Abstract
BACKGROUND & AIMS
Studies of liver cancer risk in recipients of solid organ transplants have generally been small, yielding mixed results, and little is known about biliary tract cancers among transplant recipients.
METHODS
We identified incident hepatobiliary cancers among 201,549 US recipients of solid organs, from 1987 through 2008, by linking data from the US transplant registry with 15 cancer registries. We calculated standardized incidence ratios (SIRs), comparing risk relative to the general population. We also calculated incidence rate ratios (RRs), comparing risk for hepatocellular carcinoma (HCC) and total (intrahepatic and extrahepatic) cholangiocarcinoma among subgroups of recipients.
RESULTS
Of transplant recipients, 165 developed hepatobiliary cancers (SIR, 1.2; 95% confidence interval [CI], 1.0–1.4). HCC risk was increased among liver recipients (SIR, 1.5; 95% CI, 1.0–2.2), especially 5 or more y after transplant (SIR, 1.8; 95% CI, 1.0–3.0). Cholangiocarcinoma was increased among liver (SIR, 2.9; 95% CI,1.6–4.8) and kidney recipients (SIR, 2.1; 95% CI, 1.3–3.1). HCC was associated with hepatitis B virus (RR, 3.2; 95% CI, 1.3–6.9), hepatitis C virus (RR, 10; 95% CI, 5.9–16.9), and non-insulin-dependent diabetes (RR, 2.5; 95% CI, 1.2–4.8). Cholangiocarcinoma was associated with azathioprine maintenance therapy (RR, 2.0; 95% CI, 1.1–3.7). Among liver recipients, primary sclerosing cholangitis (PSC) was associated with increased risk of cholangiocarcinoma, compared to the general population (SIR, 21; 95% CI, 8.2–42) and compared to liver recipients without PSC (RR, 12.3; 95% CI, 4.1–36.4).
CONCLUSIONS
Risks for liver and biliary tract cancer are increased among organ transplant recipients. Risk factors for these cancers include medical conditions and medications taken by recipients.
Keywords: liver disease, transplantation, epidemiology, HBV, HCV
Liver cancer, including hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC), is the third leading cause of cancer deaths worldwide.1 In the US, the incidence of HCC,2 ICC,3 and extrahepatic cholangiocarcinoma (ECC)4 is increasing, together with hepatobiliary cancer mortality rates.5
Liver cancers may be elevated after solid organ transplantation, which is associated with increased risk of infections-related cancers.6 Immunosuppressive drugs used to prevent organ rejection suppress immunologic control of infections and create imbalances in immune response. Biliary tract cancers have also been associated with hepatitis B and C viruses (HBV, HCV)7,8 and may therefore be elevated in transplant recipients.
Previous studies of liver cancer risk in transplant recipients have produced mixed results.9,10 Most studies included few (<20) liver cancer cases and did not evaluate individual hepatobiliary cancers, especially biliary tract cancers. The purpose of the current study was to compare the incidence of individual hepatobiliary cancers in solid organ transplant recipients relative to the general population and determine risk factors for these cancers among transplant recipients.
Materials and Methods
Solid Organ Transplant Recipients
With the addition of the Utah and Florida cancer registries, the recently described6 US Transplant Cancer Match Study currently accounts for ~43% of the US transplant population through 2008. In brief, the Scientific Registry of Transplant Recipients (SRTR), which has data from all US solid organ transplant recipients since 1987, was matched with 15 US population-based cancer registries. The study was approved by human subjects committees at the National Cancer Institute and participating cancer registries as required.
Among recipients, we evaluated follow-up from transplantation (or start of cancer registry coverage, whichever came last) to the first of: 1) hepatobiliary malignancy diagnosis; 2) transplanted organ failure; 3) subsequent transplant; 4) death; 5) end of cancer registry coverage. HIV-infected recipients (<300) were excluded because they are infection-immunosuppressed. Hepatobiliary cancers were identified using linked cancer registry data, based on International Classification of Disease for Oncology (ICD-O-3) topography codes C22 (primary liver cancer), C23.9 (gallbladder cancer), and C24 (other biliary tract cancers), with further refinements described in Supplemental Table 1. Major cancers of interest included HCC, total cholangiocarcinoma (ICC and ECC), gallbladder cancer, and ampulla of Vater cancer.
We initially identified 1238 hepatobiliary cancers among solid organ transplant recipients; 165 (13%) were included in the final analysis (Table 1) after the following exclusions. First, we excluded 1062 hepatobiliary cancer cases diagnosed within 6 months of a liver transplant (Supplemental Figure 1) since hepatobiliary cancers diagnosed so soon after a liver transplant were likely present in the explanted liver at the time of transplantation but looked like they occurred after transplant due to small errors in diagnosis date (median time to cancer diagnosis for these cases=7 days). Second, since some cases classified as incident in fact actually reflect regrowth of the original tumor, we excluded recipients noted by the transplant registry as having recurrent malignancy (N=632, of whom only 11 had hepatobiliary cancer documented in the cancer registry).
Table 1.
