Abstract
The literature indicates that retinoids can influence the metabolism and actions of xenobiotics and conversely that xenobiotics can influence the metabolism and actions of retinoids. We were interested in understanding the degree to which hepatic retinoid stores, accumulated over a lifetime, affect xenobiotic metabolism, and actions. To investigate this, we induced liver injury through administration of the hepatotoxin thioacetamide (TAA) to chow fed wild type (WT) mice and lecithin:retinol acyltransferase-deficient (Lrat−/−) mice that are genetically unable to accumulate hepatic retinoid stores. Within 48 h of TAA-treatment, WT mice develop liver injury as evidenced by focal necrotic areas and increases in serum ALT activity and myeloperoxidase activity in hepatic parenchyma. Simultaneously, features of hepatic encephalopathy develop, as evidenced by a 25% increase in blood ammonia and a threefold reduction of blood glucose levels. This is accompanied by reduced hepatic glutathione, and increased thiobarbituric acid reactive substances, protein carbonyl and sulfhydryl groups, and increased cytochrome P450-catalyzed hydroxylation activity and flavin-containing monooxygenase activity in microsomes prepared from WT liver. Strikingly, none of these TAA-induced effects were observed for matched Lrat−/− mice. To confirm that TAA hepatotoxicity depends on retinoid availability, we administered, over 48 h, four oral doses of 3000 IU retinyl acetate each to the mice. This led to the development of hepatotoxicity in Lrat−/− mice that was similar in extent to that observed in WT mice. Our findings establish that endogenous hepatic retinoid stores can modulate the toxicity of TAA in mice.
Keywords: vitamin A, retinoic acid, retinyl ester, hepatic stellate cell, gavage
Retinoids (vitamin A and its metabolites) are potent natural regulators of cellular activities, including cell proliferation, differentiation, and apoptosis (Gudas, 2012; Noy, 2010). Retinoic acid, the major transcriptionally active retinoid species, is reported to regulate over 500 genes (Balmer and Blomhoff, 2002). The all-trans- and 9-cis-isomers of retinoic acid regulate transcription upon binding to one of their six cognate nuclear receptors, the retinoic acid receptors (RARα, RARβ, and RARγ) and the retinoid X receptors (RXRα, RXRβ, and RXRγ) (Chawla et al., 2001; Duong and Rochette-Egly, 2011). All six of these ligand-dependent transcription factors are expressed in the liver (Wagner et al., 2011). The liver accounts for ∼80% of all retinoid that is present within the body of a healthy adult, with 80–90% of this hepatic retinoid being stored as retinyl ester within the lipid droplets of hepatic stellate cells (HSCs) (Blaner et al., 2009; Goodman et al., 1965). The ability to store retinoid within the liver relieves the organism from the obligate need to regularly acquire retinoid from the diet (Quadro et al., 1999).
Many different linkages between retinoid metabolism and actions and xenobiotic metabolism and actions have been reported in the literature. There are reports that retinoic acid treatment of both normal human keratinocytes and HepG2 cells with physiological concentrations of retinoic acid induces CYP1A1 expression in these cells (Ohno et al., 2012; Vecchini et al., 1994), probably through actions on the aryl hydrocarbon receptor nuclear translocator (Ohno et al., 2012). A number of other hepatic cytochrome P450s (CYPs) are known to be directly regulated at the transcriptional level by retinoic acid (Brtko and Dvorak, 2011; Ross et al., 2011). These include CYP26A1, CYP26B1 and CYP26C1, CYPs that are widely accepted as being responsible for retinoic acid catabolism in the body (Kedishvili, 2013). Other CYPs too have been shown to metabolize retinoic acid in vitro including CYP2E1, CYP2S1, CYP2C22, CYP2C39, CYP2C8, CYP2C9, and CYP3A4 (Qian et al., 2010; Thatcher et al., 2010). Pretreatment of mice with relatively large doses of retinol for several days prior to acetaminophen administration is reported to potentiate the hepatotoxicity of acetaminophen (Bray et al., 2001; Bray and Rosengren, 2001; Pumford et al., 1990). Eighty-five of 543 environmental toxins screened, including 16 organochlorine pesticides, 14 styrene dimers, 9 monoalkylphenols, and 6 parabens, were found to possess RAR agonist activity in reporter assays (Kamata et al., 2008). Hepatic retinoid stores are reported to plummet rapidly upon administration of either tetra- or hexachlorobiphenyl to mice or rats (Brouwer et al., 1985; Roos et al., 2011), tetrachlorodibenzo-p-dioxin to rats (Schmidt et al., 2003) or alcohol to mice (Clugston et al., 2013). It has been proposed that some of the toxic effects of polychlorbiphenyl, dioxin, and alcohol intoxication arise through disruptions of normal retinoic acid signaling pathways (Brouwer et al., 1985; Clugston et al., 2013; Roos et al., 2011; Schmidt et al., 2003). Thus, there is considerable evidence in the literature for significant retinoid-xenobiotic interactions within the body.
