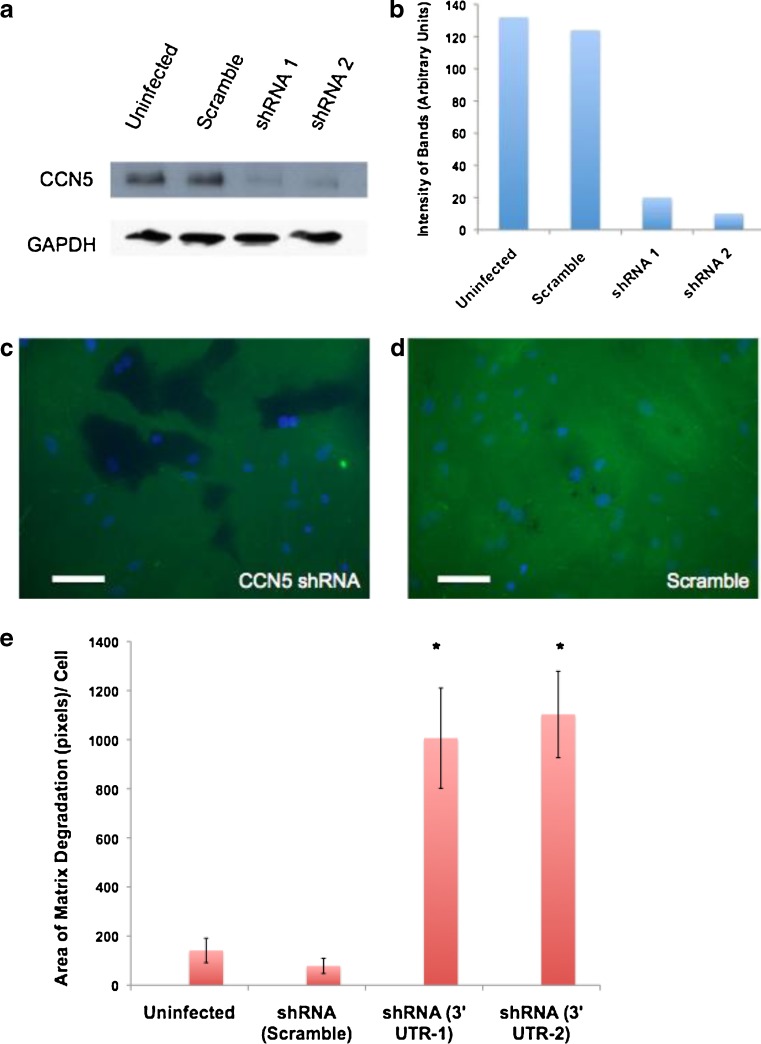
The results above correlate CCN5 levels with matrix degradation by podosomes, but do not indicate if a causal relationship exists. To demonstrate the direction of causality, we knocked down CCN5 using shRNA. VSMC were infected with lentivirus vectors containing one of two shRNAs against CCN5, or a scrambled shRNA. Infected the cells were drug-selected to ensure a pure population of infected cells. Western Blot analysis confirmed that both the shRNAs directed against CCN5 reduced CCN5 protein levels >85 %, whereas the scrambled control did not affect CCN5 levels (Fig. 8a,b).
Fig. 8.
CCN5 is Required for PDBU-Induced Podosomal Degradation of Matrix. a VSMCs were infected with either lentivirus expressing one of two shRNA against CCN5 or lentivirus expression shScramble as a control. Uninfected cells were also included as a control. Cell lysates were then prepared from a fraction of the infected cells in an NP-40 based lysis buffer. Proteins were resolved by SDS-PAGE and analyzed by Western blotting using an antibody against CCN5 or GAPDH (loading control) b The level of CCN5 from a was quantitated using densitometry. c-d VSMCs were then plated on glass coverslips coated with cross-linked fluorescein–conjugated gelatin. After 24 h, cells were treated with 2 μM phorbol ester phorbol 12,13-dibutyrate (PDBU) for 1 h to induce podosome formation. Cells were then fixed and immunofluorescence was visualized, DAPI (blue) and gelatin (green). Bars = 50 μm e Total area of matrix degradation was then quantitated using Adobe Photoshop CS. This data was then graphed. Error Bars = standard deviation, n = 3, * indicates p < 0.05 with respect to the value for either uninfected cells or cells infected with lenti-scramble shRNA
To assess the effect of CCN5 knock-down on podosome matrix degradation, VSMC were seeded onto glass slides pre-coated with cross-linked, fluoresceinated gelatin and treated for 1 h with PDBU. A group of uninfected cells were also seeded on gelatin-coated slides as a control. Following PDBU treatment, gelatin degradation was visualized as dark areas in the fluorescent matrix. To quantify this effect, three representative photographs were taken of each slide and matrix degradation assessed by measuring the total amount of black area (degraded gelatin) in the three pictures; that number was divided by the total number of cells in the three pictures. This experiment was repeated three times using different VSMC isolates and averaged. Knockdown of CCN5 with either of our two shRNAs resulted in approximately a ten-fold increase above uninfected cells; cells infected with the scrambled shRNA showed no change in matrix degradation (Fig. 8c,d,e).