Hepatobiliary cancers in the Transplant Cancer Match Study (overall and analyzed dataset)
| Cancer Group | All hepatobiliary cancers (N=1238)
|
Hepatobiliary cancers in analysis (N=165)†
|
||||||
|---|---|---|---|---|---|---|---|---|
|
Liver transplant only
|
Other transplant type
|
Liver transplant only
|
Other transplant type
|
|||||
| ≤6 months* | >6 months* | ≤6 months* | >6 months* | ≤6 months* | >6 months* | ≤6 months* | >6 months* | |
| HCC | 907 | 38 | 37 | 53 | 0 | 30 | 5 | 51 |
| ICC | 41 | 10 | 2 | 17 | 0 | 10 | 1 | 17 |
| ECC | 13 | 5 | 1 | 9 | 0 | 4 | 1 | 9 |
| Gallbladder | 20 | 0 | 0 | 8 | 0 | 0 | 0 | 8 |
| Ampulla of Vater | 0 | 1 | 0 | 11 | 0 | 1 | 0 | 11 |
| Other hepatobiliary | 45 | 4 | 6 | 10 | 0 | 4 | 3 | 10 |
| All hepatobiliary Cancers | 1026 | 58 | 46 | 108 | 0 | 49 | 10 | 106 |
Diagnosis date relative to transplant.
Excluding recurrent malignancies and cancers within 6 months of liver transplant.
Abbreviations: HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma; ECC, extrahepatic cholangiocarcinoma
Ninety-two percent of initially identified HCC cases (949/1035) were thus dropped as prevalent or recurrent, compared to 60% of ICC cases (42/70), 50% of ECC cases (14/28), 71% of gallbladder cancer cases (20/28), and 0% ampulla of Vater cases (0/12) (Table 1). We did not exclude cancers in individuals with an SRTR or cancer registry indication of liver cancer diagnosed at or before transplant because these individuals may have developed a new cancer. The final analytic cohort included 201,549 transplant recipients.
Statistical Analyses
We compared hepatobiliary cancer risk in solid organ transplant recipients to the general population using standardized incidence ratios (SIRs).6 We stratified SIRs by transplanted organ (liver versus non-liver) and time since transplant, testing for linear trend using Poisson regression. Since the gallbladder is removed from donor livers, the SIR calculations for gallbladder cancer were restricted to non-liver transplant recipients.
Risk factors for developing HCC and total cholangiocarcinoma, the most common hepatobiliary subtypes, after transplantation were examined via Poisson regression-based incidence rate ratios (RRs). All models were adjusted for age at transplant (continuous), gender, race/ethnicity (non-Hispanic white versus other), and transplant organ (liver only versus other).
Potential risk factors included HBV, HCV, body mass index (BMI), diabetes, immunosuppressive therapies, and reason for transplant. Only data from 1994 onward were used to establish HBV or HCV status because few data were available before 1994. Subjects were classified as having an active HBV infection if they were seropositive for HBV surface antigen (HBsAg), resolved infection if seropositive for antibody to HBV core antigen (anti-HBc) but negative/unknown for HBsAg, uninfected if seronegative for either antigen and the other marker was negative/unknown, and unknown if serostatus was unknown for both antigens (see Supplemental Methods).
Because the dataset included children (ages 2–19 years) and adults (ages ≥20 years), BMI was defined as follows (adults, children): underweight (15–18.4 kg/m2, <5th percentile) normal (18.5–24.9 kg/m2, 5th–85th percentile) overweight (25.0–29.9 kg/m2, 85–95th percentile) obese (≥30.0 kg/m2, ≥95th percentile).
We conducted sensitivity analyses for the risk factors to address concerns over the potential presence of residual prevalent cancers despite the exclusions described above. We excluded: 1) all HCCs (N=5) and cholangiocarcinomas (N=2) diagnosed within 6 months of transplant regardless of transplanted organ; 2) all individuals flagged in the SRTR and/or cancer registry as having liver cancer at/before transplantation (10 HCC, five cholangiocarcinomas); 3) cancers that developed among liver transplant recipients (31 HCC, 14 cholangiocarcinomas).
Results
The median time between transplant and diagnosis for the 165 hepatobiliary cancer cases was 4.2 years. Most cases were HCC (52.1%), followed by ICC (17.0%), ECC (8.5%), ampulla of Vater cancer (7.3%), and gallbladder cancer (4.8%); 10.3% were other tumor types (Supplemental Table 1). No cases had HBV- or HCV-positive donors.
The median age of hepatobiliary cancer cases and non-cases was similar (45 versus 47 years), as was the distribution of race/ethnicity and BMI (Table 2). Hepatobiliary cancer cases were more likely male and liver recipients than non-cases. The median follow-up for the cohort was 3.5 years.
Table 2.
