Abstract
In an attempt to find out a new molecular counterpart of CCN family protein 2 (CCN2), a matricellular protein with multiple functions, we performed an interactome analysis and found fibroblast growth factor (FGF) -1 as one of the candidates. Solid-phase binding assay indicated specific binding between CCN2 and FGF-1. This binding was also confirmed by surface plasmon resonance (SPR) analysis that revealed a dissociation constant (Kd) of 3.98 nM indicating strong molecular interaction between the two. RNA analysis suggested that both FGF-1 and CCN2 could be produced by chondrocytes and thus their interaction in the cartilage is possible. These findings for the first time indicate the direct interaction of CCN2 and FGF-1 and suggest the co-presence of these molecules in the cartilage microenvironment. CCN2 is a well-known promoter of cartilage development and regeneration, whereas the physiological and pathological role of FGF-1 in cartilage mostly remains unclear. Biological role of FGF-1 itself in cartilage is also suspected.
Keywords: FGF-1, CCN2, CTGF, Chondrocyte, Cartilage
Introduction
Most of the bones constructing our body develop from cartilaginous prototypes, which grow and mineralize through endochondral ossification, leaving permanent articular cartilage at the sites of joints (Takigawa, 2013). Even after growth, articular cartilage remains as an indispensable locomotive element supporting our physical activities with flexible movement. Thus, cartilage is a critical tissue for both development and maintenance of our skeleton. Cartilage development and maintenance are executed under the collaboration of multiple extracellular signaling molecules.
During endochondral ossification, chondrocytes undergo proliferative, maturating and hypertrophic stages towards programmed cell death, in order to enable the growth and gradual mineralization of the bone (Takigawa, 2013). Typically, proliferation and maturation of chondrocytes are accelerated by fibroblast growth factor (FGF)-2 and insulin-like growth factor (IGF)-I & 2 or parathyroid hormone (PTH), respectively (Takigawa, 2013; Weksler et al., 1999; Takigawa et al. 1981, 1997). Among such molecules, CCN family protein 2 (CCN2), formerly designated as connective tissue growth factor, was re-discovered as a factor that promotes all of the stages of cartilage development and endochondral ossification (Takigawa, 2013; Nakanishi et al., 2000).
CCN2 is a classical member of the CCN family characterized by the conserved modular structure and matricellular actions (Perbal and Takigawa, 2005; Rachfal and Brigstock, 2005; Leask and Abraham, 2006; Jun and Lau, 2011). Indeed, CCN2 promotes proliferation, maturation and even hypertrophic differentiation of growth plate chondrocytes that conduct endochondral ossification (Kubota and Takigawa, 2007; Takigawa, 2013). Of note, CCN2 also enhances the proliferation and maturation of articular chondrocytes without promoting ectopic hypertrophic differentiation (Nishida et al., 2002). These molecular functions of CCN2 are also confirmed in vivo. Namely, Ccn2-null mice are characterized by delayed endochondral ossification leading to major skeletal defects (Ivkovic et al., 2003; Kawaki et al., 2008). Moreover, CCN2 is known to regenerate damaged articular cartilage in experimental rat osteoarthritis (OA) models in vivo (Nishida et al., 2004; Abd El Kader et al., 2014). The multiple functionality of CCN2 in cartilage is enabled by the interaction with multiple counterparts present in the microenvironment (Kubota and Takigawa, 2007; Takigawa, 2013).
Until today, a significant number of CCN2 counterparts have been identified. For example; growth factors including bone morphogenetic proteins (BMPs)(Kubota and Takigawa, 2007; Maeda et al., 2009), vascular endothelial growth factor (VEGF)(Inoki et al., 2002), FGF-2 (Nishida et al., 2011) and transforming growth factor (TGF) ß (Kubota and Takigawa, 2007); cell surface receptors as typically represented by various integrins (Perbal and Takigawa, 2005); and extracellular matrix (ECM) molecules, such as fibronectin (Hoshijima et al., 2006) and heparan sulfate proteoglycans (Nishida et al., 2003; Gao and Brigstock, 2004; Jun and Lau, 2011). During the course of searching for additional cofactors of CCN2, we found FGF-1 as one of the candidates. Here, our subsequent study uncovered novel molecular action of FGF-1 in the presence of CCN2.
