Abstract
Purpose
The present study aimed to determine the role played by β-defensin 124 (DEFB124) in the innate immunity of prostate epithelial RWPE-1 cells during bacterial infection.
Materials and Methods
The expression of DEFB124 was examined by quantitative real-time polymerase chain reaction (PCR), Western blotting, and immunocytochemistry. Enzyme-linked immunosorbent assays and quantitative real-time PCR were performed to determine the production of cytokines and chemokines. Western blotting and chromatin immunoprecipitation studies were performed to assess the interaction between DEFB124 and nuclear factor-kappa B (NF-κB) in peptidoglycan (PGN)-stimulated RWPE-1 cells. By chemotaxis assay, we assessed the effect of DEFB124 on the migration of monocytes.
Results
Exposure to PGN induced DEFB124 upregulation and NF-κB activation through IκBα phosphorylation and IκBα degradation. Bay11-7082, an NF-κB inhibitor, blocked PGN-induced DEFB124 production. Also, NF-κB was shown to be a direct regulator and to directly bind to the -3.14 kb site of the DEFB124 promoter in PGN-treated human prostate epithelial RWPE-1 cells. When DEFB124 was overexpressed in RWPE-1 cells, interestingly, the production of cytokines (interleukin [IL] 6 and IL-12) and chemokines (CCL5, CCL22, and CXCL8) was significantly increased. These DEFB124-upregulated RWPE-1 cells markedly induced chemotactic activity for THP-1 monocytes.
Conclusions
Taken together, these results provide strong evidence for the first time that increased DEFB124 expression via NF-κB activation in PGN-exposed RWPE-1 cells enhances the production of cytokines and chemokines, which may contribute to an efficient innate immune defense.
Keywords: Chemotaxis, Cytokines/chemokines, Defensins, Innate immunity, NF-kappa B
INTRODUCTION
Antimicrobial peptides (AMPs) are endogenous small molecular weight proteins that form important components of the innate immune system and have broad-spectrum antimicrobial activity against bacteria, viruses, protozoa, yeast, and fungi [1,2]. AMPs have been shown to be important in such diverse functions as antigen presentation, angiogenesis, wound healing, and chemotaxis [3,4]. In humans and other mammals, the two main AMP families are defensins and cathelicidins [5,6]. Defensins contain six highly conserved cysteine residues, which form three cysteine pairings, connected by disulfide bonds. On the basis of sequence homology and the connectivity of the six conserved cysteine residues, defensins are classified into three structural families: α, β, and θ [7,8]. Only α- and β-defensins are present in humans [3].
Many more human β-defensins exist and are believed to be widely distributed in various types of epithelial cells [9,10]. However, only a few human β-defensins have been characterized thus far at the protein level. To date, more than 28 members of the human β-defensin family have been discovered [11]. β-Defensin 124 (DEFB124), a new member of the human β-defensin family, was identified by analysis based on hidden Markov chain models linked to BLAST searches of the whole human genome [11]. Previously, we identified a list of human β-defensins, which are differentially expressed, among the toll-like receptors (TLR) agonists in RWPE-1 cells by using the Illumina HumanHT-12 microarray. We found a significantly high level of DEFB124 expression in peptidoglycan (PGN)-stimulated RWPE-1 cells relative to other TLR agonist-stimulated RWPE-1 cells (not published). These results strongly suggested that DEFB124 expression is also inducible in response to microbial organisms and proinflammatory stimuli, as described for other members of the human β-defensins family. At present, however, the precise function of DEFB124 is not known. Therefore, the objective of this study was to examine the roles played by DEFB124 in the innate immune responses of the human prostate.
MATERIALS AND METHODS
1. Reagents and antibodies
PGN and the NF-κB inhibitor Bay11-7082 were purchased from Sigma-Aldrich and Calbiochem, respectively. Rabbit anti-IκBα antibody, rabbit antiphospho-IκBα antibody, rabbit NF-κB/p65 antibody, and normal rabbit immunoglobulin G (IgG) were purchased from Cell Signaling Technology Inc. (Danvers, MA, USA). Mouse anti-ACTB antibody and goat anti-DEFB124 antibody were purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA).
2. RWPE-1 cell culture
The immortalized human prostate epithelial cell line (RWPE-1) was obtained from the American Type Culture Collection. RWPE-1 cells were cultured in Keratinocyte-SFM (K-SFM, Invitrogen Co., Carlsbad, CA, USA) supplemented with 0.05 mg/mL bovine pituitary extract (BPE) and 5 ng/mL epidermal growth factor (EGF) in a humidified atmosphere containing 5% CO2.