Characteristics of the analytical transplant cohort
| Hepatobiliary cancer
|
||||
|---|---|---|---|---|
| Yes (N=165)
|
No (N=201384)
|
|||
| N | % | N | % | |
| Age at transplant, median (range) | 45 (2–81) | 47 (0–87) | ||
| Years of follow-up, median (range) | 4.2 (0.02–16.1) | 3.5 (0.003–22.2) | ||
| Gender | ||||
| Male | 128 | 77.6 | 122644 | 60.9 |
| Female | 37 | 22.4 | 78740 | 39.1 |
| Race/ethnicity | ||||
| White, non-Hispanic | 106 | 64.2 | 124770 | 62.0 |
| Black, non-Hispanic | 30 | 18.2 | 34181 | 17.0 |
| Hispanic | 21 | 12.7 | 31278 | 15.5 |
| Asian/Pacific Islander | 8 | 4.8 | 11155 | 5.5 |
| BMI/obesity* | ||||
| Underweight | 7 | 4.9 | 8027 | 4.8 |
| Normal | 67 | 46.9 | 71578 | 43.0 |
| Overweight | 40 | 28.0 | 52218 | 31.4 |
| Obese | 29 | 20.3 | 34676 | 20.8 |
| Transplanted organ | ||||
| Liver | 49 | 29.7 | 29023 | 14.4 |
| Kidney | 81 | 49.1 | 117271 | 58.2 |
| Heart and/or lung | 34 | 20.6 | 43333 | 21.5 |
| Other | 1 | 0.6 | 11757 | 5.8 |
Numbers do not sum to total due to missing data.
Transplant recipients had higher risk of hepatobiliary cancers than the general population (SIR,1.2; 95%CI,1.0–1.4). The HCC risk was not elevated for all solid organ transplant recipients (Table 3) or non-liver recipients (SIR,0.9; 95%CI,0.7–1.1) but was elevated among liver recipients (SIR,1.5; 95%CI,1.0–2.2). In contrast, risk tended to be increased for biliary tract cancers, especially ICC. Risk of cholangiocarcinoma was elevated in liver (SIR,2.9; 95%CI,1.6–4.8) and non-liver (SIR,1.8; 95%CI,1.2–2.6) recipients (Table 3), especially kidney recipients (SIR, 2.1; 95%CI,1.3–3.1). Among non-liver recipients, risk for gallbladder cancer was similar to the general population (SIR,0.8; 95%CI,0.3–1.6). Ampulla of Vater cancer was elevated among non-liver recipients only (SIR,1.9; 95%CI,0.9–3.4) (Table 3).
Table 3.
Standardized incidence ratios for incident hepatobiliary malignancies (overall and by transplanted organ)
| Transplant recipient group and outcome category | Overall
|
|||
|---|---|---|---|---|
| Observed | Expected | SIR | 95% CI | |
| All recipients | ||||
| HCC | 86 | 83.8 | 1.0 | 0.8–1.3 |
| Total cholangiocarcinoma | 42 | 20.6 | 2.0 | 1.5–2.8 |
| ICC | 28 | 12.0 | 2.3 | 1.6–3.4 |
| ECC | 14 | 8.6 | 1.6 | 0.9–2.7 |
| Ampulla of Vater | 12 | 7.6 | 1.6 | 0.8–2.8 |
| Liver only recipients | ||||
| HCC | 30 | 19.7 | 1.5 | 1.0–2.2 |
| Total cholangiocarcinoma | 14 | 4.9 | 2.9 | 1.6–4.8 |
| ICC | 10 | 2.9 | 3.5 | 1.7–6.4 |
| ECC | 4 | 2.0 | 2.0 | 0.5–5.0 |
| Ampulla of Vater | 1 | 1.8 | 0.56 | 0.01–3.1 |
| Non-liver only recipients | ||||
| HCC | 56 | 64.1 | 0.9 | 0.7–1.1 |
| Total cholangiocarcinoma | 28 | 15.7 | 1.8 | 1.2–2.6 |
| ICC | 18 | 9.2 | 2.0 | 1.2–3.1 |
| ECC | 10 | 6.6 | 1.5 | 0.7–2.8 |
| Gallbladder | 8 | 10.0 | 0.8 | 0.3–1.6 |
| Ampulla of Vater | 11 | 5.8 | 1.9 | 0.9–3.4 |
Abbreviations: CI, confidence interval; HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma; ECC, extrahepatic cholangiocarcinoma; SIR, standardized incidence ratio
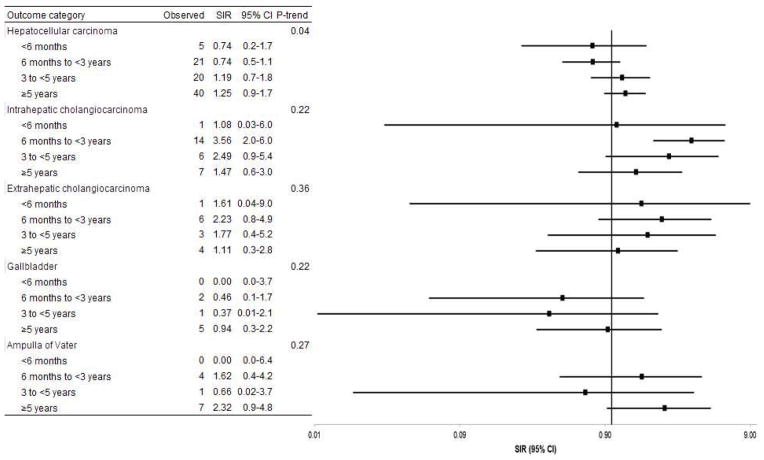
HCC risk tended to increase with time since transplant among all transplant recipients (Figure 1, p-trend, 0.04) and was borderline elevated ≥5 years after transplant among liver recipients (SIR:1.8; 95%CI:1.0–3.0). Risk for other hepatobiliary cancers did not consistently vary by time since transplant (Figure 1).