Materials and methods
Cell culture
The human chondrocytic cell line HCS-2/8, established from a human chondrosarcoma, retains chondrocytic properties exemplified by the gene expression and production of type II collagen and aggrecan (Takigawa et al., 1989). The human breast cancer cell line MCF7 and human cervical carcinoma cell line HeLa were also utilized. Human chondrocytes were isolated form the apparently intact regions of articular cartilage obtained upon total knee replacement of OA patients under the approval of the Ethical Committee for Human Research of Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences. These cells were maintained at 37 °C in Dulbecco’s modified Eagle’s medium (D-MEM) supplemented with 10 % fetal bovine serum (FBS) in 5 % CO2. The human chondrocytes were used for experiments within 3 passages in culture.
Interactome analysis
Interaction between CCN2 and more than 9,000 human proteins was comprehensively analyzed with a Proto Array Human Protein Microarray ver. 5.0 (Life Technologies, Carlsbad, CA). Experiments were professionally performed by Filgen incorporated (Nogoya, Japan) by using an oligohistidine-tagged recombinant human CCN2 fragment as a probe. This fragment was produced by a secretory production system with Brevibacillus choshinensis and contains the entire insulin-like growth factor binding protein-like module of CCN2, as described previously (Kubota et al. 2006). Data were analyzed by Proto Array Prospector (Filgen) software specifically designed for this array system.
Solid phase binding assay
Evaluation of direct interaction between CCN2 and FGF-1 on ELISA plates was performed as described previously (Aoyama, Kubota and Takigawa, 2012). Briefly, wells of ELISA plates coated with 1 μg/ml of recombinant human CCN2 (BioVendor Laboratory Medicine) or 2 % bovine serum albumin (BSA), and were blocked with 100 μl of binding buffer for 2 h at room temperature. Diluted recombinant human FGF-1 (PeproTech, Rocky Hill, NJ) was then added to the wells, and incubation was conducted for 2 h at 37 °C. Thereafter, the wells were washed and incubated primary with an anti-human FGF-1 antibody (Abnova, Taipei, Taiwan) and then with a horseradish peroxidase (HRP)-conjugated anti-human IgG antibody (Sigma Aldrich, St Louis, MO); and the bound HRP was then monitored by using 3,3,5,5 -tetramethylbenzidine (TMB) peroxidase substrate (Sigma-Aldrich).
Surface plasmon resonance (SPR) analysis
Kinetic analysis of the interaction between CCN2 and FGF-1 was analyzed by using a BIAcore X (GE HealthCare, Little Chalfont, UK). As a ligand, CCN2 was immobilized onto a CM5 sensor chip according to the manufacturer’s protocol. FGF-1 diluted in HBS-EP buffer (10 mM HEPES, 0.15 M NaCl, 3 mM EDTA and 0.005 % Tween 20; pH 7.4) at 6.25, 12.5, 25, 50 or 100 nM was perfused over the control surface or a surface bearing CCN2, and the resonance changes were monitored. The response was standardized by subtracting the one on the control from the one on the CCN2-conjugated surface. Data analysis and computation of dissociation constant (Kd) were performed by the BIAevaluation software version 4.1 with the single cycle kinetics support package (GE Healthcare)(Aoyama, Kubota and Takigawa, 2012).
RNA extraction and quantitative real-time reverse transcription polymerase chain reaction (RT-PCR)
The cells were cultured at 37 °C with 5 % CO2 in the air and were allowed to reach confluence. Then, total RNA was extracted and purified from the cells with Isogen (Nippongene, Tokyo, Japan) or RNeasy kit (Qiagen, Hilden, Germany), following the manufacturers’ instructions. Total RNA (500 ng) was reverse transcribed by avian myeloblastosis virus (AMV) reverse transcriptase (Takara, Otsu, Japan) at 42 °C for 30 min, according to the manufacturer’s protocol. Real-time PCR was performed by using TOYOBO SYBR Green PCR Master Mix (TOYOBO, Osaka, Japan) in a LightCycler™ system (Roche, Basel, Switzerland) as described previously (Moritani et al. 2005). The nucleotide sequences of the primers used are as follows: 5′- GCA GGC TAG AGA AGC AGA GC −3′ (sense) and 5′- ATG TCT TCA TGC TGG TGC AG −3′ (antisense) for CCN2; 5′-ACA AGG GAC AGG AGC GAC-3′ (sense) and 5′-TCC AGC CTT TCC AGG AAC A-3′ (antisense) for FGF1; 5′-GCC AAA AGG GTC ATC ATC TC-3′ (sense) and 5′-GTC TTC TGG GTG GCA GTG AT-3′ (antisense) for GAPDH.