Recently, we reported that Lrat-deficient (Lrat−/−) mice, a strain of mice lacking the enzyme responsible for retinyl ester formation and hence retinoid stores in HSCs (O’Byrne et al., 2005), are less susceptible to diethylnitrosamine (DEN) induced hepatic tumorigenesis than matched wild type (WT) mice (Shirakami et al., 2012). This observation was surprising to us because both dietary retinoid intake in humans and retinoic acid administration to cancer cells in culture or experimentally induced cancers in animals have often been found to convey chemoprotection against cancer development (Tang and Gudas, 2011). To assess whether this hepatic response in the total absence of retinoid stores is specific to DEN or whether it can be more generalized to other hepatic toxins, we undertook studies of thioacetamide (TAA)-induced hepatic toxicity in age-, diet-, gender-, and genetic background-matched Lrat−/− and WT mice. We chose to study specifically TAA because of its efficacy in inducing hepatic failure in rodents (Butterworth et al., 2009; Rahman and Hodgson, 2000), its high specificity for the liver, its regiospecificity for the perivenous area, and the short window of time between its necrogenic effects and liver failure (Chilakapati et al., 2007; Mehendale, 2005).
MATERIALS AND METHODS
Animal husbandry and dietary regimens
All mice employed in our studies (males weighing 20–25 g, 10–12 weeks of age) were treated and maintained according to the NIH Guide for the Care and Use of Laboratory Animals (2011). The Lrat−/− mice were derived from ones originally described on a mixed genetic background through 10 backcrosses into the C57BL/6J genetic background, rendering all mice employed in our studies congenic in this genetic background. During the breeding and lactation periods, all mice were maintained on breeder chow that contained 15 IU retinol/g diet. After weaning, mice were maintained on a standard chow diet that also contained 15 IU retinol/g diet.
Acute hepatic failure induction
Acute hepatic injury was induced by a single intraperitoneal (ip) injection of a dose of 500 mg/kg TAA (Sigma-Aldrich Co.) dissolved in saline (0.9% (wt/vol) NaCl). Control-treated mice received the same volume of saline via ip injection. Routinely, six mice per group were studied. For retinoid-supplementation studies, separate groups of mice (six for each genotype) received 3000 IU of retinyl acetate in vegetable oil by gavage at 12 h intervals after either TAA or saline administration. At the time of sacrifice, 48 h after TAA injection, mice were weighed, blood was taken from the inferior vena cava, and the liver was immediately removed. The dissected livers were rapidly weighed and either used immediately for microsomal fraction isolation or frozen in liquid N2 and stored at –80°C. Tissues were stored continuously without thaw at −80°C until analysis. Sections from the dissected livers were also fixed in 10% neutral buffered formalin for histological analysis.
Histology
For paraffin sections, livers were first fixed in neutral buffered formalin and then processed into paraffin blocks according to standard protocols (Fischer et al., 2008). The embedded tissues were cut into 6 μm slices, mounted on charged adhesive slides, and dried overnight at 50°C. Slides were deparaffinized in xylene and rehydrated in graded alcohol and distilled water. Representative sections were stained with hematoxylin and eosin (H&E) according to standard protocols (Fischer et al., 2008).
Liver function tests
Alanine aminotransferase (ALT) enzymatic activity was determined in mouse serum using a kit from Felicit Diagnostics (Felicit Diagnostics, Ukraine), according to the manufacturer's instructions.
Measurement of myeloperoxidase activity in liver tissue was performed exactly as described earlier by others (Schierwagen et al., 1990). Briefly, 50 mg of liver tissue was homogenized on ice in 500 μl of 50mM potassium phosphate (KPO4) buffer, pH 7.4, using a Potter homogenizer, followed by centrifugation at 15,000 × g for 15 min at 4°C. The pellet was washed in the same buffer and recentrifuged for 5 min at 15,000 × g, followed by resuspension in 10 volumes of 50mM KPO4 buffer, pH 6.0, containing 0.5% (wt/vol) hexadecyltrimethylammonium bromide (Fluka Chemie Buchs, Switzerland) and incubation at 60°C for 2 h. The resuspended pellet was then sonicated for 10 s. After sonication, samples were subjected to three freeze-thaw cycles and again sonicated for 10 s, followed by centrifugation at 15,000 × g at 4°C for 15 min. An aliquot of supernatant (100 μl) was mixed with 100 μl of o-diansidine dihydrochloride (10 mg/ml in KPO4 buffer; Sigma-Aldrich Co.), 0.3% hydrogen peroxide (H2O2) was added and the absorbance was measured at 405 nm for 1 min to determine myeloperoxidase specific activity.
Blood ammonia levels were measured using a standard method (Huizenga et al., 1994). Blood glucose levels were measured employing the glucose oxidase method using a kit from Felicit Diagnostics (Felicit Diagnostics), according to the manufacturer's instructions.
Oxidative damage measurement
The degree of oxidative modification of hepatic proteins was determined through assessments of the levels of protein carbonylation (Levine et al., 1990) and of protein sulfhydryl groups (Murphy and Kehrer, 1989). Lipid peroxidation in liver was determined by assessing the level of thiobarbituric acid-reactive substances (TBRAS) (Ohkawa et al., 1979). Hepatic reduced glutathione level was determined as described by Ellman (1959).