3. Reverse transcription-polymerase chain reaction
Total RNA was isolated from RWPE-1 cells by using the RNeasy kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Complementary DNA (cDNA) was synthesized from 2 µg total RNA by using 0.5 µg of oligo dT primer according to the first-strand synthesis system protocol (Promega Co., Madison, WI, USA). The following primer set was used: human DEFB124 forward 5'-ATGTGCCATCAGGGAGAAGT-3' and reverse 5'-TGCATAGGAGAGACAGCACA-3' (131 bp). The specificity of the primers was tested by use of a GenBank basic local alignment search tool. The polymerase chain reaction (PCR) conditions consisted of 1 cycle of 94℃ for 5 minutes; 35 cycles of 94℃ for 30 seconds, 64℃ for 30 seconds, and 72℃ for 30 seconds; and 1 cycle of 72℃ for 5 minutes. PCR products were electrophoresed on a 1.5% agarose gel. Gels were photographed and analyzed by use of the Bio-Rad Molecular Imager Gel Doc XR+ System (Bio-Rad Laboratories Inc., Hercules, CA, USA). Relative gene expression levels were normalized to the expression of ACTB. All experiments were repeated in triplicate.
4. Quantitative real-time RT-PCR
To measure the amount of mRNA in RWPE-1 cells, quantitative real-time reverse transcription-PCR (RT-PCR) analysis was performed by using the Rotor-Gene Q (Qiagen). The primers used to amplify the selected genes were purchased from Qiagen (QuantiTect Primer Assay). The Rotor-Gene SYBR Green PCR kit (Qiagen) was used to monitor amplification, and the results were evaluated with Rotor-Gene Q series software. Reaction mixtures were set up in a total volume of 25 µL by using 1 µL of cDNA (diluted 1:100), 12.5 µL of SYBR Green PCR master mix (Qiagen), and 20 pmol of each gene-specific primer. The reactions were performed by using a Rotor-Gene-Q machine. The cycling conditions were as follows: 95℃ for 5 minutes and 40 cycles of 95℃ for 5 seconds and 60℃ for 10 seconds with a single fluorescence measurement. Upon completion of PCR, fluorescence was monitored continuously while slowly heating the samples from 60℃ to 95℃ with a heating rate of 0.2℃/sec and a continuous fluorescence measurement. The melting curves were used to identify any nonspecific amplification products. Quantitation of gene amplification was performed by determining the cycle threshold (CT) based on the fluorescence detected within the geometric region of the semilog amplification plot. Expression of each mRNA species was normalized to the expression of β-actin (ACTB). Relative quantitation of target gene expression was evaluated by using the comparative CT method, and experiments were repeated at least three times by using different sets of RWPE-1 cells.
5. Western blot
The constituent proteins of the epithelial cell extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 10% separating gel and were then transferred to a nitrocellulose membrane (Bio-Rad Laboratories Inc.). The membrane was blocked for 1 hour in Tris-buffered saline-Tween (TBST) containing 5% nonfat dry milk. The blocked membranes were then incubated with rabbit anti-IκBα antibody (1:1000), rabbit antiphospho-IκBα antibody (1:1000), goat anti-DEFB124 antibody (1:1000), and mouse monoclonal anti-ACTB antibody (1:2000) in TBST. After incubation, membranes were incubated with horseradish-peroxidase-conjugated anti-rabbit IgG (1:2000) or antimouse IgG (1:2000) or antigoat IgG (1:4000) in TBST for 1 hour at room temperature (RT). After each step, the membranes were washed several times with TBST, and bound antibody was detected by using an enhanced chemiluminescence detection system (Amersham Biosciences Co., Piscataway, NJ, USA) according to the manufacturer's instructions.
6. Immunocytochemistry
RWPE-1 cells were grown on glass coverslips, fixed with 4% paraformaldehyde for 40 minutes, and then permeabilized with 0.1% Triton X-100 for 20 minutes at RT. Cells were blocked with 3% bovine serum albumin (BSA; Sigma-Aldrich Co., St. Louis, MO, USA) for 2 hours to block unspecific binding of the antibodies and were incubated with antibodies against DEFB124 at 4℃ overnight. After washing, the cells were incubated with FITC-conjugated anti-rabbit IgG (1:1000 dilution) for 1 hour at RT, and DNA was counterstained with propidium iodide (Sigma-Aldrich Co.). Coverslips were mounted with fluorescence mounting medium (DAKO, Carpinteria, CA, USA). Fluorescence was detected by confocal laser microscopy (Zeiss LSM 510).
7. Production of recombinant DEFB124
The vectors for DEFB124 overexpression were purchased from ORIGENE (RC217724). The cells were then transfected with either 4 µg of DEFB124-DDK-Myc vector or 4 µg of empty vector by using Lipofectamine 2000 in Opti-MEM media according to the manufacturer's directions. After 24 hours, the transfection medium was replaced with K-SFM containing BPE and EGF. After another 24 hours, RWPE-1 cells were plated at a lower density and were cultured in selection medium containing 400 µg/mL G418. The G418 concentration was reduced to 200 µg/mL for 1 week for selection and was then reduced to 100 µg/mL for maintenance. The supernatants obtained from cultured RWPE-1 cells transfected with empty vector or DEFB124-DDK-Myc were concentrated with a 3-kDa molecular mass cutoff filter (EMD Millipore, Billerica, MA, USA) and were stored at -80℃. Production of the recombinant DEFB124-DDK-Myc protein was checked by Western blot by use of anti-Myc antibody according to the manufacturer's instructions.
