Abstract
Cirrhosis patients’ comorbidities are their other diseases than cirrhosis. Comorbidities are neither causes nor consequences of cirrhosis, but they can increase mortality and are therefore clinically important. They are also an important source of confounding in epidemiologic studies. Comorbidity scoring systems have been developed as tools to measure the cirrhosis patient’s total burden of comorbidity, and they are useful in the clinic and for epidemiologic studies. The recently developed CirCom score is the only comorbidity scoring system developed specifically for cirrhosis patients, and it may be preferred over the older, generic, and more complex Charlson comorbidity index. Studies of individual comorbid diseases can provide insight into the interactions between cirrhosis and other diseases and thus into the pathophysiology of cirrhosis. This article reviews the literature on comorbidity in cirrhosis.
Keywords: Liver cirrhosis, Comorbidity, Prognosis, Epidemiology
Core tip: Cirrhosis patients’ comorbidities are their other diseases than cirrhosis. They can increase mortality and are therefore clinically important. They are also an important source of confounding in epidemiologic studies. Comorbidity scoring systems have been developed as tools to measure the cirrhosis patient’s total burden of comorbidity, and they are useful in the clinic and for epidemiologic studies. Studies of individual comorbid diseases can provide insight into the interactions between cirrhosis and other diseases and thus into the pathophysiology of cirrhosis. This article reviews the literature on comorbidity in cirrhosis.
INTRODUCTION
Cirrhosis patients’ comorbidities are their other diseases than cirrhosis[1,2]. Comorbidities increase mortality and are therefore clinically relevant[3,4]. The presence of comorbidity may also be an important source of confounding and should be accounted for in epidemiologic studies of cirrhosis patients.
Comorbidities must be distinguished from complications such as ascites, variceal bleeding, and hepatic encephalopathy. Complications are at least to some extent a consequence of the portal hypertension and loss of liver function resulting from cirrhosis, whereas comorbidities are neither causes nor consequences of cirrhosis[1]. Sometimes the distinction is difficult: for example, is hepatocellular carcinoma a complication or comorbidity to cirrhosis? Cirrhosis develops in response to a repeated injury to the hepatocytes, and hepatocellular carcinoma in a cirrhosis patient likely develops in response to the same injury[5]. Therefore it is reasonable to interpret hepatocellular carcinoma as a complication to cirrhosis although it can also develop in patients without cirrhosis. There are many diseases whose causal relationship with cirrhosis is unclear, and the categorization of a disease as a complication or comorbidity may change as our understanding of cirrhosis pathophysiology evolves.
The aim of this article is to review the evidence regarding comorbidities’ impact on the mortality of cirrhosis patients. The cirrhosis patient’s total burden of comorbidity may be assessed by a comorbidity scoring system, and such a system may be helpful for clinical decision-making and for confounder control in epidemiologic studies. The prognostic impact of individual comorbidities, on the other hand, may point to areas where cirrhosis and comorbid diseases interact. Studies of individual comorbidities may therefore improve our understanding of the pathophysiology of cirrhosis. This article reviews studies of comorbidity scoring systems and studies of the impact of individual comorbidities on the clinical course of cirrhosis.
COMORBIDITY SCORING SYSTEMS
The purpose of a comorbidity scoring system is to express a patient’s total burden of comorbidity as a single number rather than a list of diagnoses: Comorbidity scores make it easier to communicate a patient’s comorbidity burden, and they also facilitate epidemiologic studies because inclusion or exclusion criteria can be based on a comorbidity score, analyses can be stratified according to the comorbidity score, and the comorbidity score may serve as a confounding factor that can be adjusted for in the statistical analysis.
A comorbidity scoring system should reflect the combined effects of all a patient’s comorbidities. This might be complex, but for purposes of mortality prediction it appears that there is no need to consider more than two diseases for each patient[3]. It is possible to develop comorbidity scores for other outcomes than mortality, e.g., surgical risk or variceal bleeding, but existing scoring systems have been developed to predict mortality. Two comorbidity scores have been validated as predictors of mortality among cirrhosis patients: The Charlson comorbidity index and the CirCom score[3,4]. The Charlson comorbidity index and a modified version thereof, the CCI-OLT, have also been shown to predict mortality among liver transplant recipients[6,7].
Charlson comorbidity index
The Charlson comorbidity index assigns a numeric score ranging from one to six to 17 diseases according to their effect on mortality (Table 1). The sum of a patient’s scores is a measure of the total burden of comorbidity[8]. In studies of cirrhosis patients, liver disease must be excluded from the Charlson index because liver diseases cannot be considered co-morbidities.
Table 1.