Preparation of liver microsomes
Liver microsomes were prepared according to the method of Schenkman and Cinti (1978), with modifications. Briefly, livers were removed and homogenized using a Potter homogenizer in 10 volumes of ice-cold 0.25M sucrose. The liver homogenate was filtered through a 100 μm nylon mesh to remove debris and then centrifuged at 12,000 × g for 15 min at 4°C. Tris-НСl buffer (10mM, рН 7.4) containing 80mM СаCl2 and 160mM МgCl2 was added to the resulting supernatant (9 volumes of supernatant + 1 volume of buffer). The samples were gently mixed for 10 min at 4°C followed by centrifugation for 15 min at 9000 × g. The resulting pellet consisting of liver microsomes was washed twice with ice-cold 0.25M sucrose. The purity of each microsomal fraction was assessed for marker enzymes for other subcellular organelles, as described in Archakov et al. (1973). Specifically, we assessed succinate dehydrogenase (a marker enzyme of the inner mitochondrial membrane), glucose-6-phosphatase (a marker enzyme for the membrane of endoplasmic reticulum), and Na+/K+-ATPase (a marker enzyme for the plasma membrane) activities. For all of our studies, we employed only microsome preparations for which contaminating marker enzyme assays did not exceed 10% of the activity measured in the crude liver homogenate used for microsome isolation. Aliquots of liver microsomes were stored at –80°C until use. The content of microsomal protein was determined according to the Bradford method using bovine serum albumin as a standard (Bradford, 1976).
Microsomal monooxygenase activities
The aniline p-hydroxylase activity of hepatic cytochrome P450s was determined by the method of Archakov et al. (1974). The reaction mixture consisted of 40mМ Tris-НСl buffer, pH 7.3, containing 16mМ MgCl2, 3mМ NADPH, and 2 mg of microsomal protein. The reaction was initiated by adding aniline to a final concentration 3mМ. In control samples, NADPH was added after the termination of the reaction. Test and control samples were incubated at 37°C for 20 min with constant shaking. The reaction was terminated through addition of 15% trichloroacetic acid, followed by centrifugation at 3500 × g for 10 min. Following centrifugation, 10% (wt/vol) Na2CO3 and 2% (wt/vol) phenol in 0.2M NaOH were added to the supernatant. The samples were incubated in a water bath at 37°C for 30 min. To assess enzymatic activity, the absorbance was determined spectrophotometrically at 630 nm using a molar extinction coefficient for p-aminophenol of 13.3/mM/cm. Aniline p-hydroxylase activity was expressed as nmol/min/mg microsomal protein.
To assess the N-demethylase activity of hepatic cytochrome P450s, a reaction mixture consisting of 40mM Tris-HCl buffer, pH 7.6, containing 3mM NADPH, 16mМ MgCl2, and 1.5 mg microsomal protein was employed. The reaction was initiated by addition of N,N-dimethylaniline to a final concentration 6mМ. Test and control (no NADPH added) samples were incubated at 37°C for 30 min with vigorous shaking. The reaction was terminated by addition of an equal volume of 25% (wt/vol) Zn2SO4 in a saturated BaOH, followed by centrifugation at 3500 × g for 10 min. The formaldehyde content in the supernatant was determined employing the Nash color reaction (Nash, 1953). Color intensity was determined spectrophotometrically at 412 nm. The activity was calculated using a formaldehyde molar extinction coefficient of 1.5/mM/cm and expressed as nmol/min/mg microsomal protein.
Flavin-containing monooxygenase (FMO) activity was determined by the method of Ziegler & Pettit (Pettit et al., 1964) with some modifications. The reaction mixture consisted of 40mM Tris-HCl, pH 7.6, containing 3mM NADPH, 16mМ MgCl2, and 1.5 mg microsomal protein. The reaction was initiated by addition of N,N-dimethylaniline to a final concentration 6mМ. The test and control (no NADPH added) samples were incubated at 37°C for 30 min with vigorous shaking. The reaction was terminated by addition of 0.9M HClO4. Precipitated protein was pelleted by centrifugation at 3500 × g for 10 min. The clarified supernatants were then transferred to graduated test tubes and pHs were adjusted to 9.4 through addition of 1M NaOH. To eliminate unoxidized dimethylaniline, the samples were extracted three times with diethyl ether, each after rigorous shaking for 2 min. After the third extraction, the samples were left open for 20 min to allow for evaporation of the diethyl ether. After the ether had evaporated, the pH was adjusted to 2.4 through addition of several microliters of 5% trichloroacetic acid. Subsequently, 0.2 ml of 0.1M NaNO2 was added, followed by adjustment of the final volume to 3 ml through addition of citrate buffer (pH 2.4). For color development, the tubes were placed in a water bath at 60°C for 5 min. The absorbance was measured at 420 nm. To calculate enzyme specific activity expressed as nmol/min/mg microsomal protein, a molar extinction coefficient for p-nitroso-N,N-dimethylaniline of 8.2/mM/cm was employed.
Statistical analysis
All data are presented as means ± SD. Student's t-test was used to analyze differences between the control and knock-out strains. Statistical comparisons involving larger groups were first analyzed by a one-way ANOVA followed by multiple comparisons employing Tukey's HSD post hoc test. p-values <0.05 were considered statistically significant.