8. Enzyme-linked immunosorbent assay
Cytokines and chemokines were measured by using the human inflammatory cytokines multianalyte ELISArray kit (Qiagen) and human common chemokines multianalyte ELISArray kit (Qiagen) according to the manufacturers' instructions, respectively. Briefly, 50 µL of medium was incubated in a 96-well plate for 1 hour at RT, followed by washing steps, incubation with detection antibodies for 1 hour, washing, incubation with avidin-horseradish peroxidase for 30 minutes, washing, development for 15 to 30 minutes, termination of the development with stop solution, and colorimetric quantitation of cytokines or chemokines by determination of the optical density at 450 nm.
9. Chromatin immunoprecipitation
Experiments were performed with the chromatin immunoprecipitation (ChIP) assay kit (Upstate Biotechnology, Charlottesville, VA 6) according to the manufacturer's procedure. Briefly, 2×107 cells were treated with 1% formaldehyde for 10 minutes at 37℃. Subsequent procedures were performed on ice in the presence of protease inhibitors. Cross-linked cells were harvested, washed with phosphate-buffered saline, and lysed in sodium dodecyl sulfate lysis buffer (1% sodium dodecyl sulfate, 10 mM ethylenediaminetetraacetic acid [EDTA], 50 mM Tris-HCl, pH 8.1) for 10 minutes at 4℃. Chromatin was sonicated with five 10-second pulses at 30% amplitude. After centrifugation, the supernatant was diluted 10-fold with ChIP dilution buffer (0.01% sodium dodecyl sulfate, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl, pH 8.1, 167 mM NaCl). Diluted extracts were precleared in the presence of Protein G Agarose. One tenth of the diluted extract was kept for direct quantitative PCR (input). The remaining extracts were incubated for 16 hours at 4℃ in the presence of 1 µg of anti-NF-κB/p65 antibodies or normal rabbit IgG per mL, followed by incubation with Protein G Agarose for 1 hour. Following extensive washing, bound DNA fragments were eluted by means of a 30-minute incubation in elution buffer (1% sodium dodecyl sulfate, 0.1 M NaHCO3). The DNA was recovered for 4 hours at 65℃ in elution buffer containing 200 mM NaCl and was then incubated in the presence of proteinase K (20 µg/mL) for 1 hour at 45℃. DNA was extracted in the presence of phenol-chloroform and chloroform-isoamyl alcohol and was ethanol precipitated before being subjected to real-time PCR.
10. Monocyte chemotaxis assay
Chemotaxis was assayed in 24-well plates containing Transwell inserts with a pore size of 5 µm (Corning Costar, Thermo Fisher Scientific, Waltham, MA, USA). Briefly, THP-1 cells were preincubated for 24 hours in serum-free Rosewell Park Memorial Institute (RPMI) 1640 supplemented with 0.1% BSA (Sigma-Aldrich Co.). After starvation, these cells were washed twice in PBS and were resuspended in RPMI 1640 containing 0.1% BSA. For each well, 1×106 THP-1 cells were placed in the upper chamber with a polycarbonate membrane at the bottom, and a chemoattractant suspension was added in the lower chamber. Chemoattractant suspensions consisted of a 50-fold concentrated culture medium from empty vector (control) or DEFB1124-DDK-Myc transfected RWPE-1 cells. Monocyte chemoattractant protein-1 (MCP-1) was used as a positive control in the assay because MCP-1 is a well-established chemotactic substance for monocytes. After incubation at 37℃ for 24 hours, the cells that migrated to the lower chamber were quantified by trypan blue dye exclusion. Data are expressed as the fold increase over the empty vector.