General
Unless otherwise specified, all of the evaluations were performed at least twice, yielding comparable results.
Results
CCN2 partner candidates as revealed by interactome analysis
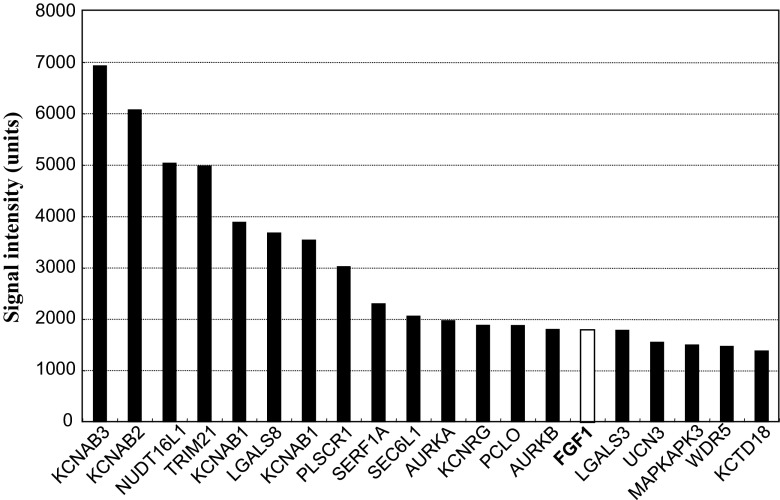
In order to find out possible counterparts of CCN2, we screened more than 9,000 proteins on a microarray and found 139 proteins giving significant binding signal to the CCN2 probe in duplicate. From these candidates, proteins that appear to be related to extracellular signaling were further selected. Figure 1 shows the summary of the signals yielded by these candidates. These proteins include several lectins and ion channels, but only a few growth factors were involved therein. Among them, FGF-1 showed the highest signals for CCN2, and thus was selected for further analysis in this study.
Fig. 1.
Twenty candidates for CCN2 cofactors that may be related to cell signaling. Interactomic analysis with a protein array revealed more than 100 proteins that may directly bind to human CCN2. Among them, 20 proteins related to cellular signaling are shown as gene symbols with mean signal intensities from 2 independent spots. FGF-1 is highlighted in bold letters with an open column
Direct and strong binding of FGF-1 to CCN2
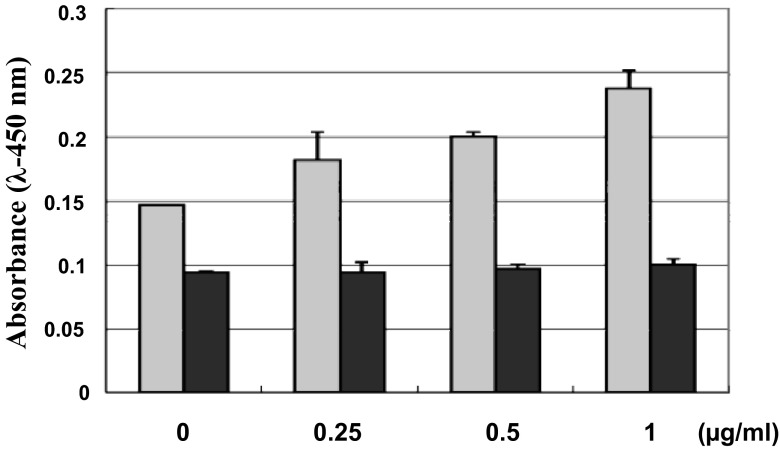
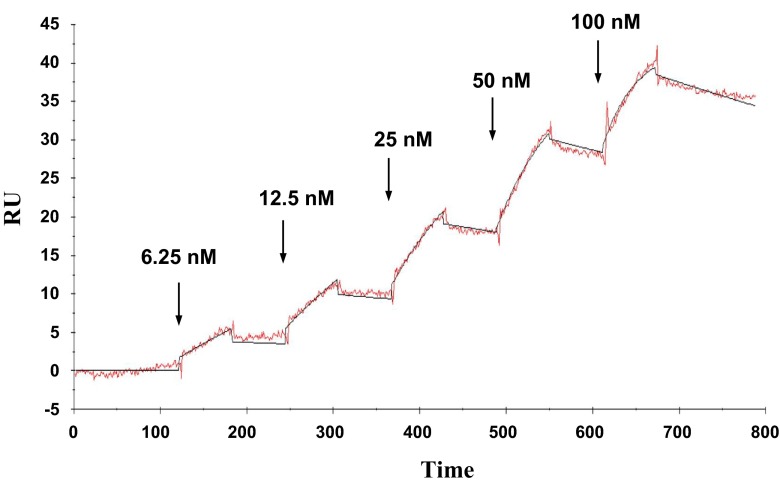
To confirm the specific binding of FGF-1 to CCN2, a conventional solid phase binding assay was initially employed. As a result, significant binding of FGF-1 to CCN2 coated on the ELISA plate was observed in a dose dependent manner, whereas that onto BSA showed only a background signal without dose response (Fig. 2), indicating specific interaction between FGF1 and CCN2. Next, molecular interaction of these 2 molecules was kinetically analyzed by an SPR methodology. As shown in Fig. 3, increasing concentrations of FGF-1 revealed dose-dependent response in the resonance units representing molecular interaction, as observed in the solid-phase binding assay. Based on the sensorgram obtained, we computed the Kd of this FGF-1-CCN2 interaction. The Kd value was 3.98 nM, which is indeed comparable to the affinity of interactions between FGFs and their canonical receptors (Aoyama, Kubota and Takigawa, 2012).