The ability of podosomes to degrade matrix is generally attributed to the presence of matrix metalloproteinases (MMPs). Furthermore, prior studies from our laboratory demonstrated that the expression of MMP-2 in VSMC is inversely correlated with expression of CCN5 (12). To examine the possibility that decreased MMP2 in the presence of CCN5 occurred at the level of the podosome, we carried out immunofluorescence studies using two different MMP2 antibodies, but were unable to detect MMP2 labeling in podosomes. No MMP2 was detected with either antibody (data not shown). Immunofluorescence labeling with an antibody to the membrane-bound form of MMP2—MT-MMP2—also failed to reveal MT-MMP2 in podosomes. The same negative labeling result was seen with an anti-MMP9 antibody. To ensure that we were capable of detecting MMPs in podosomes, we labeled SMC with an anti–MT-MMP1 antibody. MT-MMP1 labeling was seen in all podosomes whether or not CCN5 was also present in the podosome (data not shown).
Discussion
In this study we report several novel findings that shed light on the mechanism of action of CCN5 and the regulation of podosome function: 1) integrin αvβ3 is a CCN5 interacting protein; 2) CCN5 and integrin αvβ3 co-localize in podosomes; 3) CCN5 protein levels do not regulate the number of podosomes in cells; and 4) CCN5 negatively regulates matrix degradation by podosomes.
To date, a functional cell surface binding protein for CCN5 has not been identified. We have identified integrin αvβ3 as a CCN5 binding protein, based on co-immunoprecipitation of integrin αvβ3 with both endogenous and adenovirally expressed CCN5 (Fig. 2). Furthermore, CCN5 and integrin αvβ3 co-localize in podosomes using immunofluorescence (Fig. 3). Integrin αvβ3 is known to bind to a broad range of both RGD-containing and non-RGD-containing ligands (Brooks et al. 1996; Piali et al. 1995; Ruoslahti & Pierschbacher 1987). The primary sequence of CCN5 does not contain the RGD recognition motif present integrin αvβ3 ligands such as vitronectin, fibronectin, fibrinogen, thrombospondin, and osteopontin (Ruoslahti & Pierschbacher 1987). In addition to CCN5, at least three other integrin αvβ3 ligands do not contain the consensus RGD sequence: a cell surface molecule from the immunoglobulin superfamily CD31/PECAM-1 (Piali et al. 1995), the matrix metalloproteinase MMP-2 (Brooks et al. 1996), and the CCN family member CCN1 (Chen et al. 2004). Interestingly, CCN5 contains a stretch of amino acids in its von Willebrand Factor-C domain that is very similar to a known integrin αvβ3 binding site in CCN1 (Fig. 1) (Chen et al. 2004).
The finding that CCN5 interacts with integrin αvβ3 also provides a possible explanation for the ability of CCN5 to inhibit cell migration. Following the binding of extracellular ligands, integrins are known to initiate “outside-in” signaling cascades, namely the phosphorylation and activation of focal adhesion kinase (FAK), and subsequent activation of Ras/Raf/Erk, known to regulate cell proliferation and Rho/Rac/cdc42, known to regulate cell adhesion and motility (Giancotti & Ruoslahti 1999). CCN proteins have been shown to act through integrin αvβ3 in fibroblasts and endothelial cells to promote cell proliferation and migration (Grzeszkiewicz et al. 2001; Leu et al. 2002; Lin et al. 2005). Therefore, the interaction of CCN5 with integrin αvβ3 could be regulating the known anti-proliferation and anti-motility effects of CCN5 in a similar fashion.