RESULTS
1. Upregulation of PGN-induced DEFB124 through NF-κB activation
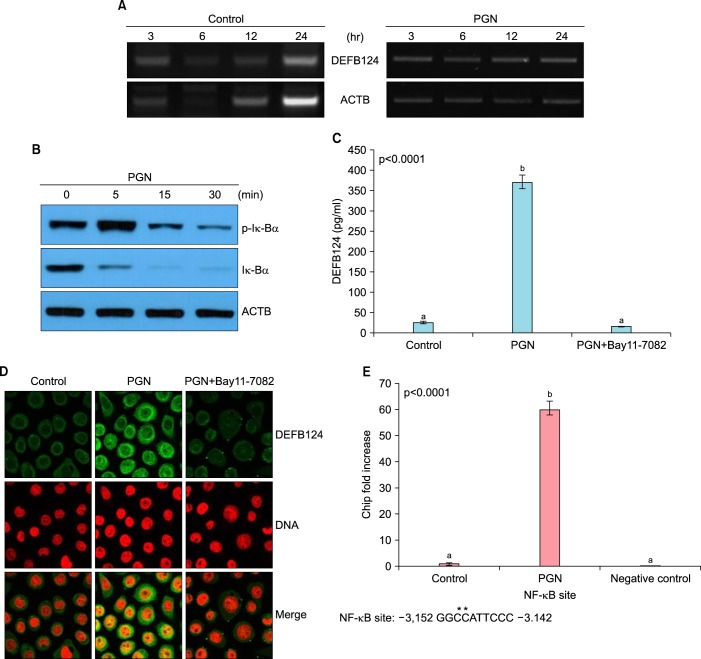
The expression of DEFB124 in RWPE-1 cells treated with PGN was evaluated by RT-PCR. The DEFB124 transcript was more highly expressed in PGN-stimulated RWPE-1 cells than in untreated RWPE-1 cells (Fig. 1A). To determine whether PGN leads to NF-κB activation in RWPE-1 cells, we performed Western blot analysis for phosphorylated IκBα and IκBα protein. The maximal phosphorylation of IκBα was detected at 5 minutes after PGN stimulation, but thereafter phosphorylation gradually decreased (Fig. 1B). In contrast, total IκBα was ubiquitinated within the same PGN stimulation periods (Fig. 1B). The results showed that PGN induced NF-κB activation. To determine the effect of NF-κB activation on the production of DEFB124, Bay11-7082 was used to block NF-κB activation. Bay11-7082 led to a decrease in PGN-induced DEFB124 production by RWPE-1 cells (Fig. 1C). The NF-κB inactivation had inhibitory effects on PGN-induced DEFB124 protein production, which were additionally confirmed by immunocytochemistry staining (Fig. 1D). Taken together, these results suggest that PGN induces increases in the expression of DEFB124 through NF-κB activation. To determine whether this regulation of expression was direct, we performed ChIP analysis. After NF-κB/p65 immunoprecipitation, binding of NF-κB/p65 protein to the DEFB124 promoter was analyzed by quantitative real-time PCR CT values of the promoter region containing a kappa-B (κB) regulatory element. The level of NF-κB/p65 binding to the DEFB124 promoter was higher in PGN-induced RWPE-1 cells relative to untreated RWPE-1 cells (Fig. 1E). The binding site for NF-κB (-3142 to -3152) in the proximal promoter region of DEFB124 was presented (Fig. 1E). These results imply that NF-κB is a direct regulator of PGN-stimulated DEFB124 production.
FIG. 1.
Peptidoglycan (PGN) induces gene expression of β-defensin 124 (DEFB124) through nuclear factor-kappa B (NF-κB) activation in RWPE-1 cells. (A) PGN induces DEFB124 mRNA expression. The RWPE-1 cells were treated with PGN (10 µg/mL) for the indicated times and DEFB124 expression was determined by reverse transcription-polymerase chain reaction. (B) PGN leads to NF-κB activation in RWPE-1 cells. The cells were stimulated with 10 µg/mL of PGN, and protein was extracted at the indicated time points. The phosphorylation and ubiquitination of IκBα were examined by western blot. ACTB was used as an internal control. (C) The activation of NF-κB is required for the upregulation of PGN-induced DEFB124. The RWPE-1 cells were treated with PGN or NF-κB inhibitor Bay11-7082 (10 µM), and the concentration of secreted DEFB124 protein was measured in the culture supernatant by enzyme-linked immunosorbent assay. Concentrations are picograms of protein per mL, and data are the mean results of three distinct experiments. Different letters indicate significant differences at p<0.0001. (D) PGN induces DEFB124 production through NF-κB activation. The RWPE-1 cells were treated with PGN or Bay11-7082, and reduced production of PGN-induced DEFB124 protein was evaluated by immunocytochemistry. RWPE-1 cells were fixed in 4% paraformaldehyde, stained with anti-DEFB124 antibody (green), and counterstained with PI (red) for DNA staining. (E) NF-κB interacts with DEFB124 chromatin structure. NF-κB directly binds at the -3.14 Kb site of DEFB124 in PGN-stimulated RWPE-1 cells. Results, normalized for the input DNA and expressed as the relative enrichment of immunoprecipitated PGN-treated RWPE-1 cells compared to the untreated control RWPE-1 cells, are the means of three independent experiments. The site difference from the consensus NF-κB binding sequences (CBS) are indicated by asterisks. CBS, GGGRNNYYCC; R, purine; Y, pyrimidine. Different letters indicate significant differences at p<0.0001.