RESULTS
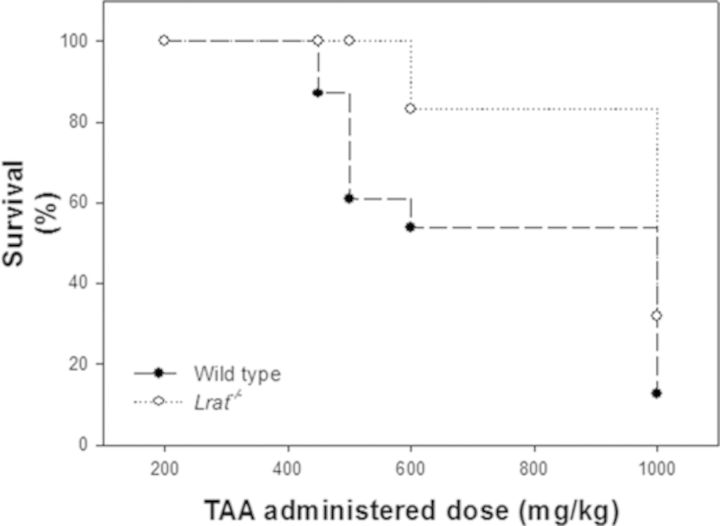
To understand whether hepatic retinoid stores influence the metabolism and toxicity of TAA, WT, and Lrat−/− mice were treated with a single ip dose of TAA, a hepatotoxin known to cause liver failure (Butterworth et al., 2009; Rahman and Hodgson, 2000). We first assessed the effects of different doses of TAA on the survival of matched WT and Lrat−/− mice, which lack any hepatic retinoid stores (O’Byrne et al., 2005). To this end, we initially employed a range of TAA concentrations (200–1000 mg/kg body weight) that is standardly used in the literature to investigate acute TAA toxicity. As can be seen from Figure 1, a dose of 450 mg TAA/kg body weight resulted in mortality in WT mice, with progressively more mortality observed with increasingly larger doses. Mortality due to TAA administration was first observed for Lrat−/− mice at a larger dose of 600 mg/kg body weight. The data provided in Figure 1 establish that the Lrat−/− mice are less susceptible to acute TAA toxicity than gender-, diet-, and genetic background-matched WT mice.
FIG. 1.
Survival of wild type and Lrat−/− mice 48 h after administration of a single intraperitoneal injection of different doses of thioacetamide.
Because 500 mg TAA/kg body weight was the highest TAA dose that did not induce mortality in Lrat−/− mice, which resulted in a >35% mortality rate in WT mice, we employed this dose in our subsequent studies. For these studies, WT and Lrat−/− mice were treated with a dose of TAA, administered as a single ip injection of 500 mg TAA/kg body weight. This treatment led to the development of extensive liver injury within 48 h in WT mice, as evidenced by the appearance of focal necrotic areas (Supplementary fig. 1) and increases, by 2 orders of magnitude, in serum ALT activity and myeloperoxidase activity in hepatic parenchyma compared with control values (Figs. 2A and 2B). This pronounced hepatic toxicity was not seen for Lrat−/− mice. These mutant mice exhibited near normal levels of serum ALT activity and no histological evidence for hepatic parenchyma damage (Fig. 2A, Supplementary fig. 1). The inflammation-associated recruitment of neutrophils was also attenuated in the livers of Lrat−/− mice (Fig. 2B).
FIG. 2.
Biochemical features of acute hepatotoxicity and hepatic encephalopathy in wild type and Lrat−/− mice administered a single intraperitoneal injection of 500 mg/kg thioacetamide. Serum ALT activity (panel A), hepatic parenchymal myeloperoxidase activity (panel B), blood ammonia concentrations (panel C), and blood glucose concentrations (panel D) were determined 48 h after injection of saline or TAA, with or without oral retinyl acetate administration. Values marked with different letters (a, b, and c) are statistically different, p < 0.05. All values are given as the mean ± 1SD, n = 6 for each group.
The hepatic parenchymal injury observed in WT mice was accompanied by a loss of liver function. Forty-eight hours after TAA administration to WT mice, features of hepatic encephalopathy were present, as evidenced by a 25% increase in blood ammonia levels and a threefold reduction of blood glucose levels (Figs. 2C and 2D). However, these changes were not observed in Lrat−/− animals whose blood ammonia levels were not statistically different from the values of the untreated group. Although a 35% reduction in blood glucose level was observed in Lrat−/− mice after TAA injection, these values were still significantly larger by 30% than for WT mice subjected to TAA administration.
Because TAA hepatotoxicity requires TAA bioactivation to highly reactive sulfoxide (TASO) and dioxide (TASO2) prooxidant species (Chilakapati et al., 2005, 2007; Hajovsky et al., 2012), we measured lipid and protein oxidative damage products in the livers of treated matched WT and Lrat−/− mice. TAA treatment of WT mice resulted in a significantly lower level of reduced hepatic glutathione, and protein sulfhydryl groups, and significantly elevated levels of protein carbonyl derivatives and thiobarbituric acid reactive substances (TBARS). For the TAA-treated Lrat−/− mice (Fig. 3), these parameters were not different than those of sham treated controls (either WT or Lrat−/− mice injected with the saline vehicle).
FIG. 3.