Podosomes are highly dynamic adhesion structures with a half-life of approximately 2–12 min (Destaing et al. 2003). They have been documented in several cells including macrophages, osteoclasts, transformed fibroblasts, dendritic cells, and VSMCs (Marchisio et al. 1987; Hiura et al. 1995; Linder & Aepfelbacher 2003; Burgstaller & Gimona 2005), and their presence has been correlated with the invasiveness and ability of these cells to move across anatomical boundaries (Marchisio et al. 1987). Subcellular position of podosomes corresponds to sites of localized proteolytic activity (Chen et al. 1984). We demonstrated that the podosome-like structures containing CCN5 were indeed podosomes, as they were labeled by the podosome marker cortactin, and their formation was inducible by the phorbol ester PDBU in a dose-dependent manner. Interestingly, we observed that unlike CCN5, CCN2 did not localize to podosomes. This lack of CCN2 in podosomes is consistent with the opposite expression patterns of CCN2 and CCN5 observed in VSMC. CCN2 over-expression induces VSMC proliferation and increases MMP-2 expression, and CCN5 over-expression reduces proliferation and MMP-2 expression. CCN5 expression decreases whereas CCN2 expression increases in VSMC during the proliferative phase of balloon angioplasty injury (Lake et al. 2003; Lake & Castellot 2003; Ando et al. 2004; Fan et al. 2000; Fan & Karnovsky 2002).
The human MMP family currently consists of 24 members, including both secreted isoforms as well as MMPs that are localized to the cell surface through membrane anchors or transmembrane stretches (MT-MMPs). MT1-MMP and MMP-9 are present at podosomes in osteoclasts whereas, MT1-MMP and MMP-2 are present in thepodosomes of endothelial cells. We were unsuccessful in showing a correlation between CCN5 and MMPs in podosomes using immunofluorescence, with the exception of MT-MMP1, which we used as a positive control. The failure of MMP2 and MMP9 to localize to podosomes is not surprising, since they are secreted MMPs and may not be present at detectable levels bound to a podosome. While our negative data suggests that MT-MMP1, MMP2, MT-MMP2, and MMP-9 are not involved in the ability of CCN5 to regulate matrix degradation in podosomes, this intriguing question deserves further exploration.
The defining characteristic of podosome structure is an actin-rich core (Marchisio et al. 1984; Pfaff & Jurdic 2001; Baldassarre et al. 2006). Proteins involved in actin nucleation including Wiskott–Aldrich syndrome protein (WASP) (Linder et al. 1999), cortactin (Bowden et al. 1999), and Arp2/3 complex (Linder et al. 2000a) are present throughout the actin-rich core. This intracellular core also contains integrins, including mostly β2 and β3 integrins (Gaidano et al. 1990) and integrin associated proteins such as talin and paxillin (Pfaff & Jurdic 2001; Bowden et al. 1999), thereby allowing the podosome to interact with matricellular proteins such as CCN5.
We investigated the role CCN5 was playing in podosomes by examining whether it regulated podosome formation. The formation of an F-actin core is necessary for podosome formation as F-actin disassembly through cytochalasins or latrunculin results in disappearance of podosomes (Destaing et al. 2003; Lehto et al. 1982; Linder et al. 2000b). This assembly of the F-actin core is catalyzed by CDC42-dependent activation of Wasp/N-WASP, which in turn activates the actin-nucleating Arp2/3 complex (Linder et al. 2000b). Given the dynamic nature of podosomes, which last only 2-12 min (Destaing et al. 2003), disassembly of the F-actin core is just as crucial as its formation. Because we previously showed that CCN5 knock-down in VSMC reduced the well-organized stress fibers observed in untreated VSMC (Lake & Castellot 2003), we hypothesized that CCN5 might play a role in the formation of podosomes. Interestingly, CCN5 overexpression (Fig. 5) or knockdown (Fig. 6) did not effect the formation of podosomes, thus corroborating the null hypothesis that CCN5 does not alter podosome formation.