2. DEFB124 upregulation induces the gene expression of cytokines and chemokines
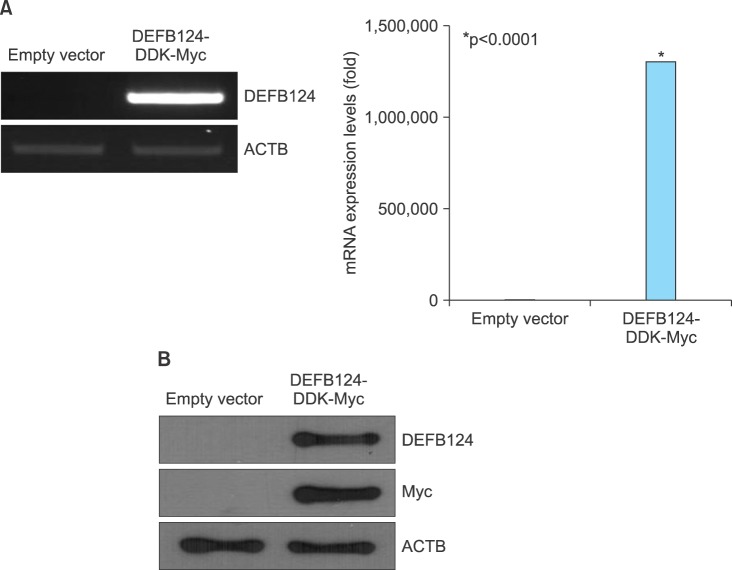
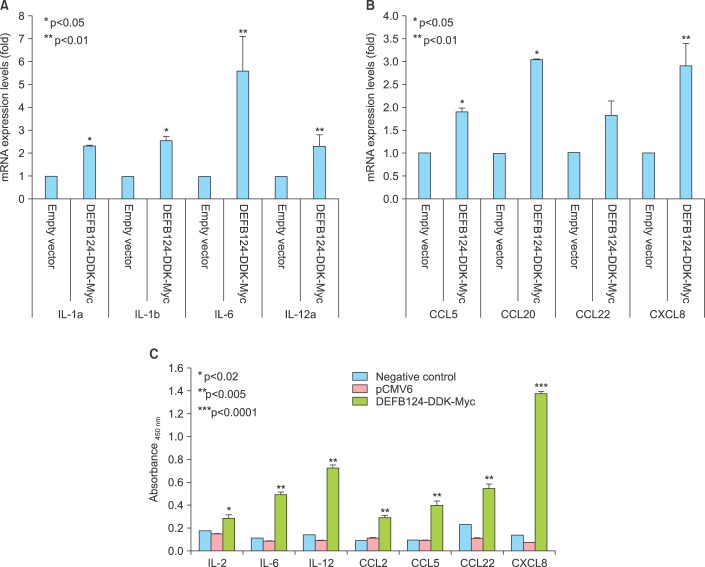
The RWPE-1 cells were transfected with DEFB124-DDK-Myc vector or empty vector, and the upregulation of DEFB124 was determined by quantitative real-time PCR and western blot. High levels of DEFB124 mRNA expression were detected in DEFB124-DDK-Myc-transfected RWPE-1 cells relative to its expression in empty vector-transfected RWPE-1 cells (Fig. 2A). Also, subsequent DEFB124 protein production was markedly increased in DEFB124-DDK-Myc-transfected RWPE-1 cells (Fig. 2B). To determine whether upregulation of DEFB124 expression leads to the production of cytokines and chemokines, we measured the expression levels by quantitative real-time PCR. Interestingly, as shown in Fig. 3A and 3B, DEFB124 upregulation significantly enhanced the expression of cytokines (interleukin [IL] 1α, IL-1β, IL-6, and IL-12) and chemokines (CCL5, CCL20, and CXCL8) compared with endogenous DEFB124 expression. Subsequently, the amount of cytokines and chemokines secreted into the culture supernatant was evaluated by enzyme-linked immunosorbent assay (ELISA). In DEFB124-increased RWPE-1 cells, the production of cytokines, such as IL-2, IL-6, and IL-12, was significantly higher than in empty-vector-transfected RWPE-1 cells (Fig. 3C). Additionally, the levels of cytokines, such as CCL2, CCL5, CCL22, and CXCL8, were also upregulated by DEFB124 overexpression (Fig. 3C).
FIG. 2.
Overexpression of β-defensin 124 (DEFB124) in RWPE-1 cells. (A) DEFB124 mRNA overexpression. The RWPE-1 cells were transfected with DEFB124-DDK-Myc vector or empty vector, and DEFB124 mRNA expression was determined by reverse transcription-polymerase chain reaction (left) and quantitative real-time polymerase chain reaction (right). ACTB was used as an internal control. Asterisk represents statistical significance at p<0.0001. (B) Upregulation of DEFB124 protein. DEFB124 protein was evaluated by western blot by using antibodies against DEFB124 and Myc. ACTB was used as an internal control.
FIG. 3.
Beta-Defensin 124 (DEFB124) upregulation induces increased production of cytokines and chemokines. DEFB124 promotes mRNA expression for cytokines (A) and chemokines (B) in DEFB124-induced RWPE-1 cells. The mRNA expression of these genes was determined by using quantitative real-time polymerase chain reaction. Relative expression levels of each gene were calculated from cycle threshold values and were normalized with ACTB, and the expression ratio was calculated against the expression of each gene in the empty vector-transfected RWPE-1 cells. Experiments were repeated at least three times, and data are expressed as the mean±standard error of the mean (SEM). Asterisks, * and **, represent statistical significance at p<0.05 and p<0.01, respectively. (C) DEFB124 is required for cytokine and chemokine production. Supernatants from empty vector- or DEFB124-DDK-Myctransfected RWPE-1 cells were collected. The concentrations of cytokines and chemokines in supernatant were measured by multianalyte enzyme-linked immunosorbent assay (ELISA). The diagram shows mean ELISA absorbance values (450 nm) for triplicates with the error bars representing the SEM. Asterisks (*, **, and ***) represent statistical significance at p<0.02, p<0.005, and p<0.0001, respectively. IL, interleukin.