Comorbidity scoring systems for patients with cirrhosis
| Target population | Charlson comorbidity index | CirCom | CCI-OLT |
| Patients with any disease | Patients with cirrhosis | Orthotopic liver transplant recipients | |
| HIV/AIDS | 6 | ||
| Cancer (metastatic) | 6 | 31 | |
| Cancer (non-metastatic or hematologic) | 2 | 11 | |
| Liver disease (mild) | 1 | ||
| Liver disease (severe) | 3 | ||
| Diabetes (no complications) | 1 | 1 | |
| Diabetes (with complications) | 2 | 1 | |
| Kidney disease | 2 | 3 | 2 |
| Hemiplegia | 2 | ||
| Peptic ulcer | 1 | ||
| Connective tissue disease | 1 | 2 | |
| Chronic obstructive lung disease | 1 | 1 | 3 |
| Dementia | 1 | ||
| Epilepsy | 1 | ||
| Cerebrovascular disease | 1 | ||
| Peripheral vascular disease | 1 | 1 | |
| Congestive heart failure | 1 | 1 | |
| Acute myocardial infarction | 1 | 11 | 2 |
| Substance abuse other than alcoholism | 1 |
Add two points if the comorbid disease is active. The numbers indicate the comorbid diseases’ weight. HIV: Human immunodeficiency virus; AIDS: Acquired immunodeficiency syndrome; CCI-OLT: Charlson comorbidity index for orthotopic liver transplantation.
The Charlson index was developed to predict mortality among hospitalized patients, but it was not developed for cirrhosis patients or for patients with any other particular index disease[8]. There are other reasons why it is probably suboptimal for use among cirrhosis patients: First, it was developed based on only 559 patients[8], so rare but severe diseases may not have been included. Second, psychiatric diseases were not considered for inclusion, but eight percent of Danish cirrhosis patients have been diagnosed with a psychiatric disease other than substance abuse[3]. Third, it does not consider the duration between the occurrence of the comorbidity and the development of cirrhosis; but the impact of, e.g., a peptic ulcer or an acute myocardial infarction decreases over time[9,10], whereas the opposite is true for cancer and diabetes[8]. Fourth, the prognostic impact of many diseases has changed since the Charlson index was developed in 1984[11-13]. Despite these shortcomings, the Charlson index has been shown to be strongly associated with mortality among cirrhosis patients in Denmark and the United Kingdom[4,14]. Moreover, it was not only associated with the risk of death from any cause, it was also associated with the risk of death from cirrhosis[4].
CirCom score
Our group recently developed a cirrhosis-specific comorbidity scoring system using data from healthcare registries on 12976 Danish cirrhosis patients, most of whom had alcoholic cirrhosis[3]. We defined 34 comorbidities on the basis of hospital discharge diagnosis codes[3]. Fifty-five percent of patients had at least one of these comorbidities at the time of cirrhosis diagnosis. The final comorbidity scoring system-the CirCom score-included nine diseases (Table 1). The prevalence of any of these nine diseases was 24.2% at the time of cirrhosis diagnosis, with the highest prevalence for chronic obstructive lung disease (7.3%), cancer (6.7%), and heart failure (5.2%).
The CirCom score is based on nine diseases of which at most two count towards a patient’s CirCom score (Figure 1). Although simpler than the Charlson index, the CirCom score was slightly better at predicting mortality: In the full cohort of 12976 cirrhosis patients the C statistic for the CirCom score was 0.6% points (95%CI: 0.3%-0.8%) higher than the C statistic for the Charlson index. The Net Reclassification Index, a newer measure of predictive ability, was 3.6% (95%CI: 2.3%-5.0%) higher for the CirCom score. In the two validation cohorts of 419 patients with alcoholic cirrhosis and 4656 patients with chronic hepatitis C infection, the CirCom score remained superior, although not by a statistically significant amount[3].
Figure 1.
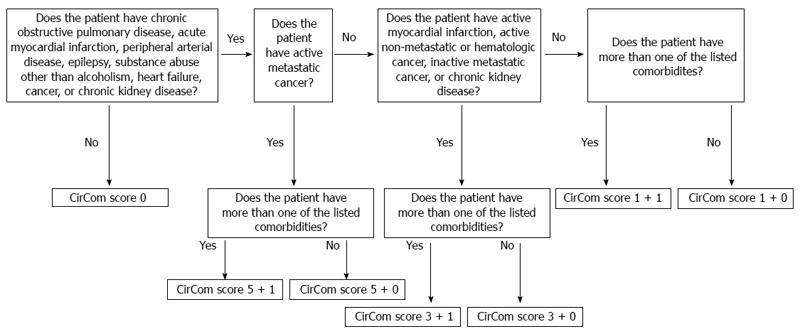
Algorithm for computing of CirCom scores[3]. Reprinted from reference 3, with permission from Elsevier.
CCI-OLT
The Charlson comorbidity index has been evaluated in a study of 221 Italian liver transplant recipients. The prevalence of comorbidity was 57%, and patients with a comorbidity score > 1 had higher risks of graft loss and death than patients with a score of 0 or 1[7]. Thus the Charlson index predicted both death and graft loss, but none of the individual comorbid diseases in the Charlson index was a statistically significant predictor of mortality, and only chronic obstructive lung disease was a statistically significant predictor of graft loss (HR = 4.71, 95%CI: 1.07-20.83).
