Abstract
Mice are resistant to aflatoxin hepatotoxicity, primarily due to high expression of glutathione S-transferases (GSTs), and in particular the GSTA3 subunit. Nuclear factor erythroid 2 related factor 2 (Nrf2) signaling, which controls a broad-based cytoprotective response, was activated either genetically or pharmacologically in an attempt to rescue GSTA3 knockout mice from aflatoxin genotoxicity. Genetic activation of Nrf2 signaling was attained in a GSTA3: hepatocyte-specific Keap1 double knockout (DKO) mouse whereas pharmacologic activation of Nrf2 was achieved through pretreatment of mice with the triterpenoid 1-[2-cyano-3-,12-dioxoleana-1,9(11)-dien-28-oyl] imidazole (CDDO-Im) prior to aflatoxin B1 exposure. Following oral treatment with aflatoxin, urine was collected from mice for 24 h and hepatic and urinary aflatoxin metabolites then quantified using isotope dilution-mass spectrometry. Although Nrf2 was successfully activated genetically and pharmacologically, neither means affected the response of GSTA3 knockout mice to chemical insult with aflatoxin. Hepatic aflatoxin B1-N7-guanine levels were elevated 120-fold in GSTA3 knockout mice compared with wild-type and levels were not attenuated by the interventions. This lack of effect was mirrored in the urinary excretion of aflatoxin B1-N7-guanine. By contrast, urinary excretion of aflatoxin B1-N-acetylcysteine was >200-fold higher in wild-type mice compared with the single GSTA3 knockout or DKO mouse. The inability to rescue GSTA3 knockout mice from aflatoxin genotoxicity through the Nrf2 transcriptional program indicates that Gsta3 is unilaterally responsible for the detoxication of aflatoxin in mice.
Keywords: Nrf2, Keap1, aflatoxin, glutathione S-transferases, DNA adducts
Aflatoxin B1 (AFB1) is a potent hepatocarcinogen produced by the mold Aspergillus flavus, which commonly grows on groundnuts and maize (Kensler et al., 2011). AFB1 is metabolized by cytochrome P450s to a reactive AFB1-epoxide that can cause genotoxic damage by binding with DNA at the N − 7 atom of guanine (Eaton and Gallagher, 1994). This AFB1-N7-guanine adduct is unstable and undergoes spontaneous depurination resulting in an abasic site in DNA leading to the excretion of the N − 7 adduct in urine. The AFB1-N7-guanine adduct can also rearrange to form a stable, ring-opened formamidopyrimidine adduct. Both forms of DNA lesions may lead to cytotoxicity and mutagenesis. The AFB1-epoxide can be detoxified by conjugation with glutathione through the catalytic actions of glutathione S-transferases (GSTs). Upon further metabolism, these thiol conjugates are excreted in urine as water soluble aflatoxin mercapturic acids (AFB1-NAC) (Busby and Wogan, 1984).
Significant disparities in sensitivity to AFB1 carcinogenesis have been reported between species with humans, rats, ducks and trout exhibiting greater sensitivity, and adult mice greater resistance to the toxic effects of AFB1 (Busby and Wogan, 1984). Interestingly, newborn mice are substantially more susceptible to hepatocarcinogenesis than adult mice (Vesselinovitch et al., 1972). In addition, newborn mice have very low hepatic GST levels compared with adult mice (Shupe and Sell, 2004). Such interspecies and age-related differences may reflect variations in the expression and catalytic activities of distinct GST isoforms toward conjugation of the aflatoxin epoxide. In particular, adult mice exhibit high constitutive levels of the GSTA3 subunit, which is very active with the AFB1-epoxide as substrate (Buetler et al., 1992). Mice in which the Gsta3 gene is specifically knocked out exhibit accentuated acute cytotoxicity and genotoxicity following AFB1 exposure (Ilic et al., 2010). Thus, GSTA3 is an important determinant of resistance to AFB1 in this species
Genetic intervention represents a means for alteration of nuclear factor erythroid 2 related factor 2 (Nrf2) signaling. The Nrf2-knockout mouse has been shown to be highly susceptible to many toxic compounds and carcinogens (reviewed in Slocum and Kensler, 2011). Correspondingly, hepatocyte-specific Keap1 (Kelch-like ECH associating protein 1) knockout mice, which exhibit constitutive up-regulation of Nrf2 signaling, demonstrate high hepatic expression of detoxication enzymes including GSTs and NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1) (Okawa et al., 2006). This mouse is resistant to the acute toxicities of many hepatotoxins (Liu et al., 2013), although its relative sensitivity or resistance to AFB1 is not known.
In addition to genetic intervention, protection from the toxic and carcinogenic effects of aflatoxins can be conferred in sensitive species such as rats by many classes of compounds, including phenolic antioxidants, dithiolethiones, isothiocyanates, and synthetic oleanane triterpenoids (Yates and Kensler, 2007). These protective actions can be attributed largely to interactions with the Keap1-Nrf2 pathway. These compounds oxidize or alkylate cysteines in Keap1, thereby allowing the transcription factor Nrf2 to escape proteasomal degradation and translocate into the nucleus. Nrf2 in the nucleus drives the transcription of its target genes, including multiple isoforms of GSTs, through antioxidant response elements in their upstream regulatory domains (Chanas et al., 2002). 1-[2-Cyano-3-,12-dioxoleana-1,9(11)-dien-28-oyl] imidazole (CDDO-Im) is among the most potent inducers of Nrf2 signaling in vitro and in vivo (Dinkova-Kostova et al., 2005; Yates et al., 2006). Cotreatment of rats with AFB1 and CDDO-Im leads to a dramatic reduction of the hepatic burden of preneoplastic lesions compared with exposure to AFB1 alone. Reductions of 40–90% of hepatic AFB1-N7-guanine adducts coupled with declines of 85 to >99% for preneoplastic foci were reported over the range of 1–100 μmol CDDO-Im/kg body weight (Yates et al., 2006). CDDO-Im is completely protective against AFB1-induced hepatocarcinogenesis (Johnson et al., forthcoming). Thus, in the rat, activation of Nrf2 signaling can affect profoundly the toxicity of aflatoxin.