Figure 1.
Standardized incidence ratios for hepatobiliary cancer stratified by time since transplant.
Abbreviations: CI, confidence interval; SIR, standardized incidence ratio
In a multivariate model, HCC risk increased with each additional 10 years of age (RR:1.5, 95%CI:1.3–1.8), non-white race/ethnicity (RR:1.4, 95%CI:0.9–2.1), and liver transplantation (RR:2.0, 95%CI:1.3–3.1), and decreased with female gender (RR:0.3, 95%CI:0.2–0.5). For cholangiocarcinoma, RRs were 1.6 (95%CI:1.3–2.1) for age (10 years), 1.3 (95%CI:0.7–2.4) for non-white race/ethnicity, 1.8 (95%CI:0.9–3.4) for liver transplants, and 0.6 (95%CI: 0.3–1.2) for gender.
Among all recipients, HCC risk was associated with HBV (RR,3.2; 95%CI,1.3–6.9), HCV (RR,10; 95%CI,5.9–17), and non-insulin-dependent diabetes (RR,2.5; 95%CI,1.2–4.8) (Table 4). Results were similar after adjusting for transplant year (Supplemental Table 2). Increasing BMI was associated with decreased HCC risk (p-trend, 0.03). Estimates of cholangiocarcinoma risk, although limited by small numbers, tended to increase with non-insulin-dependent diabetes (RR,2.0; 95%CI,0.7–5.6) and obesity (RR,1.8; 95%CI,0.9–3.8). Only one cholangiocarcinoma case was HCV seropositive, and HCV was associated with reduced risk (RR,0.1; 95%CI,0.01–0.7). No immunosuppressive medications were associated with HCC (Table 4), but azathioprine was associated with increased cholangiocarcinoma risk (RR,2.0; 95%CI,1.1–3.7) with similar results after adjusting or stratifying by year of transplant (RR,2.1; 95%CI,0.9–4.6; Supplemental Table 3). Results for immunosuppressive medication use were similar when stratified by liver versus non-liver transplant (Supplemental Table 4). A model mutually adjusted for HBV, HCV, BMI, and diabetes produced RRs for HCC similar to those from the independent models (Supplemental Table 5). It was not possible to run a mutually adjusted model for cholangiocarcinoma given the small number of cases.
Table 4.
Risk factors for hepatocellular carcinoma (HCC) and cholangiocarcinoma.
| Variable | HCC
|
Total cholangiocarcinoma
|
||||
|---|---|---|---|---|---|---|
| N cases (N=86) | RR* | 95% CI | N cases (N=42) | RR* | 95% CI | |
| HBV† | ||||||
| Uninfected | 46 | 1.0 | 23 | 1.0 | ||
| Active infection | 7 | 3.2 | 1.3–6.9 | 0 | ND | |
| Resolved infection | 10 | 1.8 | 0.8–3.4 | 6 | 2.0 | 0.8–4.8 |
| Unknown | 6 | 1.0 | 0.4–2.2 | 1 | 0.3 | 0.04–2.3 |
| HCV† | ||||||
| Seroegative | 23 | 1.0 | 27 | 1.0 | ||
| Seropositive | 38 | 10.0 | 5.9–16.9 | 1 | 0.1 | 0.01–0.7 |
| Unknown | 8 | 1.7 | 0.7–3.3 | 2 | 0.4 | 0.1–1.2 |
| BMI‡ | ||||||
| Underweight | 5 | 2.5 | 0.9–5.9 | 0 | ND | |
| Normal | 32 | 1.0 | 17 | 1.0 | ||
| Overweight | 23 | 0.8 | 0.5–1.3 | 10 | 0.9 | 0.4–1.9 |
| Obese | 10 | 0.6 | 0.3–1.2 | 12 | 1.8 | 0.9–3.8 |
| Diabetes‡ | ||||||
| No | 36 | 1.0 | 17 | 1.0 | ||
| Insulin-dependent | 9 | 1.3 | 0.6–2.5 | 1 | 0.3 | 0.04–2.2 |
| Non-insulin-dependent | 12 | 2.5 | 1.2–4.8 | 5 | 2.0 | 0.7–5.6 |
| Unknown dependency | 2 | 3.9 | 0.6–13.1 | 0 | ND | |
| Unknown | 1 | 0.3 | 0.02–1.5 | 1 | 0.7 | 0.1–5.3 |
| Any induction therapy | ||||||
| No | 59 | 1.0 | 28 | 1.0 | ||
| Yes | 27 | 0.9 | 0.6–1.5 | 14 | 1.0 | 0.5–1.9 |
| Maintenance therapy with cyclosporine | ||||||
| No | 37 | 1.0 | 17 | 1.0 | ||
| Yes | 49 | 1.4 | 0.9–2.1 | 25 | 1.5 | 0.8–2.9 |
| Maintenance therapy with tacrolimus | ||||||
| No | 56 | 1.0 | 29 | 1.0 | ||
| Yes | 30 | 0.7 | 0.4–1.1 | 13 | 0.6 | 0.3–1.1 |
| Maintenance therapy with azathioprine | ||||||