The Charlson comorbidity index has been modified for analyses of kidney transplant recipients[15], and the same modified index with only nine comorbidities has been evaluated in two studies of orthotopic liver transplant recipients followed from transplantation. In the two studies, 30% and 40% of patients had one or more of these nine comorbidities[6,16]. The first study followed 169 patients for one month after transplantation. It showed that the prevalence of the nine comorbidities was similar for those who lived or died, hence comorbidity did not predict mortality[16]. The second study followed 624 patients for up to twelve years after transplantation and found that a simplified index with five comorbidities predicted survival. This comorbidity scoring system was named CCI-OLT (Table 1).
Which comorbidity scoring system should be preferred?
For the cirrhosis patient, the choice between the CirCom score and the Charlson comorbidity index is not obvious. The CirCom score was developed in a cohort dominated by patients with alcoholic cirrhosis, and it has not been validated using data from other countries than Denmark or data obtained in the clinic[17]. The Charlson index is more extensively validated[4,14], but has limitations, as described above. Based on the available evidence, clinicians and researchers may prefer the simpler, yet slightly better cirrhosis-specific CirCom score, but more comparative studies are necessary to determine which comorbidity scoring system is better.
For the liver transplant recipient, the CCI-OLT should be preferred because it assigns transplantation-specific weights to the comorbid diseases from the Charlson index. Liver transplant recipients are a highly selected group that excludes patients with severe comorbidities, and the post-transplant immunosuppression may affect the prognosis of the comorbidities. Therefore it makes good sense to have a comorbidity index specifically for liver transplant recipients. The greater detrimental effect of chronic obstructive lung disease than of cancer highlights the importance of having a transplant-specific comorbidity index[6,7].
INDIVIDUAL COMORBIDITIES
Studies of individual comorbidities’ effect on the clinical course of cirrhosis can provide insight into the pathophysiology of cirrhosis. Unfortunately, only few such studies have been conducted[18], and all have focused on the prognosis with respect to death. This section presents the available evidence.
Diabetes
Diabetes is the best studied comorbidity to cirrhosis, but studies have reached different conclusions. Among the 12976 Danish cirrhosis patients included in the CirCom study, diabetes without complications was unassociated with mortality whereas diabetes with complications did increase mortality (Table 2)[3]. A study from the Netherlands including 226 patients diagnosed with cirrhosis in 2001-2011 found that diabetes was unassociated with all-cause and liver-related mortality[19], and a smaller Mexican study found that the reduced survival for cirrhosis patients with diabetes was due to confounding by cirrhosis severity and renal impairment[20]. Earlier studies have been reviewed by Garcia-Compean et al[21] who concluded that diabetes mellitus does increase mortality in cirrhosis, and that the excess mortality in diabetes patients is due to hepatocellular failure, not to diabetes[22]. More detailed studies are needed to clarify the interactions between cirrhosis and diabetes.
Table 2.
Effects of comorbid diseases on the mortality of patients with liver cirrhosis in the CirCom cohort of 12976 Danish cirrhosis patients. Hazard ratios are adjusted for gender and age differences[3]
| Comorbidity | Hazard ratio (95%CI) |
| Diabetes with complications | 1.16 (1.10-1.22) |
| Diabetes without complications | 1.03 (0.98-1.08) |
| Acute myocardial infarction | 1.59 (1.40-1.79) |
| Peripheral arterial disease | 1.29 (1.18-1.40) |
| Heart failure | 1.27 (1.20-1.34) |
| Valvular heart disease | 1.17 (1.04-1.30) |
| Cardiomyopathy | 1.16 (1.03-1.30) |
| Arterial hypertension with complications | 1.16 (0.98-1.37) |
| Cardiac arrhythmia | 1.14 (1.08-1.20) |
| Mesenteric vascular disease | 1.13 (0.85-1.49) |
| Ischemic heart disease without myocardial infarction | 1.13 (1.06-1.21) |
| Mesenteric vascular disease | 1.13 (0.85-1.49) |
| Cerebrovascular disease | 1.09 (1.02-1.16) |
| Venous thromboembolism | 1.20 (1.08-1.33) |
| Chronic obstructive lung disease | 1.24 (1.18-1.30) |
| Peptic ulcer with bleeding or perforation | 1.17 (1.11-1.23) |
| Chronic pancreatitis | 1.09 (1.03-1.16) |
| Chronic inflammatory bowel disease | 1.08 (0.95-1.22) |
| Peptic ulcer without complications | 1.03 (0.97-1.10) |
| Acute pancreatitis | 1.01 (0.92-1.12) |
| Chronic kidney disease | 1.59 (1.37-1.83) |
| Psoriasis | 1.05 (0.92-1.21) |
| Connective tissue disease | 0.99 (0.91-1.08) |
| Epilepsy | 1.22 (1.11-1.35) |
| Schizophrenia | 1.15 (1.00-1.32) |
| Bipolar disorder | 0.98 (0.76-1.26) |
| Depression | 1.00 (0.91-1.09) |
| Dementia | 1.04 (0.95-1.15) |
| Substance abuse other than alcoholism | 1.25 (1.14-1.37) |
| Metastatic cancer | 1.72 (1.53-1.94) |
| Non-metastatic solid cancer | 1.35 (1.27-1.43) |
| Hematologic cancer | 1.30 (1.10-1.53) |
| Human immunodeficiency virus infection | 0.79 (0.49-1.26) |
| Osteoporosis | 1.03 (0.93-1.15) |
| Obesity | 1.02 (0.92-1.12) |
Cardiovascular disease
The hyperdynamic circulation in cirrhosis provides some protection against atherosclerosis, ischemic events, and overt heart failure[23,24], but acute myocardial infarction, peripheral arterial disease, and heart failure were all strong predictors of mortality in the CirCom study. Other cardiovascular diseases were weaker predictors (Table 2)[3]. Coronary disease, defined by acute myocardial infarction or coronary disease on angiography, was also a predictor of mortality among liver transplant recipients[6]. The reasons for these associations are unclear.