We also hypothesized that CCN5 was involved in modulating the matrix degrading function of podosomes and carried out in situ zymography assays to test this idea. A negative correlation was seen between intensity of CCN5 signal in a podosome and the ability of a podosome to degrade matrix (Fig. 7). Podosomes with robust areas of matrix degradation below them always had exceedingly low intensity CCN5 staining, whereas podosomes with little or no matrix degradation below them were higher in CCN5 intensity. The direction of causality of this correlation was then investigated by knocking down CCN5 prior to performing in situ zymography assays (Fig. 8). Knockdown of CCN5 resulted in approximately a ten-fold increase in matrix degradation compared to uninfected cells or scrambled shRNA treated cells, thus strongly supporting the hypothesis that CCN5 regulates podosome function.
These results suggest the importance of CCN5 in podosome-mediated extracellular matrix degradation, a process thought to underlie the motogenic phenotype of VSMC. The involvement of CCN5 in podosomes could potentially be important in restenosis following vascular surgery. Following procedures such as angioplasty or coronary artery bypass grafts, VSMC migrate from their original location in the media of a vessel wall, through the internal elastic lamina and into the lumen, where they proliferate and block the artery in a process called restenosis. Cortactin, a classic marker of podosomes, shows high expression in neointima, implicating podosomes in the VSMC migration seen in restenosis (Minta et al. 2006). We have shown that ectopic expression of CCN5 in a mouse model for restenosis strongly suppresses VSMC migration and proliferation (unpublished work). The results of the present paper suggest the possibility that CCN5-mediated inhibition of restenosis may occur via blocking the ability of medial VSMC podosomes to degrade matrix, thus preventing migration into the intima.
Another example may be found in melanoma. CCN5 is the most highly expressed mRNA of the CCN family in normal human skin cells. UV irradiation of human skin reduced the levels of CCN5 mRNA by 50 % in a time-dependent manner and did not return to basal levels until 48 h post irradiation (Quan et al. 2009). In addition, the involvement of podosomes in the invasive capacity of human melanoma has been well documented (Tague et al. 2004; Nakahara et al. 2003).
The observations reported here raise many intriguing questions about the mechanism of action of CCN5 and its role in podosome function. Among these are: Does CCN5 bind directly to integrins, and/or are other proteins are involved? What downstream signaling pathways are controlled by CCN5? Does CCN5 block the action of specific proteolytic enzymes like MMP-2, which we have previously shown to be strongly inhibited by CCN5? Experiments designed to answer these important questions are currently underway in our laboratory.
Abreviations
- BMP
bone morphogenic protein
- CCN
Cyteine-rich 61/Connective Tissue Growth Factor/Nephroblastomaoverexpressed family of proteins
- CT
carboxy – terminal
- HSPG
heparin-sulfate proteoglycans
- IGFBP
insulin - like growth factor binding protein - like module
- MMP
matrix metalloproteinase
- PDBU
Phorbol 12,13-Dibutyrate
- RGD
Arginine-Glycine-Aspartic Acid
- shRNA
small hairpin RNA
- TSP
thrombospondin-1
- VSMC
smooth muscle cells
- VWC
von Willebrand Factor – C
- WASP
Wiskott – Aldrich Syndrome protein
Footnotes
Concise summary
In this study we report that CCN5 binds to the cell surface receptor integrin αvβ3 in vascular smooth muscle cells. Furthermore, this interaction takes place in podosomes, organelles known to degrade matrix and mediate motility. We show that CCN5 regulates the ability of podosomes to degrade matrix, but does not affect podosome formation.