To further probe the role of the Keap1-Nrf2 pathway as a modifier of chemical insult in mice, we examine whether genetic or pharmacologic activation of Nrf2 signaling could rescue the hyper-sensitive GSTA3 knockout mouse from the genotoxicity of AFB1. Such a study could serve to delineate the unilateral role of a single, highly efficient detoxication enzyme versus a broad multigenic cytoprotective response as key effectors of murine resistance to aflatoxins.
MATERIALS AND METHODS
Caution
AFB1 is a human carcinogen and dimethyldioxirane is an extremely volatile oxidant. Both should be used in properly vented areas exercising care to avoid personal exposure. Proper decontamination procedures should be followed.
Chemicals
AFB1, potassium peroxysulfate (Oxone; Dupont trademark), and N-acetylcysteine (NAC) were purchased from Sigma-Aldrich Co. (St Louis, MO). 13C15N-acetyl cysteine was purchased from Cambridge Isotope Laboratories (Tewksbury, MA). Dimethyldioxirane was prepared by the alkaline oxidation of acetone with the potassium peroxysulfate and AFB1-epoxide then prepared by oxidizing the dimethyldioxirane with AFB1. Subsequent synthesis of AFB1-N7-guanine and AFB1-N7–15N5-guanine was conducted as described (Egner et al., 2006). CDDO-Im was synthesized as described by Honda et al. (2000) and contributed by Dr. Michael Sporn, Department of Pharmacology and Toxicology, Dartmouth Medical School, Hanover, NH. All chromatographic solvents were HPLC grade and all chemicals used were of the highest purity grade possible.
Synthesis and purification of AFB1-NAC
The disodium salt of NAC was prepared by adding a small piece of sodium metal to NAC dissolved in methanol (Bartels and Timchalk, 1990). A 10-fold excess of the reaction mixture was added to freshly prepared AFB1-epoxide. After 1 min, the reaction was neutralized by the addition of 1M acetic acid. Solvents were evaporated and the crude AFB1-NAC mixture purified by HPLC (Scholl et al., 1997). A separate reaction was conducted substituting 13C15N-acetylcysteine was used to create the AFB1-13C15N-NAC. Both the AFB1-NAC and AFB1-13C15N-NAC were over 99% isotopically pure.
Animals and characterization
All procedures were approved by the Johns Hopkins University Animal Care and Use Committee. Wild-type C57BL/6J mice and B6.Cg-Tg(Alb-Cre)21Mgn/J (AlbCre) mice were obtained from The Jackson Laboratories (Bar Harbor, ME). AlbCre:Keap1flox/flox (Okawa et al., 2006) and GSTA3 knockout mice (Ilic et al., 2010) were crossed to produce compound GSTA3:Alb:Cre:Keap1flox/flox double knockout mice (DKO). Mouse genotypes were verified through analysis of tail genomic DNA using previously published PCR assays (Jowsey et al., 2003b; Okawa et al., 2006). Male mice were used in all studies.
Quantitative real-time PCR
Total RNA was extracted from frozen liver samples from untreated mice using the Trizol reagent (Life Technologies, Carlsbad, CA). RNA samples were treated with TURBO DNase (Life Technologies) to remove any possible genomic DNA contamination and were further purified using the RNeasy Mini Kit (Qiagen, Germantown, MD). RNA was quantified spectrophotometrically at 260 nm. RNA purity was estimated by the optical absorbance ratio A260 nm/A280 nm and RNA integrity was assessed by agarose gel electrophoresis. cDNA was prepared from 1 μg of each RNA sample using the qScript cDNA Synthesis Kit (Quanta Biosciences, Gaithersburg, MD). The cDNA samples were used as a template for qRT-PCR analysis using the iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) and specific primers for each gene synthesized by IDT (Coralville, IA). The primer sequences were obtained from the PrimerBank (Wang et al., 2012) and are shown in Table 1. The qRT-PCR reactions were run in technical tetraplicates for each biological replicate in a MyiQ Single Color real-time PCR system (Bio-Rad) using the following conditions; 95°C for 3 min and then 95°C for 10 s (step 1), 61°C for 30 s (step 2), go to step 1, 39 times. The correct size of the PCR products was confirmed by agarose gel electrophoresis and their purity was assessed by melt curve analysis using the MyiQ optical system software version 1.0410 (Bio-Rad). PCR efficiency was calculated from a standard curve using serial dilutions of pooled liver samples and relative mRNA levels were calculated by the comparative threshold cycle method using GAPDH as the housekeeping gene and the Pfaffl method to calculate fold changes (Pfaffl, 2001). The mean ratio was then calculated by dividing the relative mRNA levels of each target gene in the GSTA3 knockout mice by the relative mRNA levels of the same gene in the wild-type mice taking into account the error propagation that may arise by this method (Holmes and Buhr, 2007).
TABLE 1. Primers Used for Measuring GST Transcript Levels in Wild-type and GSTA3 Knockout (KO) Mouse Liver.
