INTRODUCTION
Degenerative disc disease is a common pathological disorder accompanied by both structural and biochemical changes which may be associated with aging, biomechanical insult and genetic background [1]. Disc degeneration is biologically-mediated with complex biochemical and inflammatory processes that alter the homeostasis of the disc’s extracellular matrix [2,3]. Progression of degeneration subsequently affects the composition of the disc. Reduced proteoglycan content in the disc [4] and thus decreased hydration and disc height loss, increased collagen type I in the inner one half of the anulus fibrosus (AF) and nucleus pulposus (NP) and increased collagen cross-linking [5] are all associated with degeneration progression. Mechanical factors (i.e., vibration, torsion, and compression/loading) are also implicated in the induction of disc degeneration [6,7].
Mechanical adaptations subsequently occur as a result of these biochemical changes in the disc. Specifically, as hydration levels in the NP drop, loading patterns on the disc are altered resulting in abnormal shear and compressive stresses experienced by the AF rather than the NP [8-10]. These changes in stress distribution across the disc are likely the main culprit of structural damage, including increased delamination [11], fissure formation [12], and inward lamellae buckling [13], that have been observed in the AF following degeneration. Delamination, specifically, is characterized by the separation of individual layers in the AF from each other as a result of failure of the adhesive bond. If this occurs in the disc, the integrity or strength of the AF is likely compromised thereby increasing the risk of further damage.
The focus of the current study was to examine the effect of disc degeneration on the resistance to delamination in the AF. The examination of structural changes to the disc, and in particular the AF, as a result of degeneration is of considerable importance as most structural damage is irreversible in the aging disc. This is due to significantly slowed metabolism and extracellular protein synthesis [4,14] preventing sufficient healing despite evidence of increased cytokine and matrix metalloproteinase secretion [15]. By understanding the effects of degeneration on the disc, biological repair therapies can be developed to allow for more specific target of the weakened structures. Therefore, the purpose of this study was to determine the peel strength of the inter-lamellar matrix, and thus resistance to delamination, in rabbit AF tissue following induced degeneration. A unique peel test design [16] was used to measure the delamination strength of the inter-lamellar matrix of the AF following degeneration development. In short, this test allows for the quantification of the material properties of the matrix found between layers of the AF by employing a 180-degree mechanical peel test.
METHODS
Animal Model
An established anular puncture intervertebral disc degeneration model in the New Zealand white rabbit (Western Oregon Rabbit Co., Philomath, Oregon USA) was used for the current study [17]. Specifically, eight rabbits (4.6 kg ± 0.2) underwent spinal surgery to induce disc degeneration (termed experimental animals) and four additional rabbits (4.7 kg ± 0.2) served as control animals. The study protocol was approved by the University’s Animal Care Use Committee (IACUC).
Four of the experimental animals underwent spinal surgery at eight months of age and were sacrificed one month post-surgery (nine months of age), while the remaining four experimental animals underwent surgery at six months of age and were sacrificed three months post-surgery (therefore also nine months of age at time of sacrifice). The four control animals were sacrificed at nine months of age and did not undergo any surgical operation.
Anular Puncture Disc Degeneration Model
Prior to spinal surgery, animals were anaesthetized with xylazine (5 mg/kg; Akron, Inc., Decatur, IL USA) and ketamine hydrochloride (35 mg/kg; Fort Dodge Animal Health, Fort Dodge, Iowa USA) by subcutaneous injection. Anaesthesia levels were maintained during surgery with 1-3% isoflurane (Piramal Critical Care, Boise, ID USA). Each rabbit was positioned laterally on its right side and a sagittal plane radiograph was taken to locate the L3/4 intervertebral disc. An incision (approximately 6 cm in length) was made along the postero-lateral side of the animal (right side of body) into the retroperitoneal space. Using the L3/4 disc as a guide, the L2/3 and L4/5 discs were located and were subsequently punctured with an 18-gauge needle through the antero-lateral region of the AF [17]; Figure 1A. Care was taken to ensure the needle did not puncture the posterior AF tissue. Following disc punctures, the surgical site was cleaned with sterile saline and closed using layered sutures. Each rabbit was then given a subcutaneous injection of buprenorphine hydrochloride (analgesic; 0.05 mg/kg; Reckitt Benchkiser Pharmacueticals, Richmond, VA USA) and Cefazolin (antibiotic; 22 mg/kg; Steri-Pharma, LLC, Syracuse, NY USA) and monitored every 15 minutes (heart rate, blood oxygen, body temperature, and respiratory rate). Once fully alert, the animals were returned to their cages and allowed to resume regular daily activity. Additional doses of buprenorphine hydrochloride and Cefazolin were given one and two days post-surgery. Animals were monitored each day until sacrifice.