References
- Ando H, Fukuda N, Kotani M, Yokoyama S, Kunimoto S, Matsumoto K, Saito S, Kanmatsuse K, Mugishima H. Chimeric DNA-RNA hammerhead ribozyme targeting transforming growth factor-beta 1 mRNA inhibits neointima formation in rat carotid artery after balloon injury. Eur J Pharmacol. 2004;483:207–214. doi: 10.1016/j.ejphar.2003.10.035. [DOI] [PubMed] [Google Scholar]
- Baldassarre M, Ayala I, Beznoussenko G, Giacchetti G, Machesky LM, Luini A, Buccione R. Actin dynamics at sites of extracellular matrix degradation. Eur J Cell Biol. 2006;85:1217–1231. doi: 10.1016/j.ejcb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–4449. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- Bowden ET, Coopman PJ, Mueller SC. Invadopodia: unique methods for measurement of extracellular matrix degradation in vitro. Methods Cell Biol. 2001;63:613–627. doi: 10.1016/S0091-679X(01)63033-4. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996;85:683–693. doi: 10.1016/S0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- Burgstaller G, Gimona M. Podosome-mediated matrix resorption and cell motility in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2005;288:H3001–3005. doi: 10.1152/ajpheart.01002.2004. [DOI] [PubMed] [Google Scholar]
- Burns S, Thrasher AJ, Blundell MP, Machesky L, Jones GE. Configuration of human dendritic cell cytoskeleton by Rho GTPases, the WAS protein, and differentiation. Blood. 2001;98:1142–1149. doi: 10.1182/blood.V98.4.1142. [DOI] [PubMed] [Google Scholar]
- Castellot JJ, Jr, Favreau LV, Karnovsky MJ, Rosenberg RD. Inhibition of vascular smooth muscle cell growth by endothelial cell-derived heparin, Possible role of a platelet endoglycosidase. J Biol Chem. 1982;257:11256–11260. [PubMed] [Google Scholar]
- Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41:771–783. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WT, Olden K, Bernard BA, Chu FF. Expression of transformation-associated protease(s) that degrade fibronectin at cell contact sites. J Cell Biol. 1984;98:1546–1555. doi: 10.1083/jcb.98.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Leu SJ, Todorovic V, Lam SC, Lau LF. Identification of a novel integrin alphavbeta3 binding site in CCN1 (CYR61) critical for pro-angiogenic activities in vascular endothelial cells. J Biol Chem. 2004;279:44166–44176. doi: 10.1074/jbc.M406813200. [DOI] [PubMed] [Google Scholar]
- Delmolino LM, Stearns NA, Castellot JJ., Jr COP-1, a member of the CCN family, is a heparin-induced growth arrest specific gene in vascular smooth muscle cells. J Cell Physiol. 2001;188:45–55. doi: 10.1002/jcp.1100. [DOI] [PubMed] [Google Scholar]
- Destaing O, Saltel F, Geminard JC, Jurdic P, Bard F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol Biol Cell. 2003;14:407–416. doi: 10.1091/mbc.E02-07-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan WH, Karnovsky MJ. Increased MMP-2 expression in connective tissue growth factor over-expression vascular smooth muscle cells. J Biol Chem. 2002;277:9800–9805. doi: 10.1074/jbc.M111213200. [DOI] [PubMed] [Google Scholar]
- Fan WH, Pech M, Karnovsky MJ. Connective tissue growth factor (CTGF) stimulates vascular smooth muscle cell growth and migration in vitro. Eur J Cell Biol. 2000;79:915–923. doi: 10.1078/0171-9335-00122. [DOI] [PubMed] [Google Scholar]
- Gaidano G, Bergui L, Schena M, Gaboli M, Cremona O, Marchisio PC, Caligaris-Cappio F. Integrin distribution and cytoskeleton organization in normal and malignant monocytes. Leukemia. 1990;4:682–687. [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Gray MR, Castellot JJ (2005) Function and regulation of CCN5. In: T.M.e. Perbal BV (Ed) CCN proteins: a new family of cell growth and differentiation regulators. Imperial College Press, London
- Grzeszkiewicz TM, Kirschling DJ, Chen N, Lau LF. CYR61 stimulates human skin fibroblast migration through Integrin alpha vbeta 5 and enhances mitogenesis through integrin alpha vbeta 3, independent of its carboxyl-terminal domain. J Biol Chem. 2001;276:21943–21950. doi: 10.1074/jbc.M100978200. [DOI] [PubMed] [Google Scholar]