Venous thromboembolism
In the CirCom cohort, venous thromboembolism in the form of deep venous thrombosis or pulmonary embolism increased mortality 1.20-fold (95%CI: 1.08-1.33) after adjustment for gender and age (Table 2)[3]. By contrast, both manifestations were unassociated with mortality in a study of United States veterans with cirrhosis after adjustment for gender, age, race, Charlson comorbidity index, insurance type, and presence of cirrhosis complications (HR = 1.01, 95% CI: 0.83-1.23)[25]. This could indicate that the association in the Danish cohort is due to uncontrolled confounding by cirrhosis complications, with greater risk of thromboembolism for cirrhosis patients with complications. Coagulation in liver disease is complex[26], and it remains unclear whether venous thromboembolism is a marker of severe liver function loss.
Lung disease
In the CirCom cohort, chronic obstructive lung disease increased cirrhosis patients’ mortality (Table 2)[3], and it was also the strongest predictor of mortality in the studies of liver transplant recipients[6,7]. Chronic obstructive lung disease is a relative contraindication for the non-selective beta blockers that reduce the risk of variceal bleeding[27], but endoscopic ligation is a satisfactory alternative treatment[28]. The mechanisms behind the adverse effect of chronic obstructive lung disease are therefore unclear. Smoking has also been identified as an adverse prognostic factor in patients with cirrhosis[29], but this association is unexplained, too[30].
Gastrointestinal disease
Alcohol is the dominant cirrhosis etiology in Denmark, yet the prevalence of chronic pancreatitis in the CirCom cohort was only 4.5%[3], a prevalence similar to that seen among alcohol abusers with or without cirrhosis[31]. This observation is consistent with the hypothesis that cirrhosis and chronic pancreatitis develop along different pathogenetic lines[31,32]. The same hypothesis might explain why chronic pancreatitis increased mortality only 1.09-fold (95%CI: 1.03-1.16) (Table 2)[3], despite the generally increased cancer risk and mortality in patients with chronic pancreatitis[33]. Another possible explanation to this unexpectedly weak association is that patients with chronic pancreatitis are immediately screened for cirrhosis and therefore have their cirrhosis diagnosed in an earlier stage than other cirrhosis patients. No other studies have examined chronic pancreatitis in cirrhosis.
Acute pancreatitis, chronic inflammatory bowel disease, and uncomplicated peptic ulcer had no clinically or statistically significant effect on cirrhosis patients’ mortality in the CirCom cohort (Table 2), but patients with a history of complicated peptic ulcer had a 1.17-fold (95%CI: 1.11-1.23) increased mortality (Table 2). The reasons are unclear, but cirrhosis patients’ high risk of rebleeding from peptic ulcer-26% within five years after first bleeding-is likely to have contributed[34].
Chronic kidney disease
Chronic kidney disease increases morbidity and mortality among the general population[35] and among liver transplant recipients[6]. It was also a strong predictor of mortality in the CirCom cohort (Table 2)[3]. The circulatory dysfunction that ultimately leads to the hepatorenal syndrome may worsen an existing kidney dysfunction[36], and the International Ascites Club has been involved in defining and studying the complex interplay between kidney disease and cirrhosis[37,38].
Connective tissue disease
Connective tissue disease is included in the Charlson comorbidity index and also a predictor of mortality among liver transplant recipients (HR = 2.32, 95%CI: 1.02-5.25)[6]. It was, however, unassociated with mortality in the CirCom cohort (Table 2)[3]. One possible explanation is that better treatment methods introduced after the Charlson index’s development in 1984 have improved the prognosis of connective tissue diseases, but at least in rheumatoid arthritis there seems to have been no improvements in overall survival[39]. This explanation is therefore questionable. An alternative explanation is that connective tissue diseases have a smaller impact on the mortality of cirrhosis patients than on other patients, possibly because cirrhosis patients do not survive long enough to suffer the long-term consequences of these diseases. Further studies are needed to substantiate this speculation.