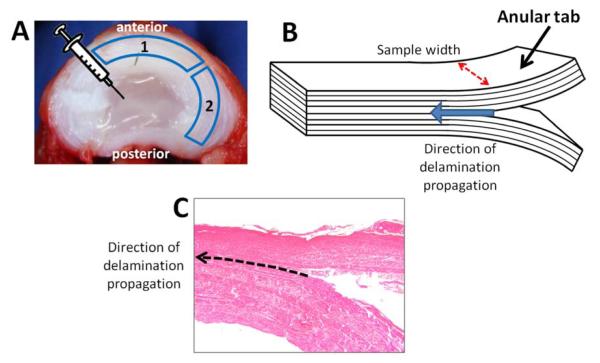
Figure 1.
Delamination strength measurement of the anulus fibrosis (AF) in the anular-puncture rabbit model of disc degeneration. A – Axial view of intervertebral disc. Needle icon represents location of AF puncture. B – Illustration of tissue sample. Anular tabs are mounted into a material testing system to propagate the peel test. Dashed arrow indicates tissue width used to calculate peel strength. Block arrow indicates direction of delamination (peel) propagation. C – Tissue sample slice showing delamination between the centre-most AF layers. Dashed arrow indicated direction of delamination propagation.
Image Analysis
Radiographs and Disc Height
Disc height was monitored by lateral radiographs taken 1, 2, 4 (for all experimental animals), 8, and 12 weeks post-op (only for the four animals that were sacrificed 3 months post-surgery) under anesthesia with ketamine hydrochloride (35mg/kg) and acepromazine maleate (1 mg/kg; VEDCO, INC. St. Joseph, MO, USA). Care was taken to ensure similar level of anaesthesia for each radiograph. All animals were sacrificed while anaesthetized via venous (marginal ear vein) injection of Euthasol (1 ml/4.54 kg; Virbac Animal Health, Fort Worth, TX USA). Disc height loss was determined by calculating the disc height index (DHI) [17] (Masuda et al., 2005) for each radiograph and comparing it to the DHI just prior to surgery.
MR Imaging
Following sacrifice, the lumbar spine and surrounding musculature were removed and immediately MR imaged at 3T on GE Signa HDx using the following parameters: spin echo sequence: TR=2000 ms, TE=10-70 ms (8 echoes; for sagittal images showing L2/3 to L4/5) or 70 ms (for axial images); FOV=80 mm, matrix=384×384, slice=2.5 mm. For T2 quantification, regions of interest representing the NP and AF were placed on sagittal images, and T2 relaxation times and T2 maps were determined using mono-exponential fitting using a Matlab program. Using the T2 values in L3/4 as internal control, normalized T2 values were determined. Following MR imaging, spines were frozen at −80°C until used for mechanical testing.
Tissue Sample Preparation and Mechanical Testing
Lumbar spines were thawed at room temperature and the L2/3 (punctured), L3/4 (non-punctured), L4/5 (punctured), and L6/7 (non-punctured) intervertebral discs were dissected from the lumbar spine and were hydrated with 10% phosphate-buffered saline. The two non-punctured discs served as internal controls; the L3/4 was adjacent to the punctured discs while the L6/7 disc was two levels away from the closest punctured disc. The same disc levels were removed from the four non-operated control rabbits (termed “external control” discs) yielding a total of 48 discs (from 12 rabbits).
Two tissue samples (containing approximately eight AF layers) were dissected from each disc: one from the anterior region of the disc and the other from the posterior-lateral region of the disc (Figure 1A). Samples could not be obtained from the complete posterior region due to size constraints. A T- (180°) peel test sample was created by manually peeling apart the two most middle layers, thus forming tabs for mechanical testing (Figure 1B and 1C); [16]. Figure 1C shows a histological view of a T-peel test sample. This histological image illustrates the delamination, or peeling, between two adjacent layers of the AF.