| No | 57 | 1.0 | 24 | 1.0 | ||
| Yes | 29 | 1.3 | 0.8–2.1 | 18 | 2.0 | 1.1–3.7 |
| Maintenance therapy with mycophenolate mofetil or MMF | ||||||
| No | 55 | 1.0 | 27 | 1.0 | ||
| Yes | 31 | 0.7 | 0.4–1.1 | 15 | 0.7 | 0.3–1.3 |
| Maintenance therapy with mTOR inhibitors | ||||||
| No | 85 | 1.0 | 41 | 1.0 | ||
| Yes | 1 | 0.2 | 0.01–1.1 | 1 | 0.5 | 0.03–2.3 |
| Maintenance therapy with steroids | ||||||
| No | 5 | 1.0 | 3 | 1.0 | ||
| Yes | 81 | 1.8 | 0.8–5.1 | 39 | 1.4 | 0.5–5.9 |
| Reason for transplant | ||||||
| Liver only recipients | ||||||
| Noncholestatic liver disease | ||||||
| No | 1 | 1.0 | 9 | 1.0 | ||
| Yes | 29 | 13 | 2.5–245 | 5 | 0.2 | 0.1–0.5 |
| Cholestatic liver disease | ||||||
| No | 29 | 1.0 | 7 | 1.0 | ||
| Yes | 1 | 0.3 | 0.01–1.2 | 7 | 9.1 | 3.1–27.1 |
| Alcoholic liver disease | ||||||
| No | 19 | 1.0 | 14 | 1.0 | ||
| Yes | 11 | 1.3 | 0.6–2.7 | 0 | ND | |
| Kidney only recipients | ||||||
| Glomerular diseases | ||||||
| No | 33 | 1.0 | 18 | 1.0 | ||
| Yes | 4 | 0.3 | 0.1–0.9 | 5 | 0.8 | 0.3–2.1 |
| Diabetes | ||||||
| No | 22 | 1.0 | 17 | 1.0 | ||
| Yes | 15 | 2.4 | 1.2–4.6 | 6 | 1.3 | 0.5–3.1 |
| Polycystic kidneys | ||||||
| No | 35 | 1.0 | 16 | 1.0 | ||
| Yes | 2 | 0.5 | 0.1–1.8 | 7 | 3.2 | 1.2–7.8 |
| Hypertensive nephrosclerosis | ||||||
| No | 28 | 1.0 | 20 | 1.0 | ||
| Yes | 9 | 1.1 | 0.5–2.2 | 3 | 0.6 | 0.1–1.7 |
RRs were adjusted for age at transplant, gender, race/ethnicity (non-white versus white, non-Hispanic), and transplanted organ (liver only versus other).
Numbers do not sum to total because only data from 1994 onward were used.
Numbers do not sum to total due to missing data.
Abbreviations: BMI, body mass index; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; RR, rate ratio
Among liver recipients, HCC risk was associated with noncholestatic liver disease (RR,13; 95%CI,2.5–245) (Table 4), which includes cirrhosis due to HBV/HCV. Of 30 liver recipients with noncholestatic liver disease who developed HCC, 70% had l HBV-related liver disease (N=3), HCV (N=16), or both (N=2). Liver recipients with alcoholic liver disease as reason for transplant had borderline increased HCC risk compared to the general population (SIR,1.9; 95%CI,0.9–3.3).
Cholestatic liver disease was associated with increased cholangiocarcinoma risk (RR,9.1; 95%CI,3.1–27) and noncholestatic liver disease with decreased risk (RR,0.2; 95%CI,0.1–0.5) among liver recipients (Table 4). Cholestatic liver disease includes primary sclerosing cholangitis (PSC) and primary biliary cirrhosis (PBC). Of 14 liver recipients with cholangiocarcinoma, 7 (50%) had PSC and 1 (7%) had PBC. Additionally, 10 of these 14 cholangiocarcinomas (five with PSC) were intrahepatic and therefore developed in the transplanted liver. The remaining four cholangiocarcinomas (two with PSC) were extrahepatic. The SIR for cholangiocarcinoma among liver recipients with PSC was 21 (95%CI,8.2–42), and the RR for cholangiocarcinoma among PSC versus non-PSC liver recipients was 12 (95%CI,4.1–36). However, after excluding people with PSC, cholangiocarcinoma risk remained elevated among transplant recipients compared to the general population (SIR,1.7; 95%CI,1.4–2.4).
Among kidney recipients (Table 4), HCC risk was associated with diabetes (RR,2.4; 95%CI,1.2–4.6) and inversely with glomerular disease (RR,0.3; 95%CI,0.1–0.9) as reason for transplant. Cholangiocarcinoma risk increased with polycystic kidney disease (RR,3.2; 95%CI,1.2–7.8).