Epilepsy
Idiopathic epilepsy-i.e., epilepsy not due to brain tumor, vascular disease, alcoholism, or metabolic disease-has been found to increase mortality 1.6-fold in the general population[40]. Even so, we had not expected epilepsy to increase mortality as much as it did in the CirCom cohort, the hazard ratio being 1.22 (95%CI: 1.11-1.35), on par with chronic obstructive lung disease (Table 2)[3]. The reasons for this strong association are unclear. It is possible that hepatic encephalopathy may manifest as status epilepticus[41], or that some patients given a diagnosis of non-convulsive epilepsy did in fact have hepatic encephalopathy. In both instances a diagnosis of epilepsy would be a marker of cirrhosis with hepatic encephalopathy, and this complication has a very poor prognosis[42]. St Germaine-Smith et al[43] previously constructed an epilepsy-specific comorbidity index. It included cirrhosis without complications in the ‘‘mild liver disease’’ category which was unassociated with mortality, whereas cirrhosis with complications was in the ‘‘severe liver disease’’ category which was associated with a three-fold increase in mortality. These findings suggest that cirrhosis and epilepsy do not always interact to cause a poor prognosis, supporting the hypothesis that epilepsy is a marker of severe cirrhosis, not a cause. It is also possible that epilepsy promotes the development of hepatic encephalopathy or vice versa; that treatments for epilepsy are detrimental to cirrhosis patients; or that the explanation lies in alcohol which is a cause of status epilepticus[44] and also an adverse prognostic factor in cirrhosis. Further research is clearly needed.
Psychiatric disease
In developing the CirCom score we had expected psychiatric disease to be a strong predictor of mortality in cirrhosis patients due to its association with substance abuse and suicide risk[45]. Schizophrenia was indeed an adverse prognostic factor, whereas bipolar disorder and depression were unassociated with mortality (Table 2). No other studies have examined the prognostic impact of psychiatric diseases in cirrhosis.
Substance abuse
Alcohol abuse is highly prevalent among cirrhosis patients in the Western world, but since it is a cause of liver disease it should not be considered a comorbid condition. Substance abuse other than alcoholism increased mortality in the CirCom cohort (Table 2)[3], possibly because it is a marker of adverse lifestyle factors, malnutrition, and low socioeconomic status[46].
Non-hepatic cancer
Cirrhosis patients have an increased risk of non-hepatic cancer[47,48]. The mechanisms are unclear, but lifestyle factors associated with both cirrhosis and cancer development-primarily alcohol consumption and tobacco-are clearly important. Unsurprisingly, non-hepatic cancer increased the mortality of the CirCom cohort (Table 2)[3]. Some cancer forms may aggravate ascites formation and portal hypertension, e.g., by causing portal vein thrombosis, and patients with advanced cirrhosis may not tolerate chemotherapy[49]. These two mechanisms indicate that cirrhosis and non-hepatic cancer may worsen each other’s prognosis. Reuken et al[50] have previously reported that cancer increases mortality among cirrhosis patients with urinary tract infections, and Gundling et al[51] have shown that, among cirrhosis patients in general, metastatic cancer was a stronger predictor of mortality than was non-metastatic cancer.
Miscellaneous comorbid diseases
In the CirCom cohort, human immunodeficiency virus (HIV) infection did not increase mortality (Table 2), whereas in the Charlson index HIV infection has the same weight as metastatic cancer (Table 1). This discrepancy is likely the result of the considerable progress made in the clinical management of HIV infection[52]. Finally, in the CirCom cohort osteoporosis and obesity did not affect mortality (Table 2).
CONCLUSION
Comorbidity affects the prognosis of cirrhosis patients. Measures of a patient’s total burden of comorbidity are important for epidemiologic studies and for clinical use. The CirCom score may be the preferred comorbidity scoring system because it is simpler yet slightly better than the Charlson comorbidity index, but more comparative studies are needed. Studies aiming to update the CirCom score to other cirrhosis populations will also improve its clinical value and credibility[53].
The available evidence of interactions between cirrhosis and individual comorbid diseases is sparse. A better understanding of such interactions will improve our understanding of cirrhosis pathophysiology, and clinical epidemiologic studies may help by answering questions like these: (1) does the comorbid disease increase the risk of developing cirrhosis complications? That would provide evidence that the comorbid disease affects portal pressure or liver function loss, or that it reduces the efficacy of cirrhosis treatments; (2) is there prognostic synergy between cirrhosis and the comorbid disease? That would provide evidence that the comorbid disease is more detrimental to cirrhosis patients than to others due to a pathophysiological interaction with cirrhosis; (3) is cirrhosis a risk factor for developing the comorbid disease? That would providence evidence that cirrhosis may affect the pathogenesis of the comorbid disease; or (4) are cirrhosis patients with decompensated as opposed to compensated cirrhosis at greater risk of developing the comorbid disease? That would provide evidence that portal hypertension and/or loss of liver function facilitates the development of the comorbid disease.