Tissues were mounted at the tabs created via manual peeling and were further peeled at a rate of 0.5 mm/sec using a material testing apparatus (5565A, Instron, Norwood, MA USA) [16]. This test accomplished delamination (peeling) between the two middle AF layers. The resulting force-displacement curves showed the characteristic plateau or “leveling-off” observed during peel tests of adhesive materials [16]. From this peel test curve, the peel strength (averaged over the plateau region, normalized to tissue sample width (Figure 1B), units N/mm), was obtained for each test.
Statistics
Data were collapsed across sacrifice date (one and three months post-surgery measurements were pooled) as this was not found to have a significant effect on the peel strength of the degenerated anulus samples (p=0.57). Subsequently, a two-way repeated measures ANOVA was conducted on condition (external control (non-punctured discs from non-operated control animals); internal control (non-punctured discs from operated animals); and punctured (punctured discs from operated animals)) and AF location (anterior; posterior-lateral) on the variable peel strength. A t-test was conducted on condition (punctured L2/3 and L4/5 discs from operated animals versus non-punctured L2/3 and L4/5 discs from non-operated animals) on normalized T2 relaxation times. Statistical significance was set at p < 0.05.
RESULTS
Effect of Puncture on Degeneration
Disc Height Loss observed in X-Ray Images
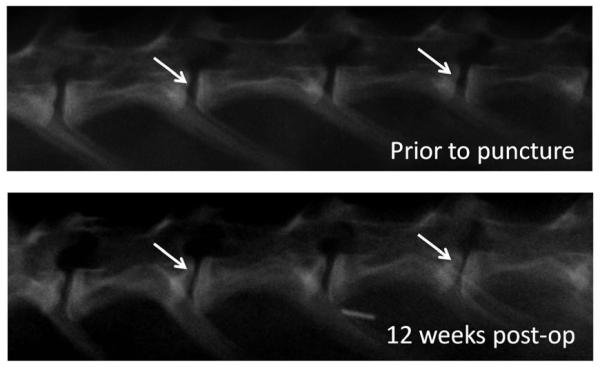
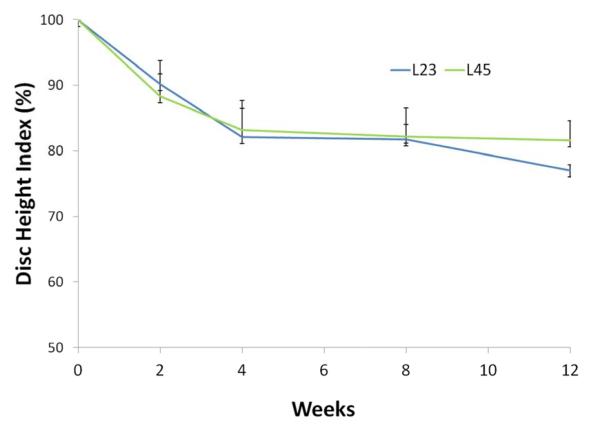
Punctured discs showed a substantial decrease in disc height following puncture (Figure 2 and 3) in the X-Ray images taken. Specifically, four weeks post-surgery, the DHI for L2/3 discs was an average (± standard error) of 82.1 ± 4.0% (i.e. 17.9% less disc height than internal control) and for L4/5 an average of 83.1 ± 4.2%. Twelve weeks post-surgery, the average DHI for L2/3 and L4/5 was 77.0 ± 0.9% and 81.6 ± 3.0%, respectively (Figure 3)
Figure 2.
Sagittal X-Ray images of the rabbit lumbar spine prior to and 12 weeks following anular puncture. The arrows indicate the punctured discs. Note the narrowing of the disc space 12 weeks post-surgery as compared to before the spinal surgery.
Figure 3.
Disc height index scores at 2, 4, 8 and 12 weeks following anular-puncture in the rabbit. Disc height index values are relative to the L3/4 internal control (100% disc height index shown by dashed line).