| Gene symbol | Forward primer | Reverse primer |
|---|---|---|
| GAPDH | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
| GSTα1 | AAGCCCGTGCTTCACTACTTC | GGGCACTTGGTCAAACATCAAA |
| GSTα3 | AGATCGACGGGATGAAACTGG | CAGATCCGCCACTCCTTCT |
| GSTα4 | TGATTGCCGTGGCTCCATTTA | CAACGAGAAAAGCCTCTCCGT |
| GSTM1 | ATACTGGGATACTGGAACGTCC | AGTCAGGGTTGTAACAGAGCAT |
| GSTM2 | ACACCCGCATACAGTTGGC | TGCTTGCCCAGAAACTCAGAG |
| GSTM3 | GCGGACTGACTCACTCCATC | CCCCATGACATATCTCTTCTCCT |
| GSTM4 | CTGAAGGTGGAATACTTGGAGC | GCCCAGGAACTGTGAGAAGA |
| GSTM5 | TCATCCAAGTCTATGGTTCTGGG | CCACAGATGTACCGTTTCTCCT |
| GSTM6 | ACAGGTCATGGACACTCGAAT | TGGCTTCCGTTTCTCAAAGTC |
| GSTP1 | ATGCCACCATACACCATTGTC | GGGAGCTGCCCATACAGAC |
| GSTT1 | AGGCTCGTGCTCGTGTAGA | CAGGGAACATCACCTTATGCC |
| GSTT2 | TGCCCAAGTCCACGAATACC | CCATTCTATCTCTGTTCCGTTCC |
Treatment protocol
Approximately 9-week-old male mice were treated by gavage with three doses of vehicle or 30 μmol/kg of CDDO-Im every other day (Monday, Wednesday, and Friday). This dose and schedule provides maximal induction of Nrf2 target genes in vivo in multiple murine tissues (Yates et al., 2007) and completely protects against AFB1-induced hepatocarcinogenesis in rats (Johnson et al., forthcoming). The vehicle used for CDDO-Im was 10% DMSO (dimethyl sulfoxide), 10% Cremophor-EL and PBS. Twenty-four hours after receiving the last dose, a single oral 0.8 mg/kg dose of AFB1 dissolved in DMSO was administered. Urine was collected from these mice by placing four mice in a glass metabolic cage for 24 h between administration of AFB1 and euthanasia. Urine volumes were recorded and aliquots were frozen for analysis. Identical protocols were followed for experiments using DKO mice; however, without pretreatment with CDDO-Im or vehicle. Livers were removed, snap frozen in liquid nitrogen, and stored at −80°C prior to analysis. Hepatic AFB1-N7-guanine, urinary AFB1-N7-guanine, and AFB1-NAC levels were measured by isotope-dilution mass spectrometry. A separate cohort of mice, treated identically but sacrificed 2 h after treatment with AFB1 was used for NQO1 enzyme activity and immunoblot analyses.
Protein preparation and immunoblot analyses
Tissue was homogenized in radioimmunoprecipitation assay buffer (RIPA-I), which contained protease inhibitor cocktail (Roche, Indianapolis, IN). An equal volume of 2× SDS sample buffer was added, followed by denaturation via boiling for 5 min. Samples were run through a SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to an Amersham Hybond-C Extra nitrocellulose membrane (GE Life Sciences, Piscataway, NJ). Membranes were blocked with tris-buffered saline containing 0.1% Tween 20 and 5% skim milk followed by treatment with primary antibody. NQO1 antibody (ab80588) was obtained from Abcam (Cambridge, MA), GAPDH (NB300-221) antibody was sourced from Novus Biologicals (Littleton, CO) and anti-GSTA3 antibody was kindly provided by Dr John D. Hayes, University of Dundee, UK. Membranes were then reacted with the appropriate secondary antibodies conjugated to horseradish peroxidase (Bio-Rad, 170-6515, 170-5047). The immunocomplexes were visualized with enhanced chemiluminescence.
Quantification of NQO1 enzyme activity
NQO1 enzyme activity was measured according to the method of Prochaska and Santamaria (1988) in homogenates prepared from livers of mice pretreated with three doses of CDDO-Im or vehicle and then sacrificed 2 h after dosing with AFB1. NQO1 levels were normalized to protein concentration (Pierce Protein BCA Kit no. 23225, ThermoFisher Scientific, Rockford, IL).
Analysis of AFB1 adducts and conjugates
Liver DNA was isolated 24 h after AFB1 treatment and the AFB1-N7-guanine adducts isolated as previously described (Kensler et al., 1985). AFB1-15N5-guanine was used as an internal standard for both DNA adduct analyses as well as the urinary AFB1-N7-guanine determinations. All mass spectrometric conditions have been previously reported (Egner et al., 2006). AFB1-NAC excreted into the urine was measured under the same chromatographic conditions using AFB1-13C15N-NAC as an internal standard. Retention time for the AFB1-N7-guanine was ∼4.0 min while the AFB1-NAC eluted at 6.2 min. Limits of detection for AFB1-N7-guanine and AFB1-NAC were 0.2 and 2 pmol/mg creatinine, respectively.
RESULTS
Generation and Characterization of Knockout and Transgenic Mice
The GST isoforms shown to be expressed in the liver of mice by Knight et al. (2008) were measured using real-time PCR methods to determine the impact, if any, of disruption of Gsta3 on either the compensatory expression or off-target disruption of other hepatic GST isoforms. As shown in Figure 1A, ratios (Gsta3 knockout/wild-type) of transcript levels of Gsta1, Gsta4, Gstm1, Gstm2, Gstm3, Gstm4, Gstm5, Gstm6, Gstp1, Gstt1, and Gstt2 demonstrated only modest differences in expression by genotype. By comparison, transcripts for Gsta3 were undetectable in the knockout mice. Collectively, these results indicate that the primary genotype and phenotype of these mice include normal expression of all hepatic GSTs except for Gsta3 in the GSTA3 knockout mice.
FIG. 1.