A better understanding of the interactions between cirrhosis and individual comorbid diseases is the first step towards clinical advances, e.g., the possibility to tailor cirrhosis treatments to specific comorbidity patterns. Currently, our understanding of the impact of comorbidities in cirrhosis is in its infancy.
Footnotes
Supported by A grant from the Danish Council for Independent Research under the Danish Agency for Science, Technology and Innovation No. 10-081838/FSS
P- Reviewers: Fialla AD, Martinez-Esparza M, Savopoulos CG, Yang ZX S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
References
- 1.Ording AG, Sørensen HT. Concepts of comorbidities, multiple morbidities, complications, and their clinical epidemiologic analogs. Clin Epidemiol. 2013;5:199–203. doi: 10.2147/CLEP.S45305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feinstein AR. The pre-therapeutic classification of co-morbidity in chronic disease. J Chronic Dis. 1970;23:455–468. doi: 10.1016/0021-9681(70)90054-8. [DOI] [PubMed] [Google Scholar]
- 3.Jepsen P, Vilstrup H, Lash TL. Development and validation of a comorbidity scoring system for patients with cirrhosis. Gastroenterology. 2014;146:147–156; quiz e15-16. doi: 10.1053/j.gastro.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Jepsen P, Vilstrup H, Andersen PK, Lash TL, Sørensen HT. Comorbidity and survival of Danish cirrhosis patients: a nationwide population-based cohort study. Hepatology. 2008;48:214–220. doi: 10.1002/hep.22341. [DOI] [PubMed] [Google Scholar]
- 5.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 6.Volk ML, Hernandez JC, Lok AS, Marrero JA. Modified Charlson comorbidity index for predicting survival after liver transplantation. Liver Transpl. 2007;13:1515–1520. doi: 10.1002/lt.21172. [DOI] [PubMed] [Google Scholar]
- 7.Grosso G, di Francesco F, Vizzini G, Mistretta A, Pagano D, Echeverri GJ, Spada M, Basile F, Gridelli B, Gruttadauria S. The Charlson comorbidity index as a predictor of outcomes in liver transplantation: single-center experience. Transplant Proc. 2012;44:1298–1302. doi: 10.1016/j.transproceed.2012.01.131. [DOI] [PubMed] [Google Scholar]
- 8.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 9.Koek HL, Soedamah-Muthu SS, Kardaun JW, Gevers E, de Bruin A, Reitsma JB, Bots ML, Grobbee DE. Short- and long-term mortality after acute myocardial infarction: comparison of patients with and without diabetes mellitus. Eur J Epidemiol. 2007;22:883–888. doi: 10.1007/s10654-007-9191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lassen A, Hallas J, Schaffalitzky de Muckadell OB. Complicated and uncomplicated peptic ulcers in a Danish county 1993-2002: a population-based cohort study. Am J Gastroenterol. 2006;101:945–953. doi: 10.1111/j.1572-0241.2006.00518.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang YR, Richter JE, Dempsey DT. Trends and outcomes of hospitalizations for peptic ulcer disease in the United States, 1993 to 2006. Ann Surg. 2010;251:51–58. doi: 10.1097/SLA.0b013e3181b975b8. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt M, Jacobsen JB, Lash TL, Bøtker HE, Sørensen HT. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. BMJ. 2012;344:e356. doi: 10.1136/bmj.e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harjutsalo V, Forsblom C, Groop PH. Time trends in mortality in patients with type 1 diabetes: nationwide population based cohort study. BMJ. 2011;343:d5364. doi: 10.1136/bmj.d5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming KM, Aithal GP, Card TR, West J. All-cause mortality in people with cirrhosis compared with the general population: a population-based cohort study. Liver Int. 2012;32:79–84. doi: 10.1111/j.1478-3231.2011.02517.x. [DOI] [PubMed] [Google Scholar]
- 15.Jassal SV, Schaubel DE, Fenton SS. Baseline comorbidity in kidney transplant recipients: a comparison of comorbidity indices. Am J Kidney Dis. 2005;46:136–142. doi: 10.1053/j.ajkd.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Wasilewicz M, Raszeja-Wyszomirska J, Wunsch E, Wójcicki M, Milkiewicz P. Modified Charlson Comorbidity Index in predicting early mortality after liver transplantation. Transplant Proc. 2009;41:3117–3118. doi: 10.1016/j.transproceed.2009.07.097. [DOI] [PubMed] [Google Scholar]