Excluding cancers diagnosed within 6 months of transplant and liver cancers at the time of transplant did not substantially change the results. For example, the RRs for active HBV and HCC with these exclusions were 3.3 (95%CI,1.2–7.1) and 4.4 (95%CI,1.6–9.7), respectively. Similarly, the RRs for azathioprine and cholangiocarcinoma were 2.0 (95%CI,1.0–3.8) and 1.8 (95%CI,0.9–3.5), respectively. Restricting to cancers that developed in non-liver recipients, the RRs were at least as strong as for all cases combined (e.g. RR:7.4, 95%CI,2.2–18 for active HBV and HCC risk among non-liver recipients). The exception was BMI, although the trend was still inverse among non-liver recipients: RR=2.1 (95%CI,0.5–5.8) for underweight, 0.45 (95%CI 0.2–0.9) for overweight, 0.62 (95%CI,0.2–1.4) for obese versus normal. For cholangiocarcinoma, the association with azathioprine was at least as strong among non-liver transplant recipients (RR 2.4; 95%CI,1.1–5.1) as overall.
Discussion
In >200,000 solid organ transplant recipients, we found increased risk of hepatobiliary cancers, including HCC among liver recipients, cholangiocarcinoma among liver and non-liver recipients, and ampulla of Vater cancer among non-liver recipients.
The associations of HCC with HBV/HCV support a true increased risk since these infections are expected to cause incident HCC after transplant. Furthermore, 88% of the 165 cases occurred ≥1 year after transplantation, and >63% occurred ≥3 years after transplantation. The SIR for HCC among liver recipients was elevated ≥5 years after transplant, suggesting that the increased HCC risk is not due to prevalent cancers. While cancers may recur late after transplant, new cancers may also develop in the transplanted organ due to prolonged immunosuppression and/or HBV/HCV infection. For cholangiocarcinoma, incidence was elevated in both liver and kidney recipients compared to the general population, again supporting that the elevated SIRs reflect truly increased risk.
To our knowledge, this report presents the first evaluation of risk factors for cholangiocarcinoma after solid organ transplant, as well as an expanded analysis of risk factors for HCC. In addition to known risk factors for HCC and cholangiocarcinoma (e.g., HBV, HCV, non-insulin-dependent diabetes), there were novel findings, such as the associations with reason for transplant. For liver recipients, noncholestatic liver disease was associated with increased HCC risk. The inverse RR for noncholestatic liver disease and cholangiocarcinoma likely reflects that individuals with one condition typically do not have the other. To determine whether the increased HCC risk in transplant recipients is due entirely to a higher prevalence of HBV/HCV in this population or whether immunosuppression due to transplant also plays a role, we would ideally compare HBV/HCV-positive transplant recipients with HBV/HCV-positive individuals in the general population. Unfortunately, we do not have HBV/HCV information for the general population.
Cholestatic liver disease (largely PSC) among liver recipients was strongly associated with increased cholangiocarcinoma risk. Cholangiocarcinoma develops in 10–30% of PSC patients (10-year cumulative incidence=7–9%).12 PSC is the 5th leading reason for transplantation in the US.13 Up to 25% of PSC patients who receive a liver transplant may develop recurrent PSC within 5–10 years.12 We do not have data on PSC recurrence in our study but found that PSC-related liver recipients were 20 times more likely than the general population to develop cholangiocarcinoma and 12 times more likely than other liver recipients. The increased risk of cholangiocarcinoma among transplant recipients was not entirely due to PSC, however, since cholangiocarcinoma risk was elevated in kidney and liver recipients. It is unclear whether the increase in cholangiocarcinoma is due to a higher prevalence of risk factors among these patients or transplant-related immunosuppression.
Most cholangiocarcinoma cases in liver recipients were intrahepatic, reflecting carcinogenesis in the donor liver. For the four liver recipients with extrahepatic cholangiocarcinoma, it is unknown whether cancer developed in the donor extrahepatic duct joining the transplanted liver to the small intestine or in the stump of the native extrahepatic bile duct attached to the pancreas. Two cases had PSC as the reason for transplant. PSC patients commonly have hepaticojejunostomy rather than choledocholedochostomy and therefore do not have residual extrahepatic bile duct present after liver transplantation. Thus, the two extrahepatic cholangiocarcinoma cases with PSC are likely de novo cases.
Among kidney recipients, transplant-related glomerular disease was associated with decreased HCC risk, possibly due to chance. Polycystic kidney disease was associated with increased risk of cholangiocarcinoma, in accordance with several case reports of cholangiocarcinoma in individuals with polycystic kidney disease.14–16
The increased risk of cholangiocarcinoma associated with azathioprine was intriguing. PSC is treated with azathioprine, raising concerns over potential confounding due to treatment for PSC. However, the association was particularly strong among non-liver recipients. Although azathioprine is no longer a standard immunosuppressive medication, this association is not likely to reflect an effect of transplantation era since adjusting for year of transplant did not change the results. Azathioprine has been associated with skin cancer and lymphoma in solid organ transplant recipients.17,18 One recent study of 180 patients with autoimmune hepatitis found no significant association between azathioprine treatment and HCC 19 but had limited power since only 6 patients developed HCC. Given evidence that azathioprine is hepatotoxic in humans,20 it seems plausible that azathioprine may increase risk of cholangiocarcinoma in solid organ transplant recipients.
The trend toward decreasing HCC risk with increasing BMI was unexpected. The elevated RR for HCC in underweight transplant recipients may suggest that these patients had wasting due to the advanced stage of their disease. A recent study among liver transplant recipients found that the prevalence of muscle wasting (cachexia) increased dramatically with decreasing BMI.21 The observed association between BMI and risk of HCC may also reflect cachexia/muscle mass.