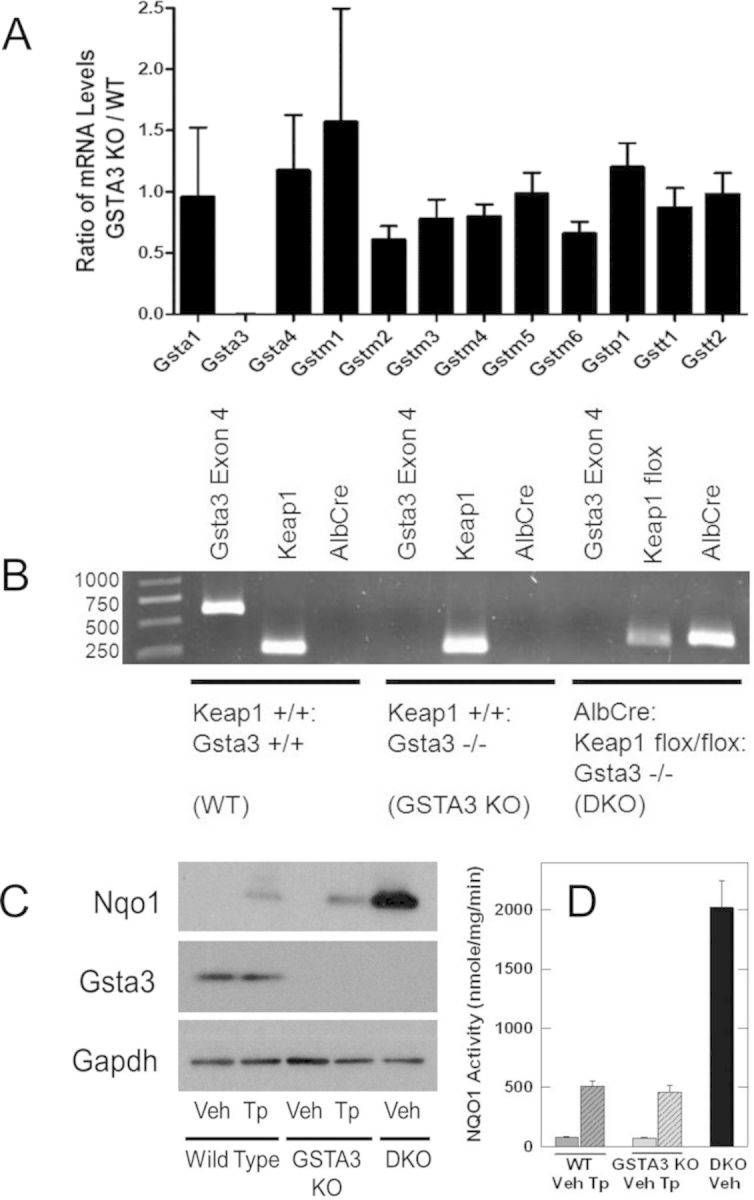
Genotypic and phenotypic characterization of wild-type (WT), GSTA3 knockout (GSTA3 KO), and GSTA3:AlbCre:Keap1flox/flox double knockout (DKO) mice. (A) Ratio of mRNA levels of hepatic GST isoforms between GSTA3KO and WT mice. Bars show mean ± SD. (B) Genotypic characterization of mice by PCR. Gsta3 was only present in the WT mice (576 bp). WT and GSTA3 KO mice demonstrated WT Keap1(250 bp) while DKO mice demonstrated floxed Keap1 (350 bp). Albumin Cre recombinase was present only in the DKO mice (356 bp). (C) NQO1 and GSTA3 protein expression by Western blot. Veh, vehicle; Tp, triterpenoid (CDDO-Im). (D) Hepatic NQO1 activity. Values are the mean ± SE, n = 4.
GSTA3 knockout mice and hepatocyte-specific Keap1 knockout mice were mated to generate a AlbCre:Keap1flox/flox::GSTA3−/− double knockout mouse. PCR assays were utilized to verify this DKO genotype (Fig. 1B). Both male and female DKO mice appear healthy and demonstrate no phenotypic abnormalities. Immunoblot analyses substantiated that neither GSTA3 knockout nor DKO mice expressed the GSTA3 protein (Fig. 1C). Upon treatment with the triterpenoid CDDO-Im, hepatic protein and activity levels for NQO1, an enzyme not involved in AFB1 metabolism (Eaton and Gallagher, 1994), increased in both wild-type and GSTA3 knockout mice (Figs. 1C and D). In addition, NQO1 activity was approximately fourfold greater in livers of DKO mice as compared to wild-type or GSTA3 knockout mice receiving CDDO-Im (Figs. 1C and D). As Nqo1 is regulated by Nrf2 (Nioi et al., 2003), these analyses confirm that efforts to genetically and pharmacologically activate Nrf2 signaling were successful.
Effect of Nrf2 Activation on Hepatic Aflatoxin B1-N7-Guanine Levels
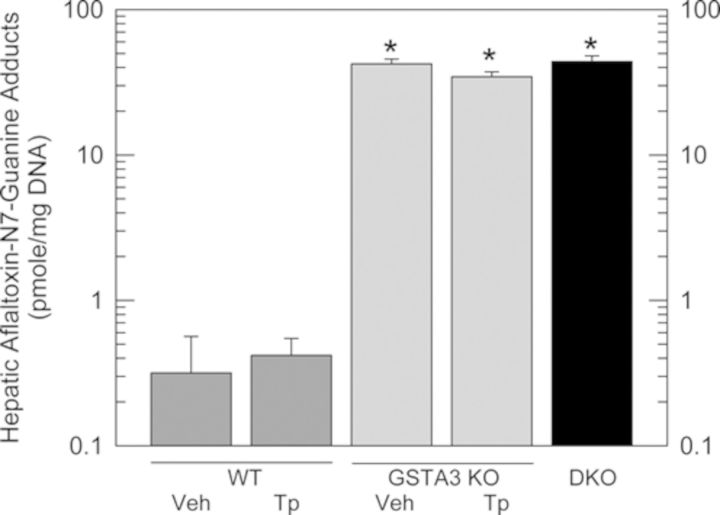
Comparison of AFB1-N7-guanine adduct levels in livers collected 24 h after administration of AFB1 across genotypes and treatments indicates that neither genetic nor pharmacologic activation of Nrf2 signaling significantly altered the hepatic adduct burden in GSTA3 knockout mice (Fig. 2). GSTA3 knockout mice receiving vehicle pretreatment had a mean 42.3 ± 3.1 pmol/mg DNA adducts as compared with 0.3 ± 0.0 pmol/mg DNA among wild-type mice receiving the vehicle pretreatment. Pretreatment with the triterpenoid CDDO-Im had insignificant effects on both GSTA3 knockout and wild-type mice, with a mean adduct burden of 34.5 ± 2.7 pmol AFB1-N7-guanine adducts/mg DNA among GSTA3 knockout mice and 0.4 ± 0.1 pmol/mg DNA among wild-type mice receiving CDDO-Im. Hepatocyte-specific disruption of Keap1 to enhance Nrf2 signaling likewise did not alter the adduct burden of the GSTA3 knockout mice as there was a mean 44.0 ± 4.0 pmol AFB1-N7-guanine adducts/mg DNA among DKO mice. The great disparity in hepatic aflatoxin genotoxicity between GSTA3 knockout and wild-type mice (Ilic et al., 2010) was not reduced through pharmacologic or genetic activation of Nrf2 signaling.