Fig. 2.
Direct binding of FGF-1 to CCN2 as evaluated by solid phase binding assay. Binding of FGF-1 to CCN2 (gray columns) and BSA (black columns) coated onto an ELISA plate surface was colorimetrically evaluated. Concentrations of FGF-1 applied onto the ELISA plate are indicated on the abscissa
Fig. 3.
Kinetic analysis of direct binding between FGF-1 and CCN2 by SPR methodology. Interaction of FGF-1 as an analyte and CCN2 immobilized to the sensor chip was kinetically analyzed with Biacore X system. The experiment and analysis were performed following the single kinetics protocol with sequential injection of increasing concentrations of the analyte, as shown on the graph. RU: resonance units
Specific expression of CCN2 and FGF-1 in chondrocytic cells
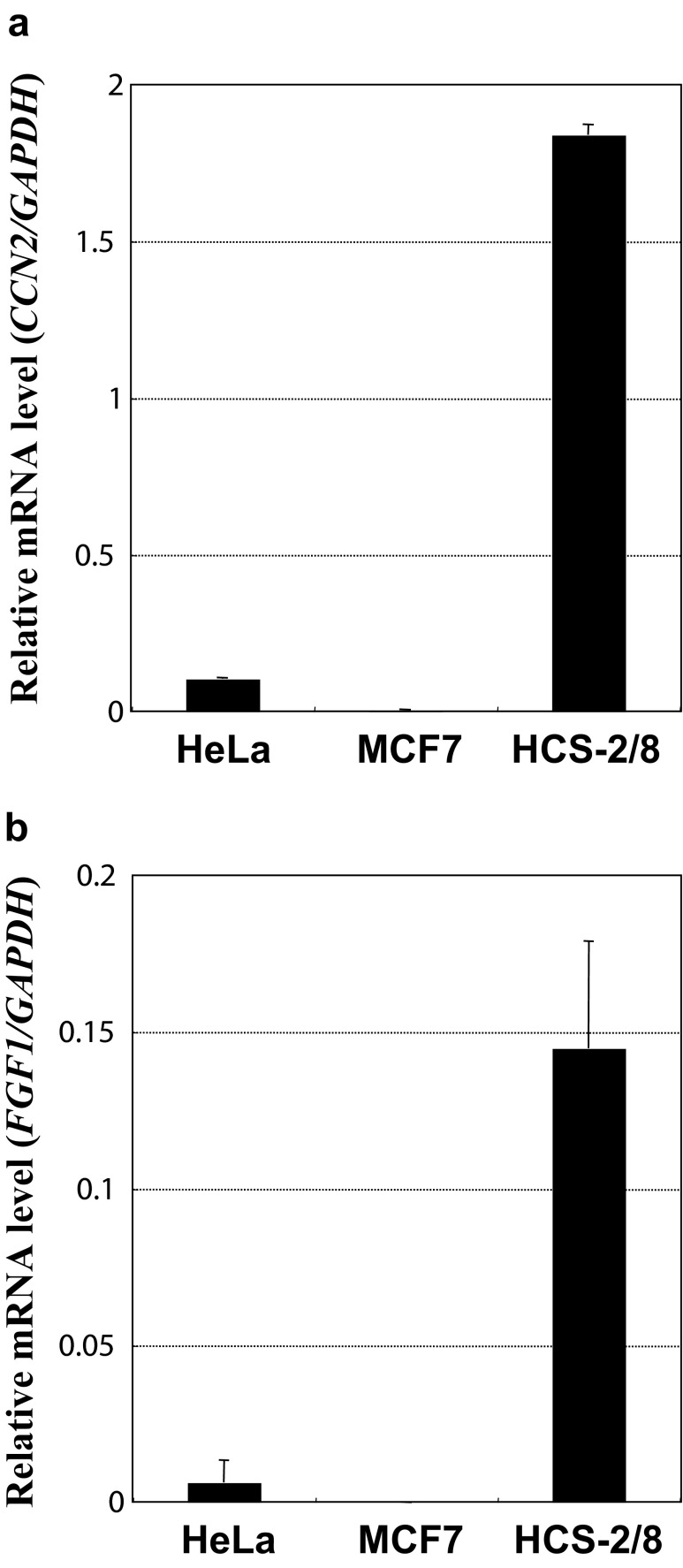
Although FGF-1 was capable of binding to CCN2, it is necessary for these molecules to actually coexist in biological microenvironments in order to have interactions. In our previous studies, vigorous production of CCN2 by a human chondrocytic cell line, HCS-2/8, was observed; however, it remained to be examined whether FGF-1 is produced therein. Suspecting possible co-existence and molecular interaction of FGF-1 and CCN2, we evaluated the gene expression of FGF-1 in those cells. Quantitative real-time RT-PCR analysis indicated strong gene expression of both CCN2 and FGF-1 in HCS-2/8 cells, whereas human cell lines from different origins showed much lower expression (Fig. 4). These results suggest specific production of FGF-1 by chondrocytes and possible interaction between FGF-1 and CCN2 in cartilage.
Fig. 4.