3. DEFB124-induced cytokines and chemokines promote THP-1 monocyte chemotaxis
To determine whether increased DEFB124-induced cytokines and chemokines led to chemotaxis in THP-1 monocytes, we tested the direct effects of DEFB124-induced cytokines and chemokines on monocyte migration by using Transwell assays in which THP-1 monocytes added to the upper chamber migrated in response to chemotactic stimuli added to the lower chamber. As expected, DEFB124-induced cytokines and chemokines effectively increased monocyte migration by 2.6-fold compared with the empty-vector-induced stimuli-treated control group (Fig. 4).
FIG. 4.
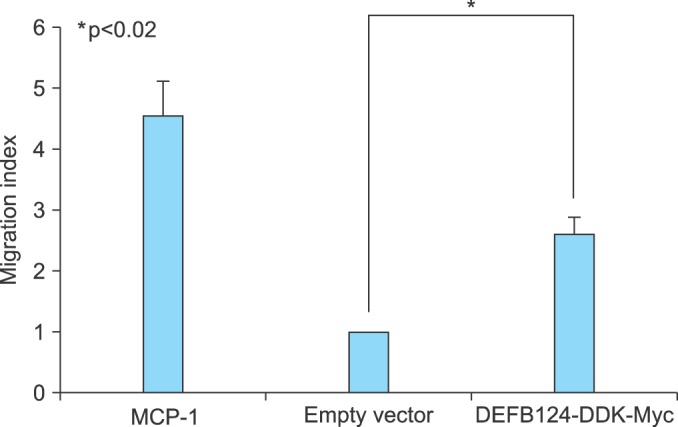
Beta-Defensin 124 (DEFB124)-mediated upregulation of cytokines and chemokines promote chemotactic response of THP-1 monocytes. DEFB124- or DEFB124-mediated cytokines and chemokines induce chemotaxis for THP-1 monocytes. THP-1 cells (1×106 cells) were added to the upper chamber, and the lower chamber contained supernatants from either the empty vector- or DEFB124-DDK-Myc-transfected RWPE-1 cells. The results are presented as a migration index denoting the fold increase of cell migration over the empty vector. Monocyte chemoattractant protein-1 (MCP-1) (100 ng/mL) was used as a positive control. Results are representative of three independent experiments. Asterisk represents statistical significance at p<0.02.
DISCUSSION
Human β-defensins are one of the key components of innate immunity, which is the first defense against infection. Besides their common function in the inhibition of bacterial growth, all β-defensins show marked differences in tissue expression, gene regulation, and bioactivities of the human innate immune system [12,13]. By use of bioinformatics approaches, the members of the β-defensins family were identified according to a conserved pattern of six cysteine residues. To date, more than 28 members have been assigned [11]. Our previous research on human β-defensins by use of Illumina HumanHT-12 microarray showed that various microbial compounds induce the various human β-defensins (data not published). Among the β-defensins, we found a significantly high level of DEFB124 expression in PGN-stimulated RWPE-1 cells (Fig. 1A). In the present study, we examined the intracellular signaling pathways and nuclear response in prostate epithelial cells that contribute to the gene induction of DEFB124 upon stimulation with PGN. Also, we examined the effects of DEFB124 on innate immunity in the prostate, such as monocyte chemotaxis.
Until now, the regulation of DEFB124 expression in prostate epithelial cells has not been reported. Therefore, we analyzed the regulation of expression of prostate epithelial DEFB124. We provide evidence that PGN induces the activation of the NF-κB transcription factor in RWPE-1 cells (Fig. 1B). NF-κB is an essential intracellular signal in both innate and adaptive immunity, which induces the activation of β-defensins [14,15]. Signal transduction through NF-κB is initiated through the binding of ligands to the cell membrane receptors, which leads to activation of the IκB kinase (IKK) complex and subsequent phosphorylation of NF-κB inhibitors (IκBα or IκBβ). Phosphorylation targets the inhibitor to ubiquitination and proteasomal degradation, thus activating NF-κB. Active NF-κB translocates into the nucleus where it binds to the promoter region of genes [16,17]. Our IκBα Western blotting data demonstrated that PGN induced NF-κB activation (Fig. 1B), and the requirement for PGN-induced DEFB124 expression was confirmed by NF-κB inhibitor experiment. Bay11-7082, a chemical inhibitor of NF-κB, is an inhibitor of IKK that almost completely silenced DEFB124 expression, as induced by PGN (Fig. 1C, D). These findings showed the necessity of NF-κB for DEFB124 production. Several other studies have demonstrated that NF-κB consensus sequences are identified in β-defensins in the proximal promoter region [18,19,20]. We provide evidence that the binding site for the transcription factor NF-κB is found in the proximal promoter of the DEFB124 gene (Fig. 1E). Consequently, we conclude that the proximal NF-κB site is required for the induction of the DEFB124 gene in response to PGN.