FIG. 2.
Quantification of hepatic AFB1-N7-guanine adducts (pmol/mg DNA) by isotope-dilution mass spectrometry for wild-type (WT) and GSTA3 KO mice receiving CDDO-Im (Tp) or vehicle (Veh) and in DKO mice 24 h after treatment with AFB1. Values are the mean ± SE, n = 8. (*) Differs from WT vehicle, p < 0.05.
Effect of Nrf2 Activation on Urinary Aflatoxin Metabolite Levels
Analyses of aflatoxin metabolites in urine collected for 24 h after administration of 0.8 mg/kg of AFB1 corroborated the failure of activation of Nrf2 signaling to enhance aflatoxin detoxication in GSTA3 knockout mice (Table 2). Pretreatment with CDDO-Im failed to significantly reduce the urinary levels of excreted AFB1-N7-guanine adducts among GSTA3 knockout mice, which exhibited greater than 100-fold higher urinary concentrations of adducts than the wild-type mice. Correspondingly, the wild-type mice excreted several hundred-fold greater amounts of the mercapturic acid, AFB1-NAC, suggesting a greater aflatoxin detoxication capacity than for the GSTA3 knockout mice. Hepatocyte-specific Keap1 disruption similarly did not reduce the level of urinary AFB1-N7-guanine adducts or increase excretion of AFB1-NAC among GSTA3 knockout mice. Indeed, the disparity in aflatoxin detoxication capacity between GSTA3 knockout and wild-type mice is highlighted by nondetectable levels of AFB1-N7-guanine in the urine of wild-type mice and nondetectable levels of AFB1-NAC in the urine of GSTA3 knockout mice.
TABLE 2. Urinary Excretion of AFB1-N7-Guanine and AFB1-NAC.
| Genotype | Pretreatment | AFB1-N7-guanine (pmol/mg creatinine) | AFB1-NAC (pmol/mg creatinine) |
|---|---|---|---|
| Wild-type | Vehicle | <0.2a | 471 ± 81 |
| Triterpenoid | <0.2 | 655 ± 83 | |
| GSTA3 KO | Vehicle | 71.2 ± 14.8 | <2 |
| Triterpenoid | 60.3 ± 2.4 | <2 | |
| DKO | None | 69.2 ± 12.0 | <2 |
aFour pools of four mice each were used for the urinary analyses. Values are mean ± S.E.
DISCUSSION
Aflatoxin B1 is metabolized by cytochrome P450s to AFB1-epoxides, which can bind with DNA or undergo detoxication. In mice, the principal pathway for detoxication is through conjugation of the epoxide with glutathione mediated by GSTs. Murine GSTs exhibit substantially greater conjugative activity with the AFB1-epoxide than those in rats or other species with higher susceptibility to aflatoxin toxicity (Eaton and Gallagher, 1994). Alpha class GSTs are believed to play the primary role in aflatoxin detoxication and constitutive expression of the Gsta3 subunit accounts for 35% of all GSTs in the livers of male mice (Mitchell et al., 1997). Upon transfection into hamster V79 cells, murine Gsta3 reduced AFB1 genotoxicity by 70–80% and increased resistance to cytotoxicity 4.6-fold (Fields et al., 1999).
There is robust evidence that many murine GSTs, are regulated through the Keap1-Nrf2 signaling pathway, including Gsta1, Gsta2, Gsta4, Gstm1, Gstm2, Gstm3, Gstm4, and Gstm5 (Chanas et al., 2002; Hayes et al., 2000; Knight et al., 2008; McWalter et al., 2004). However, evidence for the regulation of Gsta3 by an antioxidant response element in the Keap1-Nrf2 pathway in mice is ambiguous. Heightened levels of GSTA3 were observed when mouse Hepa-1c1c7 cells were treated with the isothiocyanate, sulforaphane, a potent Nrf2 inducer. (Jowsey et al., 2003a; McWalter et al., 2004). Additionally, these studies reported that Nrf2−/− mice demonstrated lower constitutive expression of GSTA3 than wild-type, and that GSTA3 was not inducible in these transgenic mice. Other studies have indicated that there was no reduction of basal GSTA3 in Nrf2−/− mice, and likewise Nrf2 inducers such as butylated hydroxyanisole, oltipraz, and ethoxyquin had no effect on hepatic GSTA3 expression (Chanas et al., 2002; Hayes et al., 2000; Knight et al., 2008). In this study, treatment with CDDO-Im had no effect on hepatic GSTA3 expression in wild-type C57BL/6J mice, lending credence to the hypothesis that Gsta3 is not regulated by the Keap1-Nrf2 pathway in the mouse. Nevertheless, given the arsenal of phase II enzymes, including other GSTs, which are regulated through the Nrf2 signaling pathway, it would be expected that they could compensate for the loss of GSTA3 in mitigating AFB1 genotoxicity. However, no reduction in AFB1 genotoxicity was attained in the GSTA3 knockout mice despite successful activation of the Keap1-Nrf2 signaling pathway achieved either pharmacologically or genetically. These data indicate that Gsta3 is unilaterally responsible for the detoxication of AFB1 in the mouse. Such a single deterministic outcome defining natural resistance to an environmental carcinogen in mammals is unusual, but not unprecedented. Pi class GST knockout mice are more sensitive to skin, lung and colon carcinogenesis (Henderson et al., 1998; Ritchie et al., 2007, 2009). However, whether such mice can be rescued by members of the Keap1-Nrf2 gene battery is not known.
Other attempts to define unilateral determinants of resistance to AFB1 genotoxicity have failed. The epoxide-derived dialdehyde of AFB1 has been hypothesized to contribute indirectly to the carcinogenic effects of AFB1 via protein adduction and subsequent hepatotoxicity. This aldehyde is reduced through the activity of aflatoxin aldehyde reductases (AKR7A1), which is the most highly inducible gene yet described in rat liver by CDDO-Im and other inducers known to affect Nrf2 signaling (Knight et al., 1999; Yates et al., 2006). Rats are a species with greater vulnerability to the effects of AFB1 than mice. However, AKR7A1 transgenic rats still maintained congruent susceptibility to hepatocarcinogenesis as wild-type rats despite robust expression of the transgene and demonstrable alterations in the disposition of the aflatoxin dialdehyde in vivo (Roebuck et al., 2009). The mechanism of protection in the rat likely lies in the induction of GSTA5, which is the orthologous GST to murine GSTA3 and has a very high catalytic activity towards the AFB1 epoxide (Buetler et al., 1995).