Specific expression of FGF-1 and CCN2 in human chondrocytic HCS-2/8 cells. Distinct gene expression of CCN2 a and FGF-1 b was observed in chondrocytic HCS-2/8 cells, whereas the other non-chondrocytic cell lines showed almost no expression of those genes. Gene expression levels are represented by relative values against those of GAPDH with error bars of standard deviations
Co-expression of CCN2 and FGF-1 in human chondrocytes from OA patients
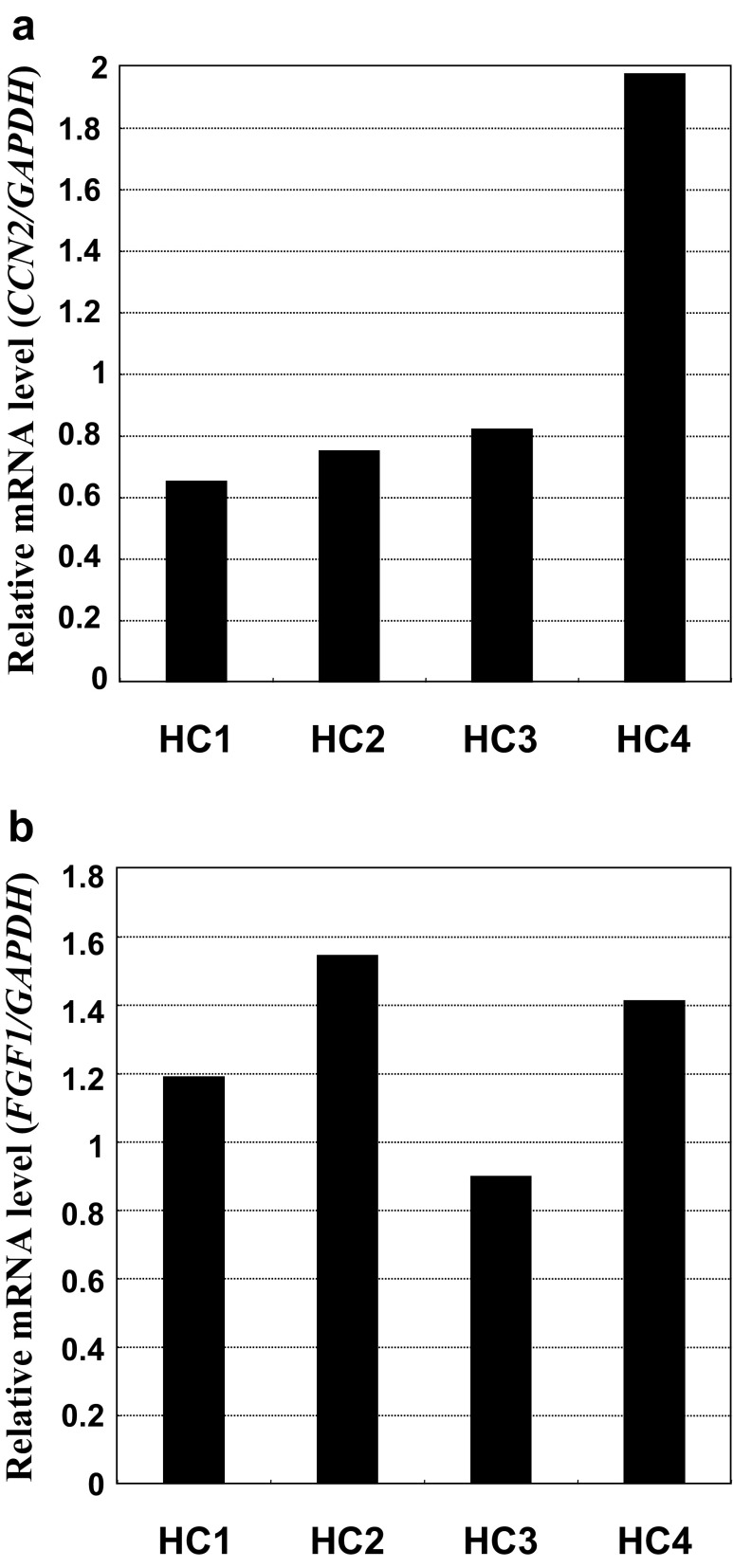
Osteoarthritis (OA) is a major locomotive disorder of joints that seriously affects the quality of life of patients. Although CCN2 is known to be produced in OA cartilage, no report described the co-expression of CCN2 and FGF1 in chondrocytes from OA patients. As a final step of our present research, we performed the same analysis as we employed for Fig. 4 with OA chondrocytes. As displayed in Fig. 5, expression of these genes was distinctly observed in chondrocytes from the articular cartilage of OA patients, suggesting the presence of FGF-1 and CCN2-FGF-1 interaction in articular cartilage.
Fig. 5.
Concomitant expression of FGF-1 and CCN2 in human articular chondrocytes from OA patients. Expression of CCN2 a and FGF-1 b in human chondrocytes obtained from knee joints upon total knee replacement therapy of OA. Values obtained from each individual (HC1 - 4) are shown. Gene expression levels are represented by relative values against those of GAPDH
Discussion
In this study, we, for the first time, found that FGF-1 and CCN2 specifically and directly interact with high affinity. Recently, interaction of FGF signaling and CCN2 has been attracting the interest of the scientists in the relevant fields. In fact, FGF-2, another classical member of the FGF family, binds to CCN2 and thus affects the phenotype of chondrocytes (Nishida et al., 2011). Moreover, direct interaction between FGF