MRI T2 values
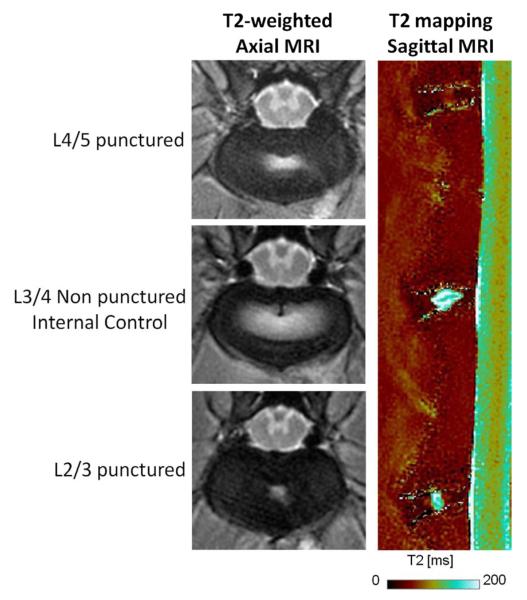
T2 values were obtained from the MR images of the experimental spines following harvest (Figure 4). NP T2 values were found to be significantly lower in the punctured discs as compared to non-punctured control discs (p<0.001) suggesting lower hydration levels in the NP of punctured discs. Specifically, the average (± standard error) normalized T2 values were 0.63 ± 0.12 and 1.04 ± 0.04 for punctured and non-punctured discs, respectively. However, no significant difference in the AF T2 values were observed between the punctured and non-punctured discs (p=0.86).
Figure 4.
Axial MR images of the two punctured discs (L2/3 and L4/5) as well as one internal control between (L3/4) in the anular-puncture rabbit model of disc degeneration. Note the reduced white area in the punctured discs. This indicates lower hydration of the NP region. Sagittal MR image also shows significantly lower T2 times in the punctured discs as compared to the internal control disc (p=0.002).
Effect of Puncture-induced Degeneration on Material Properties of Anulus Fibrosus
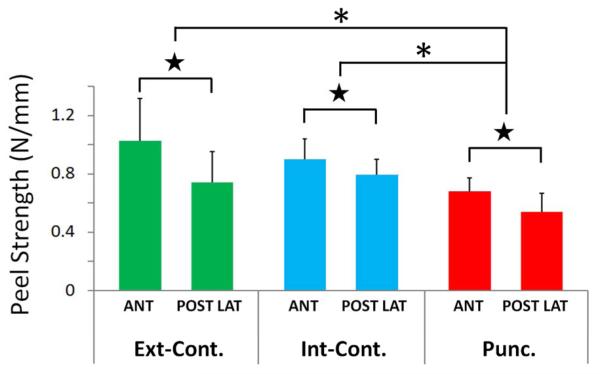
Punctured discs exhibited significantly lower peel strength in the inter-lamellar matrix of the AF as compared to non-punctured discs (p=0.024); Figure 5. This was found in comparison to both internal and external control discs. However, peel strength did not vary between internal and external control discs (p=0.74). Specifically, peel strength of the AF from punctured discs (mean 0.62 N/mm ± 0.16 S.E.) was found to be 27% lower than from non-punctured internal control discs (0.85 N/mm ± 0.17 S.E.) and 30% lower than the peel strength of AF obtained from external control discs (0.88 N/mm ± 0.26 S.E.).
Figure 5.
Peel strength measurements of the anulus fibrosis (AF) in the anular-puncture rabbit model of disc degeneration. Average peel strength values (N/mm) for the external control disc samples (Ext-Cont.), internal control disc samples (Int-Cont.) and punctured disc samples (Punc.). A significant drop in peel strength values was observed in the punctured disc samples as compared to both in internal and external control samples (denoted by asterisks; p=0.024). Further, samples obtained from the posterior-lateral AF (POST LAT) exhibited significantly lower peel strength values than samples obtained from the anterior AF (ANT), denoted by stars (p=0.028).
Effect of Location on Mechanical Properties of Anulus Fibrosus
The location of the AF from which the tissue sample was obtained had a significant effect on the peel strength values. Specially, the peel strength of tissues obtained from the anterior region of the AF was, on average 0.88 N/mm (± 0.13 S.E.), compared to 0.70 N/mm (± 0.11 S.E.) in the posterior-lateral region of the AF (p = 0.028); Figure 5.
DISCUSSION
Anular puncture successfully induced intervertebral disc degeneration, as previously reported [17], evidenced by the disc height loss and reduced NP hydration observed in the current study. Further, this degeneration also significantly affected the material properties of the AF tissue, in particular the peel strength, or resistance to delamination. Disc degeneration specifically reduced the AF’s ability to resist delamination and effectively reduced the inter-lamellar matrix integrity.