Disruption of Nrf2 has been shown to enhance the sensitivity of mice to chemical carcinogenesis in models targeting the forestomach with benzo[a]pyrene (Ramos-Gomez et al., 2001), the bladder with N-nitrosobutyl(4-hydroxybutyl)amine (Iida et al., 2007), the skin with dimethylbenz[a]anthracene (Xu et al., 2006), and the liver with 2-amino-3-methylimidazo[4,5]quinoline (Kitamura et al., 2007). Correspondingly, Keap1flox/flox mice are more resistant to tongue and esophageal carcinogenesis following treatment with 4-nitroquinoline-1-oxide than wild-type (Okhoshi et al., 2013). The cytoprotective transcriptional program regulated through Nrf2 consists of several hundred genes, many but not all of which encode enzymes engaged in the detoxication of chemicals. Thus, there is a presumption that multiple gene products contribute to this broad sweep of protection. In this context, it is remarkable that a single gene, Gsta3, has evolved to determine sensitivity of mice to AFB1. The extent to which GSTs determine human sensitivity to AFB1, and their inducibility in either Nrf2-dependent or -independent manners is not resolved. Human alpha class GST proteins that are constitutively expressed in the liver (hGSTA1 and hGSTA2) have little, if any activity toward the AFB1-epoxide, although mu class GSTs may afford some protection (Eaton et al., 2001). Early chemoprevention trials with the Nrf2 inducer oltipraz provoked enhanced rates of urinary excretion of the AFB1-NAC conjugate compared to placebo treated participants (Wang et al., 1999). Collectively, studies across species highlight the importance of GSTs in the detoxication of aflatoxin and as possible targets for induction by chemopreventive agents.
FUNDING
National Institutes of Health (R01 CA39416 to T.W.K., P01 ES006052 to J.D.G., R01 CA161649 to S.S.).
Acknowledgments
The authors wish to express their gratitude to Dr John D. Hayes (University of Dundee) for his donation of the anti-GSTA3 antibody and Dr Michael B. Sporn (Dartmouth Medical School) for the CDDO-Im.
REFERENCES
- Bartels M. J., Timchalk C. 1,2-Dichloropropane: investigation of the mechanism of mercapturic acid formation in the rat. Xenobiotica. 1990;20:1035–1042. doi: 10.3109/00498259009046824. [DOI] [PubMed] [Google Scholar]
- Bartels M. J., Miner V. W. Synthesis of stable isotopic-labelled analogs of the cysteine and N-acetyl cysteine conjugates of tetrachloroethylene. J. Labelled Compounds Radiopharm. 1989;28:209–214. [Google Scholar]
- Buetler T. M., Gallagher E. P., Wang C.-H.., Stahl D. L., Hayes J. D., Eaton D. L. Induction of phase I and phase II drug metabolizing enzyme mRNA, protein and activity by BHA, ethoxyquin and oltipraz. Toxicol. Appl. Pharm. 1995;135:45–57. doi: 10.1006/taap.1995.1207. [DOI] [PubMed] [Google Scholar]
- Buetler T. M, Slone D., Eaton D. L. Comparison of the aflatoxin B1–8,9-epoxide conjugating activities of two bacterially expressed alpha class glutathione S-transferase isozymes from mouse and rat. Biochem. Biophys. Res. Commun. 1992;188:597–603. doi: 10.1016/0006-291x(92)91098-b. [DOI] [PubMed] [Google Scholar]
- Busby W. F., Wogan G. N. Aflatoxins. In: Searle C., editor. Chemical Carcinogens. Washington, D.C: American Chemical Society; 1984. pp. 945–1136. [Google Scholar]
- Chanas S. A., Jiang Q., McMahon M., McWalter G. K., McLellan L. I., Elcombe C. R., Henderson C. J., Wolf C. R., Moffat G. J., Itoh K., et al. Loss of the Nrf2 transcription factor causes a marked reduction in constitutive and inducible expression of the GST Gsta1, Gsta2, Gstm1, Gstm3, and Gstm4 genes in the livers of male and female mice. Biochem. J. 2002;365:405–416. doi: 10.1042/BJ20020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova A. T., Liby K. T., Stephenson K. K., Holtzclaw W. D., Gao X., Suh N., Williams C., Risingsong R., Honda T., Gribble G. W., et al. Extremely potent triterpenoid inducers of the phase 2 response: Correlations of protection against oxidant and inflammatory stress. Proc. Natl. Acad. Sci. U.S.A. 2005;102:4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D. L., Bammler T. K., Kelly E. J. Interindividual differences in response to chemoprotection against aflatoxin-induced hepatocarcinogenisis: Implications for human biotransformation enzyme polymorphisms. Adv. Exp. Med. Biol. 2001;500:559–576. doi: 10.1007/978-1-4615-0667-6_85. [DOI] [PubMed] [Google Scholar]
- Eaton D. L., Gallagher E. P. Mechanisms of aflatoxin carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1994;34:135–172. doi: 10.1146/annurev.pa.34.040194.001031. [DOI] [PubMed] [Google Scholar]
- Egner P. A., Groopman J. D., Wang J. S., Kensler T. W., Friesen M. Quantification of aflatoxin-B1-N7-Guanine in human urine by high-performance liquid chromatography and isotope dilution tandem mass spectrometry. Chem. Res. Toxicol. 2006;19:1191–1195. doi: 10.1021/tx060108d. [DOI] [PubMed] [Google Scholar]