receptor 2 (FGFR2) and CCN2 is also indicated, which results in the modification of intracellular signaling in osteoblastic cells (Aoyama, Kubota and Takigawa, 2012). Together with the present data, these findings suggest an intimate relationship between CCN family and FGF signaling. Therefore, mutual interaction has to be considered, whenever the functionality of each member of CCN and FGF families is characterized.
In relation to cartilage biology, molecular function and biological role have been extensively characterized, particularly in FGF-2 and FGF-18 (Ellman, et al., 2008). In the case of FGF-18, its anabolic effects on chondrocytes are firmly supported by a number of experimental data including increased proteoglycan synthesis and type II collagen production by FGF-18 (Ellman, et al., 2008). On the contrary, a number of previous publications indicate that FGF-2 enhances proliferation of chondrocytes, whereas ECM degradation is accelerated simultaneously (Ellman, et al., 2008). Although much less information is available, the functionality of FGF-1 in cartilage described in a few previous studies appears contradictory. One report shows the induction of proliferation of sheep chondrocytes (Acosta et al., 2006), while another indicates its repression in rodent chondrocytes mediated by the STAT pathway (Sahni et al., 1999). The discrepancy between these results is anticipated to result from the difference in the molecular background of the experimental systems used, in which CCN2 may be involved.
Since FGF-1 is structurally and functionally similar to FGF-2, FGF-1 itself and CCN2-FGF-1 interaction are suspected to play some roles in the biology of articular cartilage. Investigation to clarify this topic is currently in progress.