Hepatic oxidative damage products in wild type and Lrat−/− mice administered a single intraperitoneal injection of 500 mg/kg thioacetamide. Levels of reduced glutathione (panel A), protein thiol groups (panel B) and carbonyl derivatives (panel C), and thiobarbituric acid reactive substances (panel D) were determined in livers of mice 48 h after injection of saline or TAA. Values marked with different letters (a, b, and c) are statistically different, p < 0.05. All values are given as the mean ± 1SD, n = 6 for each group.
We hypothesized that the absence of lipid and protein oxidative damage products in livers of TAA-treated Lrat−/− mice might be as a result of defective drug metabolism and activation of the TAA, owing to the lack of hepatic retinoid stores in Lrat−/− mice. We assessed hepatic drug detoxification activities for both WT and Lrat−/− mice. After TAA administration, we observed both lower CYP hydroxylation activity and lower FMO oxygenase activity in microsomes prepared from Lrat−/− livers compared with activities determined for WT microsomes (Fig. 4).
FIG. 4.
Hepatic monooxygenase system activities in wild type and Lrat−/− mice administered a single intraperitoneal injection of 500 mg/kg thioacetamide. p-Hydroxylase (panel A), N-demethylase (panel B), and N-oxygenase (panel C) activities were determined for microsomes isolated from livers of mice at 48 h after injection of saline or TAA, either with or without oral retinyl acetate administration. Values marked with different letters (a, b, and c) are statistically different, p < 0.05. All values are given as the mean ± 1SD, n = 6 for each group.
To confirm that TAA hepatotoxicity depends on hepatic retinoid availability, we administered orally 3000 IU retinyl acetate at each of four 12 h intervals to the mice after TAA administration. This is a relatively large dose of retinoid given that our mice consuming a chow diet would only be consuming 45–50 IU/day. When retinyl acetate was administered alone not in conjunction with TAA, we did not observe any adverse effect, including hepatic injury or oxidative damage, in Lrat−/− mice for the short period (48 h) of supplementation employed in our studies. Nor did we observe an effect in WT mice after supplementation (data not shown). However, following TAA injection, retinyl acetate administration led to the development of acute hepatotoxicity in Lrat−/− mice, including the appearance of necrotic areas in parenchyma (Supplementary fig. 1), an increase in serum ALT and hepatic myeloperoxidase activities, accompanied by the development of hepatic encephalopathy (Fig. 2). Upon retinyl acetate supplementation, CYP hydroxylation and FMO oxygenase activities were increased in microsomes of Lrat−/− livers (Fig. 4) and protein and lipid oxidation markers were detected as well (Fig. 3), at levels which were identical to those observed for TAA-treated WT mice. Furthermore, many parameters of acute hepatotoxicity were further aggravated in TAA-injected WT mice following administration of retinyl acetate (Figs. 2–4).
DISCUSSION
Our data convincingly establish that the complete absence of hepatic retinoid stores in Lrat−/− mice results in diminished TAA-induced hepatic toxicity compared with matched WT mice. This protection was lost upon oral administration of retinyl acetate to Lrat−/− mice. The Lrat−/− mice, when dosed with retinyl acetate, displayed a similar degree of hepatic toxicity to that observed for TAA-treated WT mice. This provides strong support for the unexpected conclusion that hepatic retinoid stores accumulated over a lifetime from the diet help to facilitate liver toxicity associated with TAA exposure. This conclusion is in agreement with our earlier published finding that mice lacking hepatic retinoid stores are less susceptible to DEN-induced hepatocellular carcinoma (Shirakami et al., 2012). The present finding raises the possibility that hepatic retinoid stores may more broadly contribute to chemically induced liver injury.
What are the molecular processes that underlie the association between hepatic retinoids and chemically induced hepatoxicity? Our data establish that TAA treatment of WT mice results in a significantly decreased level of reduced hepatic glutathione and increased levels of oxidized proteins and TBARS compared with Lrat−/− mice. This was accompanied by lower levels of CYP hydroxylation activity and FMO oxygenase activity in livers of Lrat−/− mice. When mice are treated with retinyl acetate, these differences are completely abolished. Thus, the presence of hepatic retinoids must be contributing to increase levels of oxidative stress within the liver as well as increases in the activity levels of the CYP and FMO systems. Because TAA is bioactivated by the hepatic CYP and/or FMO systems and the products formed upon bioactivation account for the hepatic injury associated with TAA (Kang et al., 2008; Kim et al., 2000; Wang et al., 2000), our findings are in agreement with the literature. Moreover, this literature suggests that CYP2E1 is centrally involved in mediating TAA induced oxidative stress and liver injury (Kang et al., 2008; Wang et al., 2000). Although we did not specifically assess CYP2E1 mRNA or protein levels in this study, we earlier showed that both CYP2E1 mRNA and protein levels were lower 4, 24, and 48 h after administration of DEN to Lrat−/− mice compared with treated WT mice (Shirakami et al., 2012). Thus, we hypothesize that hepatic retinoids may be needed to maintain high levels of CYP2E1 and other CYPs and FMOs involved in xenobiotic metabolism in the liver. Although retinoic acid is known to regulate the rate of transcription of a number of CYP enzymes (Kedishvili, 2013), as far as we are aware, there is no information regarding whether retinoic acid contributes to the transcriptional regulation of CYP2E1.