- Fields W. R., Morrow C. S., Doehmer J., Townsend J. D. Expression of stably transfected murine glutathione s transferase A3-3 protects against nucleic acid alkylation and cytotoxicity by aflatoxin B1 in hamster V79 cells expressing rat cytochrome P450-2B1. Carcinogenisis. 1999;20:1121–1125. doi: 10.1093/carcin/20.6.1121. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Chanas S. A., Henderson C. J., McMahon M., Sun C., Moffat G. J., Wolf C. R., Yamamoto M. The Nrf2 transcription factor contributes to the basal expression of glutathione S-transferases in mouse liver and to their induction by the chemopreventive synthetic antioxidants, butylated hydroxyanisole and ethoxyquin. Biochem. Soc. Trans. 2000;28:33–41. doi: 10.1042/bst0280033. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Judah D. J., McLellan L. I., Neal G. E. Contribution of the glutathione S-transferase to the mechanisms of resistance to aflatoxin B1. Pharmacol. Ther. 1991;58:443–472. doi: 10.1016/0163-7258(91)90053-o. [DOI] [PubMed] [Google Scholar]
- Henderson C. J., Smith A. G., Ure J., Brown K., Bacon E. J., Wolf C. R. Increased skin tumorigenesis in mice lacking pi class glutathione S-transferases. Proc. Natl. Acad. Sci. U.S.A. 1998;95:5275–5280. doi: 10.1073/pnas.95.9.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. T., Buhr K. A. Error propagation in calculated ratios. Clin. Biochem. 2007;40:728–734. doi: 10.1016/j.clinbiochem.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Honda T., Rounds B. V., Bore L., Finlay H. J., Favaloro F. G., Jr, Suh N., Wang Y., Sporn M. B., Gribble G. W. Synthetic oleanane and ursane triterpenoids with modified rings A and C: A series of highly active inhibitors of nitric oxide production in mouse macrophages. J. Med. Chem. 2000;43:4233–4246. doi: 10.1021/jm0002230. [DOI] [PubMed] [Google Scholar]
- Iida K., Itoh K., Maher J. M., Kumagai Y., Oyasu R., Mori Y., Shimazui T., Akaza H., Yamamoto M. Nrf2 and p53 cooperatively protect against BBN-induced urinary bladder carcinogenesis. Carcinogenesis. 2007;28:2398–2403. doi: 10.1093/carcin/bgm146. [DOI] [PubMed] [Google Scholar]
- Ilic Z., Crawford D., Vakharia D., Egner P. A., Sell S. Glutathione S-transferase A3 knockout mice are sensitive to acute cytotoxic and genotoxic effects of aflatoxin B1. Toxicol. Appl. Pharm. 2010;242:241–246. doi: 10.1016/j.taap.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson N. M., Egner P. A., Baxter V.K., Sporn M. B., Wible R. S., Sutter T. R., Groopman J. D., Kensler T. W., Roebuck B. D. Complete protection against aflatoxin B1-induced liver cancer with triterpenoid: DNA adduct dosimetry, molecular signature and genotoxicity threshold. Cancer Prev. Res. doi: 10.1158/1940-6207.CAPR-13-0430. doi:10.1158/1940-6207.CAPR-13-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jowsey I. R., Jiang Q., Itoh K., Yamamoto M., Hayes J. D. Expression of the aflatoxin B1-8,9-epoxide-metabolizing murine glutathione S-transferase A3 subunit is regulated by the Nrf2 transcription factor through an antioxidant response element. Mol. Pharmacol. 2003a;64:1018–1028. doi: 10.1124/mol.64.5.1018. [DOI] [PubMed] [Google Scholar]
- Jowsey I. R., Smith S. A., Hayes J. D. Expression of the murine glutathione S-transferase alpha3 (GSTA3) subunit is markedly induced during adipocyte differentiation: Activation of the GSTA3 gene promoter by the pro-adipogenic eicosanoid 15d-PGJ2. Biochem. Biophys. Res. Commun. 2003b;312:1226–1235. doi: 10.1016/j.bbrc.2003.11.068. [DOI] [PubMed] [Google Scholar]
- Kensler T. W., Egner P. A., Trush M. A., Bueding E., Groopman J. D. Modification of aflatoxin B1 binding to DNA in vivo in rats fed phenolic antioxidants, ethoxyquin and a dithiothione. Carcinogenesis. 1985;6:759–763. doi: 10.1093/carcin/6.5.759. [DOI] [PubMed] [Google Scholar]
- Kensler T. W., Roebuck B. D., Wogan G. N., Groopman J. D. Aflatoxin: A 50 year odyssey of mechanistic and translational toxicology. Toxicol. Sci. 2011;120:28–48. doi: 10.1093/toxsci/kfq283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y., Umemura T., Kanki K., Kodama Y., Kitamoto S., Saito K., Itoh K., Yamamoto M., Masegi T., Nishikawa A., et al. Increased susceptibility to hepatocarcinogenicity of Nrf2-deficient mice exposed to 2-amino-3-methylimidizo[4,5]quinoline. Cancer Sci. 2007;98:19–24. doi: 10.1111/j.1349-7006.2006.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight T. R., Choudhuri S., Klaassen C. D. Induction of hepatic glutathione S-transferases in male mice by prototypes of various classes of microsomal enzyme inducers. Toxicol. Sci. 2008;106:329–338. doi: 10.1093/toxsci/kfn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight L. P., Primiano T., Groopman J. D., Kensler T. W., Sutter T. R. cDNA cloning, expression and activity of a second human aflatoxin B1-metabolizing member of the aldo-keto reductase superfamily, AKR7A3. Carcinogenesis. 1999;20:1215–1223. doi: 10.1093/carcin/20.7.1215. [DOI] [PubMed] [Google Scholar]
- Liu J., Wu K. C., Lu Y. F., Ekuase E., Klaassen C. D. Nrf2 protection against liver injury produced by various hepatotoxicants. Oxid. Med. Cell Longev. 2013;2013:305861. doi: 10.1155/2013/305861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWalter G. K., Higgins L. G., McLellan L. I., Henderson C. J., Song L., Thornalley P. J., Itoh K., Yamamoto M., Hayes J. D. Transcription factor Nrf2 is essential for induction of NAD(P)H:quinone oxidoreductase 1, glutathione S-transferases, and glutamate cysteine ligase by broccoli seeds and isothiocyanates. J. Nutr. 2004;134:3499S–3506S. doi: 10.1093/jn/134.12.3499S. [DOI] [PubMed] [Google Scholar]