As NP hydration levels decrease, loading patterns on the disc are altered resulting in abnormal shear and compressive stresses migrating towards the AF from the NP [8-10]. In turn, these stresses lead to weakening of the inter-lamellar strength and consequent delamination between the AF layers [12,18]. Given that the current study measured peel strength, and thus resistance to delamination, disc degeneration appears to weaken the inter-lamellar matrix which would presumably increase the likelihood of AF layers delaminating from one another. Interestingly, the hydrations levels of the AF did not change as a result of the puncture. This suggests that significant hydration had not been lost post-puncture. However, changes in the biomechanics of the AF were observed, suggesting that degeneration had begun even prior to changes in hydration.
The inter-lamellar matrix has been previously examined in terms of its structural composition. Specifically, this matrix, which exists between the individual lamellae of the AF, contains type VI collagen fibres [19,20], proteoglycans [21], and elastin [22,23]. Each of these components plays a vital role in ensuring matrix integrity and resistance to delamination. Being able to resist this separation of AF layers is of particular importance as it is been hypothesized that a certain degree of delamination must be present in order for intervertebral disc herniation, a potential precursor of degeneration, to progress through the AF [24]. Furthermore, delamination resistance has previously been found to be lower in the deep layers of the AF in human discs as compared to the outer layers of the AF [16], which may already predispose the disc to herniation. If delamination can be prevented, then it is possible that herniation progress can also be slowed or averted.
Limiting the potential for disc herniation, characterized by the progression of NP through the AF, is important even after degeneration has occurred, as currently there are various methods which target re-growth of the NP following degeneration. A recent review identified growth factors, NP cell transplantation, protein injection and gene transfer as all potential therapy strategies for the re-growth of NP material [25]. While many of the studies reviewed by Masuda [25] showed positive results for these therapeutic strategies, the long-term efficacy is largely unknown. If the AF is not strong enough to effectively contain this newly grown material, or is already weakened to delamination as the current study suggests possible, then the potential of reherniation or loss of nuclear material and thus disc height in the long-term is high. Further, recent work by Michalek and colleagues [26] observed significant, and potentially detrimental, changes in the shear properties of AF tissue following anular puncture. The authors state that these unfavorable changes need to be considered when administering certain biological repair therapies, as these are often injected into the disc via disc puncture. Although the size of the rabbit disc is much smaller in comparison to the human disc, and thus the insult created in the current study is larger in relation that what might be observed when administering biological repair therapies, it is important to acknowledge that needle puncture in general can elicit degeneration progression. Further work is required to determine the degree of AF damage and disc degeneration as a result of the needle puncture created as a result of the injection of therapeutic drugs. Additionally, given the limited degree of vascularization in the AF (blood vessels tend to only be found in the outer 1-2mm of the AF [27,28]), it is unlikely that the delamination strength changes observed in the current study would have been repaired if given sufficient time post-puncture, making it even more important to understand the damage that results from needle puncture.
The observed reduction in peel strength in degenerated discs observed in the current study also supports the notion that the state of the AF and its ability to contain NP material must be considered when examining such re-growth therapies. Further, the NP should not be the only structure targeted when developing these therapies, and rather both the NP and AF should be treated together. As a result, it is possible that regeneration therapies that include both the AF and the NP may be more effective that replacing the NP alone with an implant or with therapeutic gel injections.
One important aspect of this study was to establish a quantitative and functional assessment of AF tissue in a rabbit model. Comprehensive methods have previously been established to assess structure and function of the disc (e.g., biomechanical testing of bone-disc-bone units, quantitative MRI evaluation, X-ray, histology and biochemical analyses) in various animal models of disc degeneration and repair. However, minimal research has focused on the assessment of components of the disc, namely AF tissues. Importantly, the methods used in the current study can be used to determine the effectiveness of disc re-growth therapies applied to animal models, such as the rabbit model used here.
The study was limited by a number of factors. First, the degeneration induced in this study was more rapid that natural degeneration in the human disc. As a result, the biomechanical properties of the AF may be slightly altered in the surgically induced model. However, other studies have also successfully induced clinically-relevant degeneration using similar methodology [17, 29-34]. Second, due to the quadrupedal nature of rabbits, the loading within the lumbar spine may not be directly comparable to that of a human, altering the degeneration process and properties of the AF. Last, due to the small size of the rabbit intervertebral disc, only one tissue sample could be dissected from the anterior region, and one dissected from the posterior-lateral region, of the AF. Therefore, the effect of AF depth (superficial versus deep) could not be addressed. Future considerations include examining the peel strength of the AF following local therapeutic disc treatments targeting re-growth of the NP.