The literature indicates that repeated administration of relatively high doses of retinol (∼5000 IU) to mice for 4 days prior to administration of acetaminophen potentiates the acute hepatotoxicity of this compound (Bray et al., 2001; Bray and Rosengren 2001; Pumford et al., 1990). Because the mice employed in these published studies were WT mice that were also maintained on a chow diet throughout life, our finding that oral retinyl acetate administration to WT mice enhances a number of features of the hepatoxicity associated with TAA administration is consistent with the earlier work. Hence, we should not view the findings from our work as being too surprising. (We remind the reader that oral retinyl acetate will be hydrolyzed in the gut and absorbed as retinol.) Strikingly though, our studies establish that the absence of hepatic retinoid stores arising from the ablation of the Lrat gene completely abolishes the hepatic toxicity associated with TAA administration. Within the liver, the great majority of hepatic retinoid is found within the lipid droplets of HSCs (Blaner et al., 2009). This suggests a dynamic role for HSC retinoids in the TAA-induced disease process that also involves the other cell types present in the liver.
What is the practical significance of our findings for understanding TAA-induced injury? Our data indicate that the severity of the effects of chemicals used to induce hepatic injury, ones like DEN and TAA, may be different depending on the retinoid status of the liver. Greater toxicity would likely be observed when hepatic retinoid stores are large, or dietary retinoid intake is high. Hepatic retinoid stores reflect dietary retinoid intake over the entire lifetime. This point may need to be considered in the design of experiments and/or in the interpretation of data obtained from studies of chemically induced liver injury. Our observations may also hold some therapeutic values to patients who have been acutely exposed to hepatotoxins like TAA. Based on our data, it would seem reasonable that such patients might be advised to immediately limit their dietary intake of retinoid.
In summary, hepatic retinoid stores and/or newly administered dietary retinoid potentiates TAA-induced liver injury. Hepatotoxicity was associated with higher levels of hepatic CYP and FMO activities and greater bioactivation of TAA. The bioactivated metabolites being formed increase the levels of oxidative stress experienced by the liver, facilitating disease development. These observations are however counterintuitive because retinoids are normally seen as agents promoting optimal health, and the presence of substantial hepatic stores as being beneficial. This raises an issue as to the extent to which retinoid stores and dietary intake need to be considered in the design and interpretation of studies involving TAA and possibly related compounds.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
U.S. Public Health Services, National Institutes of Health (RC2 AA019413, R01 DK068437, R21 AA02131136).
Supplementary Material
REFERENCES
- Archakov A. I., Karuzina I. I, Tveritinov V. N., Kokareva I. S. Hydroxylation of aniline and aminoantipyrine (1-phenyl-2,3-dimethyl-aminopyrasolon-5) derivatives in liver endoplasmatic reticulum. Biochem. Pharmacol. 1974;23:1053–1063. doi: 10.1016/0006-2952(74)90005-7. [DOI] [PubMed] [Google Scholar]
- Archakov A. I., Panchenko L. F., Kapitanov A. B., Efron I. I., Knyazeva T. I., Zherebkova N. S. A quantitative estimation of degree of purity of preparations of subcellular structures. Anal. Biochem. 1973;54(1):223–233. doi: 10.1016/0003-2697(73)90266-2. [DOI] [PubMed] [Google Scholar]
- Balmer J. E., Blomhoff R. Gene expression regulation by retinoic acid. J. Lipid Res. 2002;43:1773–1808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- Blaner W. S., O’Byrne S. M., Wongsiriroj N., Kluwe J., D’Ambrosio D. M., Jiang H., Schwabe R. F., Hillman E. M., Piantedosi R., Libien J. Hepatic stellate cell lipid droplets: A specialized lipid droplet for retinoid storage. Biochim. Biophys. Acta. 2009;1791:467–473. doi: 10.1016/j.bbalip.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bray B. J., Goodin M. G., Inder R. E., Rosengren R. J. The effect of retinol on hepatic and renal drug-metabolising enzymes. Food Chem. Toxicol. 2001;39:1–9. doi: 10.1016/s0278-6915(00)00110-1. [DOI] [PubMed] [Google Scholar]
- Bray B. J., Rosengren R. J. Retinol potentiates acetaminophen-induced hepatotoxicity in the mouse: Mechanistic studies. Toxicol. Appl. Pharmacol. 2001;173:129–136. doi: 10.1006/taap.2001.9170. [DOI] [PubMed] [Google Scholar]
- Brouwer A., van den Berg K. J., Kukler A. Time and dose responses of the reduction in retinoid concentrations in C57BL/Rij and DBA/2 mice induced by 3,4,3′,4′-tetrachlorobiphenyl. Toxicol. Appl. Pharmacol. 1985;78:180–189. doi: 10.1016/0041-008x(85)90282-0. [DOI] [PubMed] [Google Scholar]