Data from previous studies of liver cancer after transplant are mixed.9–11,22–24 In our study, increased HCC risk of was limited to liver recipients, as seen previously.11 Few studies have reported on biliary tract cancer. Vajdic et al reported increased gallbladder cancer risk among kidney transplant recipients,22 and Engels et al reported increased intrahepatic cholangiocarcinoma and gallbladder cancer risk among all solid organ recipients.6 The Engels et al results were based on an earlier version of the SRTR/cancer matched data but, unlike the present analyses, did not incorporate exclusions to eliminate hepatobiliary cancers that may have been prevalent or recurrent.
Strengths of this study include the representative population covering ~43% of transplant recipients and the largely complete case ascertainment through population-based cancer registries. Since liver cancer itself is an indication for liver transplant, we made special efforts to exclude prevalent cases. Although one recent study addressed this problem by assuming that all liver cancers reported after liver transplantation were not new cancers,23 this approach would incorrectly exclude any true de novo tumors. Given 88% of the hepatobiliary cancers analyzed in this study were diagnosed >1 year after transplant, it seems likely that most were truly incident. Additionally, sensitivity analyses excluding all cases diagnosed within 6 months of transplant and all subjects with liver cancer at the time of transplant did not change the results. Finally, the study was large enough to conduct in-depth analyses of HCC and cholangiocarcinoma. However, the number of cases was limited in some analyses, leading to imprecision in the point estimates. Some associations could be due to change given multiple comparisons. Furthermore, we had limited ability to control for potential confounders (e.g., smoking), HCV diagnosis was based only on antibody positivity without PCR confirmation, and we did not have data on dosage or duration of use of azathioprine or other immunosuppressive agents. Finally, we note that the US Transplant Cancer Match Study does not include all US solid organ transplant recipients, potentially limiting generalizability, although transplant recipients included in this study have been previously shown to be comparable to transplant recipients who were not included.6
In conclusion, this report provides the first in-depth analysis of both liver and biliary tract cancers after solid organ transplant. Its novelty stems from the large study population size, which allowed us to characterize the incidence of and risk factors for individual types of hepatobiliary cancers. Cholangiocarcinoma risk was elevated among liver and kidney recipients, and HCC risk was elevated among liver recipients. Consistent with studies in non-transplant populations, we found that conditions like viral hepatitis infection and diabetes were associated with increased risk. Given the success of new HCV treatments, our results highlight the importance of better therapies to treat HBV/HCV infections before transplant and to prevent or treat recurrent infection after transplant. Finally, although the results must be interpreted with caution, the associations with PSC and azathioprine use are intriguing and require further evaluation.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the support and assistance provided by individuals at the Health Resources and Services Administration (Monica Lin), the SRTR (Ajay Israni, Bertram Kasiske, Paul Newkirk, Jon Snyder), and the following cancer registries: the states of California (Tina Clarke), Colorado (Jack Finch), Connecticut (Lou Gonsalves), Georgia (Rana Bayakly), Hawaii (Brenda Hernandez), Iowa (Charles Lynch), Illinois (Lori Koch), Michigan (Glenn Copeland), New Jersey (Xiaoling Niu), New York (Amy Kahn), North Carolina (Chandrika Rao), Texas (Melanie Williams), and Utah (Janna Harrell), and the Seattle-Puget Sound area of Washington (Margaret Madeleine). We also thank analysts at Information Management Services for programming support (David Castenson, Matthew Chaloux, Michael Curry, Ruth Parsons).
The views expressed in this paper are those of the authors and should not be interpreted to reflect the views or policies of the National Cancer Institute, Health Resources and Services Administration, SRTR, cancer registries, or their contractors. This research was supported in part by the Intramural Research Program of the National Cancer Institute.
During the initial period when registry linkages were performed, the SRTR was managed by Arbor Research Collaborative for Health in Ann Arbor, MI (contract HHSH234200537009C); beginning in September 2010, the SRTR was managed by Minneapolis Medical Research Foundation in Minneapolis, MN (HHSH250201000018C). The following cancer registries were supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention: California (agreement 1U58 DP000807-01), Colorado (U58 DP000848-04), Georgia (5U58DP003875-01), Illinois (5658DP000805-04), Michigan (5U58DP000812-03), New Jersey (1US58/DP0039311-01), New York (U58DP0038789), North Carolina (U58DP000832), and Texas (5U58DP000824-04). The following cancer registries were supported by the SEER Program of the National Cancer Institute: California (contracts HHSN261201000036C, HHSN261201000035C, and HHSN261201000034C), Connecticut (HHSN261201000024C), Hawaii (HHSN261201000037C, N01-PC-35137, and N01-PC-35139), Iowa (HSN261201000032C and N01-PC-35143), New Jersey (HHSN261201000027C, N01-PC-2010-0027), Seattle-Puget Sound (N01-PC-35142), and Utah (HHSN261201000026C). Additional support was provided by the states of California, Colorado, Connecticut, Illinois, Iowa, New Jersey, New York (Cancer Surveillance Improvement Initiative 14-2491), Texas, and Washington, as well as the Fred Hutchinson Cancer Research Center in Seattle, WA.