Acknowledgments
The authors thank Dr. Emilio S. Hara, Dr. Takako Hattori and Mr. Tsutomu Kidate for their helpful support in experiments, as well as Ms. Yoshiko Miyake for her secretarial assistance. This study was supported by grants from the program Grants-in-aid for Scientific Research (B) [#24390415 to M.T.], and (C) [#25462886 to S.K.] from the Japan Society for the Promotion of Science.
Contributor Information
Satoshi Kubota, Phone: +81-86-235-6645, Email: kubota1@md.okayama-u.ac.jp.
Masaharu Takigawa, Phone: +81-86-235-6645, Email: takigawa@md.okayama-u.ac.jp.
References
- Abd El Kader T, Kubota S, Nishida T, Hattori T, Aoyama E, Janune D, Hara ES, Ono M, Tabata Y, Kuboki T, Takigawa M. The regenerative effects of CCN2 independent modules on chondrocytes in vitro and osteoarthritis models in vivo. Bone. 2014;59:180–188. doi: 10.1016/j.bone.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Acosta CA, Izal I, Ripalda P, Douglas-Price AL, Forriol F. Gene expression and proliferation analysis in young, aged, and osteoarthritic sheep chondrocytes effect of growth factor treatment. J Orthop Res. 2006;24:2087–94. doi: 10.1002/jor.20245. [DOI] [PubMed] [Google Scholar]
- Aoyama E, Kubota S, Takigawa M. CCN2/CTGF binds to fibroblast growth factor receptor 2 and modulates its signaling. FEBS Lett. 2012;586:4270–4275. doi: 10.1016/j.febslet.2012.10.038. [DOI] [PubMed] [Google Scholar]
- Ellman MB, An HS, Muddasani P, Im HJ. Biological impact of the fibroblast growth factor family on articular cartilage and intervertebral disc homeostasis. Gene. 2008;420:82–89. doi: 10.1016/j.gene.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Brigstock DR. Connective tissue growth factor (CCN2) induces adhesion of rat activated hepatic stellate cells by binding of its C-terminal domain to integrin alpha(v)beta(3) and heparan sulfate proteoglycan. J Biol Chem. 2004;279:8848–8855. doi: 10.1074/jbc.M313204200. [DOI] [PubMed] [Google Scholar]
- Hoshijima M, Hattori T, Inoue M, Araki D, Hanagata H, Miyauchi A, Takigawa M. CT domain of CCN2/CTGF directly interacts with fibronectin and enhances cell adhesion of chondrocytes through integrin alpha5beta1. FEBS Lett. 2006;580:1376–1382. doi: 10.1016/j.febslet.2006.01.061. [DOI] [PubMed] [Google Scholar]
- Inoki I, Shiomi T, Hashimoto G, Enomoto H, Nakamura H, Makino K, Ikeda E, Takata S, Kobayashi K, Okada Y. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J. 2002;16:219–221. doi: 10.1096/fj.01-0332fje. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:9459–9463. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaki H, Kubota S, Suzuki A, Lazar N, Yamada T, Matsumura T, Ohgawara T, Maeda T, Perbal B, Lyons KM, Takigawa M. Cooperative regulation of chondrocyte differentiation by CCN2 and CCN3 shown by a comprehensive analysis of the CCN family proteins in cartilage. J Bone Miner Res. 2008;23:1751–1764. doi: 10.1359/jbmr.080615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S, Takigawa M. Role of CCN2/CTGF/Hcs24 in bone growth. Int Rev Cytol. 2007;257:1–41. doi: 10.1016/S0074-7696(07)57001-4. [DOI] [PubMed] [Google Scholar]
- Kubota S, Kawaki H, Kondo S, Yosimichi G, Minato M, Nishida T, Hanagata H, Miyauchi A, Takigawa M. Multiple activation of mitogen-activated protein kinases by purified independent CCN2 modules in vascular endothelial cells and chondrocytes in culture. Biochimie. 2006;88:1973–1981. doi: 10.1016/j.biochi.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- Maeda A, Nishida T, Aoyama E, Kubota S, Lyons KM, Kuboki T, Takigawa M. CCN family 2/connective tissue growth factor modulates BMP signalling as a signal conductor, which action regulates the proliferation and differentiation of chondrocytes. J Biochem. 