CONCLUSION
Disc degeneration induced via intervertebral disc puncture in a live rabbit model showed lower resistance to AF delamination than non-punctured control discs. This finding suggests that degeneration increases the potential for delamination between AF layers. Given this substantial change to the integrity of the AF following degeneration, clinical treatments should not only target rehydration or re-growth of the NP, but should also target repair and strengthening of the AF in order to support the NP.
Acknowledgments
The authors wish to acknowledge NIH for funding (NIH P01AR48152). Diane Gregory was further supported by an NSERC Postdoctoral Fellowship and the ISSLS McNab-Larocca Fellowship for this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].An HS, Masuda K, Inoue N. Intervertebral disc degeneration: biological and biomechanical factors. J Orthop Sci. 2006;11(5):541–52. doi: 10.1007/s00776-006-1055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lotz JC, Haughton V, Boden SD, et al. New treatments and imaging strategies in degenerative disease of the intervertebral disks. Radiology. 2012;264:6–19. doi: 10.1148/radiol.12110339. [DOI] [PubMed] [Google Scholar]
- [3].Chan WCW, Sze KL, Samartzis D, et al. Structure and biology of the intervertebral disk in health and disease. Orthop Clin N Am. 2011a;42:447–64. doi: 10.1016/j.ocl.2011.07.012. [DOI] [PubMed] [Google Scholar]
- [4].Antoniou J, Steffen T, Nelson F, et al. The human lumbar intervertebral disc: Evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Duance VC, Crean JK, Sims TJ, et al. Changes in collagen cross-linking in degenerative disc disease and scoliosis. Spine. 1998;23:2545–51. doi: 10.1097/00007632-199812010-00009. [DOI] [PubMed] [Google Scholar]
- [6].Chan SCW, Ferguson SJ, Gantenbein-Ritter B. The effects of dynamic loading on the intervertebral disc. Eur Spine J. 2011b;20(11):1796–812. doi: 10.1007/s00586-011-1827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hadjipavlou AG, Tzermiadianos MN, Bogduk N, et al. The pathophysiology of disc degeneration: a critical review. J Bone Joint Surg Br. 2008;90(10):1261–70. doi: 10.1302/0301-620X.90B10.20910. [DOI] [PubMed] [Google Scholar]
- [8].Adams MA, McMillan DW, Green TP, et al. Sustained loading generates stress concentrations in lumbar intervertebral discs. Spine. 1996a;21:434–8. doi: 10.1097/00007632-199602150-00006. [DOI] [PubMed] [Google Scholar]
- [9].Adams MA, McNally DS, Dolan P. ‘Stress’ distributions inside intervertebral discs. J Bone Joint Surg Br. 1996b;78-B:965–72. doi: 10.1302/0301-620x78b6.1287. [DOI] [PubMed] [Google Scholar]
- [10].McNally DS, Adams MA. Internal intervertebral disc mechanics as revealed by stress profilometry. Spine. 1992;17:66–73. doi: 10.1097/00007632-199201000-00011. [DOI] [PubMed] [Google Scholar]
- [11].Boos N, Weissbach S, Rohrbach H, et al. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27:2631–44. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- [12].Osti OL, Vernon-Roberts B, Moore R, et al. Annular tears and disc degeneration in the lumbar spine. A post-mortem study of 135 discs. J Bone Joint Surg Br. 1992;74:678–82. doi: 10.1302/0301-620X.74B5.1388173. [DOI] [PubMed] [Google Scholar]
- [13].Gunzburg R, Parkinson R, Moore R, et al. A cadaveric study comparing discography, magnetic resonance imaging, histology, and mechanical behavior of the human lumbar disc. Spine. 1992;17:417–26. doi: 10.1097/00007632-199204000-00007. [DOI] [PubMed] [Google Scholar]
- [14].Maeda S, Kokubun S. Changes with age in proteoglycan synthesis in cells cultured in vitro from the inner and outer rabbit annulus fibrosus, Responses to interleukin-1 and interleukin-1 receptor antagonist protein. Spine. 2000;25:166–9. doi: 10.1097/00007632-200001150-00005. [DOI] [PubMed] [Google Scholar]
- [15].Handa T, Ishihara H, Ohshima H, et al. Effects of hydrostatic pressure on matrix synthesis and matrix metalloproteinase production in the human lumbar intervertebral disc. Spine. 1997;22(10):1085–91. doi: 10.1097/00007632-199705150-00006. [DOI] [PubMed] [Google Scholar]