- Hai CM, Hahne P, Harrington EO, Gimona M. Conventional protein kinase C mediates phorbol-dibutyrate-induced cytoskeletal remodeling in a7r5 smooth muscle cells. Exp Cell Res. 2002;280:64–74. doi: 10.1006/excr.2002.5592. [DOI] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiura K, Lim SS, Little SP, Lin S, Sato M. Differentiation dependent expression of tensin and cortactin in chicken osteoclasts. Cell Motil Cytoskeleton. 1995;30:272–284. doi: 10.1002/cm.970300405. [DOI] [PubMed] [Google Scholar]
- Kireeva ML, Lam SC, Lau LF. Adhesion of human umbilical vein endothelial cells to the immediate-early gene product Cyr61 is mediated through integrin alphavbeta3. J Biol Chem. 1998;273:3090–3096. doi: 10.1074/jbc.273.5.3090. [DOI] [PubMed] [Google Scholar]
- Kumar S, Hand AT, Connor JR, Dodds RA, Ryan PJ, Trill JJ, Fisher SM, Nuttall ME, Lipshutz DB, Zou C, Hwang SM, Votta BJ, James IE, Rieman DJ, Gowen M, Lee JC. Identification and cloning of a connective tissue growth factor-like cDNA from human osteoblasts encoding a novel regulator of osteoblast functions. J Biol Chem. 1999;274:17123–17131. doi: 10.1074/jbc.274.24.17123. [DOI] [PubMed] [Google Scholar]
- Lake AC, Castellot JJ., Jr CCN5 modulates the antiproliferative effect of heparin and regulates cell motility in vascular smooth muscle cells. Cell Commun Signal. 2003;1:5. doi: 10.1186/1478-811X-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake AC, Bialik A, Walsh K, Castellot JJ., Jr CCN5 is a growth arrest-specific gene that regulates smooth muscle cell proliferation and motility. Am J Pathol. 2003;162:219–231. doi: 10.1016/S0002-9440(10)63813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LF, Lam SC (2005) Stephen Integrin-Mediated CCN Functions. In: T.M.e. Perbal BV (Ed) CCN proteins: a new family of cell growth and differentiation regulators. Imperial College Press, London
- Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- Lehto VP, Hovi T, Vartio T, Badley RA, Virtanen I. Reorganization of cytoskeletal and contractile elements during transition of human monocytes into adherent macrophages. Lab Invest. 1982;47:391–399. [PubMed] [Google Scholar]
- Leu SJ, Lam SC, Lau LF. Pro-angiogenic activities of CYR61 (CCN1) mediated through integrins alphavbeta3 and alpha6beta1 in human umbilical vein endothelial cells. J Biol Chem. 2002;277:46248–46255. doi: 10.1074/jbc.M209288200. [DOI] [PubMed] [Google Scholar]
- Lin CG, Chen CC, Leu SJ, Grzeszkiewicz TM, Lau LF. Integrin-dependent functions of the angiogenic inducer NOV (CCN3): implication in wound healing. J Biol Chem. 2005;280:8229–8237. doi: 10.1074/jbc.M404903200. [DOI] [PubMed] [Google Scholar]
- Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/S0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- Linder S, Nelson D, Weiss M, Aepfelbacher M. Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc Natl Acad Sci U S A. 1999;96:9648–9653. doi: 10.1073/pnas.96.17.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S, Higgs H, Hufner K, Schwarz K, Pannicke U, Aepfelbacher M. The polarization defect of Wiskott-Aldrich syndrome macrophages is linked to dislocalization of the Arp2/3 complex. J Immunol. 2000;165:221–225. doi: 10.4049/jimmunol.165.1.221. [DOI] [PubMed] [Google Scholar]
- Linder S, Hufner K, Wintergerst U, Aepfelbacher M. Microtubule-dependent formation of podosomal adhesion structures in primary human macrophages. J Cell Sci. 2000;113(Pt 23):4165–4176. doi: 10.1242/jcs.113.23.4165. [DOI] [PubMed] [Google Scholar]
- Marchisio PC, Cirillo D, Naldini L, Primavera MV, Teti A, Zambonin-Zallone A. Cell-substratum interaction of cultured avian osteoclasts is mediated by specific adhesion structures. J Cell Biol. 1984;99:1696–1705. doi: 10.1083/jcb.99.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchisio PC, Cirillo D, Teti A, Zambonin-Zallone A, Tarone G. Rous sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interactions. Exp Cell Res. 1987;169:202–214. doi: 10.1016/0014-4827(87)90238-2. [DOI] [PubMed] [Google Scholar]