Cytokines are key regulators of inflammation and immunity, and modulation of their function has enormous potential for therapeutic benefit in the treatment of numerous diseases and autoimmune pathologies [21,22]. In addition, chemokines play a crucial role in coordinating adaptive immune responses [23,24]. Several studies have demonstrated that not only do β-defensins act as chemoattractants themselves, but each human β-defensin induces unique patterns of cytokine and chemokine induction. Although β-defensin-stimulated secretion of cytokines and chemokines is reported, the effectiveness of DEFB124 to elicit cytokine and chemokine responses has not been examined. Therefore, a comprehensive study is needed to understand the role of DEFB124 in stimulating cytokine and chemokine production in RWPE-1 cells.
In our investigation, we created DEFB124-expressing RWPE-1 cells (Fig. 2). Like other β-defensins tested so far, DEFB124 upregulation caused cytokine and chemokine induction (Fig. 3). Although quantitative real-time PCR analysis indicated an increased level of cytokines and chemokines in DEFB124-expressing RWPE-1 cells, ELISA analysis concluded that the results obtained from the quantitative real-time PCR analysis were not entirely reliable (Fig. 3). We observed that the mRNA expression and protein secretion of IL-6 and IL-12 were upregulated in DEFB124-expressing RWPE-1 cells (Fig. 3). IL-6 is structurally homologous to IL-12 [22] but its function varies from that of IL-12. IL-6 plays an important role in the stimulation of B lymphocytes for antibody production, and together with tumor necrosis factor-α, it may boost the proliferation and differentiation of B cells [25]. In addition, previous reports have shown that IL-12 may play an important role in inducing the development of autoimmunity [21,26]. These results suggest that DEFB124-expressing RWPE-1 cells secrete cytokines, such as IL-6 and IL-12, to activate and regulate the inflammatory and immune responses of both innate and adaptive immunity.
Our observations also demonstrated that DEFB124 induces or upregulates various other chemokines, such as CCL5, CCL22, and CXCL8 (Fig. 3). Chemokines are mainly produced by lymphocytes, monocytes, macrophages, and epithelial cells, but are especially produced by activated NK cells [24,27,28]. Chemokines regulate the migration of antigen-presenting cells, including dendritic cells, macrophages, and monocytes [23,29]. This work suggests that DEFB124-expressing RWPE-1 cells recruit immune cells to the site of infection by secreting chemokines and chemokines, such as IL-6, IL-12, CCL5, CCL22, and CXCL8.
Numerous studies have previously demonstrated that β-defensins can function as potent immune regulators, altering host gene expression, acting as chemokines or inducing chemokine production, promoting wound healing, and modulating the responses of immune cells of the adaptive immune response [8,9]. To complete our study, we investigated the chemotaxis action of DEFB124- and DEFB124-induced cytokines and chemokines. Our results clearly showed that DEFB124-induced cytokines and chemokines, as revealed by quantitative real-time PCR and ELISA analysis, correlated with the induction of chemotactic activity in DEFB124-expressing RWPE-1 cells (Fig. 4). We recently identified DEFB124 as being responsible for the chemotactic effect of DEFB124-induced cytokines and chemokines on monocyte THP-1 cells (Fig. 4). The chemotactic activities of DEFB124 indicate that they contribute to the recruitment of immune cells within sites of infection. These studies suggest that DEFB124 might function as a bridge between innate and adaptive immunity by recruiting inflammatory cells.
CONCLUSIONS
Our study presents the first report of the association of DEFB124 with innate immunity in the prostate. In this study, we observed that DEFB124 expression was markedly increased through NF-κB activation in combating infection, such as PGN stimulation. Also, our results demonstrated that increased DEFB124 induced the upregulation of cytokines and chemokines in RWPE-1 cells, and the subsequent chemotactic response of immune cells, which suggests that DEFB124 may affect the promotion of innate immunity in the prostate epithelium. Taken together, our results suggest DEFB124 as an essential immune regulator that is pivotal for promotion of innate immunity in the prostate.
ACKNOWLEDGMENTS
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (2008-313-E00243).
Footnotes
The authors have nothing to disclose.