- Mitchell A., Morin D., Lakritz J., Jones A. D. Quantitative profiling of tissue and gender related expression of GST isoenzymes in the mouse. Biochem. J. 1997;325:207–216. doi: 10.1042/bj3250207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nioi P., McMahon M., Itoh K., Yamamoto M., Hayes J. D. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone reductase 1 gene: Reassessment of the ARE consensus sequence. Biochem. J. 2003;374:337–348. doi: 10.1042/BJ20030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okawa H., Motohashi H., Kobayashi A., Aburatani H., Kensler T. W., Yamamoto M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem. Biophys. Res. Commun. 2006;339:79–88. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- Okhoshi A., Suzuki T., Ono M., Kobayashi T., Yamamoto M. Roles of Keap1-Nrf2 system in upper aerodigestive tract carcinogenesis. Cancer Prev. Res. 2013;6:149–159. doi: 10.1158/1940-6207.CAPR-12-0401-T. [DOI] [PubMed] [Google Scholar]
- Pfaffl M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska H. J., Santamaria A. B. Direct measurement of NAD(P)H:quinone reductase from cells cultured in microtiter wells: A screening assay for anticarcinogenic enzyme inducers. Anal. Biochem. 1988;169:328–336. doi: 10.1016/0003-2697(88)90292-8. [DOI] [PubMed] [Google Scholar]
- Ramos-Gomez M., Kwak M. K., Dolan P. M., Itoh K., Yamamoto M., Talalay P., Kensler T. W. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie K. J., Henderson C. J., Wang X. J., Vassieva O., Carrie D., Farmer P. B., Gaskell M., Park K., Wolf C. R. Glutathione transferase pi plays a critical role in the development of lung carcinogenesis following exposure to tobacco-related carcinogens and urethane. Cancer Res. 2007;67:9248–9257. doi: 10.1158/0008-5472.CAN-07-1764. [DOI] [PubMed] [Google Scholar]
- Ritchie K. J., Walsh S., Sansom O. J., Henderson C. J., Wolf C. R. Markedly enhanced colon tumorigenesis in Apc(Min) mice lacking glutathione S-transferase Pi. Proc. Natl. Acad. Sci. U.S.A. 2009;106:20859–20864. doi: 10.1073/pnas.0911351106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebuck B. D., Johnson D. N., Sutter C. H., Egner P. A., Scholl P. F., Friesen M. D., Baumgartner K. J., Ware N. M., Bodreddigari S. B., Groopman J. D., et al. Transgenic expression of aflatoxin aldehyde reductase (AKR7A1) modulates aflatoxin B1 metabolism but not hepatic carcinogenesis in the rat. Toxicol. Sci. 2009;109:41–49. doi: 10.1093/toxsci/kfp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl P. F., Musser S. M., Groopman J. D. Synthesis and characterization of aflatoxin B1 mercapturic acids and their identification in rat urine. Chem. Res. Toxicol. 1997;10:1144–1151. doi: 10.1021/tx960161+. [DOI] [PubMed] [Google Scholar]
- Shupe T., Sell S. Low hepatic S-transferase and increased hepatic DNA adduction contribute to increased tumorigenicity of aflatoxin B1 in newborn and partially hepatectomized mice. Toxicol. Lett. 2004;148:1–9. doi: 10.1016/j.toxlet.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Slocum S. L., Kensler T. W. Nrf2: Control of sensitivity to carcinogens. Arch. Toxicol. 2011;85:273–284. doi: 10.1007/s00204-011-0675-4. [DOI] [PubMed] [Google Scholar]
- Vesselinovitch S. D., Mihailovich N., Wogan G. N., Lombard L. S., Rao K. V.N. Aflatoxin B1, a hepatocarcinogen in the infant mouse. Cancer Res. 1972;32:2289–2291. [PubMed] [Google Scholar]
- Wakabayashi N., Itoh K., Wakabayashi J., Motohashi H., Noda S., Takahashi S., Imakado S., Kotsuji T., Otsuka F., Roop D. R., et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- Wang J. S., Shen X., He X., Zhu Y. R., Zhang B. C., Wang J. B., Qian G. S., Kuang S. Y., Zarba A., Egner P. A., et al. Protective alterations in phase 1 and 2 metabolism of aflatoxin B1 by oltipraz in residents of Qidong, People's Republic of China. J. Natl. Cancer Inst. 1999;91:347–354. doi: 10.1093/jnci/91.4.347. [DOI] [PubMed] [Google Scholar]
- Wang X., Spandidos A., Wang H., Seed B. PrimerBank: A PCR primer database for quantitative gene expression analysis, 2012 update. Nucleic Acids Res. 2012;40:D1144–D1149. doi: 10.1093/nar/gkr1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Huang M. T., Shen G., Yuan X., Lin W., Khor T. O., Conney A. H., Kong A. N. Inhibition of 7,12-dimethylbenza[a]anthracene-induced skin tumorigenesis in C57BL6/J mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer Res. 2006;66:8293–8296. doi: 10.1158/0008-5472.CAN-06-0300. [DOI] [PubMed] [Google Scholar]
- Yates M. S., Kensler T. W. Keap1 eye on the target: chemoprevention of liver cancer. Acta Pharmacol. Sin. 2007;28:1331–1342. doi: 10.1111/j.1745-7254.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- Yates M. S., Kwak M., Egner P. A., Groopman J. D., Bodreddigari S., Sutter T. R., Baumgartner K. J., Roebuck B. D., Liby K. T., Yore M. M., et al. Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3–12-dioxooleana-1,9(11)-dien-28-ol = yl]imidazole. Cancer Res. 2006;66:2488–2494. doi: 10.1158/0008-5472.CAN-05-3823. [DOI] [PubMed] [Google Scholar]
- Yates M. S., Tauchi M., Katsuoka F., Flanders K. C., Liby K. L., Honda T., Gribble, Johnson D. A., Johnson J. A., Burton N. C., et al. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol. Cancer Ther. 2007;6:154–162. doi: 10.1158/1535-7163.MCT-06-0516. [DOI] [PubMed] [Google Scholar]