- 17.Younossi ZM, Henry L, Stepanova M. A new comorbidity model for predicting mortality in patients with cirrhosis: does it work? Gastroenterology. 2014;146:19–24. doi: 10.1053/j.gastro.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 18.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Wlazlo N, van Greevenbroek MM, Curvers J, Schoon EJ, Friederich P, Twisk JW, Bravenboer B, Stehouwer CD. Diabetes mellitus at the time of diagnosis of cirrhosis is associated with higher incidence of spontaneous bacterial peritonitis, but not with increased mortality. Clin Sci (Lond) 2013;125:341–348. doi: 10.1042/CS20120596. [DOI] [PubMed] [Google Scholar]
- 20.Quintana JO, García-Compean D, González JA, Pérez JZ, González FJ, Espinosa LE, Hernández PL, Cabello ER, Villarreal ER, Rendón RF, et al. The impact of diabetes mellitus in mortality of patients with compensated liver cirrhosis-a prospective study. Ann Hepatol. 2011;10:56–62. [PubMed] [Google Scholar]
- 21.Garcia-Compean D, Jaquez-Quintana JO, Gonzalez-Gonzalez JA, Maldonado-Garza H. Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol. 2009;15:280–288. doi: 10.3748/wjg.15.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bianchi G, Marchesini G, Zoli M, Bugianesi E, Fabbri A, Pisi E. Prognostic significance of diabetes in patients with cirrhosis. Hepatology. 1994;20:119–125. doi: 10.1016/0270-9139(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 23.Lee SS, Liu H. Cardiovascular determinants of survival in cirrhosis. Gut. 2007;56:746–748. doi: 10.1136/gut.2006.112169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berzigotti A, Bonfiglioli A, Muscari A, Bianchi G, Libassi S, Bernardi M, Zoli M. Reduced prevalence of ischemic events and abnormal supraortic flow patterns in patients with liver cirrhosis. Liver Int. 2005;25:331–336. doi: 10.1111/j.1478-3231.2005.01002.x. [DOI] [PubMed] [Google Scholar]
- 25.Ali M, Ananthakrishnan AN, McGinley EL, Saeian K. Deep vein thrombosis and pulmonary embolism in hospitalized patients with cirrhosis: a nationwide analysis. Dig Dis Sci. 2011;56:2152–2159. doi: 10.1007/s10620-011-1582-5. [DOI] [PubMed] [Google Scholar]
- 26.Northup PG, Caldwell SH. Coagulation in liver disease: a guide for the clinician. Clin Gastroenterol Hepatol. 2013;11:1064–1074. doi: 10.1016/j.cgh.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 27.Boyer TD. The patient who cannot receive beta-blockers. In: Groszmann RJ, Bosch J, editors. Portal hypertension in the 21st century. 1st ed. Netherlands: Springer; 2004. pp. 301–307. [Google Scholar]
- 28.Gluud LL, Krag A. Banding ligation versus beta-blockers for primary prevention in oesophageal varices in adults. Cochrane Database Syst Rev. 2012;8:CD004544. doi: 10.1002/14651858.CD004544.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pessione F, Ramond MJ, Peters L, Pham BN, Batel P, Rueff B, Valla DC. Five-year survival predictive factors in patients with excessive alcohol intake and cirrhosis. Effect of alcoholic hepatitis, smoking and abstinence. Liver Int. 2003;23:45–53. doi: 10.1034/j.1600-0676.2003.01804.x. [DOI] [PubMed] [Google Scholar]
- 30.Altamirano J, Bataller R. Cigarette smoking and chronic liver diseases. Gut. 2010;59:1159–1162. doi: 10.1136/gut.2008.162453. [DOI] [PubMed] [Google Scholar]
- 31.Aparisi L, Sabater L, Del-Olmo J, Sastre J, Serra MA, Campello R, Bautista D, Wassel A, Rodrigo JM. Does an association exist between chronic pancreatitis and liver cirrhosis in alcoholic subjects? World J Gastroenterol. 2008;14:6171–6179. doi: 10.3748/wjg.14.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura Y, Kobayashi Y, Ishikawa A, Maruyama K, Higuchi S. Severe chronic pancreatitis and severe liver cirrhosis have different frequencies and are independent risk factors in male Japanese alcoholics. J Gastroenterol. 2004;39:879–887. doi: 10.1007/s00535-004-1405-y. [DOI] [PubMed] [Google Scholar]
- 33.Nøjgaard C, Bendtsen F, Becker U, Andersen JR, Holst C, Matzen P. Danish patients with chronic pancreatitis have a four-fold higher mortality rate than the Danish population. Clin Gastroenterol Hepatol. 2010;8:384–390. doi: 10.1016/j.cgh.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 34.Hsu YC, Lin JT, Chen TT, Wu MS, Wu CY. Long-term risk of recurrent peptic ulcer bleeding in patients with liver cirrhosis: a 10-year nationwide cohort study. Hepatology. 2012;56:698–705. doi: 10.1002/hep.25684. [DOI] [PubMed] [Google Scholar]