References
- 1.Boman HG. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 2.Nizet V. Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr Issues Mol Biol. 2006;8:11–26. [PubMed] [Google Scholar]
- 3.Izadpanah A, Gallo RL. Antimicrobial peptides. J Am Acad Dermatol. 2005;52(3 Pt 1):381–390. doi: 10.1016/j.jaad.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen LT, Haney EF, Vogel HJ. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011;29:464–472. doi: 10.1016/j.tibtech.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Ganz T. The role of antimicrobial peptides in innate immunity. Integr Comp Biol. 2003;43:300–304. doi: 10.1093/icb/43.2.300. [DOI] [PubMed] [Google Scholar]
- 6.Ganz T. Defensins: antimicrobial peptides of vertebrates. C R Biol. 2004;327:539–549. doi: 10.1016/j.crvi.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Tang YQ, Yuan J, Miller CJ, Selsted ME. Isolation, characterization, cDNA cloning, and antimicrobial properties of two distinct subfamilies of alpha-defensins from rhesus macaque leukocytes. Infect Immun. 1999;67:6139–6144. doi: 10.1128/iai.67.11.6139-6144.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 10.Wei G, de Leeuw E, Pazgier M, Yuan W, Zou G, Wang J, et al. Through the looking glass, mechanistic insights from enantiomeric human defensins. J Biol Chem. 2009;284:29180–29192. doi: 10.1074/jbc.M109.018085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schutte BC, Mitros JP, Bartlett JA, Walters JD, Jia HP, Welsh MJ, et al. Discovery of five conserved beta -defensin gene clusters using a computational search strategy. Proc Natl Acad Sci U S A. 2002;99:2129–2133. doi: 10.1073/pnas.042692699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bajaj-Elliott M, Fedeli P, Smith GV, Domizio P, Maher L, Ali RS, et al. Modulation of host antimicrobial peptide (beta-defensins 1 and 2) expression during gastritis. Gut. 2002;51:356–361. doi: 10.1136/gut.51.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motzkus D, Schulz-Maronde S, Heitland A, Schulz A, Forssmann WG, Jubner M, et al. The novel beta-defensin DEFB123 prevents lipopolysaccharide-mediated effects in vitro and in vivo. FASEB J. 2006;20:1701–1702. doi: 10.1096/fj.05-4970fje. [DOI] [PubMed] [Google Scholar]
- 14.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Jung JR, Kim HJ, Lee SY, Chang IH, Lee TJ, et al. Expression of human β-defensin-2 in the prostate. BJU Int. 2011;107:144–149. doi: 10.1111/j.1464-410X.2010.09469.x. [DOI] [PubMed] [Google Scholar]
- 16.Andresen L, Jorgensen VL, Perner A, Hansen A, Eugen-Olsen J, Rask-Madsen J. Activation of nuclear factor kappaB in colonic mucosa from patients with collagenous and ulcerative colitis. Gut. 2005;54:503–509. doi: 10.1136/gut.2003.034165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wehkamp J, Harder J, Wehkamp K, Wehkamp-von Meissner B, Schlee M, Enders C, et al. NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect Immun. 2004;72:5750–5758. doi: 10.1128/IAI.72.10.5750-5758.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steubesand N, Kiehne K, Brunke G, Pahl R, Reiss K, Herzig KH, et al. The expression of the beta-defensins hBD-2 and hBD-3 is differentially regulated by NF-kappaB and MAPK/AP-1 pathways in an in vitro model of Candida esophagitis. BMC Immunol. 2009;10:36. doi: 10.1186/1471-2172-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mburu YK, Abe K, Ferris LK, Sarkar SN, Ferris RL. Human β-defensin 3 promotes NF-κB-mediated CCR7 expression and anti-apoptotic signals in squamous cell carcinoma of the head and neck. Carcinogenesis. 2011;32:168–174. doi: 10.1093/carcin/bgq236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watford WT, Moriguchi M, Morinobu A, O'Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14:361–368. doi: 10.1016/s1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 22.Jones LL, Vignali DA. Molecular interactions within the IL-6/IL-12 cytokine/receptor superfamily. Immunol Res. 2011;51:5–14. doi: 10.1007/s12026-011-8209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luster AD. The role of chemokines in linking innate and adaptive immunity. Curr Opin Immunol. 2002;14:129–135. doi: 10.1016/s0952-7915(01)00308-9. [DOI] [PubMed] [Google Scholar]
- 24.Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002;71:173–183. [PubMed] [Google Scholar]
- 25.Amerio P, Frezzolini A, Abeni D, Teofoli P, Girardelli CR, De Pita O, et al. Increased IL-18 in patients with systemic lupus erythematosus: relations with Th-1, Th-2, pro-inflammatory cytokines and disease activity. IL-18 is a marker of disease activity but does not correlate with pro-inflammatory cytokines. Clin Exp Rheumatol. 2002;20:535–538. [PubMed] [Google Scholar]
- 26.Maczynska I, Millo B, Ratajczak-Stefanska V, Maleszka R, Szych Z, Kurpisz M, et al. Proinflammatory cytokine (IL-1beta, IL-6, IL-12, IL-18 and TNF-alpha) levels in sera of patients with subacute cutaneous lupus erythematosus (SCLE) Immunol Lett. 2006;102:79–82. doi: 10.1016/j.imlet.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Berin MC, Dwinell MB, Eckmann L, Kagnoff MF. Production of MDC/CCL22 by human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1217–G1226. doi: 10.1152/ajpgi.2001.280.6.G1217. [DOI] [PubMed] [Google Scholar]
- 28.Murdoch C, Read RC, Zhang Q, Finn A. Choline-binding protein A of Streptococcus pneumoniae elicits chemokine production and expression of intercellular adhesion molecule 1 (CD54) by human alveolar epithelial cells. J Infect Dis. 2002;186:1253–1260. doi: 10.1086/344527. [DOI] [PubMed] [Google Scholar]
- 29.Kim CH, Broxmeyer HE. Chemokines: signal lamps for trafficking of T and B cells for development and effector function. J Leukoc Biol. 1999;65:6–15. doi: 10.1002/jlb.65.1.6. [DOI] [PubMed] [Google Scholar]