Grant Support: This research was supported by General Funds from the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- ECC
extrahepatic cholangiocarcinoma
- HCC
hepatocellular carcinoma
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- ICC
intrahepatic cholangiocarcinoma
- ICD-O-3
International Classification of Disease for Oncology, 3rd edition
- PBC
primary biliary cirrhosis
- PSC
primary sclerosing cholangitis
- SIR
standardized incidence ratio
- RR
rate ratio
Footnotes
Disclosures: No conflicts of interest exist for any author.
Author Contributions: JK – conception and design of current project, data analysis, interpretation of data, preparation of manuscript. KAM – conception and design of current project, interpretation of data, revision of manuscript. EAE – conception and design of current project and US Transplant Cancer Match Study, interpretation of data, manuscript revision. KP, MG – data collection, interpretation of data, revision of manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–91. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Oncology. 2006;15:1198–203. doi: 10.1158/1055-9965.EPI-05-0811. [DOI] [PubMed] [Google Scholar]
- 4.Castro FA, Koshiol J, Hsing AW, Devesa SS. Biliary tract cancer incidence in the United States- demographic and temporal variations by anatomic site. doi: 10.1002/ijc.28161. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–87. e1–3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsing AW, Zhang M, Rashid A, et al. Hepatitis B and C virus infection and the risk of biliary tract cancer: a population-based study in China. Int J Cancer. 2008;122:1849–53. doi: 10.1002/ijc.23251. [DOI] [PubMed] [Google Scholar]
- 8.Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173–84. doi: 10.1002/hep.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adami J, Gabel H, Lindelof B, et al. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. 2003;89:1221–7. doi: 10.1038/sj.bjc.6601219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villeneuve PJ, Schaubel DE, Fenton SS, Shepherd FA, Jiang Y, Mao Y. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant. 2007;7:941–8. doi: 10.1111/j.1600-6143.2007.01736.x. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann CJ, Subramanian AK, Cameron AM, Engels EA. Incidence and risk factors for hepatocellular carcinoma after solid organ transplantation. Transplantation. 2008;86:784–90. doi: 10.1097/TP.0b013e3181837761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta A, Bowlus CL. Primary sclerosing cholangitis: etiopathogenesis and clinical management. Frontiers in bioscience. 2012;4:1683–705. doi: 10.2741/e490. [DOI] [PubMed] [Google Scholar]
- 13.Portincasa P, Vacca M, Moschetta A, et al. Primary sclerosing cholangitis: updates in diagnosis and therapy. World J Gastroenterol. 2005;11:7–16. doi: 10.3748/wjg.v11.i1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonck C, Chauveau D, Gagnadoux MF, Pirson Y, Grunfeld JP. Autosomal recessive polycystic kidney disease in adulthood. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2001;16:1648–52. doi: 10.1093/ndt/16.8.1648. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki M, Katayanagi K, Watanabe K, Takasawa K, Nakanuma Y. Intrahepatic cholangiocarcinoma arising in autosomal dominant polycystic kidney disease. Virchows Archiv: an international journal of pathology. 2002;441:98–100. doi: 10.1007/s00428-002-0635-8. [DOI] [PubMed] [Google Scholar]
- 16.Vauthey JN, Maddern GJ, Blumgart LH. Adult polycystic disease of the liver. The British journal of surgery. 1991;78:524–7. doi: 10.1002/bjs.1800780505. [DOI] [PubMed] [Google Scholar]
- 17.Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Current treatment options in oncology. 2012;13:354–76. doi: 10.1007/s11864-012-0195-3. [DOI] [PubMed] [Google Scholar]
- 18.Bugelski PJ, Volk A, Walker MR, Krayer JH, Martin P, Descotes J. Critical review of preclinical approaches to evaluate the potential of immunosuppressive drugs to influence human neoplasia. International journal of toxicology. 2010;29:435–66. doi: 10.1177/1091581810374654. [DOI] [PubMed] [Google Scholar]
- 19.Hino-Arinaga T, Ide T, Kuromatsu R, et al. Risk factors for hepatocellular carcinoma in Japanese patients with autoimmune hepatitis type 1. Journal of gastroenterology. 2012;47:569–76. doi: 10.1007/s00535-011-0519-2. [DOI] [PubMed] [Google Scholar]
- 20.Bjornsson ES, Bergmann OM, Bjornsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. 25 e1–3. doi: 10.1053/j.gastro.2013.02.006. quiz e19–20. [DOI] [PubMed] [Google Scholar]
- 21.Cruz RJ, Jr, Dew MA, Myaskovsky L, et al. Objective Radiologic Assessment of Body Composition in Patients with End-Stage Liver Disease: Going Beyond the BMI. Transplantation. 2013;95:617–22. doi: 10.1097/TP.0b013e31827a0f27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vajdic CM, McDonald SP, McCredie MR, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–31. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 23.Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010;10:1889–96. doi: 10.1111/j.1600-6143.2010.03181.x. [DOI] [PubMed] [Google Scholar]
- 24.Busnach G, Piselli P, Arbustini E, et al. Immunosuppression and cancer: A comparison of risks in recipients of organ transplants and in HIV-positive individuals. Transplant Proc. 2006;38:3533–5. doi: 10.1016/j.transproceed.2006.10.144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.