- [16].Gregory DE, Bae WC, Sah RL, et al. Anular delamination strength of human lumbar intervertebral disc. Eur Spine J. 2012;21(9):1716–23. doi: 10.1007/s00586-012-2308-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Masuda K, Aota Y, Muehleman C, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 2005;30:5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- [18].Adams MA, Roughly PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31(18):2151–61. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- [19].Melrose J, Smith SM, Appleyard RC, et al. Aggrecan, versican and type VI collagen are components of annulus translamellar cross-bridges in the intervertebral disc. Eur Spine J. 2008;17:314–24. doi: 10.1007/s00586-007-0538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schollum ML, Robertson PA, Broom ND. ISSLS prize winner: microstructure and mechanical disruption of the lumbar disc annulus part I: a microscopic investigation of the translamellar bridging network. Spine. 2008;33:2702–10. doi: 10.1097/BRS.0b013e31817bb92c. [DOI] [PubMed] [Google Scholar]
- [21].Adams MA, Green TP. Tensile properties of the annulus fibrosus. I. The contribution of fibre-matrix interactions to tensile stiffness and strength. Eur Spine J. 1993;2:203–8. doi: 10.1007/BF00299447. [DOI] [PubMed] [Google Scholar]
- [22].Yu J, Fairbank JCT, Roberts S, et al. The elastic fiber network of the anulus fibrosus of the normal and scoliotic human intervertebral disc. Spine. 30:1815–20. doi: 10.1097/01.brs.0000173899.97415.5b. [DOI] [PubMed] [Google Scholar]
- [23].Yu J, Perter C, Roberts S, et al. Elastic fibre organization in the intervertebral discs of the bovine tail. J Anatomy. 2002;201:465–75. doi: 10.1046/j.1469-7580.2002.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Adams MA, Hutton WC. Gradual disc prolapsed. Spine. 1985;10:524–31. doi: 10.1097/00007632-198507000-00006. [DOI] [PubMed] [Google Scholar]
- [25].Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J. 2008;17(Suppl 4):S441–51. doi: 10.1007/s00586-008-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Michalek AJ, Buckley MR, Bonassar LJ, Cohen I, Iatridis JC. The effects of needle puncture injury on microscale shear strain in the intervertebral disc annulus fibrosus. Spine. 2010;10(12):1098–105. doi: 10.1016/j.spinee.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hassler O. The human intervertebral disc. A micro-angiographical study of its vascular supply at various ages. Acta Orthop Scand. 1970;40:765–72. doi: 10.3109/17453676908989540. [DOI] [PubMed] [Google Scholar]
- [28].Rudert M, Tillmann B. Detection of lymph and blood vessels in the human intervertebral disc by histochemical and immunohistochemical methods. Ann Anat. 1993;175:237–42. doi: 10.1016/s0940-9602(11)80009-9. [DOI] [PubMed] [Google Scholar]
- [29].Key JA, Ford LT. Experimental intervertebral-disc lesions. J Bone Joint Surg Am. 1948;30:621–30. [PubMed] [Google Scholar]
- [30].Lipson SJ, Muir H. Experimental intervertebral disc degeneration: morphologic and proteoglycan changes over time. Arthritis Rheum. 1981a;24:12–21. doi: 10.1002/art.1780240103. [DOI] [PubMed] [Google Scholar]
- [31].Lipson SJ, Muir H. Volvo award in basic science. Proteoglycans in experimental intervertebral disc degeneration. Spine. 1981b;6:194–210. doi: 10.1097/00007632-198105000-00002. [DOI] [PubMed] [Google Scholar]
- [32].Osti OL, Vernon RB, Fraser RD. Volvo Award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine. 1990;15:762–7. doi: 10.1097/00007632-199008010-00005. [DOI] [PubMed] [Google Scholar]
- [33].Kaigle AM, Holm SH, Hansson TH. 1997 Volvo Award winner in biomechanical studies. Kinematic behavior of the porcine lumbar spine: a chronic lesion model. Spine. 1997;22(24):2796–2806. doi: 10.1097/00007632-199712150-00002. [DOI] [PubMed] [Google Scholar]
- [34].Zhang Y, Drapeau S, An HS, et al. Histological features of the degenerating intervertebral disc in a goat disc-injury model. Spine. 2011;36(19):1519–27. doi: 10.1097/BRS.0b013e3181f60b39. [DOI] [PMC free article] [PubMed] [Google Scholar]