- Mason HR, Lake AC, Wubben JE, Nowak RA, Castellot JJ., Jr The growth arrest-specific gene CCN5 is deficient in human leiomyomas and inhibits the proliferation and motility of cultured human uterine smooth muscle cells. Mol Hum Reprod. 2004;10:181–187. doi: 10.1093/molehr/gah028. [DOI] [PubMed] [Google Scholar]
- Mason HR, Grove-Strawser D, Rubin BS, Nowak RA, Castellot JJ., Jr Estrogen induces CCN5 expression in the rat uterus in vivo. Endocrinology. 2004;145:976–982. doi: 10.1210/en.2003-0823. [DOI] [PubMed] [Google Scholar]
- Minta JO, Yun JJ, Kabiawu O, Jones J. mRNA differential display identification of vascular smooth muscle early response genes regulated by PDGF. Mol Cell Biochem. 2006;281:63–75. doi: 10.1007/s11010-006-0524-6. [DOI] [PubMed] [Google Scholar]
- Nakahara H, Otani T, Sasaki T, Miura Y, Takai Y, Kogo M. Involvement of Cdc42 and Rac small G proteins in invadopodia formation of RPMI7951 cells. Genes Cells. 2003;8:1019–1027. doi: 10.1111/j.1365-2443.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- Osiak AE, Zenner G, Linder S. Subconfluent endothelial cells form podosomes downstream of cytokine and RhoGTPase signaling. Exp Cell Res. 2005;307:342–353. doi: 10.1016/j.yexcr.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Pennica D, Swanson TA, Welsh JW, Roy MA, Lawrence DA, Lee J, Brush J, Taneyhill LA, Deuel B, Lew M, Watanabe C, Cohen RL, Melhem MF, Finley GG, Quirke P, Goddard AD, Hillan KJ, Gurney AL, Botstein D, Levine AJ. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci U S A. 1998;95:14717–14722. doi: 10.1073/pnas.95.25.14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- Perbal BV. CCN Proteins: a new family of cell growth and differentiation regulators. London: Imperial College Press; 2005. [Google Scholar]
- Pfaff M, Jurdic P. Podosomes in osteoclast-like cells: structural analysis and cooperative roles of paxillin, proline-rich tyrosine kinase 2 (Pyk2) and integrin alphaVbeta3. J Cell Sci. 2001;114:2775–2786. doi: 10.1242/jcs.114.15.2775. [DOI] [PubMed] [Google Scholar]
- Piali L, Hammel P, Uherek C, Bachmann F, Gisler RH, Dunon D, Imhof BA. CD31/PECAM-1 is a ligand for alpha v beta 3 integrin involved in adhesion of leukocytes to endothelium. J Cell Biol. 1995;130:451–460. doi: 10.1083/jcb.130.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Shin S, Qin Z, Fisher GJ. Expression of CCN family of genes in human skin in vivo and alterations by solar-simulated ultraviolet irradiation. J Cell Commun Signal. 2009;3:19–23. doi: 10.1007/s12079-009-0044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachfal AW, Brigstock DR. Structural and functional properties of CCN proteins. Vitam Horm. 2005;70:69–103. doi: 10.1016/S0083-6729(05)70003-0. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Tague SE, Muralidharan V, D’Souza-Schorey C. ADP-ribosylation factor 6 regulates tumor cell invasion through the activation of the MEK/ERK signaling pathway. Proc Natl Acad Sci U S A. 2004;101:9671–9676. doi: 10.1073/pnas.0403531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarone G, Cirillo D, Giancotti FG, Comoglio PM, Marchisio PC. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985;159:141–157. doi: 10.1016/S0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- Tatin F, Varon C, Genot E, Moreau V. A signalling cascade involving PKC, Src and Cdc42 regulates podosome assembly in cultured endothelial cells in response to phorbol ester. J Cell Sci. 2006;119:769–781. doi: 10.1242/jcs.02787. [DOI] [PubMed] [Google Scholar]
- Wei L, McKeon F, Russo JW, Lemire J, Castellot J. Domain-and species-specific monoclonal antibodies recognize the Von Willebrand Factor-C domain of CCN5. J Cell Commun Signal. 2009;3:65–77. doi: 10.1007/s12079-009-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Averboukh L, Zhu W, Zhang H, Jo H, Dempsey PJ, Coffey RJ, Pardee AB, Liang P. Identification of rCop-1, a new member of the CCN protein family, as a negative regulator for cell transformation. Mol Cell Biol. 1998;18:6131–6141. doi: 10.1128/mcb.18.10.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]