- 35.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 36.Hartleb M, Gutkowski K. Kidneys in chronic liver diseases. World J Gastroenterol. 2012;18:3035–3049. doi: 10.3748/wjg.v18.i24.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong F, Nadim MK, Kellum JA, Salerno F, Bellomo R, Gerbes A, Angeli P, Moreau R, Davenport A, Jalan R, et al. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011;60:702–709. doi: 10.1136/gut.2010.236133. [DOI] [PubMed] [Google Scholar]
- 38.Angeli P, Sanyal A, Moller S, Alessandria C, Gadano A, Kim R, Sarin SK, Bernardi M. Current limits and future challenges in the management of renal dysfunction in patients with cirrhosis: report from the International Club of Ascites. Liver Int. 2013;33:16–23. doi: 10.1111/j.1478-3231.2012.02807.x. [DOI] [PubMed] [Google Scholar]
- 39.Gabriel SE, Michaud K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther. 2009;11:229. doi: 10.1186/ar2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cockerell OC, Johnson AL, Sander JW, Hart YM, Goodridge DM, Shorvon SD. Mortality from epilepsy: results from a prospective population-based study. Lancet. 1994;344:918–921. doi: 10.1016/s0140-6736(94)92270-5. [DOI] [PubMed] [Google Scholar]
- 41.Delanty N, French JA, Labar DR, Pedley TA, Rowan AJ. Status epilepticus arising de novo in hospitalized patients: an analysis of 41 patients. Seizure. 2001;10:116–119. doi: 10.1053/seiz.2000.0482. [DOI] [PubMed] [Google Scholar]
- 42.Jepsen P, Ott P, Andersen PK, Sørensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010;51:1675–1682. doi: 10.1002/hep.23500. [DOI] [PubMed] [Google Scholar]
- 43.St Germaine-Smith C, Liu M, Quan H, Wiebe S, Jette N. Development of an epilepsy-specific risk adjustment comorbidity index. Epilepsia. 2011;52:2161–2167. doi: 10.1111/j.1528-1167.2011.03292.x. [DOI] [PubMed] [Google Scholar]
- 44.Pilke A, Partinen M, Kovanen J. Status epilepticus and alcohol abuse: an analysis of 82 status epilepticus admissions. Acta Neurol Scand. 1984;70:443–450. doi: 10.1111/j.1600-0404.1984.tb00850.x. [DOI] [PubMed] [Google Scholar]
- 45.Hoang U, Stewart R, Goldacre MJ. Mortality after hospital discharge for people with schizophrenia or bipolar disorder: retrospective study of linked English hospital episode statistics, 1999-2006. BMJ. 2011;343:d5422. doi: 10.1136/bmj.d5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jepsen P, Vilstrup H, Andersen PK, Sørensen HT. Socioeconomic status and survival of cirrhosis patients: a Danish nationwide cohort study. BMC Gastroenterol. 2009;9:35. doi: 10.1186/1471-230X-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalaitzakis E, Gunnarsdottir SA, Josefsson A, Björnsson E. Increased risk for malignant neoplasms among patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:168–174. doi: 10.1016/j.cgh.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 48.Sørensen HT, Friis S, Olsen JH, Thulstrup AM, Mellemkjær L, Linet M, Trichopoulos D, Vilstrup H, Olsen J. Risk of liver and other types of cancer in patients with cirrhosis: a nationwide cohort study in Denmark. Hepatology. 1998;28:921–925. doi: 10.1002/hep.510280404. [DOI] [PubMed] [Google Scholar]
- 49.Cabibbo G, Palmeri L, Palmeri S, Craxì A. Should cirrhosis change our attitude towards treating non-hepatic cancer? Liver Int. 2012;32:21–27. doi: 10.1111/j.1478-3231.2011.02629.x. [DOI] [PubMed] [Google Scholar]
- 50.Reuken PA, Stallmach A, Bruns T. Mortality after urinary tract infections in patients with advanced cirrhosis - Relevance of acute kidney injury and comorbidities. Liver Int. 2013;33:220–230. doi: 10.1111/liv.12029. [DOI] [PubMed] [Google Scholar]
- 51.Gundling F, Seidl H, Schmidtler F, Löffler N, Strassen I, Wolf P, Pehl C, Schmidt T, Schepp W. Nonhepatic cancer in liver cirrhosis: a retrospective study of prevalence, complication rate after specific oncological treatment, follow-up and prognostic predictors of outcome in 354 patients with cirrhosis. Anticancer Res. 2011;31:2931–2938. [PubMed] [Google Scholar]
- 52.Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P, d’Arminio Monforte A, Yust I, Bruun JN, Phillips AN, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet. 1998;352:1725–1730. doi: 10.1016/s0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- 53.Moons KG, Altman DG, Vergouwe Y, Royston P. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ. 2009;338:b606. doi: 10.1136/bmj.b606. [DOI] [PubMed] [Google Scholar]