- Brtko J., Dvorak Z. Role of retinoids, rexinoids and thyroid hormone in the expression of cytochrome p450 enzymes. Curr. Drug Metab. 2011;12:71–88. doi: 10.2174/138920011795016881. [DOI] [PubMed] [Google Scholar]
- Butterworth R. F., Norenberg M. D., Felipo V., Ferenci P., Albrecht J., Blei A. T. Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int. 2009;29:783–788. doi: 10.1111/j.1478-3231.2009.02034.x. [DOI] [PubMed] [Google Scholar]
- Chawla A., Repa J. J., Evans R. M., Mangelsdorf D. J. Nuclear receptors and lipid physiology: Opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- Chilakapati J., Korrapati M. C., Hill R. A., Warbritton A., Latendresse J. R., Mehendale H. M. Toxicokinetics and toxicity of thioacetamide sulfoxide: A metabolite of thioacetamide. Toxicology. 2007;230:105–116. doi: 10.1016/j.tox.2006.11.050. [DOI] [PubMed] [Google Scholar]
- Chilakapati J., Shankar K., Korrapati M. C., Hill R. A., Mehendale H. M. Saturation toxicokinetics of thioacetamide: Role in initiation of liver injury. Drug Metab. Dispos. 2005;33:1877–1885. doi: 10.1124/dmd.105.005520. [DOI] [PubMed] [Google Scholar]
- Clugston R. D., Jiang H., Lee M. X., Berk P. D., Goldberg I. J., Huang L. S., Blaner W. S. Altered hepatic retinyl ester concentration and acyl composition in response to alcohol consumption. Biochim. Biophys. Acta. 2013;1831:1276–1286. [PubMed] [Google Scholar]
- Duong V., Rochette-Egly C. The molecular physiology of nuclear retinoic acid receptors. From health to disease. Biochim. Biophys. Acta. 2011;1812:1023–1031. doi: 10.1016/j.bbadis.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Ellman G. L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fischer A. H., Jacobson K. A., Rose J., Zeller R. Paraffin embedding tissue samples for sectioning. CSH Protoc. 2008 doi: 10.1101/pdb.prot4989. 2008: pdb prot4989. [DOI] [PubMed] [Google Scholar]
- Goodman D. W., Huang H. S., Shiratori T. Tissue distribution and metabolism of newly absorbed vitamin a in the rat. J. Lipid Res. 1965;6:390–396. [PubMed] [Google Scholar]
- Gudas L. J. Emerging roles for retinoids in regeneration and differentiation in normal and disease states. Biochim. Biophys. Acta. 2012;1821:213–221. doi: 10.1016/j.bbalip.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajovsky H., Hu G., Koen Y., Sarma D., Cui W., Moore D. S., Staudinger J. L., Hanzlik R. P. Metabolism and toxicity of thioacetamide and thioacetamide S-oxide in rat hepatocytes. Chem. Res. Toxicol. 2012;25:1955–1963. doi: 10.1021/tx3002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizenga J. R., Tangerman A., Gips C. H. Determination of ammonia in biological fluids. Ann. Clin. Biochem. 1994;31:529–543. doi: 10.1177/000456329403100602. [DOI] [PubMed] [Google Scholar]
- Kamata R., Shiraishi F., Nishikawa J., Yonemoto J., Shiraishi H. Screening and detection of the in vitro agonistic activity of xenobiotics on the retinoic acid receptor. Toxicol. In Vitro. 2008;22:1050–1061. doi: 10.1016/j.tiv.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Kang J. S., Wanibuchi H., Morimura K., Wongpoomchai R., Chusiri Y., Gonzalez F. J., Fukushima S. Role of CYP2E1 in thioacetamide-induced mouse hepatotoxicity. Toxicol. Appl. Pharmacol. 2008;228:295–300. doi: 10.1016/j.taap.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Kedishvili N. Y. Enzymology of retinoic acid biosynthesis and degradation. J. Lipid Res. 2013;54:1744–1760. doi: 10.1194/jlr.R037028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. H., Bae J. H., Cha S. W., Han S. S., Park K. H., Jeong T. C. Role of metabolic activation by cytochrome P450 in thioacetamide-induced suppression of antibody response in male BALB/c mice. Toxicol. Lett. 2000;114:225–235. doi: 10.1016/s0378-4274(00)00168-5. [DOI] [PubMed] [Google Scholar]
- Levine R. L., Garland D., Oliver C. N., Amici A., Climent I., Lenz A. G., Ahn B. W., Shaltiel S., Stadtman E. R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- Mehendale H. M. Tissue repair: An important determinant of final outcome of toxicant-induced injury. Toxicol. Pathol. 2005;33:41–51. doi: 10.1080/01926230590881808. [DOI] [PubMed] [Google Scholar]
- Murphy M. E., Kehrer J. P. Oxidation state of tissue thiol groups and content of protein carbonyl groups in chickens with inherited muscular dystrophy. Biochem. J. 1989;260:359–364. doi: 10.1042/bj2600359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem. J. 1953;55:416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (US) Committee. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- Noy N. Between death and survival: Retinoic acid in regulation of apoptosis. Annu. Rev. Nutr. 2010;30:201–217. doi: 10.1146/annurev.nutr.28.061807.155509. [DOI] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Ohno M., Ikenaka Y., Ishizuka M. All-trans retinoic acid inhibits the recruitment of ARNT to DNA, resulting in the decrease of CYP1A1 mRNA expression in HepG2 cells. Biochem. Biophys. Res. Commun. 2012;417:484–489. doi: 10.1016/j.bbrc.2011.11.146. [DOI] [PubMed] [Google Scholar]