2009;145:207–216. doi: 10.1093/jb/mvn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritani NH, Kubota S, Sugahara T, Takigawa M. Comparable response of ccn1 with ccn2 genes upon arthritis: an in vitro evaluation with a human chondrocytic cell line stimulated by a set of cytokines. Cell Commun Signal. 2005;3:6. doi: 10.1186/1478-811X-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi T, Nishida T, Shimo T, Kobayashi K, Kubo T, Tamatani T, Tezuka K, Takigawa M. Effects of CTGF/Hcs24, a product of a hypertrophic chondrocyte-specific gene, on the proliferation and differentiation of chondrocytes in culture. Endocrinology. 2000;141:264–273. doi: 10.1210/endo.141.1.7267. [DOI] [PubMed] [Google Scholar]
- Nishida T, Kubota S, Nakanishi T, Kuboki T, Yosimichi G, Kondo S, Takigawa M. CTGF/Hcs24, a hypertrophic chondrocyte-specific gene product, stimulates proliferation and differentiation, but not hypertrophy of cultured articular chondrocytes. J Cell Physiol. 2002;192:55–63. doi: 10.1002/jcp.10113. [DOI] [PubMed] [Google Scholar]
- Nishida T, Kubota S, Fukunaga T, Kondo S, Yosimichi G, Nakanishi T, Takano-Yamamoto T, Takigawa M (2003) CTGF/Hcs24, hypertrophic chondrocyte-specific gene product, interacts with perlecan in regulating the proliferation and differentiation of chondrocytes. J Cell Physiol 196:265-275 [DOI] [PubMed]
- Nishida T, Kubota S, Kojima S, Kuboki T, Nakao K, Kushibiki T, Tabata Y, Takigawa M. Regeneration of defects in articular cartilage in rat knee joints by CCN2 (connective tissue growth factor) J Bone Miner Res. 2004;19:1308–1319. doi: 10.1359/JBMR.040322. [DOI] [PubMed] [Google Scholar]
- Nishida T, Kubota S, Aoyama E, Janune D, Maeda A, Takigawa M. Effect of CCN2 on FGF2-induced proliferation and MMP9 and MMP13 productions by chondrocytes. Endocrinology. 2011;182:4232–4241. doi: 10.1210/en.2011-0234. [DOI] [PubMed] [Google Scholar]
- Perbal B, Takigawa M (2005) CCN proteins -A new family of cell growth and differentiation regulators. Imperial College Press, London
- Rachfal AW, Brigstock DR. Structural and functional properties of CCN proteins. Vitam Horm. 2005;70:69–103. doi: 10.1016/S0083-6729(05)70003-0. [DOI] [PubMed] [Google Scholar]
- Sahni M, Ambrosetti DC, Mansukhani A, Gertner R, Levy D, Basilico C. FGF signaling inhibits chondrocyte proliferation and regulates bone development through the STAT-1 pathway. Genes Dev. 1999;13:1361–1366. doi: 10.1101/gad.13.11.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takigawa M. CCN2: a master regulator of the genesis of bone and cartilage. J Cell Commun Signal. 2013;7:191–201. doi: 10.1007/s12079-013-0204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takigawa M, Takano T, Suzuki F. Effects of parathyroid hormone and cyclic AMP analogues on the activity of ornithine decarboxylase and expression of the differentiated phenotype of chondrocytes in culture. J Cell Physiol. 1981;106:259–268. doi: 10.1002/jcp.1041060212. [DOI] [PubMed] [Google Scholar]
- Takigawa M, Tajima K, Pan HO, Enomoto M, Kinoshita A, Suzuki F, Takano Y, Mori Y. Establishment of a clonal human chondrosarcoma cell line with cartilage phenotypes. Cancer Res. 1989;49:3996–4002. [PubMed] [Google Scholar]
- Takigawa M, Okawa T, Pan H, Aoki C, Takahashi K, Zue J, Suzuki F, Kinoshita A. Insulin-like growth factors I and II are autocrine factors in stimulating proteoglycan synthesis, a marker of differentiated chondrocytes, acting through their respective receptors on a clonal human chondrosarcoma-derived chondrocyte cell line, HCS-2/8. Endocrinology. 1997;138:4390–4400. doi: 10.1210/endo.138.10.5265. [DOI] [PubMed] [Google Scholar]
- Weksler NB, Lunstrum GP, Reid ES, Horton WA. Differential effects of fibroblast growth factor (FGF) 9 and FGF2 on proliferation, differentiation and terminal differentiation of chondrocytic cells in vitro. Biochem J. 1999;342:677–682. doi: 10.1042/0264-6021:3420677. [DOI] [PMC free article] [PubMed] [Google Scholar]