- O’Byrne S. M., Wongsiriroj N., Libien J., Vogel S., Goldberg I. J., Baehr W., Palczewski K., Blaner W. S. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT) J. Biol. Chem. 2005;280:35647–35657. doi: 10.1074/jbc.M507924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit F. H., Orme-Johnson W., Ziegler D. M. The requirement for flavin adenine dinucleotide by a liver microsmal oxygenase catalyzing the oxidation of alkylaryl amines. Biochem. Biophys. Res. Commun. 1964;16:444–448. doi: 10.1016/0006-291x(64)90373-0. [DOI] [PubMed] [Google Scholar]
- Pumford N. R., Roberts D. W., Benson R. W., Hinson J. A. Immunochemical quantitation of 3-(cystein-S-yl)acetaminophen protein adducts in subcellular liver fractions following a hepatotoxic dose of acetaminophen. Biochem. Pharmacol. 1990;40:573–579. doi: 10.1016/0006-2952(90)90558-3. [DOI] [PubMed] [Google Scholar]
- Qian L., Zolfaghari R., Ross A. C. Liver-specific cytochrome P450 CYP2C22 is a direct target of retinoic acid and a retinoic acid-metabolizing enzyme in rat liver. J. Lipid Res. 2010;51:1781–1792. doi: 10.1194/jlr.M002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadro L., Blaner W. S., Salchow D. J., Vogel S., Piantedosi R., Gouras P., Freeman S., Cosma M. P., Colantuoni V., Gottesman M. E. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. 1999;18:4633–4644. doi: 10.1093/emboj/18.17.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman T. M., Hodgson H. J. Animal models of acute hepatic failure. Int. J. Exp. Pathol. 2000;81:145–157. doi: 10.1046/j.1365-2613.2000.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos R., Andersson P. L., Halldin K., Hakansson H., Westerholm E., Hamers T., Hamscher G., Heikkinen P., Korkalainen M., Leslie H. A., et al. Hepatic effects of a highly purified 2,2′,3,4,4′,5,5′-heptachlorbiphenyl (PCB 180) in male and female rats. Toxicology. 2011;284:42–53. doi: 10.1016/j.tox.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Ross A. C., Cifelli C. J., Zolfaghari R., Li N. Q. Multiple cytochrome P-450 genes are concomitantly regulated by vitamin A under steady-state conditions and by retinoic acid during hepatic first-pass metabolism. Physiol. Genomics. 2011;43:57–67. doi: 10.1152/physiolgenomics.00182.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman J. B., Cinti D. L. Preparation of microsomes with calcium. Methods Enzymol. 1978;52:83–89. doi: 10.1016/s0076-6879(78)52008-9. [DOI] [PubMed] [Google Scholar]
- Schierwagen C., Bylund-Fellenius A. C., Lundberg C. Improved method for quantification of tissue PMN accumulation measured by myeloperoxidase activity. J. Pharmacol. Methods. 1990;23:179–186. doi: 10.1016/0160-5402(90)90061-o. [DOI] [PubMed] [Google Scholar]
- Schmidt C. K., Hoegberg P., Fletcher N., Nilsson C. B., Trossvik C., Hakansson H., Nau H. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) alters the endogenous metabolism of all-trans-retinoic acid in the rat. Arch. Toxicol. 2003;77:371–383. doi: 10.1007/s00204-003-0457-8. [DOI] [PubMed] [Google Scholar]
- Shirakami Y., Gottesman M. E., Blaner W. S. Diethylnitrosamine-induced hepatocarcinogenesis is suppressed in lecithin:retinol acyltransferase-deficient mice primarily through retinoid actions immediately after carcinogen administration. Carcinogenesis. 2012;33:268–274. doi: 10.1093/carcin/bgr275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X. H., Gudas L. J. Retinoids, retinoic acid receptors, and cancer. Annu. Rev. Pathol. 2011;6:345–364. doi: 10.1146/annurev-pathol-011110-130303. [DOI] [PubMed] [Google Scholar]
- Thatcher J. E., Zelter A., Isoherranen N. The relative importance of CYP26A1 in hepatic clearance of all-trans retinoic acid. Biochem. Pharmacol. 2010;80:903–912. doi: 10.1016/j.bcp.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchini F., Lenoir-Viale M. C., Cathelineau C., Magdalou J., Bernard B. A., Shroot B. Presence of a retinoid responsive element in the promoter region of the human cytochrome P4501A1 gene. Biochem. Biophys. Res. Commun. 1994;201:1205–1212. doi: 10.1006/bbrc.1994.1833. [DOI] [PubMed] [Google Scholar]
- Wagner M., Zollner G., Trauner M. Nuclear receptors in liver disease. Hepatology. 2011;53:1023–1034. doi: 10.1002/hep.24148. [DOI] [PubMed] [Google Scholar]
- Wang T., Shankar K., Ronis M. J., Mehendale H. M. Potentiation of thioacetamide liver injury in diabetic rats is due to induced CYP2E1. J. Pharmacol. Exp. Ther. 2000;294:473–479. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.